Abstract
Symptomatic giant extracranial internal carotid artery (ICA) aneurysm is a rare and surgically intractable disease. Several authors have described successful treatments for extracranial ICA aneurysm. None, however, have described a perioperative evaluation of cerebral perfusion or a postoperative complication of cerebral hyperperfusion syndrome (CHS). We present a rare case of CHS after endovascular covered stent grafting for a giant extracranial ICA aneurysm. The CHS was successfully managed on the basis of hemodynamic monitoring. CHS can appear after endovascular reconstruction of an extracranial ICA aneurysm, and perioperative repeated evaluation of cerebral perfusion allows safe and effective management of CHS.
Cerebral hyperperfusion syndrome (CHS) is reportedly caused by rapidly increased cerebral blood flow (CBF) in a chronically hypoperfused area.1, 2 Various revascularization procedures are known to induce CHS, but CHS after the surgical obliteration of an extracranial internal carotid artery (ICA) aneurysm has not been previously described.1, 2 Extracranial ICA aneurysms are rare and tend to be manifested with pulsatile cervical masses, cranial nerve palsy, or thromboembolic stroke.3 Recent reports have advocated endovascular covered stent grafting as a safe and effective alternative to direct surgery.4, 5 We report a rare case of CHS after endovascular covered stent grafting for a giant extracranial ICA aneurysm and discuss the perioperative management of CHS. The patient gave consent to publish the data.
Case report
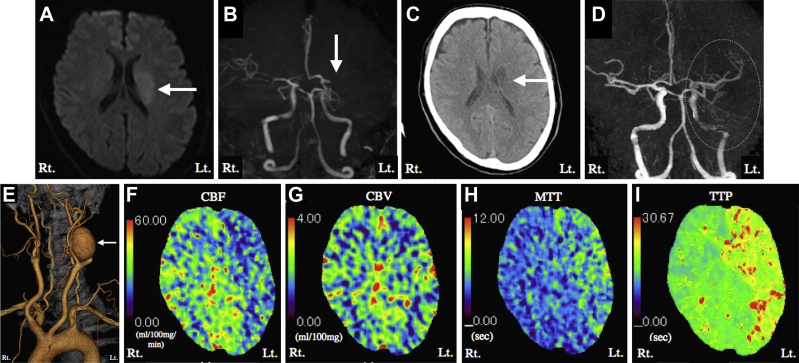
A 45-year-old man with no previous medical history presented with an acute onset of right hemiparesis and dysarthria. Magnetic resonance diffusion-weighted imaging and magnetic resonance angiography revealed a hyperintense lesion in the left corona radiata and occlusion of the left middle cerebral artery (MCA), respectively (Fig 1, A and B). The patient's initial National Institutes of Health Stroke Scale score was 8. Intravenous systemic thrombolysis was administered 90 minutes after onset, and the patient's National Institutes of Health Stroke Scale score improved from 8 to 2. One day later, computed tomography (CT) images showed an infarction in the left corona radiata (Fig 1, C). CT angiography revealed a left giant extracranial ICA aneurysm (5.5 cm in diameter) with recanalization but poor visualization of the left MCA and ICA, probably linked to stagnant blood flow in the aneurysm (Fig 1, D and E). CT perfusion imaging 3 weeks after stroke onset to minimize the effect of reperfusion of the occluded MCA demonstrated decreased ipsilateral CBF, cerebral blood volume (CBV) in the normal range, delayed mean transit time, and delayed time to peak (Fig 1, F-I). We surmised that the regional hypoperfusion resulted from stagnant blood flow within the aneurysm and that the MCA occlusion resulted from a thromboembolism from the aneurysm.
Fig 1.
