Abstract
We report the case of a 67-year-old patient with an anatomically complex aneurysm of the aortic arch treated by fenestrated thoracic endovascular aortic repair with subclavian-carotid extrathoracic bypass. We used the Najuta thoracic stent graft, which was approved for use in January 2013 in Japan and successfully excluded the aneurysm. Our case shows that the Najuta stent graft procedure is a feasible treatment if open repair is unsuitable for cases of aortic arch aneurysm with a challenging compromised seal zone.
A major issue in thoracic endovascular aortic repair (TEVAR) of aortic arch aneurysm with a short proximal neck is coverage of the origin of arch branches to achieve an adequate proximal landing zone.1 However, in aortic arch aneurysm with a short proximal neck, stent graft coverage of all arch branches, including the innominate artery, is required. A fenestrated stent graft in the aortic arch could achieve a better sealing zone in the challenging compromised proximal seal zone. Hybrid fenestrated TEVAR using the Najuta thoracic stent2, 3 graft has been successfully performed. Here, we present a similar case from our facility and relevant literature review. The consent of the patient was obtained to publish this report.
Case report
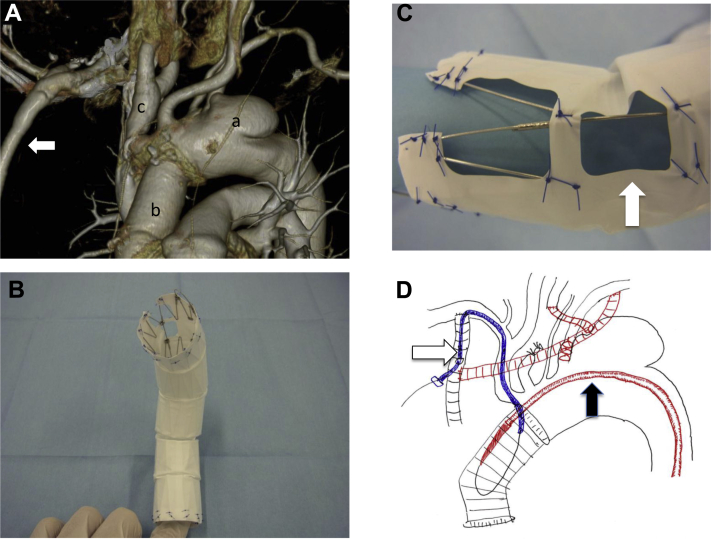
A 67-year-old woman had undergone replacement of an ascending thoracic aorta at another institution in 2014 for treatment of an acute type A dissection caused by a cardioplegia needle after aortic valve replacement for an aortic valve disease. She had also undergone right axillofemoral (Ax-F) bypass because of intraoperative right external iliac artery injury during surgery for ovarian cancer. In February 2015, follow-up computed tomography (CT) revealed a rapidly growing aneurysm in the distal arch involving the left subclavian artery (LSCA) with a short proximal neck and extreme proximal and innominate artery angulation (Fig 1, A). The patient also had an anomalous origin of the left vertebral artery from the aortic arch. Because of careful imaging of the right vertebral artery up to the basilar artery, we concluded that sufficient posterior cerebral circulation would be maintained if the left vertebral artery was closed without surgical revascularization.4 Because of two prior sternotomies, it would be a high surgical risk, and a redo operation was unsuitable for this patient; she was treated by fenestrated TEVAR using the Najuta thoracic stent graft (Kawasumi Laboratories, Inc, Tokyo, Japan; Fig 1, B and C) and a debranching bypass.
Fig 1.
A, Preoperative contrast-enhanced computed tomography (CT) showed a rapidly growing aneurysm in the distal arch involving the left subclavian artery (LSCA) with a short proximal neck and right axillofemoral (Ax-F) bypass (arrow). a, Aneurysm; b, Dacron tube graft; c, innominate artery. B, The Najuta fenestrated stent graft is a customized fenestrated device comprising a self-expandable stainless steel Z-stent and an expanded polytetrafluoroethylene graft. C, A fenestration of the Najuta to the innominate artery (arrow). D, Surgical strategy (schema); white arrow, guiding sheath; black arrow, delivery sheath of the Najuta.
