Abstract
Background
Romiplostim is a thrombopoietin‐mimetic peptibody for adult refractory chronic immune thrombocytopenia (ITP). We aimed to describe ITP patients receiving romiplostim, platelet counts and romiplostim usage in UK clinical practice.
Methods
This was a retrospective cohort study of patients in the UKITP Registry who received romiplostim between October 2009 and January 2015, including data up to 6 months before romiplostim initiation through follow‐up.
Results
Of 1440 patients in the UKITP Registry, 118 adults with primary ITP were eligible. Before romiplostim, 22% had splenectomy, 12% received platelet transfusion, 97% received ≥ 1 different ITP medication and 77% received ≥ 3. Most patients (73%) initiated romiplostim ≥ 1 year after ITP diagnosis (chronic phase). The mean duration of romiplostim treatment was 5.7 (SE 0.9) months, and the median was 1.4 months (IQR: 0.2, 6.5). Mean platelet count before romiplostim was 38 × 109/L, rising to 103 × 109/L within 1 month, and remaining 50‐150 × 109/L through up to 3 years of follow‐up. After romiplostim, 4% of patients had splenectomy, 6% received platelet transfusion, and 57% received just one ITP medication other than romiplostim.
Conclusion
The study provides valuable insights into the real‐world use of romiplostim in primary ITP in routine practice and highlighted the timing of romiplostim initiation at different ITP disease phases.
Keywords: bleeding, idiopathic thrombocytopenic purpura, platelet, primary immune thrombocytopenia, romiplostim, thrombopoietin receptor agonists
1. INTRODUCTION
Primary immune thrombocytopenia (previously known as idiopathic thrombocytopenic purpura; ITP) is a rare disorder characterised by peripheral blood platelet count below 100 × 109/L in the absence of any detectable underlying cause.1, 2 ITP, particularly when platelet counts are < 30 × 109/L, is associated with an increased risk of bleeding which can be major and, at times, fatal; bleeding risk increases with increasing age.3 The annual incidence of newly diagnosed ITP in adults is estimated to range from approximately 1.6 to 3.9 per 100 000 persons.4, 5, 6, 7, 8 Persistence or chronicity of the disorder is common and was reported to develop in approximately 67% of incident primary adult ITP patients after a mean follow‐up period of 18 months.5 The one‐year period prevalence of diagnosed ITP in adults (aged older than 16 or 18 years) is estimated to range from 4.6 to 12.1 per 100 000 persons.9, 10, 11
Current first‐line treatment for ITP includes corticosteroids and intravenous immunoglobulin (IVIg), while second‐line and subsequent treatments include splenectomy, thrombopoietin receptor agonists (TPO‐RAs), rituximab, immunosuppressants (azathioprine, cyclosporine and mycophenolate), cyclophosphamide, danazol and dapsone.2, 12 Complications of treatment, such as thromboembolic and bleeding risks after splenectomy, and infections caused by immunodeficiency‐inducing therapies (immunosuppressive drugs, splenectomy), contribute to mortality and morbidity.13, 14, 15 TPO‐RAs, romiplostim 16 and eltrombopag,17 are approved for use in chronic ITP in adults in whom ITP is refractory to other treatments. As shown in randomised double‐blind trials,16, 17, 18, 19, 20, 21, 22 TPO‐RAs stimulate megakaryopoiesis and increase platelet counts, resulting in fewer bleeding episodes and reduction in rescue medication use. Long‐term responses in patients who are no longer receiving TPO‐RAs (ie sustained remission) have been reported in the range of 10%‐32% of patients in clinical trials 22 and observational studies.23, 24, 25 Adverse events of interest for TPO‐RAs include bone marrow reticulin fibrosis, thromboembolic events, neutralising antibodies (romiplostim only), increased liver enzymes and cataracts (eltrombopag only).26, 27, 28, 29, 30
Romiplostim is a thrombopoietin‐mimetic peptibody licensed in the EU for use in splenectomised and non‐splenectomised patients with chronic ITP that is refractory to other treatments (eg corticosteroids, intravenous immunoglobulins).31 Romiplostim was first licensed in the EU in 2009 for use in splenectomised patients whose condition is refractory to other treatments; in 2014, the indication was expanded to include non‐splenectomised patients for whom surgery is contraindicated. The indication was restricted to adults during the conduct of this study, but romiplostim is currently approved in patients over 1 year of age. Romiplostim was first recommended by the National Institute for Health and Care Excellence (NICE) in 2011. Differing from the above approved label, NICE currently recommends romiplostim as an option for treating adults with chronic ITP if their condition is refractory to standard active treatments and rescue therapies, or they have severe disease and a high risk of bleeding that needs frequent courses of rescue therapies.32
The United Kingdom Adult Immune Thrombocytopenia (UKITP) Registry retrospectively and prospectively collects demographic and ITP‐related clinical data on adult patients with primary ITP enrolled by consent through a network of centres throughout the UK. 26, 33, 34 Treatment patterns associated with the use of romiplostim for ITP treatment in the clinical practice setting have been reported for Europe,25 but are not currently well understood for the UK. The aims of the study were to describe the use of romiplostim in patients with primary ITP in routine clinical practice in the UK, to describe the demographic and clinical characteristics of patients with ITP receiving romiplostim in the UK, and to report the use of ITP medications, pattern of platelet counts and bleeding events 6 months before romiplostim initiation and 6 months after romiplostim initiation.