A, Axial magnetic resonance diffusion-weighted imaging on admission showed a slightly hyperintense lesion in the left corona radiata (arrow). B, Magnetic resonance angiography on admission revealed occlusion of a left M1 segment of the middle cerebral artery (MCA; arrow). C, Axial computed tomography (CT) scan on the day after intravenous thrombolysis showed no hemorrhagic change or new ischemic lesion other than the infarction of the left corona radiata (arrow). D, Cerebral CT angiography revealed recanalized but poorly visualized left MCA and internal carotid artery (ICA; dotted circle) on the day after intravenous thrombolysis. E, Cervical CT angiography revealed a left giant extracranial ICA aneurysm (arrow). F-I, CT perfusion imaging 3 weeks after stroke onset demonstrated decreased ipsilateral cerebral blood flow (CBF;F), cerebral blood volume (CBV) in the normal range (G), delayed mean transit time (MTT;H), and delayed time to peak (TTP;I).
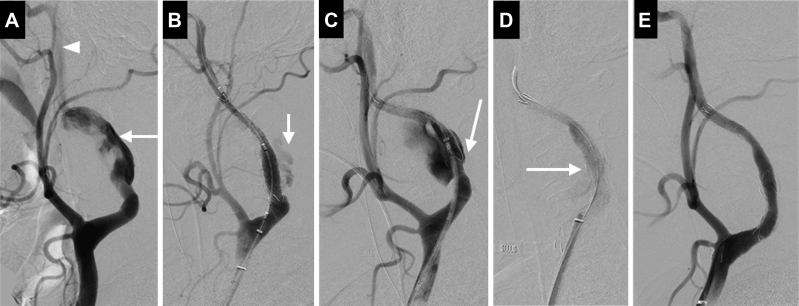
The patient received 100 mg of aspirin and 75 mg of clopidogrel daily for 1 week before the intervention. We were concerned, however, that dramatic improvement of the anterograde ICA flow after aneurysm removal would heighten the risk of postreperfusion CHS. We therefore performed the endovascular surgery under general anesthesia for better control of the patient's hemodynamic and mental status. The tissue oxygen index (TOI) was continuously measured with a NIRO-200NX spectrophotometer (Hamamatsu Photonics KK, Tokyo, Japan). Preoperatively, the ipsilateral TOI and contralateral TOI were 71% and 85%, respectively. Systemic anticoagulation with 5000 units of heparin was initiated. No embolic protection devices were used during the procedure as no significant atherosclerotic changes in the left ICA or thrombus formation within the aneurysm appeared on CT angiography. After right femoral puncture, a 9F ultralong sheath was placed at the origin of the left common carotid artery, and a 0.035-inch Radifocus guidewire (Terumo, Tokyo, Japan) was gently advanced to the distal ICA. An 8- × 50-mm Niti-S combi covered stent (Taewoong Medical, Seoul, Korea) was deployed to cover the orifice of the aneurysm (Fig 2, A and B). The covered stent appeared to be shortened because of ICA tortuosity immediately after deployment with major endoleakage (Fig 2, C). The second covered stent, a 10- × 50-mm Niti-S combi, was deployed to a position overlapping the proximal end of the first covered stent to cover the remnant orifice, but minor endoleakage persisted between the first and second covered stents despite repeated balloon angioplasty (Fig 2, D). The third covered stent, an 8- × 50-mm Niti-S combi, was deployed to cover the point of endoleakage. Subsequent angiography showed complete occlusion of the aneurysm and restoration of the anterograde ICA flow (Fig 2, E). The ipsilateral TOI and contralateral TOI rose from 71% to 76% and fell from 85% to 73%, respectively, immediately after the third covered stent implantation. We decided to keep the patient sedated until a reduction of CHS risk could be confirmed by CT perfusion as any occurrence of CHS could reap devastating consequences, such as intracranial hemorrhage.
Fig 2.
