Abstract
Aim
Ethnic Chinese women are one of the populations at high risk of gestational diabetes mellitus (GDM) internationally. This systematic review aimed to determine which dietary intervention strategies were found to be effective in improving glycaemic control and pregnancy outcomes among ethnic Chinese women with GDM.
Methods
The review protocol was registered with PROSPERO (CRD42016043585). Eight English and four Chinese language databases were searched for randomised controlled trials and cohort studies of dietary intervention among ethnic Chinese women with GDM. Review Manager 5.3 and GRADE criteria were used in meta‐analysis and assessment of quality of evidence.
Results
Included studies comprised 3944 women in 29 eligible studies. Compared to standard treatment, low glycaemic index (GI) diets, low glycaemic load (GL) diets and fibre‐enriched diets were associated with a reduction in fasting plasma glucose, 2‐hour plasma glucose and HbA1c, and improved neonatal outcomes. Low GL diets were associated with reduced caesarean section risk.
Conclusions
In ethnic Chinese women with GDM, low GI diets, low GL diets and fibre‐enriched diets were associated with improved glycaemic control and pregnancy outcomes. Given the lack of direct comparison of these three diets, future direct comparison trials are necessary to determine optimal dietary intervention strategies.
Keywords: diabetes, diet, gestational, glycaemic load, pregnancy outcome, self‐management
Introduction
Gestational diabetes mellitus (GDM), defined as onset or first recognition of glucose intolerance during pregnancy,1 adversely affects the health of both mother and offspring (with the potential to produce a perpetuating cycle of negative outcomes).2 Currently, approximately 10–30% of pregnant women in Australia are diagnosed with GDM depending on diagnostic criteria and ethnicity.3, 4 A 20% increase in prevalence of GDM in Australia occurred prior to changes in diagnostic criteria and was associated with increased GDM risk factors.5 Subsequently, use of the more inclusive diagnostic criteria further increased GDM prevalence by up to 30%.6 Women with GDM are at increased risk of short‐ and long‐term maternal complications, such as pre‐eclampsia and type 2 diabetes respectively.7 Macrosomia is the principal adverse neonatal outcome leading to increased risk of caesarean section, birth trauma and metabolic disease in later life.7, 8 However, adverse pregnancy outcomes are reduced with optimal glycaemic control.9, 10, 11, 12 Improvements in glycaemic control and pregnancy outcomes are viewed as key indicators of effective GDM management.
Despite the need for effective GDM management, examination of recent systematic reviews indicates there is limited evidence available in the English language literature to support clinicians in determining which specific dietary intervention is the most effective in optimising both glycaemic control and pregnancy outcomes.13, 14 Given dietary practices differ across cultures,15 culturally acceptable dietary intervention is viewed as critical to the optimal management of GDM.9, 10, 11, 12, 16
One of the largest groups of migrants arriving in western countries is those of ethnic Chinese background.17, 18 Their global migration patterns have contributed to an increased GDM prevalence in western countries, as ethnic Chinese women are known to be at high risk of GDM.10 In Australia, approximately 12% of family migration currently is from China.17 As Chinese cultural practices seem to be maintained irrespective of the country in which Chinese women currently live, it has been recommended that health professionals, regardless of the country in which they practise, would benefit by increased awareness of Chinese dietary habits.15, 19, 20, 23, 24, 25
Although a few systematic reviews have focused on ethnic Chinese women with GDM and shown the need for culturally acceptable dietary interventions,26, 27 no systematic review in English and/or Chinese language literature has evaluated which dietary intervention strategies are clinically effective for this population worldwide. Therefore, the aim of the present study was to complete a systematic review to determine which dietary intervention strategies were found to be effective in improving glycaemic control and pregnancy outcomes among ethnic Chinese women living with GDM.