2. METHODS
2.1. UK adult ITP registry
The UK Adult ITP Registry is a population‐based registry at the Royal London Hospital and Queen Mary University of London that collects data on ITP management from participating centres (National Research Ethic Service reference 07/H0718/57). Data are collected from hospital records (paper and electronic) and general practitioners’ records. Time points for data collections are at the time of registry enrolment and at least once annually during follow‐up. Data extraction takes place at local sites and is entered into a central database. Rigorous data checks are performed centrally in which outliers, missing data and potential erroneous entries are identified and rechecked against original sources from participating centres. This data checking process provides the registry with the highest achievable data quality and completeness. Data are additionally obtained from the UK National Health Service`s data provider NHS digital,35 through a data linkage and integrated with the data collected from medical notes and general practitioners records. NHS digital data sets consist of Hospital Episode Statistics (HES) data sets (admissions, outpatients, accident and emergency, and critical care) and mortality data from the Office of National Statistics (ONS). For this study, data utilised from the NHS digital consisted of demographic details, patients’ diagnoses, treatments (including blood and platelet transfusion, splenectomy, IVIg, anti‐D and plasmapheresis) and date of death. These were combined with the patients’ data collected from hospital and general practice records to form the overall data set which was analysed for this study.
2.2. Study design
A retrospective study of a cohort of ITP patients included in the adult UKITP Registry from October 2009 to 31 January 2015 was performed. The procedures were in accordance with the Helsinki Declaration.
2.3. Inclusion criteria
All adults (≥ 18 years) within the UK Adult ITP Registry diagnosed with primary ITP and who had received at least one dose of romiplostim after it became available in the UK in October 2009 were included in the study. Patients with prior involvement in clinical trials for romiplostim were excluded.
The first recorded use of romiplostim was defined as the index date. The pre‐index period was defined as a maximum of 6 months prior to the index date, except for comorbidities, which were included up to 12 months before index. For the present study, follow‐up started at the first exposure to romiplostim (index date) and continued until withdrawal from the registry, death (utilising data from ONS), loss to follow‐up or the end of the study period (31 January 2015), whichever was earlier.
2.4. Statistical analyses
To describe the cohort's characteristics, frequencies and percentages for categorical variables and the mean (SD) and median (interquartile range [IQR], minimum and maximum value) for continuous variables were used. ITP medication prior to romiplostim initiation was assessed from the time of diagnosis of ITP until romiplostim initiation, while ITP medication after romiplostim initiation was assessed from romiplostim initiation until end of follow‐up (the observation period). We note that these treatments may have been initiated after the romiplostim was stopped for any reason or may have been offered concurrently. Bleeding rates were summarised using event rates per 100 patient‐years during the 6 months before the index date and during 6 months after the index date. Patient‐time was variable in the 6 months before romiplostim initiation as some patients had started romiplostim within 6 months of the diagnosis of ITP. Summary statistics were used to describe platelet counts, duration of romiplostim therapy and maximum weekly dose. For summarising the platelet counts, all counts that occurred within a period were included to account for multiple records from repeat measures undertaken while monitoring individual patients due to potential inaccuracies of haematology analysers in platelet counting in severe thrombocytopenia. For measuring rate of bleeding events in person‐time, the number of events during the time at risk was divided by the total person‐time at risk. A 95% confidence interval (CI) was generated for each estimate.
3. RESULTS
3.1. Patients
Out of 1440 patients with primary ITP in the UK Adult ITP Registry, 118 adult patients were eligible for inclusion in this study. The baseline demographic and clinical characteristics of the study population are shown in Table 1. The median age at diagnosis was 58.5 years (IQR 35.8, 73.1), and 39% of patients were aged 65 or older. Forty‐nine per cent of patients were women, and the majority (81%) were Caucasian. The most common comorbidities were hypertension (36%), type 2 diabetes (18%) and other autoimmune disease (13%).
Table 1.