The procedure was performed under general anesthesia. First, an 8-mm heparin-bonded expanded polytetrafluoroethylene (Propaten; W. L. Gore & Associates, Flagstaff, Ariz) extrathoracic bypass (Ax-F graft-left common carotid artery [LCCA]-LSCA bypass) was performed. The proximal LCCA was ligated (Fig 1, D).
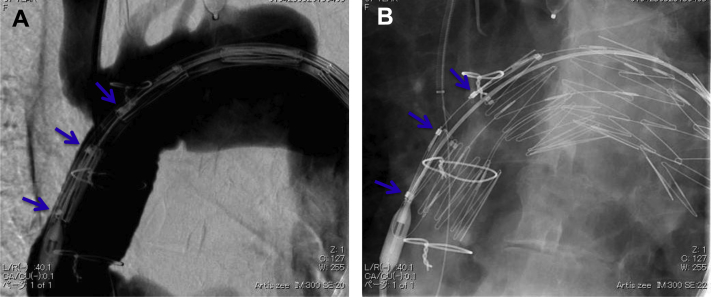
The left femoral artery was exposed, and a 6F guiding sheath was inserted from the Ax-F bypass graft into the innominate artery (Fig 1, D). A 0.032-inch Radifocus guidewire (Terumo, Tokyo, Japan) was then pulled through from the right subclavian artery to the left femoral artery. The proximal LSCA was occluded with an Amplatzer vascular plug (St. Jude Medical, St. Paul, Minn) to prevent a type II endoleak (Fig 1, D). The Najuta was delivered with a 22F J-shaped sheath maintained under continuous strain by traction at both wire ends (body floss technique).2, 5, 6, 7 Because of a severe angulation and tortuosity of the ascending aorta and innominate artery due to prior ascending aorta replacement, the delivery of the system through the arch was difficult by only a body floss technique. Therefore, the guiding sheath through the Ax-F bypass was deeply inserted into the origin of the innominate artery, and it supported the delivery of the stent graft. The Najuta was then delivered and deployed at a zone 0 proximal site with delicate positional adjustment of the fenestration of the Najuta to the innominate artery. In this case, the stent graft had two fenestrations (Fig 1, B and C). The first fenestration was opened from the first marker to the second marker, and the second fenestration was opened between the second marker and the third marker (Fig 2, A). The third marker was accurately put on the distal end of the innominate artery, and the fenestration was fitted for the branch automatically without rotation (Fig 2, B). The LCCA, LSCA, and left vertebral artery were excluded. We exchanged the wire from a 0.032-inch Radifocus wire to a Lunderquist extrastiff wire (Cook Medical, Bloomington, Ind). In addition, the Relay stent graft (Bolton Medical, Barcelona, Spain) was deployed at a zone 1 proximal site. The proximal bare stent of the Relay stent graft was inserted into the prosthetic graft of the ascending aorta.
Fig 2.
The first fenestration was opened from the first marker to the second marker, and the second fenestration was opened between the second marker and the third marker. A, Before deployment (arrows, markers). B, After deployment (arrows, markers).
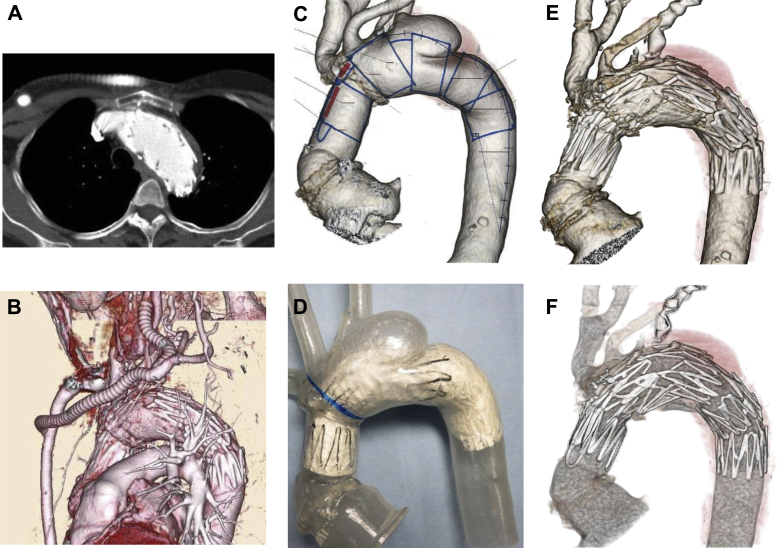
Completion angiography showed complete exclusion of the aneurysm and confirmed preservation of the innominate artery and debranching bypass flow. The patient's postoperative course was uneventful. Six months after the procedure, postoperative CT revealed that the aneurysm had been completely excluded (Fig 3, A and B). She recovered without any complications.