Methods
This systematic review was conducted in accordance with the Cochrane Handbook guidelines28 and was registered and updated in the PROSPERO International prospective register of systematic reviews (CRD42016043585). This review examined eight English language medical, maternal, nursing and social science databases (EBM Reviews, Medline, EMBASE, Scopus, PsycINFO, CINAHL, Maternity & Infant Care and Web of Science) and four Chinese language biomedical databases (Wangfang, VIP Information, Chinese National Knowledge Infrastructure and Chinese Medical Current Content). The systematic search in English databases was developed based on the population of interest (‘ethnic Chinese’ and ‘GDM’) as used in the selection criteria and illustrated in PICO (shown in Table 1) using MeSH and EMTREE terms as well as text words in OVID Medline and OVID Embase, respectively, and translated to other databases as appropriate. Because of the small number of relevant publications found after searching for any studies related to Chinese women with GDM, the search strategy was modified to extend to any GDM studies of Asian populations that analysed Asian subgroups which included a Chinese ethnic subgroup.
Table 1.
Determination of selection criteria based on PICO of research question ‘which dietary intervention strategies were found to be effective in improving glycaemic control and pregnancy outcomes among ethnic Chinese women with gestational diabetes mellitus’
| Participants (P) | Intervention (I) | Comparison (C) | Outcomes (O) | Study type | Limits | |
|---|---|---|---|---|---|---|
| Inclusion criteria |
Chinese women with gestational diabetes, including Chinese women from China, Hong Kong, Macau, Taiwan, and other Asian and western countries. Any age |
Any dietary intervention strategies different from the standard dietary intervention | Standard dietary intervention in a clinical setting |
Glycaemic control included fasting plasma glucose, 2‐hour plasma glucose and HbA1c Any gestational diabetes‐related maternal and neonatal outcomes |
Randomised controlled trials Cohort studies |
Only including studies written in English or Chinese Any years |
| Exclusion criteria |
Multiple pregnancies, pre‐existing diabetes in pregnancy, type 1 and type 2 diabetes Not ethnic specified |
Case–control studies Editorial Narrative review |
The key search terms were ‘gestational diabetes’ and a list of Asian countries. An example of a full search strategy in Ovid Medline is shown in Table S1, Supporting Information. The Chinese translation of GDM is ‘diabetes during pregnancy’ in Chinese characters and is the only commonly used phrase in Mandarin. GDM in English and traditional and simplified Chinese were used as key search terms in the Chinese databases. Using ‘gestational diabetes’ as the only keyword search in Chinese databases was particularly sensitive. Another key search term ‘intervention’ (in English and Chinese) was used in Chinese databases to search for studies related to GDM and intervention. The full search strategy was checked by a senior systematic review researcher and a university librarian, who regularly conducts and provides assistance with systematic reviews. Initially, all databases were searched with no date and language restrictions from inception to 28 July 2016. Prior to submission for publication, an updated literature search was conducted on 3 June 2018 to ensure inclusion of all recent relevant studies. Titles, abstracts and keywords of retrieved articles were screened by a bilingual researcher according to the selection criteria detailed below and in Table 1. Duplicates were deleted. Full texts of the articles were retrieved for further assessment if information given in abstracts suggested that studies met the selection criteria. If there was any doubt regarding article inclusion based on the information given in the title and abstract, the full article was retrieved for clarification. The bibliographies of included studies were further searched for additional studies. Endnote X7 was used to manage search results.
All English and Chinese cohort studies and randomised controlled trials (RCTs), comparing a dietary intervention strategy with local standard dietary care among ethnic Chinese singleton pregnancies in women with GDM were included. Given the possible variation in classifications of what constituted ‘standard dietary care’ and ‘intervention strategy’ between countries and hospitals, all dietary strategies were then categorised by the reviewers according to the description given in the included papers. Inclusion of cohort studies was sought to avoid exclusion of relevant human nutrition evidence that could be found in a non‐randomised observational design (classified as high level evidence according to NHMRC guidelines).29 Ethnic Chinese women were defined as women of Chinese descent from China, Hong Kong, Macau and Taiwan, and Chinese specified migrants in western and other Asian countries. Studies were excluded if patients had pre‐existing diabetes (type 1 and type 2 diabetes) or pregnancies with multiple offspring. There was no restriction on length of dietary intervention.