Baseline demographic and clinical characteristics of the study population
| Splenectomiseda (N = 26) | Non‐splenectomiseda (N = 92) | All (N = 118) | |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 15 (58) | 43 (47) | 58 (49) |
| Age at primary ITP diagnosis | 52.0 | 61.6 | 58.5 |
| Median (IQR) | (35.8, 64.4) | (35.8, 73.8) | (35.8, 73.1) |
| Age group at primary ITP diagnosis (n, %)b | |||
| 18 to < 30 y | 4 (15) | 16 (17) | 20 (17) |
| 30 to < 45 y | 5 (19) | 15 (16) | 20 (17) |
| 45 to < 65 y | 11 (42) | 21 (23) | 32 (27) |
| ≥65 y | 6 (23) | 40 (43) | 46 (39) |
| Time period of ITP diagnosisb | |||
| 1980‐1989 | 3 (12) | 1 (1) | 4 (3) |
| 1990‐1999 | 7 (27) | 4 (4) | 11 (9) |
| 2000‐2009 | 11 (42) | 33 (36) | 44 (37) |
| 2010‐2014 | 5 (19) | 54 (59) | 59 (50) |
| Ethnicity (n, %)b | |||
| Caucasian | 18 (69) | 78 (85) | 96 (81) |
| African/Caribbean | 0 | 0 | 0 |
| Asian | 4 (15) | 4 (4) | 8 (7) |
| Other ethnic group | 0 | 3 (3) | 3 (3) |
| Not specified | 4 (15) | 7 (8) | 11 (9) |
| Comorbiditiesc, n (%) | |||
| Autoimmune disease | 5 (19) | 10 (11) | 15 (13) |
| Candida infection | 1 (4) | 3 (3) | 4 (3) |
| Cataracts | 2 (8) | 6 (7) | 8 (7) |
| Chronic liver disease | 1 (4) | 1 (1) | 2 (2) |
| Depression/anxiety | 2 (8) | 8 (9) | 10 (8) |
| Helicobacter pylori infection | 2 (8) | 4 (4) | 6 (5) |
| Hypercholesterolemia/dyslipidemia | 2 (8) | 3 (3) | 5 (4) |
| Hypertension | 8 (31) | 34 (37) | 42 (36) |
| Malignancy | |||
| Solid | 2 (8) | 8 (9) | 10 (8) |
| Haematological | 4 (15) | 7 (8) | 11 (9) |
| Miscarriage | 1 (4) | 2 (2) | 3 (3) |
| Peptic ulcer | 1 (4) | 3 (3) | 4 (3) |
| Pneumonia | 4 (15) | 8 (9) | 12 (10) |
| Splenomegaly | ‐ | 3 (3) | 3 (3) |
| Renal failure | 1 (4) | 10 (11) | 11 (9) |
| Thyroid disease | 1 (4) | 10 (11) | 11 (9) |
| Thromboembolism | |||
| Venous | 1 (4) | 2 (2) | 3 (3) |
| Arterial | 2 (8) | 6 (7) | 8 (7) |
| Diabetes | |||
| Type 1 | 1 (4) | 2 (2) | 3 (3) |
| Type 2 | 2 (8) | 19 (21) | 21 (18) |
ITP, immune thrombocytopenia; IQR, interquartile range.
Before romiplostim initiation.
May not sum to 100% due to rounding.
Exceeds 100% due to multiple comorbidities in individual patients.
3.2. Treatment of ITP prior to romiplostim initiation
ITP therapies received before the initiation of romiplostim are shown in Table 2. Ninety‐seven per cent of patients received at least one prior treatment, and 77% received more than three treatments before romiplostim. Twenty‐two per cent of patients had previously undergone splenectomy. The most common ITP medications prior to romiplostim initiation were steroids (90%), IVIg (77%), rituximab (57%) and immunosuppressants (51%). Platelet transfusions were administered in 35% of patients.
Table 2.
ITP therapies during the 6 mo before romiplostim initiation
| Type of ITP therapy (n, %) | (N = 118) |
|---|---|
| Steroidsa | 106 (90) |
| Intravenous immunoglobulin | 91 (77) |
| Rituximab | 67 (57) |
| Immunosupressantsb | 60 (51) |
| Transfusion | 41 (35) |
| Splenectomy | 26 (22) |
| Danazol/dapsone | 19 (16) |
| Chemotherapyc | 14 (12) |
| Anti‐D | 13 (11) |
| Eltrombopag | 9 (8) |
| Number of different ITP medications used (n, %)d | |
| 0 | 3 (3) |
| 1 | 3 (3) |
| 2 | 21 (18) |
| ≥3 | 91 (77) |
Prednisolone, methylprednisolone, dexamethasone.
Azathioprine, mycophenolate, cyclosporine.
Cyclophosphamide, vinca alkaloids.
Exceeds 100% due to rounding.
