Abstract
Osler-Weber-Rendu syndrome is a rare genetic disorder that commonly features high-flow arteriovenous malformations (AVM) within the pulmonary, intracranial, and visceral circulation. We present a patient with a unique case of Osler-Weber-Rendu syndrome featuring a high-flow pelvic AVM in addition to fibromuscular dysplasia affecting multiple vascular beds. This required a unique modification of our embolic therapeutic approach for adequate treatment of the AVM.
Osler-Weber-Rendu (OWR) syndrome is a rare genetic disorder that commonly features high-flow arteriovenous malformations (AVM) within the pulmonary, intracranial, and visceral circulation. We present a patient with a unique case of OWR syndrome featuring a high-flow pelvic AVM in addition to fibromuscular dysplasia (FMD) affecting multiple vascular beds. Consent to publish this case report was obtained from patient.
Case report
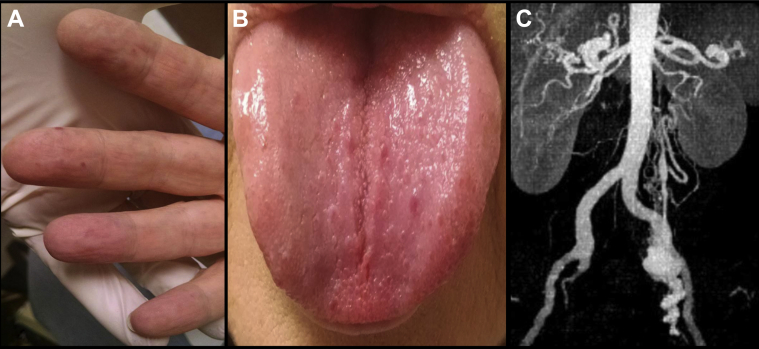
A 59-year-old woman with a history of OWR syndrome was evaluated in consultation for a symptomatic left pelvic AVM discovered on a computed tomography (CT) scan. Symptoms included a dull, throbbing ache, waxing and waning in severity, with exacerbation of symptoms after activity. She also reported a personal and first-degree relative history of chronic, persistent epistaxis. Her physical examination was remarkable for multiple, widespread erythematous macules (telangiectasias) throughout the volar aspect of the acral digits, tongue, and buccal mucosa (Fig 1, A and B). Left lower quadrant tenderness to deep palpation and a faint local bruit was appreciated.
Fig 1.
Characteristic (A) volar digital and (B) tongue telangiectasias as well as (C) a high-flow arteriovenous malformation (AVM) within the pelvic circulation strongly suggestive of Osler-Weber-Rendu (OWR) syndrome.
The CT scan confirmed the presence of large-caliber, tortuous arterial feeders supplying the nidus of a high-flow left pelvic AVM with prompt shunting into an aneurysmal draining vein, which eventually emptied into the left gonadal venous system (Fig 1, C). The arterial supply seemed to arise from the hypogastric or the gonadal arteries, or both. Results of the laboratory evaluation were unremarkable aside from mild microcytic anemia.
The patient was offered a diagnostic angiography with the option for transcatheter embolization of this symptomatic, high-flow AVM.
Technical details
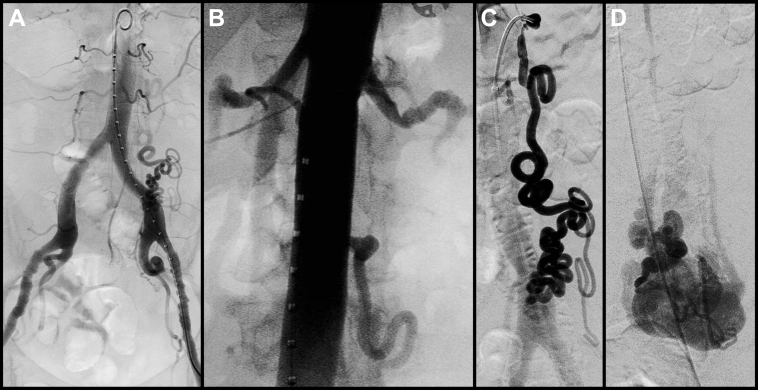
The procedure was performed in a hybrid operating suite, with the patient under general anesthesia. No systemic anticoagulation was given; however, generous and frequent heparinized saline irrigation of sheaths and catheters was performed throughout the case. Flush aortography confirmed the presence of a high-flow AVM supplied by an enlarged, redundant, and extremely tortuous left gonadal artery, with prompt shunting into aneurysmal draining veins, which eventually emptied into the left gonadal venous system. No significant left renal vein hemodynamic abnormalities were noted. Selective catheterization of the left hypogastric artery revealed no association with the AVM. Also noted were diffuse, medial fibrodysplastic lesions with a characteristic chain of beads appearance involving the bilateral external iliac arteries and the left renal artery (Fig 2, A and B).