Left common carotid angiography during endovascular surgery. A, Left common carotid angiography demonstrated that the aneurysm (arrow) arose from a tortuous left internal carotid artery (ICA), whose distal portion (arrowhead) was poorly visualized because of stagnant flow in the aneurysm. B, After deployment of the first covered stent (Niti-S combi stent 8 × 50 mm). Minor endoleakage (arrow) was observed. C, The first covered stent showed shortening immediately after placement with major endoleakage (arrow) in the proximal end of the aneurysm. D, After deployment of the second covered stent (Niti-S combi stent 10 × 50 mm). Some endoleakage (arrow) between the first and second covered stents was observed. E, After deployment of the third covered stent (Niti-S combi stent 8 × 50 mm). Subsequent angiography showed complete occlusion of the aneurysm and restoration of the anterograde ICA flow.
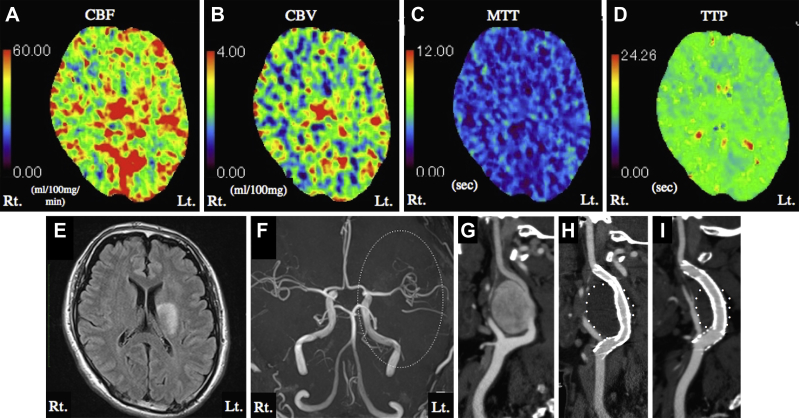
Postoperatively, the patient was mechanically ventilated under deep sedation (target: −4 points on the Richmond Agitation-Sedation Scale, −4 with daily sedation interruption), and systolic blood pressure was maintained at <120 mm Hg. CT perfusion on postoperative day (POD) 1 to reduce the high risk of CHS revealed increased ipsilateral CBF and CBV and shortened mean transit time and time to peak (Fig 3, A-D). Strict blood pressure control and sedation were continued as these findings were potentially suggestive of CHS. The patient went into general convulsions on POD 3, when the sedation was interrupted to evaluate his neurologic status. Suspecting that the convulsions were caused by CHS, we kept him sedated until improved CHS could be assessed. CT perfusion on POD 5 showed diminished CBF laterality. The ipsilateral TOI remained higher than the contralateral TOI but gradually normalized up to POD 6. The patient awoke on POD 6 after the systemic management under deep sedation was withdrawn. Magnetic resonance imaging on POD 7 showed improved left ICA flow and no new ischemic or hemorrhagic lesions (Fig 3, E and F). The patient was discharged without neurologic deficit and remained on dual antiplatelet therapy at home. CT angiography performed 8 months after treatment revealed patency of the stent grafts and a marked reduction of the aneurysm size compared with the size before treatment or on POD 1 (Fig 3, G-I).
Fig 3.
A-D, Computed tomography (CT) perfusion imaging on postoperative day (POD) 1 showed increased ipsilateral cerebral blood flow (CBF;A) and cerebral blood volume (CBV;B) and shortened mean transit time (MTT;C) and time to peak (TTP;D). E, Axial magnetic resonance fluid attenuation inversion recovery images on POD 7 showed no new ischemic or hemorrhagic lesions. F, Magnetic resonance angiography on POD 7 revealed improved left internal carotid artery (ICA) flow (dotted circle). G-I, Left common carotid CT angiography before placement of the covered stent (G), on POD 1 (H), and 8 months after treatment (I). CT angiography performed 8 months after treatment revealed no evidence of stent graft stenosis or thrombus formation. The aneurysm was markedly smaller than the size measured before treatment and on POD 1. The white points indicate the outlines of the thrombosed aneurysm.