3.3. Romiplostim treatment
The median time from ITP diagnosis to romiplostim initiation was 3.3 years (IQR: 0.9, 8.1) (Table 3). Almost three‐quarters of patients (73%) initiated romiplostim 1 year or more after ITP diagnosis, and 27% of patients initiated romiplostim within 1 year of ITP diagnosis (12% within 3 months, 6% between 3 and 6 months and 9% between 6 months and 1 year). The median time from ITP diagnosis to romiplostim initiation for those diagnosed between 2010 and 2014 was 0.9 years (IQR: 0.3, 1.9). The median maximum weekly dose of romiplostim was 3.0 μg/kg (IQR: 2.0, 6.0).
Table 3.
Romiplostim usage
| (N = 118) | |
|---|---|
| Age at romiplostim initiation (y), median (IQR) | 64.7 (43.2, 75.9) |
| Time from ITP diagnosis to romiplostim initiation (y), median (IQR) | 3.3 (0.9, 8.1) |
| Time period from ITP diagnosis to romiplostim initiation, n (%) | |
| <3 mo | 14 (12) |
| 3 to < 6 mo | 7 (6) |
| 6 mo to < 1 y | 11 (9) |
| 1 y to < 5 y | 39 (33) |
| ≥5 y | 47 (40) |
| Year of romiplostim initiation, n (%) | |
| 2009 | 1 (1) |
| 2010 | 5 (4) |
| 2011 (NICE recommendation) | 29 (25) |
| 2012 | 26 (22) |
| 2013 | 31 (26) |
| 2014 | 26 (22) |
| Duration of romiplostim administration, months | |
| Mean (SE) | 5.7 (0.9) |
| Median (IQR) | 1.4 (0.2, 6.5) |
| Median (IQR) maximum weekly dose of romiplostim, mcg/kg | 3.0 (2.0, 6.0) |
| At least 6 mo of follow‐up after the last romiplostim dose, n (%) | 84 (71) |
| Did not have romiplostim for more than 6 mo after receiving the last dose, n (%) | 45 (38) |
| Time from romiplostim initiation to the last dose for those who discontinued, n (%)a | |
| N | 84 |
| <1 mo | 37 (44) |
| 1 to < 3 mo | 8 (10) |
| 3 to < 6 mo | 10 (12) |
| 6 to < 12 mo | 10 (12) |
| >12 mo | 19 (23) |
NICE, National Institute for Health and Care Excellence.
Exceeds 100% due to rounding.
The mean duration of romiplostim treatment was 5.7 (SE: 0.9) months, and the median was 1.4 months (IQR: 0.2, 6.5). A total of 84 patients (71%) had at least 6 months follow‐up after the last recorded dose of romiplostim. The total duration of romiplostim use, defined as the time from romiplostim initiation to the last recorded dose of romiplostim, was estimated in these 84 patients and was ≥ 12 months in 19 patients (23%), ≥ 6 months in 29 patients (35%), ≥ 3 months in 39 patients (46%), ≥ 1 month in 47 patients (56%) and < 1 month in 37 patients (44%). A permissible gap for treatment interruption was not specified in this study. To assess discontinuation, the absence of any record of romiplostim was assessed ≥ 6 months after the last recorded dose of romiplostim. Out of the 84 patients with ≥ 6 months follow‐up after the last recorded dose of romiplostim, 45 patients had no record of romiplostim for more than 6 months after the last romiplostim dose was given and these patients were considered as having discontinued romiplostim therapy during the observed period.
3.4. Other ITP treatment during the observation period
During the observation period (after initiation of romiplostim until the end of follow‐up), 4 patients underwent splenectomy (3% of total patients or 4% of those who were non‐splenectomised before romiplostim [n = 92]) within a median (IQR) of 0.5 (0.3, 1.9) years, 6% received a platelet transfusion and 57% received only one additional ITP medication (other than romiplostim). The use of three or more ITP medications occurred in only 18% of patients. The most frequently administered ITP medications were steroids (32%), IVIg (27%), eltrombopag (25%) and immunosuppressants (20%) (Table 4). We were not able to establish whether these medications were used as rescue medications or for routine standard of care later in the course of ITP care. Of the 29 patients who received eltrombopag after initiation of romiplostim, the median time from romiplostim initiation to eltrombopag use was 22.7 months (IQR: 7.6, 29.0), with a reduced time to initiation in non‐splenectomised vs splenectomised patients (14.6 vs 28.4 months, respectively).
Table 4.
ITP therapies (other than romiplostim) during the observation period (from romiplostim initiation through end of follow‐up)
| Type of ITP therapy (n, %) | (N = 118) |
|---|---|
| Steroidsa | 38 (32) |
| Intravenous immunoglobulin | 32 (27) |
| Eltrombopag | 29 (25) |
| Transfusion | 24 (20) |
| Immunosupressantsb | 23 (19) |
| Chemotherapyc | 9 (8) |
| Rituximab | 7 (6) |
| Danazol/dapsone | 5 (4) |
| Splenectomyd | 4 (3) |
| Anti‐D | 2 (2) |
| Number of different other ITP therapies used (n, %) | |
| 0 | 0 |
| 1 | 67 (57) |
| 2 | 30 (25) |
| ≥3 | 21 (18) |
Prednisolone, methylprednisolone, dexamethasone.