Fig 2.
Diffuse fibrodysplastic lesions affecting the (A) external iliac, (B) renal, and (C) proximal left gonadal arteries. C, Along with the extreme tortuosity of the distal left gonadal artery, precluded superselective microcatheterization of the (D) nidus of the arteriovenous malformation (AVM) from transarterial route.
Selective catheterization of the ostium of the left gonadal artery also revealed proximal fibrodysplastic lesions. This lesion, along with the extreme tortuosity of the feeding artery, precluded transarterial microcatheter delivery of our embolic agent into the AVM nidus (Fig 2, C and D).
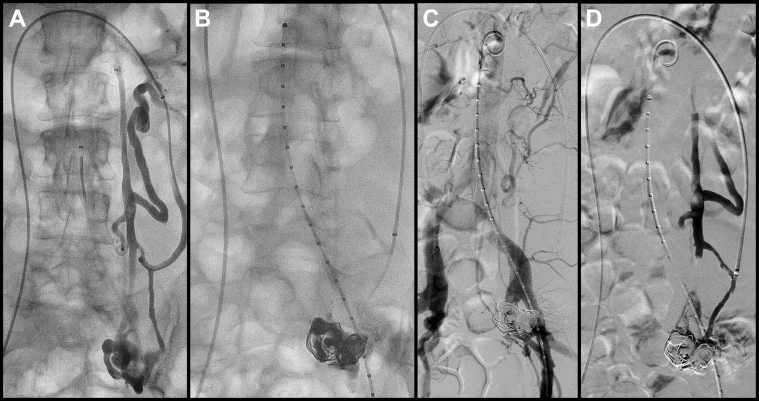
At this point, venous access was obtained, and the left renal vein was catheterized. A selective venogram demonstrated two major venous channels draining the AVM nidus. The smaller of the two venous conduits was accessed, followed by insertion of a 6F Raabe sheath (Cook Medical, Bloomington, Ind; Fig 3, A). A venogram was performed, and under roadmap guidance, retrograde superselective microcatheterization of the AVM nidus was performed with a 2.4F tipped microcatheter on a 0.014-inch platform (Fig 3, B). Two giant detachable framing coils, 24 mm × 55 cm and 32 mm × 60 cm (Ruby; Penumbra, Alameda, Calif), were placed into the nidus extending into the aneurysmal draining veins. This provided a scaffold for further embolization using Onyx-18 and Onyx-34 (Covidien, Plymouth, Minn; Fig 3, C).
Fig 3.
A, Transvenous catheterization of draining gonadal vein with sheath insertion. B, Superselective microcatheter access into arteriovenous malformation (AVM) nidus with deposition of detachable giant framing coils and Onyx (Covidien, Plymouth, Minn) cast. C and D, Completion angiography and venography demonstrates devascularization of the AVM nidus with no further arteriovenous shunting and preservation of gonadal artery flow and venous drainage.
Completion venograms and angiograms revealed complete obliteration of the nidus, with cessation of arteriovenous shunting and preservation of ovarian arterial inflow and venous drainage (Fig 3, C and D). There was no evidence of nontarget embolization.
Procedural outcome
The patient tolerated the procedure well, without any complications, and was discharged home in stable condition, without symptoms, on postoperative day 1. She was seen in follow-up at 1 month, with no further abdominal or pelvic discomfort. She will be monitored with repeat CT angiography at 6 months and then annually thereafter.