Discussion
Extracranial ICA aneurysm is rare, accounting for only 0.4% to 1% of all arterial aneurysms.6, 7 Although it is usually asymptomatic, the aneurysm may be manifested mainly as thromboembolic stroke.3, 4 Surgical intervention is considered mandatory as conservative management is associated with high mortality due to thromboembolic stroke.8, 9 The standard therapeutic option for extracranial ICA aneurysms is thought to be direct surgery, but the operation is invasive and poses a high risk of complications linked to technical difficulties.3, 9, 10, 11 As an alternative to direct surgery, endovascular covered stent grafting has been reported as a safe and effective therapeutic option with several advantages as a first-line therapy: the procedure is less invasive, has a low rate of complications, confers long-term patency, and maintains anterograde blood flow.3, 5, 12, 13 We therefore decided to perform covered stent placement even though the procedure had to be performed in an off-label fashion.
CHS is defined as an increased CBF compared with preoperative or baseline values and is thought to occur when perfusion is rapidly increased in a chronically hypoperfused area.1, 2, 14 The common clinical presentations of CHS are ipsilateral headache, hypertension, focal seizure, and neurologic deficit.1 CHS is a serious postoperative complication of carotid endarterectomy or carotid artery stenting.13, 14 Surgical obliteration of intracranial giant aneurysm is also known to cause CHS.1, 15, 16, 17 Yet no earlier reports have described CHS after obliteration of an extracranial ICA aneurysm or the importance of perioperative CBF studies to assess the CHS risk in the treatment of extracranial ICA aneurysms. CHS in such cases probably results from a sudden restoration of blood flow to a chronically hypoperfused area with unbalanced autoregulation, as a giant aneurysm traps and reduces anterograde distal blood flow.16, 17 The same mechanism may apply to our case. Whatever the underlying etiology, our pathophysiologic characterization of CHS would be a rapidly increased perfusion in a chronically hypoperfused area. CHS is a reasonable concern in light of the poor perfusion to the distal ICA and MCA and the expected improvement of blood flow once the aneurysm is removed. Preoperative CT perfusion confirmed the hemodynamics responsible for the hypoperfused area on the ipsilateral side of the aneurysm and alerted us to the risk of CHS after the endovascular obliteration of the aneurysm. We therefore took rigorous steps against CHS based on repeated CT perfusion studies, and the patient attained a good clinical outcome. The perioperative monitoring of TOI was also useful for managing CHS in a less invasive fashion. The clinical presentations of CHS after endovascular covered stent placement were confirmed by increased ipsilateral CBF and CBV on postoperative CT perfusion and general convulsions during postoperative management. The CT perfusion values on POD 1 may not have been typical of CHS, but the patient was managed with strict blood pressure control under deep sedation throughout the endovascular surgery and postoperative period to prevent CHS. We therefore suspect that the CT perfusion findings on POD 1 were underestimated.
Conclusions
CHS can appear after endovascular reconstruction of an extracranial ICA aneurysm. Preoperative CBF evaluation is a vital preliminary step when blood flow is expected to increase after revascularization, and perioperative repeated CBF evaluations lead to good clinical outcome.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.van Mook W.N., Rennenberg R.J., Schurink G.W., van Oostenbrugge R.J., Mess W.H., Hofman P.A. Cerebral hyperperfusion syndrome. Lancet Neurol. 2005;4:877–888. doi: 10.1016/S1474-4422(05)70251-9. [DOI] [PubMed] [Google Scholar]