Azathioprine, mycophenolate, cyclosporine.
Cyclophosphamide, vinca alkaloids.
Denominator includes 26 patients who underwent splenectomy before romiplostim initiation.
3.5. Platelet count
The mean platelet count within 2 weeks before romiplostim initiation was 38 × 109/L (95% CI: 27, 49). Within 1 month of initiating romiplostim, the mean platelet count was 103 × 109/L (95% CI: 89, 116) and was maintained in the range of > 50 to 150 × 109/L through up to 3 years of follow‐up (Figure 1).
Figure 1.
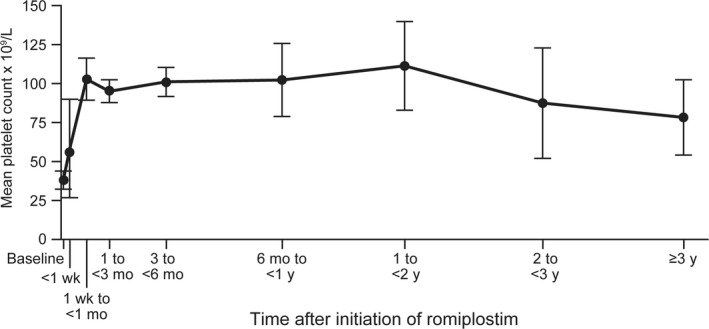
Mean (95% CI) platelet count (x 109/L) following romiplostim initiation
3.6. Bleeding
Ninety‐two (78%) patients had at least one bleeding event at any site (seven events per patient‐year during the up to 6‐month period before the initiation of romiplostim). Of 118 patients, the most frequent bleeding manifestations observed before romiplostim initiation were cutaneous (56 [48%] patients), epistaxis (28 [24%] patients), haematoma (24 [20%]), intraocular (24 [20%]) and oral (22 [18%]). One episode of intracranial haemorrhage before romiplostim initiation occurred in a splenectomised patient with a presenting platelet count < 30 × 109/L.
During the observational period within 6 months after romiplostim initiation, 34 patients (29%) had at least one bleeding event at any site (two events per patient‐year). Of 118 patients, the most frequent bleeding manifestations during the observational period were cutaneous (22 [19%]), epistaxis (10 [9%]), other gastrointestinal (non‐oral; 7 [6%]), haematoma (6 [5%]) and oral (6 [5%]).
4. DISCUSSION
This retrospective observational study in adults with primary ITP participating in the UKITP Registry was conducted to provide a better understanding of the treatment of primary ITP patients with romiplostim in routine clinical practice in the UK. The number of different types of ITP therapies used before and after romiplostim initiation within this UKITP cohort highlights the complexity of ITP treatment in routine practice and also likely reflects the high proportion of chronic, difficult‐to‐treat relapsed or refractory patients who are referred to the registry. Steurer et al25 reported a similar complexity of ITP treatment in a European observational study. The use of platelet transfusions was higher in the present study than in Steurer et al (35% vs 17%), which may reflect a higher proportion of patients with resistant disease and possible international variation in practice.
The timing of initiation of romiplostim during the study relative to the ITP diagnosis was affected by the time point in the patient's clinical history in which romiplostim became available. Although a few patients might have obtained romiplostim through individual funding requests prior to NICE guidance in 2011, most patients (95%) initiated romiplostim afterwards. In this study, for those diagnosed between 2010 and 2014 (consisting 50% of the romiplostim cohort), the median time from ITP diagnosis to romiplostim initiation was 0.9 years [IQR: 0.3, 1.9], shorter than the 3.3 years reported for the entire cohort. In Steurer et al, the median time from ITP diagnosis to romiplostim initiation was also 3.3 years, indicating that most patients may also have been diagnosed long before romiplostim availability.25
In line with NICE guidance, the majority of patients (98%) had received at least one standard ITP treatment prior to initiation with romiplostim. Of note, not all patients were in the chronic disease phase (currently defined as ≥ 1 year since ITP diagnosis), with 27% receiving romiplostim sooner than 1 year after ITP diagnosis, including 12% who were newly diagnosed (< 3 months since ITP diagnosis). An International Working Group changed the definition of chronic ITP from 6 months to 12 months post ITP diagnosis in 2009, which may explain at least in part the use of romiplostim in patients with <1 year since ITP diagnosis.1 A separate analysis of the UKITP Registry showed that platelet concentration < 50 × 109/L and bleeding within 3 months of ITP diagnosis were predictive factors for receipt of both first‐ and second‐line ITP therapies.34 Therefore, the use of romiplostim in patients who were not yet in the chronic phase may also reflect the selection of patients who were deemed likely to benefit from romiplostim based on their individual risk profiles.
