Abstract
Pseudoxanthoma elasticum (PXE) is an inherited disease characterized by skin lesions, central blindness, and progressive peripheral occlusive disease. Severe claudication is a frequent symptom for which angioplasty represents a possible therapeutic avenue. We report the outcomes of four patients with PXE treated by angioplasty and stenting of the superficial femoral artery in two centers. These patients exhibited an abnormal failure rate for angioplasty and stenting of the superficial femoral artery, suggesting an as yet unknown susceptibility in such patients. In the absence of further evidence, we do not recommend arterial angioplasty with stenting as a primary surgical approach in PXE patients with femoral artery lesions.
Three women and one man with pseudoxanthoma elasticum (PXE) diagnosed previously on the basis of skin lesions, angioid streaks, skin biopsy,1 and genotyping were referred for intravascular procedures for peripheral artery obstructive disease (PAOD). They suffered from severe intermittent claudication with walking distances of <250 m, and medical attempts to treat PAOD had failed in each case. All patients gave their informed consent to publication of this report.
Case reports
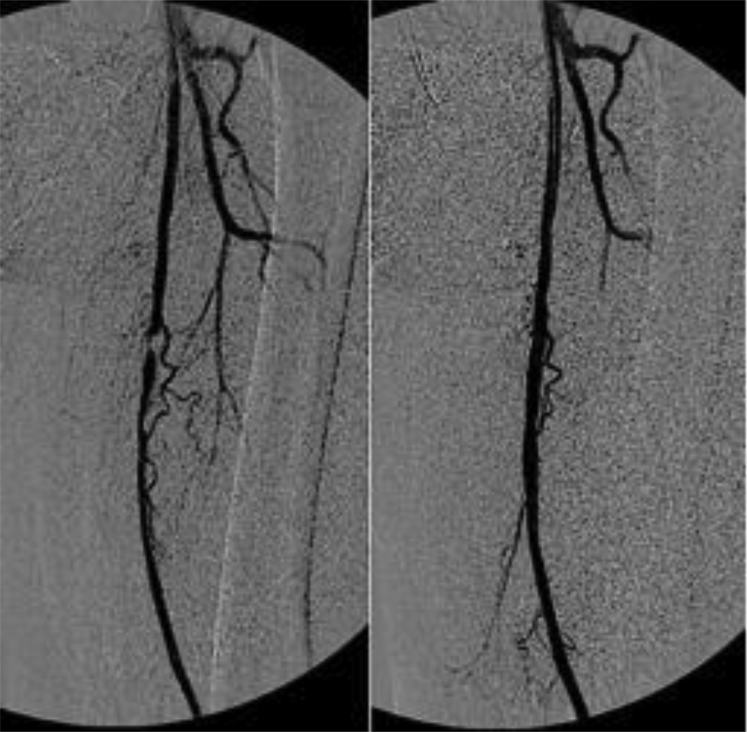
Patient 1 (a woman, 68 years old) was diagnosed with PXE at 18 years of age. She was referred with a long history of lower limb claudication with an ankle-brachial pressure index (ABI) at rest of 0.69 on the right and 0.85 on the left. Duplex scan disclosed bilateral calcified short stenosis (>70%) of the superficial femoral arteries (SFAs). Primary angioplasty with stenting of the right SFA was performed (Fig 1), followed 2 days later by angioplasty using a nitinol stent (LifeStent; Bard, Tempe, Ariz) on the left side. The patient was discharged with combined oral antiplatelet medication (aspirin and clopidogrel). Four weeks later, intermittent claudication recurred, and duplex scanning disclosed bilateral thrombosis of the stented SFAs. The patient is currently stable and does not wish to have further treatment.
Fig 1.
Intraoperative angiography demonstrating stenosis of the left superficial femoral artery (SFA) and the result after angioplasty and stenting.