This systematic review aimed to compare effectiveness of dietary intervention strategies on glycaemic control and reduction of adverse maternal and neonatal outcomes, as the indicators of improved GDM self‐management among ethnic Chinese women. The specific outcomes of interest included maternal glycaemic control parameters (fasting plasma glucose, 2‐hour plasma glucose and HbA1c levels), maternal events (pre‐eclampsia; caesarean section) and neonatal clinical outcomes of GDM (macrosomia: birth weight ≥ 4 kg; large for gestational age: birth weight > 90th percentile; small for gestational age: birth weight < 10th percentile; preterm birth: <37 weeks’ gestation; neonatal intensive care unit admission; hypoglycaemia; jaundice; respiratory distress) as defined by the International Association of Diabetes in Pregnancy Study Group, Working Group on Outcome Definitions.30
Quality assessment was conducted using the Cochrane Collaboration tool for assessing risk of bias of RCT, where bias was classified into six domains: selection, performance, detection, attrition, reporting and other bias.28, 31 The ‘other’ domain referred to dietary intervention compliance. Dietary compliance was classified as low risk when the study design appropriately considered and evaluated participants’ adherence to assigned dietary regimen. Meal or fibre supplement provided by researchers with dietary intake assessment was rated as low risk of bias in dietary intervention compliance. Weekly or fortnightly regular telephone or face‐to‐face dietary review by dietitians, and dietitian‐initiated intervention if necessary, were also rated as low risk of bias in the compliance domain. Any study that reported no dietary follow up was rated as high risk of bias in the compliance domain. Studies with no report on dietary follow up appointments were rated as unknown dietary compliance. ROBINS‐I tool was used for assessing risk of bias of cohort studies with a rating from low, moderate or serious to critical.32 Because of the limited Chinese language literacy of the second reviewer, only English language papers were re‐assessed by the second reviewer (RA). Detailed discussions between the two reviewers took place to review interpretive inconsistencies until consensus was achieved.
Data extraction was completed using a Cochrane data collection form for intervention reviews28 which included first author's name, year of publication, location of study, participants’ demographics, number of participants, details of study design, duration of intervention, intervention diet characteristics, study design associated with dietary compliance, compliance assessment, and outcomes of interest (glycaemic control and pregnancy outcomes). Statistical analysis was carried out using Review Manager 5.3 software33 and interpretation and presentation of the results were confirmed with a statistician. Clinical homogeneity was satisfied when participants, interventions, outcome measures and timing of outcome measurement were considered to be similar. Results of clinically and statistically homogenous trials were pooled and analysed using random‐effects meta‐analysis to provide estimates of the efficacy of the interventions. Taking into account possible changes in Chinese dietary habits after migration to western countries, results were pooled according to migration status where necessary. Continuous outcomes were expressed as weighted mean differences with a 95% confidence interval (CI), whereas dichotomous outcomes were expressed as relative risks with a 95% CI.