Most patients (82%) initiated romiplostim more than 6 months after ITP diagnosis and nearly one‐fifth of patients (18%) initiated romiplostim within 6 months of ITP diagnosis. Although romiplostim was not yet indicated for use in non‐splenectomised patients prior to 2014, 78% of patients in this study were non‐splenectomised prior to romiplostim initiation. Similarly, in the Steurer et al25 European observational study, 66% of those who were started on romiplostim had not undergone prior splenectomy.
The mean duration of romiplostim use was 5.7 (SE: 0.9) months, while the median was shorter (1.4 months [IQR: 0.2, 6.5]). Despite the relatively short median duration of treatment, we note that a substantial proportion of patients had prolonged use, with 46% receiving at least 3 months of romiplostim treatment, while 35% received at least 6 months, and 23% received at least 1 year. The registry does not systematically collect information on the rationale for the treatment decisions; therefore, we were unable to determine the reasons for romiplostim discontinuation in the context of this study. In addition, this study did not define a permissible treatment gap. In Steurer et al, romiplostim discontinuation was due to the need to receive an alternative therapy in 27% and adverse drug reaction in 9% of patients. Based on prior literature, the observed patterns of romiplostim use may be due to perioperative administration 36 and/or attempts to limit costs for patients with good responses,37 reflecting patient heterogeneity, response heterogeneity and reactive therapy guided by platelet counts. Further studies are required to understand influences on duration of treatment. Despite the relatively short exposure to romiplostim and limited use of ITP medications after romiplostim initiation, patients continued to maintain platelet concentrations within the normal range (after administration of romiplostim, mean platelet counts were maintained in the range of 50 to 150 × 109/L for up to 3 years). Although remission rates of approximately 30% have previously been reported,22, 38 we were not able to address remission in this study due to the limitations of the data collection. The registry was not designed to facilitate evaluation of remission rates. Similarly, we caution that the design of the study precludes any conclusions on the effects of romiplostim on platelet concentrations and bleeding rates.
An important limitation of the study is the statistical precision of the estimates due to a small sample size compared to the total size of the registry. As the more severe and chronic cases of ITP are more likely to receive romiplostim based on the product labelling, the results may not be fully extrapolated to the overall primary ITP population, many of whom have milder disease phenotypes. Another limitation of the study is the lack of information on the severity of thrombocytopenia of ITP. Confounding for treatment remains the main challenge to the interpretation of this observational study due to changes during the course of the study in the romiplostim indication, allowing for second‐line use as well as newly diagnosed patients having access earlier in their treatment pathway compared to prevalent patients in this study. An additional confounder is the evolution in physician experience in prescribing romiplostim that occurred during the course of the study.
In conclusion, the results of this study highlight the complexity of ITP treatment in routine practice, with a wide number of different types of ITP therapeutic treatments used within this UKITP cohort. In particular, the study provides insight into the use of romiplostim in patients with primary ITP in routine practice within the UK. Initiation with romiplostim occurred at different ITP disease phases, though mostly during the chronic disease phase, and mostly after receipt of two or more other ITP therapies. Following romiplostim administration, most patients received two or fewer ITP medications with mean platelet count levels being maintained in the > 50 to 150 × 109/L range through up to 3 years of follow‐up. Despite limitations such as potential selection bias into the registry, the small sample size and the heterogeneous nature of the selected cohort, the UKITP Registry provides valuable insight into the real‐world ITP patient population prescribed with romiplostim in the UK.
AUTHOR CONTRIBUTION
Indraraj Umesh Doobaree, Adrian Newland, Drew Provan and Raghava Nandigam were part of the UKITP Registry team and were employed by Queen Mary, University of London, during the conduct of this work. Indraraj Umesh Doobaree was previously employed by GSK. Adrian Newland has received research support from Amgen, GSK and Novartis; he has participated on advisory boards for Amgen, Argenx, Dova Pharmaceuticals, Novartis and Shionogi, and speakers bureaus for Amgen and Novartis. Vickie McDonald is currently part of the UKITP Registry and is employed by Queen Mary, University of London; she has received a travel grant from Novartis. Drew Provan has received honoraria from Amgen and Novartis. Anouchka Seesaghur, Hitan Patel and Sally Wetten are employees of Amgen and hold Amgen stock. Lesley Mensah was an Amgen employee during the conduct of this work and holds Amgen stock. Sandrine Leroy was a contract worker funded by Amgen during the conduct of this work. The UKITP Registry has received funding from GSK, Novartis, Amgen, the ITP Support Association (UK) and Barts Health Charity.