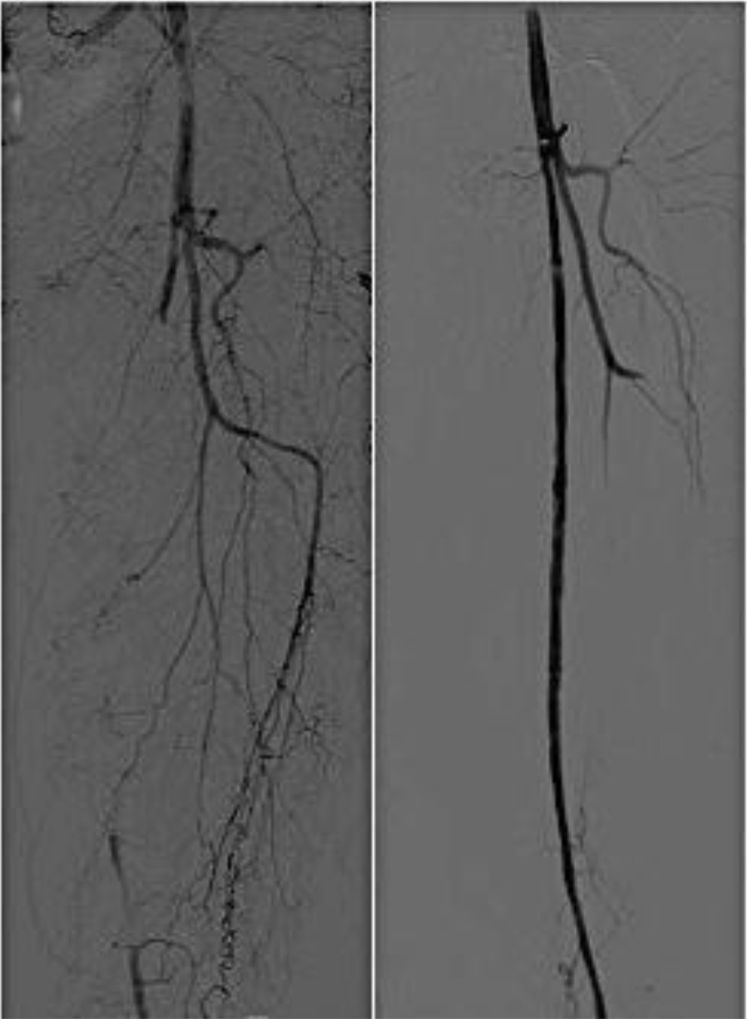
Patient 2 (a woman, 65 years old) was diagnosed with PXE at 20 years of age. She was referred for severe claudication of the left leg (<150 m) with an ABI of 0.51 and 0.68 on the right. Duplex scans disclosed significant stenosis (>70%) of the left SFA, and angioplasty of the left SFA with a nitinol stent (LifeStent; Bard) was performed after failure of medical treatment. One day later, duplex scanning confirmed reocclusion (ABI, 0.46) of the stented SFA. After patency was recovered by fibrinolysis (Fig 2), a second stent was deployed, and the patient was discharged initially with low-molecular-weight heparin and clopidogrel. She was later switched to ticagrelor alone. A month later, the intermittent claudication recurred, and occlusion of the stented artery was evidenced by duplex scan. A femoral-popliteal saphenous bypass was finally performed, and the patient has remained asymptomatic.
Fig 2.
Angiography showing acute superficial femoral artery (SFA) thrombosis and result after intra-arterial in situ thrombolysis.
Patient 3 was diagnosed with PXE at 8 years of age. At 38 years, the patient underwent her first angioplasty with stenting (S.M.A.R.T. stent; Cordis, Bridgewater, NJ) for stenosis of the right SFA. The patient was discharged with phenprocoumon (3 mg). One year later, a duplex scan disclosed a subtotal stenosis in the right SFA, which was treated by femoral-popliteal saphenous bypass. Nine years later, her claudication worsened on the left leg, and angioplasty of the SFA was performed. Twelve months later, in-stent stenosis of the left SFA was found, again treated by angioplasty. After 6 months, she was still suffering from claudication and was referred finally for a femoral-popliteal saphenous bypass.
Patient 4 was diagnosed with PXE at 41 years of age. At 44 years, he complained of severe claudication, and duplex scans showed severe stenosis of both SFAs. Bilateral angioplasty with stenting (S.M.A.R.T. stent; Cordis) was performed, and he was discharged with aspirin (80 mg/d). Three years later, his maximum walking distance had decreased; duplex scans revealed restenosis on both sides, and bilateral angioplasty was repeated. Restenosis of the right SFA was found shortly after, and a third angioplasty was performed. Three years later, his maximum walking distance had declined further (<150 m), and computed tomography angiography revealed significant restenosis of the left SFA. Since the patient had developed a dense network of collaterals in both legs, a watch and wait policy was decided.