- 2.Moulakakis K.G., Mylonas S.N., Sfyroeras G.S., Andrikopoulos V. Hyperperfusion syndrome after carotid revascularization. J Vasc Surg. 2009;49:1060–1068. doi: 10.1016/j.jvs.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q., Duan Z.Q., Xin S.J., Wang X.W., Dong Y.T. Management of extracranial carotid artery aneurysms: 17 years' experience. Eur J Vasc Endovasc Surg. 1999;18:162–165. doi: 10.1053/ejvs.1999.0876. [DOI] [PubMed] [Google Scholar]
- 4.Li Z., Chang G., Yao C., Guo L., Liu Y., Wang M. Endovascular stenting of extracranial carotid artery aneurysm: a systematic review. Eur J Vasc Endovasc Surg. 2011;42:419–426. doi: 10.1016/j.ejvs.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Bergeron P., Khanoyan P., Meunier J.P., Graziani J.N., Gay J. Long-term results of endovascular exclusion of extracranial internal carotid artery aneurysms and dissecting aneurysm. J Interv Cardiol. 2004;17:245–252. doi: 10.1111/j.1540-8183.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- 6.Welling R.E., Taha A., Goel T., Cranley J., Krause R., Hafner C. Extracranial carotid artery aneurysms. Surgery. 1983;93:319–323. [PubMed] [Google Scholar]
- 7.Zwolak R.M., Whitehouse W.M., Jr., Knake J.E., Bernfeld B.D., Zelenock G.B., Cronenwett J.L. Atherosclerotic extracranial carotid artery aneurysms. J Vasc Surg. 1984;1:415–422. [PubMed] [Google Scholar]
- 8.Rossi P., Mirallie E., Pittaluga P., Chaillou P., Patra P. Bilateral extracranial aneurysms of the internal carotid artery. A case report. J Cardiovasc Surg (Torino) 1997;38:27–31. [PubMed] [Google Scholar]
- 9.Rosset E., Roche P.H., Magnan P.E., Branchereau A. Surgical management of extracranial internal carotid artery aneurysms. Cardiovasc Surg. 1994;2:567–572. [PubMed] [Google Scholar]
- 10.Angiletta D., Pulli R., Marinazzo D., Frotino P., Maiellaro L., Regina G. Surgical and endovascular treatment of extracranial carotid artery aneurysms: early and long-term results of a single center. Ann Vasc Surg. 2014;28:659–664. doi: 10.1016/j.avsg.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Rosset E., Albertini J.N., Magnan P.E., Ede B., Thomassin J.M., Branchereau A. Surgical treatment of extracranial internal carotid artery aneurysms. J Vasc Surg. 2000;31:713–723. doi: 10.1067/mva.2000.104102. [DOI] [PubMed] [Google Scholar]
- 12.Maras D., Lioupis C., Magoufis G., Tsamopoulos N., Moulakakis K., Andrikopoulos V. Covered stent-graft treatment of traumatic internal carotid artery pseudoaneurysms: a review. Cardiovasc Intervent Radiol. 2006;29:958–968. doi: 10.1007/s00270-005-0367-7. [DOI] [PubMed] [Google Scholar]
- 13.Amar A.P., Teitelbaum G.P., Giannotta S.L., Larsen D.W. Covered stent-graft repair of the brachiocephalic arteries: technical note. Neurosurgery. 2002;51:247–252. doi: 10.1097/00006123-200207000-00040. discussion: 252-3. [DOI] [PubMed] [Google Scholar]
- 14.Coutts S.B., Hill M.D., Hu W.Y. Hyperperfusion syndrome: toward a stricter definition. Neurosurgery. 2003;53:1053–1058. doi: 10.1227/01.neu.0000088738.80838.74. discussion: 1058-60. [DOI] [PubMed] [Google Scholar]
- 15.Chiu A.H., Wenderoth J. Cerebral hyperperfusion after flow diversion of large intracranial aneurysms. J Neurointerv Surg. 2013;5:e48. doi: 10.1136/neurintsurg-2012-010479.rep. [DOI] [PubMed] [Google Scholar]
- 16.Sugino T., Ohtaki M., Wanibuchi M., Kin S., Houkin K. Hyperperfusion syndrome after clipping an unruptured cerebral aneurysm: two case reports. Neurol Med Chir (Tokyo) 2010;50:306–309. doi: 10.2176/nmc.50.306. [DOI] [PubMed] [Google Scholar]
- 17.Ecker R.D., Murray R.D., Seder D.B. Hyperperfusion syndrome after stent/coiling of a ruptured carotid bifurcation aneurysm. Neurocrit Care. 2013;18:54–58. doi: 10.1007/s12028-012-9733-x. [DOI] [PubMed] [Google Scholar]