ACKNOWLEDGEMENTS
This study was supported by Amgen (Europe) GmbH. We are grateful to our collaborators at their respective institution (s) for their contribution in recruiting participants and collecting data. The list of centre directors contributing to the adult ITP registry can be found at: http://www.ukitpforum.org/index.php/en/itp-clinical-centres/2-uncategorised/46-itp-centre-directors Editorial assistance was funded by Amgen (Europe) GmbH and provided by Wanda Krall, PhD, of Wanda Krall Medical Communications.
Doobaree IU, Newland A, McDonald V, et al. Primary immune thrombocytopenia (ITP) treated with romiplostim in routine clinical practice: retrospective study from the United Kingdom ITP Registry. Eur J Haematol. 2019;102:416–423. 10.1111/ejh.13221
This work was sponsored by Amgen (Europe) GmbH.
REFERENCES
- 1. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386‐2393. [DOI] [PubMed] [Google Scholar]
- 2. Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168‐186. [DOI] [PubMed] [Google Scholar]
- 3. Cohen YC, Djulbegovic B, Shamai‐Lubovitz O, Mozes B. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Intern Med. 2000;160(11):1630‐1638. [DOI] [PubMed] [Google Scholar]
- 4. Neylon AJ, Saunders PW, Howard MR, Proctor SJ, Taylor PR, Northern Region Haematology G . Clinically significant newly presenting autoimmune thrombocytopenic purpura in adults: a prospective study of a population‐based cohort of 245 patients. Br J Haematol 2003;122(6):966‐974. [DOI] [PubMed] [Google Scholar]
- 5. Moulis G, Palmaro A, Montastruc JL, Godeau B, Lapeyre‐Mestre M, Sailler L. Epidemiology of incident immune thrombocytopenia: a nationwide population‐based study in France. Blood. 2014;124(22):3308‐3315. [DOI] [PubMed] [Google Scholar]
- 6. Abrahamson PE, Hall SA, Feudjo‐Tepie M, Mitrani‐Gold FS, Logie J. The incidence of idiopathic thrombocytopenic purpura among adults: a population‐based study and literature review. Eur J Haematol. 2009;83(2):83‐89. [DOI] [PubMed] [Google Scholar]
- 7. Frederiksen H, Schmidt K. The incidence of idiopathic thrombocytopenic purpura in adults increases with age. Blood. 1999;94(3):909‐913. [PubMed] [Google Scholar]
- 8. Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85(3):174‐180. [DOI] [PubMed] [Google Scholar]
- 9. Terrell DR, Beebe LA, Neas BR, Vesely SK, Segal JB, George JN. Prevalence of primary immune thrombocytopenia in Oklahoma. Am J Hematol. 2012;87(9):848‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bennett D, Hodgson ME, Shukla A, Logie JW. Prevalence of diagnosed adult immune thrombocytopenia in the United Kingdom. Adv Ther. 2011;28(12):1096‐1104. [DOI] [PubMed] [Google Scholar]
- 11. Landgren O, Gridley G, Fears TR, Caporaso N. Immune thrombocytopenic purpura does not exhibit a disparity in prevalence between African American and White veterans. Blood. 2006;108(3):1111‐1112. [DOI] [PubMed] [Google Scholar]
- 12. Provan D, Newland AC. Current Management of Primary Immune Thrombocytopenia. Adv Ther. 2015;32(10):875‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boyle S, White RH, Brunson A, Wun T. Splenectomy and the incidence of venous thromboembolism and sepsis in patients with immune thrombocytopenia. Blood. 2013;121(23):4782‐4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Portielje JE, Westendorp RG, Kluin‐Nelemans HC, Brand A. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood. 2001;97(9):2549‐2554. [DOI] [PubMed] [Google Scholar]
- 15. Langeberg WJ, Schoonen WM, Eisen M, Gamelin L, Stryker S. Thromboembolism in patients with immune thrombocytopenia (ITP): a meta‐analysis of observational studies. Int J Hematol. 2016;103(6):655‐664. [DOI] [PubMed] [Google Scholar]
- 16. Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double‐blind randomised controlled trial. Lancet. 2008;371(9610):395‐403. [DOI] [PubMed] [Google Scholar]
- 17. Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6‐month, randomised, phase 3 study. Lancet. 2011;377(9763):393‐402. [DOI] [PubMed] [Google Scholar]
- 18. Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2009;373(9664):641‐648. [DOI] [PubMed] [Google Scholar]
- 19. Yang R, Li J, Jin J, et al. Multicentre, randomised phase III study of the efficacy and safety of eltrombopag in Chinese patients with chronic immune thrombocytopenia. Br J Haematol. 2017;176(1):101‐110. [DOI] [PubMed] [Google Scholar]