The quality of the body of evidence of each meta‐analysis (with at least two studies) was assessed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.34 The quality of evidence of each outcome was determined by criteria that might indicate problems with quality (including risk of bias, inconsistency, indirectness, imprecision and publication bias) or adequacy of the quality of evidence which relates to cohort studies only (including large magnitude of effect, dose response and effect of all plausible confounding factors).34 The assessment of overall risk of bias of the body of evidence for each outcome was used to determine whether there were limitations for GRADE.34 ‘Inconsistency’ was assessed using the forest plot and the I 2 test, where I 2 values over 40% indicated moderate to high heterogeneity. ‘Indirectness’ was related to the uncertainty about the applicability of the evidence to the research question. ‘Imprecision’ was defined as small sample sizes across the body of evidence or wide CIs to indicate uncertainty about the effect. ‘Possible publication bias’ was assessed using a funnel plot of each study's effect size against standard error if there were more than 10 studies in a meta‐analysis, as test power was too low to distinguish chance from real asymmetry if there were fewer than 10 studies.28
Results
An initial search of both English and Chinese databases resulted in identification of 13 537 studies. After duplicates were removed, 8256 studies were further screened based on title and abstract, resulting in 103 papers for full text screening. Of those, 78 papers were excluded, as per Figure 1. Variation in reasons for exclusion between English language and Chinese language studies was observed. The key reasons for exclusion of English language papers were lack of differentiation between the ethnic Chinese population and other groups included in the data analysis (number of studies = 32); inclusion of all types of diabetes during pregnancy (n = 6); study designs other than cohort studies or RCTs (n = 3) and focus on evaluation of individualised interventions (n = 1). The major reasons for excluding Chinese language papers were: did not meet study design inclusion requirements (n = 19), inclusion of other intervention strategies in addition to dietary strategies (n = 6) and other major foci of interest regarding dietary intervention strategies such as energy calculation, diet quality and lipid profile (n = 3). After updating the search on 3 June 2018, four more publications conducted in China and written in Chinese were found. One Chinese language systematic review examining the effectiveness of glycaemic load education on pregnancy outcomes among Chinese women with GDM was also identified.35 Full texts of studies included in that systematic review were obtained and matched with included studies in this review. No additional study was identified for inclusion. Finally, 29 studies (languages written: 4 English and 25 Chinese) were included in this review, all conducted in China across cities with different levels of prosperity. The strategy of including Asian populations in the literature search instead of the Chinese ethnic subgroup alone, allowed inclusion of studies that did not specify Chinese women as the primary focus of interest. A detailed flow diagram of study selection using the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement28 is shown in Figure 1.
Figure 1.
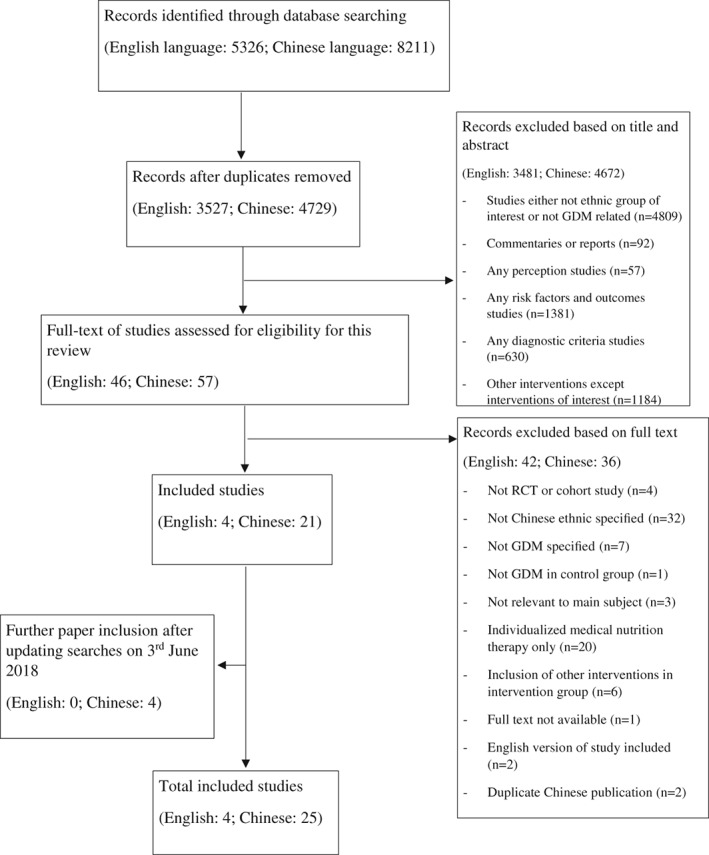
Flow diagram of the study selection.