Discussion
OWR syndrome, also known as hereditary hemorrhagic telangiectasia (HHT), is a genetic disorder affecting one in 5000 to 8000 individuals.1, 2 High-flow AVMs are a hallmark feature of this disorder owing to ectatic changes involving the precapillary arterioles that can cause secondary AV shunting.3 These AVMs are formed during development but generally do not become symptomatic until adolescence or later.4 The architecture of the AVMs is fragile, and they are thus easily perturbed, leading to frequent bruising and bleeding.1, 5 The most common symptom of OWR syndrome, therefore, is frequent and persistent epistaxis, mucosal, palmar, and plantar telangiectasias, and visceral, cranial, or pulmonary AVMs.1 The HHT Foundation International Inc has set the clinical diagnostic criteria for a “definite” diagnosis as presence of at least three symptoms from (1) persistent epistaxis, (2) telangiectasias, (3) AVMs, and (4) history of a first-degree relative with HHT.6 The presence of at least two symptoms and fewer than two symptoms can be indicative of “likely/possible” and “unlikely” diagnoses, respectively.6
The gene mutations seen in OWR syndrome are part of the transforming growth factor-β (TGF-β) signaling cascade.7 Endoglin (ENG) and activin-receptor like kinase 1 (ALK1) are two genes affected in OWR syndrome and are thought to be responsible for downstream induction of endothelial proliferation and migration in angiogenesis.1, 7
TGF-β involvement is of particular interest in this patient due to the concurrent observation of FMD affecting multiple vascular beds. Although the mechanisms of involvement have not been completely delineated, Ganesh et al8 found evidence of significantly increased levels of TGF-β in patients with FMD. No direct correlation has been shown to OWR syndrome, but FMD has been implicated in connections to a number of connective tissue and cell growth diseases, including neurofibromatosis, Alport syndrome, Ehlers-Danlos syndrome, Marfan syndrome, and Takayasu arteritis.9 Whether alterations in TGF-β expression in OWR syndrome can cause secondary fibrodysplastic lesions remains an interesting question to be answered by larger retrospective observational studies and warrants closer biomolecular scrutiny.
In this patient, superselective arterial access into the AVM nidus proved extremely difficult owing to extreme tortuosity and proximal fibrodysplastic changes of the left gonadal artery. However, because the angioarchitecture of the AVM, including its precise pattern of venous drainage, had been ascertained on delayed-phase diagnostic angiography, a transvenous route to the AVM nidus via a coaxial microcatheter-based system was proposed and performed successfully. Retrograde approaches to high-flow AVM embolization have been described mostly within the neurointerventional literature.10, 11, 12, 13
Almost all reported cases involving the use of a polymerizing embolic agent describe use of adjunctive techniques, such as balloon occlusion control of the inflow artery or outflow vein, or both, to minimize risk of nontarget embolization into the catheterized draining vein.14, 15, 16, 17 Conway et al18 recently reported a series of five patients with high-flow pelvic AVMs in whom transvenous coil or plug embolization was used as an adjunctive measure to transarterial or direct stick glue embolization, or both, of the AVM nidus.
Our approach was unique in that the entire treatment was performed purely from a transvenous route. Furthermore, careful evaluation of the venous drainage pattern identified two major draining veins emptying into the left renal venous circulation. Selection of the smaller of the two draining veins and subsequent sheath insertion sufficiently halted flow through that conduit and eliminated the need for adjunctive techniques to control flow. Therefore, once microcatheterization of the nidus was achieved, coil and Onyx embolization was performed with excellent deposition and no evidence of nontarget embolization. We initially created a scaffold using two giant framing coils and higher-viscosity Onyx-34. This provided a foundation for further embolization with lower-viscosity Onyx-18 for further devascularization and elimination of the AVM nidus as well as the aneurysmal portion of the draining veins.
Conclusions
