dear editor, Moderate‐to‐severe atopic dermatitis (AD) can affect different parts of the body and some adults have more localized AD, such as face and neck involvement.1 Compared with nonexposed areas, such as the trunk, exposed areas such as the face, neck and hands could be more vulnerable to environmental allergens and irritants, and can be more refractory to topical therapies. Local adverse effects of topical therapies are more likely to occur on the face and neck.1 Additional pathologies, such as contact dermatitis, can complicate the clinical picture and response of exposed areas to treatment. Microbial factors (e.g. Malassezia spp.) in specific regions may also influence AD severity on the face and neck.2
Dupilumab, a fully human VelocImmune® ‐derived monoclonal antibody against interleukin (IL)‐4Rα that inhibits signalling of IL‐4 and IL‐13,3, 4 is approved for treatment of inadequately controlled moderate‐to‐severe AD in adults. The efficacy and safety of dupilumab has been assessed in adults with moderate‐to‐severe AD in several clinical trials, including two phase III trials of dupilumab monotherapy (LIBERTY AD SOLO 1, LIBERTY AD SOLO 2)5 and with concomitant topical corticosteroids (TCS; LIBERTY AD CAFÉ, LIBERTY AD CHRONOS).6, 7 These large trials showed that dupilumab significantly improved the severity and extent of AD, as measured by the Eczema Area and Severity Index (EASI), vs. placebo.
The EASI is a composite score of four anatomical regions: head/neck; upper extremities; trunk; lower extremities.8 Herein, we report the disease burden by anatomical region at baseline and assess the impact of dupilumab treatment on EASI for each region in the SOLO 1, SOLO 2, CAFÉ and CHRONOS trials.
Study methodologies have been reported previously.5, 6, 7 The studies were conducted in accordance with the Declaration of Helsinki, (see Supporting Information for full ethics statement). In this post‐hoc analysis, the efficacy of subcutaneous dupilumab 300 mg every 2 weeks (q2w) or every week (qw) vs. placebo was evaluated by assessing the least‐squares (LS) mean percentage change from baseline in EASI by anatomical region at weeks 4, 16 (all trials) and 52 (CHRONOS only). SOLO 1 and SOLO 2 data were pooled by treatment group. Patients in CAFÉ and CHRONOS received a standardized concomitant TCS regimen. The full analysis set was analysed. The last‐observation‐carried‐forward method was implemented to impute data missing or censored after rescue medication usage. Data were not adjusted for multiplicity; therefore, P‐values are nominal and based on treatment difference (dupilumab vs. placebo) in LS mean percentage change, using an ancova model with baseline measurement as covariate and treatment, region and baseline IgA strata as fixed factors. Additional fixed factors included study identifier in SOLO 1 and SOLO 2, and prior ciclosporin A use (yes/no) in CAFÉ.
At baseline, EASI levels for each anatomical region were generally comparable throughout treatment groups in each trial (Fig. 1); relative regional contributions to total scores were lowest for head/neck and highest for lower extremities. This partly reflects relative differences in percentage body surface area (%BSA) among different regions. When absolute extent and severity of lesions were compared in different regions (i.e. without correcting for relative %BSA), EASI levels remained lowest in the head/neck area and were highest in the upper extremities. Considering these baseline differences, comparative assessments of EASI across anatomical regions are better achieved using percentage change rather than absolute change.
Figure 1.