Fig 3.
A and B, Follow-up contrast-enhanced computed tomography (CT) showed accurate device fixation, no major endoleak, and patency of the innominate artery and debranching bypass. C and D, A customized full-scale Najuta stent graft model was deployed to the plaster model before thoracic endovascular aneurysm repair (TEVAR). E and F, The final appearance of the Najuta.
Discussion
The hybrid repair of arch lesions for zone 0 is feasible in patients unsuitable for open repair.8 On the other hand, endografting in the aortic arch is associated with a higher mortality and morbidity than in zone 4 TEVAR.9 Vallejo et al10 reported that perioperative mortality was 25% and stroke occurred in 25% of patients by performing hybrid TEVAR for zones 0 and 1 with extra-anatomic bypass. Recent investigations have demonstrated that in situ fenestration is a novel option for endovascular aortic arch repair.11, 12 In our case, in situ fenestration of the innominate artery appeared to be highly difficult because of the angulation and tortuosity of the vessels. Furthermore, the chimney technique was also presumed to be unsuitable in our case. Gutter endoleak also remains a major issue with the chimney technique.
The Najuta thoracic stent graft is a customized fenestrated device comprising a self-expandable stainless steel Z-stent and an expanded polytetrafluoroethylene graft, which was approved for use in Japan in January 2013.13 In the process of preparing the Najuta stent graft, the patient's CT angiography Digital Imaging and Communications in Medicine data were converted to a three-dimensional (3D) manufactured patient-specific model of the aortic arch. The edited model was used for producing a physical plaster model in a 3D printer (Eden350V; Stratasys Ltd, Eden Prairie, Minn). A customized full-scale stent graft model was deployed to the plaster model before TEVAR. The use of an anatomic plaster model produced by the 3D printer is effective for obtaining a geometric analysis, eg, for bird-beaking and fitting (Fig 3, C and D). Moreover, it took only 6 hours to create one 3D model.
The Najuta stent graft enables accurate deployment and may be able to prevent migration. The Najuta makes it possible to obtain a long proximal sealing length because of its fenestration. Moreover, the 3D skeleton of the Najuta is a preshaped and rigid structure, and all Z-stents are linked with two struts. Therefore, it is able to fit a greater curvature of the aortic arch and stent well.
On the other hand, the Najuta has no Z-stent between the first and second stents. To support sealing force of this part, we used the Relay stent graft (Relay's proximal bare stent supports the part of the Najuta's second fenestration from inside).6
Moreover, the Relay system has a superior delivery sheath and fits to the curvature of the aortic arch.6 Each device was well fitted and adequate for landing for the compromised seal zone, with no endoleaks or complications (Fig 3, E and F).
Recent studies have demonstrated encouraging results of branched TEVAR.14, 15 On the other hand, significant angulation is now appreciated to be a contraindication to branched TEVAR. The indication for branched TEVAR is the presence of a stable, long, and straight proximal sealing zone. Thus, we conclude that the fenestrated Najuta thoracic stent graft may be a good treatment option in patients with a challenging compromised seal zone.