Discussion
PXE is a rare autosomal recessive inherited disorder (1/25,000) characterized by abnormal calcified elastic fibers in the internal elastic lamina of medium-sized arteries, leading to premature PAOD. The lesions develop mainly in the femoral segments, starting at a young age with long-lasting symptoms of claudication.2 For unestablished reasons, PXE disease predominates in woman.
The treatment of PAOD in PXE currently remains empirical, and there are few reports on endovascular treatment. Donas et al performed balloon angioplasty for infrarenal aortic and iliac stenosis in one PXE patient, still patent at 12 months' of follow-up.3 Siskos et al reported 6 months of patency after angioplasty with stenting at the iliac level in a 58-year-old patient.4 Zimmo et al also reported patency after endovascular surgery in a PXE patient treated for a renal artery aneurysm.5
We here describe four patients with PXE treated with angioplasty and stenting for the early development of SFA stenosis. Angioplasty was decided as the first option after all medical treatment had failed (ie, management of cardiovascular risk factors, daily walking training). All procedures failed because of either thrombosis (two patients) or restenosis (two patients). Catheterization was performed by experienced operators according to conventional techniques, and no specific technical problems occurred, patency being confirmed with final angiography after stenting. Although oral antiplatelet therapy was administered postoperatively as recommended for this type of procedure, the follow-up with ultrasound examination was not identical in both centers. The lack of a relationship between claudication and arterial occlusion after angioplasty might explain the different time intervals in detecting patency failure.
Given the rarity of the disease, the reasons for this abnormal rate and repeated failures in patients from two different centers suggest specific traits in the arterial remodeling after stenting in PXE. The early thrombosis despite antiplatelet treatment in two patients suggests a prothrombotic propensity, although standard tests did not reveal abnormalities in coagulation and hemostasis or increased inflammatory state (high-sensitivity C-reactive protein). Hypocoagulability is more often reported in PXE.5 In the general population, the 12-month' patency of the SFA with nitinol stenting reached 76.1%.6 Therefore, the chance that the rate of occlusion in PXE in two different centers would be 100% is very unlikely. Although a bypass represents an alternative to angioplasty, the fact that the calcification process could develop in a graft cannot be excluded.
Conclusions
On the basis of our experience and in the absence of further studies to elucidate the cause of such an abnormal failure rate, we do not recommend angioplasty and stenting of the SFA as a primary method for treatment of claudication in PXE.
Footnotes
Part of this work was funded by PXE France, a French association for patients with pseudoxanthoma elasticum.
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Chassaing N., Martin L., Calvas P., Le Bert M., Hovnanian A. Pseudoxanthoma elasticum: a clinical, pathophysiological and genetic update including 11 novel ABCC6 mutations. J Med Genet. 2005;42:881–892. doi: 10.1136/jmg.2004.030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leftheriotis G., Omarjee L., Le Saux O., Henrion D., Abraham P., Prunier F. The vascular phenotype in pseudoxanthoma elasticum and related disorders: contribution of a genetic disease to the understanding of vascular calcification. Front Genet. 2013;4:4. doi: 10.3389/fgene.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donas K.P., Schulte S., Horsch S. Balloon angioplasty in the treatment of vascular lesions in pseudoxanthoma elasticum. J Vasc Interv Radiol. 2007;18:457–459. doi: 10.1016/j.jvir.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Siskos D., Giannakakis S., Makris S., Pirgakis K., Psyllas A., Maltezos C. Case report of a patient with iliac occlusive disease due to pseudoxanthoma elasticum and review of the bibliography. Ann Vasc Surg. 2012;26:278.e11–278.e14. doi: 10.1016/j.avsg.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Zimmo L., Rudarakanchana N., Thompson M., Hamady M.S., Cheshire N.J., Bicknell C.D. Renal artery aneurysm formation secondary to pseudoxanthoma elasticum. J Vasc Surg. 2013;57:842–844. doi: 10.1016/j.jvs.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Nasser F., Kambara A., Abath C., Cavalcanti D., Barros I., Pires N. Safety and efficacy of the EPIC Nitinol Vascular Stent System for the treatment of lesions located in the superficial femoral artery: prospective and multicentric trial. J Cardiovasc Surg (Torino) 2015 March 3 doi: 10.23736/S0021-9509.16.08471-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]