This represents yet another effective approach to the treatment of complex AVMs in the setting of compromised inflow access. An alternative option would have been direct stick embolization of the AVM nidus under roadmap guidance after initial diagnostic angiography. However, given the particular location and angioarchitecture of this specific AVM, this approach would have proven difficult with potential for adjacent organ injury.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Govani F.S., Shovlin C.L. Hereditary haemorrhagic telangiectasia: a clinical and scientific review. Eur J Hum Genet. 2009;17:860–871. doi: 10.1038/ejhg.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nassiri N., Rachakonda A., Carroccio A., Rosen R. Scattered arteriovenous malformations in Osler-Weber-Rendu syndrome. J Vasc Surg. 2012;56:227. doi: 10.1016/j.jvs.2011.09.089. [DOI] [PubMed] [Google Scholar]
- 3.Nassiri N., Rosen R.J. Pulmonary arteriovenous malformations. In: Kipshidze N., Fareed Rosen R.T., Dangas G., Serruys P., editors. Urgent interventional therapies. 1st edition. West Sussex, UK: Wiley-Blackwell; 2014. pp. 410–418. [Google Scholar]
- 4.McDonald J., Pyeritz R.E. Hereditary hemorrhagic telangiectasia. In: Pagon R.A., Adam M.P., Ardinger H.H., Bird T.D., Dolan C.R., Fong C.T., editors. GeneReviews. University of Washington, Seattle; Seattle, WA: 2014. http://www.ncbi.nlm.nih.gov/books/NBK1351/ Available at: [Google Scholar]
- 5.Berg A.M., Amirbekian S., Mojibian H., Trow T.K., Smith S.J., White R.I. Hemothorax due to rupture of pulmonary arteriovenous malformation: an interventional emergency. Chest. 2010;137:705–707. doi: 10.1378/chest.09-0344. [DOI] [PubMed] [Google Scholar]
- 6.Shovlin C.L., Guttmacher A.E., Buscarini E., Faughnan M.E., Hyland R.H., Westermann C.J. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome) Am J Med Genet. 2000;91:66–67. doi: 10.1002/(sici)1096-8628(20000306)91:1<66::aid-ajmg12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 7.Jonker L. TGF-β & BMP receptors endoglin and ALK1: overview of their functional role and status as antiangiogenic targets. Microcirculation. 2014;21:93–103. doi: 10.1111/micc.12099. [DOI] [PubMed] [Google Scholar]
- 8.Ganesh S.K., Morissette R., Xu Z., Schoenhoff F., Griswold B.F., Yang J. Clinical and biochemical profiles suggest fibromuscular dysplasia is a systemic disease with altered TGF-β expression and connective tissue features. FASEB J. 2014;28:3313–3324. doi: 10.1096/fj.14-251207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slovut D.P., Olin J.W. Fibromuscular dysplasia. N Engl J Med. 2004;350:1862–1871. doi: 10.1056/NEJMra032393. [DOI] [PubMed] [Google Scholar]
- 10.Albuquerque F.C., Ducruet A.F., Crowley R.W., Bristol R.E., Ahmed A., McDougall C.G. Transvenous to arterial Onyx embolization. J Neurointerv Surg. 2014;6:281–285. doi: 10.1136/neurintsurg-2012-010628. [DOI] [PubMed] [Google Scholar]
- 11.Consoli A., Renieri L., Nappini S., Limbucci N., Mangiafico S. Endovascular treatment of deep hemorrhagic brain arteriovenous malformations with transvenous Onyx embolization. AJNR Am J Neuroradiol. 2013;34:1805–1811. doi: 10.3174/ajnr.A3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dabus G., Linfante I., Lin E., Benenati J., Martínez-Galdámez M. P-025 interventional treatment of high flow craniofacial vascular malformations. J Neurointerv Surg. 2014;6(Suppl 1):A33–A34. doi: 10.1136/neurintsurg-2016-012315. [DOI] [PubMed] [Google Scholar]
- 13.Martínez-Galdámez M., Saura P., Saura J., Muñiz J., Albisua J., Pérez-Higueras A. Transvenous Onyx embolization of a subependymal deep arteriovenous malformation with a single drainage vein: technical note. J Neurointerv Surg. 2014;6:e20. doi: 10.1136/neurintsurg-2012-010603.rep. [DOI] [PubMed] [Google Scholar]
- 14.Mitsuzaki K., Yamashita Y., Utsunomiya D., Sumi S., Ogata I., Takahashi M. Balloon-occluded retrograde transvenous embolization of a pelvic arteriovenous malformation. Cardiovasc Intervent Radiol. 1999;22:518–520. doi: 10.1007/s002709900443. [DOI] [PubMed] [Google Scholar]
- 15.Kishino M., Miyasaka N., Takeguchi Y., Ohashi I. Retrograde transvenous obliteration for uterine arteriovenous malformation. Obstet Gynecol. 2014;123:427–430. doi: 10.1097/AOG.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 16.Koganemaru M., Abe T., Iwamoto R., Suenaga M., Matsuoka K., Hayabuchi N. Pelvic arteriovenous malformation treated by superselective transcatheter venous and arterial embolization. Jpn J Radiol. 2012;30:526–529. doi: 10.1007/s11604-012-0081-8. [DOI] [PubMed] [Google Scholar]
- 17.Erbahceci Salik A., Islim F., Akgul A., Cil B.E. Concomitant transarterial and transvenous embolization of a pelvic arteriovenous malformation using a new liquid embolic agent, Squid-12 and detachable coils. Case Rep Vasc Med. 2014;2014:972870. doi: 10.1155/2014/972870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conway A.M., Drury J., Rosen R. VS6 Transvenous embolization of high-flow arteriovenous malformations with a dominant outflow vein. J Vasc Surg. 2014;59:35S. doi: 10.1016/j.jvsv.2014.12.003. [DOI] [PubMed] [Google Scholar]