Conclusions
We report the case of a patient with an anatomically complex aneurysm of the aortic arch treated by fenestrated TEVAR using the Najuta stent graft with subclavian-carotid extrathoracic bypass.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Samura M., Zempo N., Ikeda Y., Hidaka M., Kaneda Y., Suzuki K. Endovascular repair of distal arch aneurysm with double-chimney technique. Ann Thorac Surg. 2013;95:1778–1780. doi: 10.1016/j.athoracsur.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 2.Iwakoshi S., Ichihashi S., Itoh H., Tabayashi N., Sakaguchi S., Yoshida T. Clinical outcomes of thoracic endovascular aneurysm repair using commercially available fenestrated stent graft (Najuta endograft) J Vasc Surg. 2015;62:1473–1478. doi: 10.1016/j.jvs.2015.06.224. [DOI] [PubMed] [Google Scholar]
- 3.Mangialardi N., Ronchey S., Malaj A., Lachat M., Serrao E., Alberti V. Case report of an endovascular repair of a residual type A dissection using a not CE not FDA-approved Najuta thoracic stent graft system. Medicine (Baltimore) 2015;94:e436. doi: 10.1097/MD.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manninen H., Tulla H., Vanninen R., Ronkainen A. Endangered cerebral blood supply after closure of left subclavian artery: postmortem and clinical imaging studies. Ann Thorac Surg. 2008;85:120–125. doi: 10.1016/j.athoracsur.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 5.Krycińska R., Trznadel A., Kuchalska P., Lis M., Dołęga-Kozierowski B., Dyś K. Brachiocephalic vein stenting and body-floss technique as a treatment of CVD in dialysis-dependent patient— case report and literature Review. Pol J Radiol. 2015;80:247–251. doi: 10.12659/PJR.893358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nomura Y., Yuri K., Kimura N., Okamura H., Itoh S., Matsumoto H. Endovascular repair of thoracic aortic aneurysm associated with right-sided aortic arch: report of two cases. Gen Thorac Cardiovasc Surg. 2014 doi: 10.1007/s11748-014-0514-7. December 20;[Epublication ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi S., Yokoi Y., Shimazaki T., Koide K., Matsumoto M., Shigematsu H. Thoracic endovascular aneurysm repair in Japan: experience with fenestrated stent grafts in the treatment of distal arch aneurysms. J Vasc Surg. 2008;48(Suppl):24S–29S. doi: 10.1016/j.jvs.2008.08.037. discussion: 29S. [DOI] [PubMed] [Google Scholar]
- 8.Kanaoka Y., Ohki T., Toya N., Ishida A., Tachihara H., Hirayama S. Technical challenges in endovascular repair of complex thoracic aortic aneurysms. Ann Vasc Dis. 2012;5:21–29. doi: 10.3400/avd.oa.11.01011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez J.A., Olsen D.M., Shtutman A., Lucas L.A., Wheatley G., Alpern J. Application of endograft to treat thoracic aortic pathologies: a single center experience. J Vasc Surg. 2007;46:413–420. doi: 10.1016/j.jvs.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 10.Vallejo N., Rodriguez-Lopez J.A., Heidari P., Wheatley G., Caparrelli D., Ramaiah V. Hybrid repair of thoracic aortic lesions for zone 0 and 1 in high-risk patients. J Vasc Surg. 2012;55:318–325. doi: 10.1016/j.jvs.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 11.Malina M., Sonesson B. In situ fenestration: a novel option for endovascular aortic arch repair. J Cardiovasc Surg (Torino) 2015;56:355–362. [PubMed] [Google Scholar]
- 12.Tse L.W.H., Lerouge S., Bui B.T., Therasse E., Héon H., Soulez G. Radiofrequency perforation system for in vivo antegrade fenestration of aortic stent-grafts. J Endovasc Ther. 2010;17:192–198. doi: 10.1583/09-2903.1. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguchi S., Shimizu H., Yoshitake A., Shimazaki T., Iwahashi T., Ogino H. Endovascular stent graft repair for thoracic aortic aneurysms: the history and the present in Japan. Ann Vasc Dis. 2013;6:129–136. doi: 10.3400/avd.ra.12.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haulon S., Greenberg R.K., Spear R., Eagleton M., Abraham C., Lioupis C. Global experience with an inner branched arch endograft. J Thorac Cardiovasc Surg. 2014;148:1709–1716. doi: 10.1016/j.jtcvs.2014.02.072. [DOI] [PubMed] [Google Scholar]
- 15.Lu Q., Feng J., Zhou J., Zhao Z., Li H., Teng Z. Endovascular repair by customized branched stent-graft: a promising treatment for chronic aortic dissection involving the arch branches. J Thorac Cardiovasc Surg. 2015;150:1631–1635. doi: 10.1016/j.jtcvs.2015.08.032. [DOI] [PubMed] [Google Scholar]