- 20. Tarantino MD, Bussel JB, Blanchette VS, et al. Romiplostim in children with immune thrombocytopenia: a phase 3, randomised, double‐blind, placebo‐controlled study. Lancet. 2016;388(10039):45‐54. [DOI] [PubMed] [Google Scholar]
- 21. Shirasugi Y, Ando K, Miyazaki K, et al. Romiplostim for the treatment of chronic immune thrombocytopenia in adult Japanese patients: a double‐blind, randomized Phase III clinical trial. Int J Hematol. 2011;94(1):71‐80. [DOI] [PubMed] [Google Scholar]
- 22. Newland A, Godeau B, Priego V, et al. Remission and platelet responses with romiplostim in primary immune thrombocytopenia: final results from a phase 2 study. Br J Haematol. 2016;172(2):262‐273. [DOI] [PubMed] [Google Scholar]
- 23. Gonzalez‐Lopez TJ, Pascual C, Alvarez‐Roman MT, et al. Successful discontinuation of eltrombopag after complete remission in patients with primary immune thrombocytopenia. Am J Hematol. 2015;90(3):E40‐E43. [DOI] [PubMed] [Google Scholar]
- 24. Cervinek L, Mayer J, Doubek M. Sustained remission of chronic immune thrombocytopenia after discontinuation of treatment with thrombopoietin‐receptor agonists in adults. Int J Hematol. 2015;102(1):7‐11. [DOI] [PubMed] [Google Scholar]
- 25. Steurer M, Quittet P, Papadaki HA, et al. A large observational study of patients with primary immune thrombocytopenia receiving romiplostim in European clinical practice. Eur J Haematol. 2017;98(2):112‐120. [DOI] [PubMed] [Google Scholar]
- 26. Rizvi H, Butler T, Calaminici M, et al. United Kingdom immune thrombocytopenia registry: retrospective evaluation of bone marrow fibrosis in adult patients with primary immune thrombocytopenia and correlation with clinical findings. Br J Haematol. 2015;169(4):590‐594. [DOI] [PubMed] [Google Scholar]
- 27. Kuter DJ, Bussel JB, Newland A, et al. Long‐term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol. 2013;161(3):411‐423. [DOI] [PubMed] [Google Scholar]
- 28. Kuter DJ, Mufti GJ, Bain BJ, Hasserjian RP, Davis W, Rutstein M. Evaluation of bone marrow reticulin formation in chronic immune thrombocytopenia patients treated with romiplostim. Blood. 2009;114(18):3748‐3756. [DOI] [PubMed] [Google Scholar]
- 29. Saleh MN, Bussel JB, Cheng G, et al. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long‐term, open‐label EXTEND study. Blood. 2013;121(3):537‐545. [DOI] [PubMed] [Google Scholar]
- 30. Ghanima W, Geyer JT, Lee CS, et al. Bone marrow fibrosis in 66 patients with immune thrombocytopenia treated with thrombopoietin‐receptor agonists: a single‐center, long‐term follow‐up. Haematologica. 2014;99(5):937‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000942/WC500039537.pdf. Nplate Summary of Product Characteristics 2018. Accessed May 4, 2018.
- 32. https://www.nice.org.uk/guidance/ta221/chapter/1-Guidance. Romiplostim for the treatment of chronic immune (idiopathic) thrombocytopenic purpura. National Institute for Health and Care Excellence 2014;Technology appraisal guidance [TA221].
- 33. Sarpatwari A, Provan D, Erqou S, Sobnack R, David Tai FW, Newland AC. Autologous 111 In‐labelled platelet sequestration studies in patients with primary immune thrombocytopenia (ITP) prior to splenectomy: a report from the United Kingdom ITP Registry. Br J Haematol. 2010;151(5):477‐487. [DOI] [PubMed] [Google Scholar]
- 34. Doobaree IU, Conway K, Nandigam R, Provan D. Clinical characteristics and treatment pattern of newly diagnosed primary immune thrombocytopenia cases in the United Kingdom immune thrombocytopenia (ADULT) registry. Haematologica. 2016;101:139‐140. [Google Scholar]
- 35.Copyright © 2017, Re‐used with the permission of The Health & Social Care Information Centre.
- 36. Ramakrishna R, Rehman A, Ramakrishna S, Alexander W, Yeo WW. Use of romiplostim in patients with chronic idiopathic thrombocytopenic purpura during perioperative period. Intern Med J. 2015;45(7):718‐724. [DOI] [PubMed] [Google Scholar]
- 37. Mitrovic M, Elezovic I, Suvajdzic‐Vukovic N. ‘On‐demand’ romiplostim therapy in immune thrombocytopenia. J Clin Pharm Ther. 2016;41(3):351‐353. [DOI] [PubMed] [Google Scholar]
- 38. Marshall AL, Scarpone R, De Greef M, Bird R, Kuter DJ. Remissions after long‐term use of romiplostim for immune thrombocytopenia. Haematologica. 2016;101(12):e476‐e478 [DOI] [PMC free article] [PubMed] [Google Scholar]