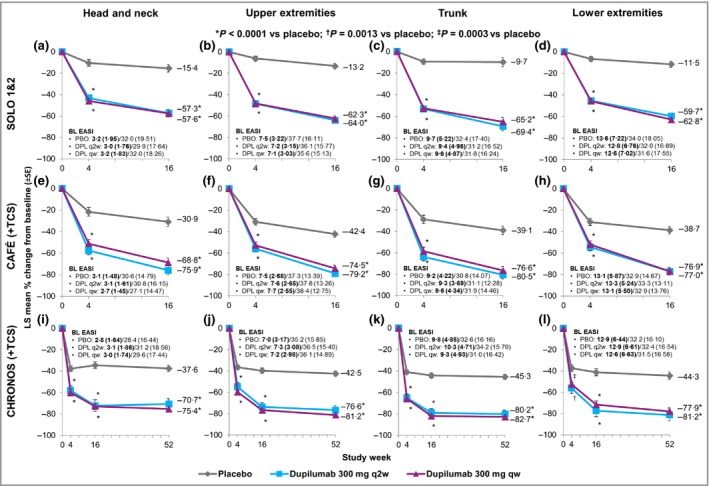
Least‐squares (LS) mean percentage change in Eczema Area and Severity Index (EASI) from baseline, over time, by anatomical region. EASI was obtained for the head/neck, upper extremities, trunk and lower extremities in four phase III clinical trials: (a–d) SOLO 1 and SOLO 2 (pooled), (e–h) CAFÉ and (i–l) CHRONOS. Patients in CAFÉ and CHRONOS received concomitant topical corticosteroids. Baseline EASI data are given as mean (SD). Numbers in bold and nonbold are baseline relative scores with weighting for percentage body surface area (%BSA) (i.e. corrected by the coefficient 0·1 for head/neck, 0·2 for upper extremities, 0·3 for trunk and 0·4 for lower extremities) and absolute scores without %BSA weighting, respectively. The analyses reported here are based on percentage reduction (i.e. improvement) from baseline, which is the same whether or not the %BSA weighting is applied. BL, baseline; PBO, placebo; DPL, dupilumab; q2w, every two weeks; qw, every week.
Compared with placebo, dupilumab treatment was associated with a significantly greater percentage improvement in EASI from baseline to week 16 across all anatomical regions in each trial (Fig. 1). Similar results were seen at week 4 in all trials (Fig. 1) and at week 52 in CHRONOS (Fig. 1i–l). Safety data were previously reported for the overall study populations.5, 6, 7 Overall rates of adverse events (AE) were similar across treatment groups in each study; the most common for dupilumab were injection‐site reactions and conjunctivitis.
In summary, treatment with dupilumab 300 mg q2w (approved) or qw (not approved) significantly improved disease severity in all anatomical regions in patients with moderate‐to‐severe AD. These data suggest that all anatomical regions are equally responsive to dupilumab. Significant improvements were observed as early as week 4 for all anatomical regions. Week 52 data from CHRONOS indicate that improvement is maintained with continuous treatment in most patients and throughout all anatomical regions. Dupilumab had an overall acceptable safety profile in all four trials.
Supporting information
Appendix S1 Author affiliations; Conflicts of interest; full ethics statement; and Acknowledgments.
Acknowledgments
Funding sources: this study was funded by Sanofi and Regeneron Pharmaceuticals.
Conflicts of interest: see Supporting Information.
See Supporting Information for author affiliations.
References
- 1. Draelos ZD. Use of topical corticosteroids and topical calcineurin inhibitors for the treatment of atopic dermatitis in thin and sensitive skin areas. Curr Med Res Opin 2008; 24:985–94. [DOI] [PubMed] [Google Scholar]
- 2. Darabi K, Hostetler SG, Bechtel MA, Zirwas M. The role of Malassezia in atopic dermatitis affecting the head and neck of adults. J Am Acad Dermatol 2009; 60:125–36. [DOI] [PubMed] [Google Scholar]
- 3. MacDonald LE, Karow M, Stevens S et al Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci USA 2014; 111:5147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL‐4/IL‐13 pathway in atopic diseases. Expert Rev Clin Immunol 2017; 13:425–37. [DOI] [PubMed] [Google Scholar]
- 5. Simpson EL, Bieber T, Guttman‐Yassky E et al Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375:2335–48. [DOI] [PubMed] [Google Scholar]
- 6. de Bruin‐Weller M, Thaçi D, Smith CH et al Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo‐controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol 2018; 178:1083–101. [DOI] [PubMed] [Google Scholar]
- 7. Blauvelt A, de Bruin‐Weller M, Gooderham M et al Long‐term management of moderate‐to‐severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1‐year, randomised, double‐blinded, placebo‐controlled, phase 3 trial. Lancet 2017; 389:2287–303. [DOI] [PubMed] [Google Scholar]
- 8. Hanifin JM, Thurston M, Omoto TM et al The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. Exp Dermatol 2001; 10:11–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Author affiliations; Conflicts of interest; full ethics statement; and Acknowledgments.