Among the 29 included studies in which different dietary intervention strategies were compared, 7 types of dietary intervention strategy were identified and categorised according to descriptions outlined in Table 2. They included individualised dietary intervention, food exchange education about all food groups, low glycaemic index (GI) diets, low glycaemic load (GL) diets, fibre‐enriched diets, dietary approaches to stop hypertension (DASH) diet and a polyunsaturated fatty acid (PUFA) rich diet. Because either individualised diet or food exchange education was used in the control groups of these studies, these 7 types of intervention strategy were assessed using 8 different comparisons as shown in Table S2. Wang (2016)86 was the only study that compared two dietary interventions (which were low GI and low GL diets) using food exchange education as the control (see Table S2). Table 3 summarises the characteristics of included studies and is presented according to category of intervention comparison. Among the 29 studies, 5 were cohort studies, with 2 examining food exchange intervention studies64, 65 and the remaining 3 examining a low GI diet,68 a low GL diet82 and a fibre‐enriched diet.78
Table 2.
Outline of dietary intervention strategies
| Dietary intervention | Description |
|---|---|
| Individualised dietary intervention | Individualised dietary advice and meal plans according to the calculation of daily energy requirement and macronutrient composition distribution from individual anthropometry information. Small frequent meals are recommended |
| Food exchange education of all food groups | Serving sizes needed in all food groups are calculated according to recommended daily energy intake and macronutrient composition. Spread required serving sizes throughout the day. Use of food exchange table to decide food type and quantity. One serving size of food is 377 kJ or 90.1 kcal in studies which have specifically defined serving sizes60 |
| Low GI diet | GI is a measurement of carbohydrate quality of a food relative to the same quantities of carbohydrate in the reference food. Choosing lower GI category food within the same food group. Low GI food has GI less than 55. Medium and high GI foods are defined as having GI between 55–75 and greater than 75, respectively38 |
| Low GL diet | GL is an extended concept of GI with consideration of the amount of carbohydrate in particular foods. Choosing lower GL food in one serving size within the same food group in the food exchange table. GL is calculated by GI and carbohydrate content in the food38 |
| Fibre‐enriched diet | Increase fibre intake by replacing a proportion of refined rice with buckwheat or wheat bran supplementation |
| DASH diet | Diet abundant in fruits, vegetables, whole grains and low fat dairy products, and low in saturated fat, cholesterol, refined grains and sweets61 |
| PUFA‐rich diet | Oil‐rich diet with 45–50 g sunflower oil used daily as cooking oil. Energy composition of carbohydrate and fat are 50–54% and 31–35%, respectively, as compared to 55–60% and 25–30%, respectively, in control group62 |
DASH diet, Dietary Approaches to Stop Hypertension; GI, glycaemic index; GL, glycaemic load; PUFA, polyunsaturated fatty acid.
Table 3.
Characteristics of included studies according to the comparison of intervention strategies
| Author, year (Study design) | Country (Municipality/Province, City) [Recruitment period] | Participant characteristics | Number of participants | Additional dietary intervention details on intervention group (duration) | Compliancea | Outcome of interest |
|---|---|---|---|---|---|---|
| Comparison: Food exchange of all food groups versus individualised dietary intervention | ||||||
| Chen (2017)63 (RCT) | China (Foshan, Guangdong) [May 2015–July 2016] |
Age: [I] 28.2 ± 4.8 [C] 28.1 ± 4.6 Week gestation: [I] 27.3 ± 1.1 [C] 27.1 ± 1.2 |
[I] 59 [C] 59 |
Since diagnosis | — | FG, 2‐hour BG and HbA1c |
| Ma (2011)60 (RCT) | China (Xinjiang, Ürümqi) [January 2009–December 2010] | First GDM diagnosis, no insulin treatment at baseline, no other medical conditions |
[I] 43 [C] 43 |
Macronutrients composition: CHO: 45–50%, fat: 20–30% and protein: 20–25% (since diagnosis) |
Review appointment till delivery. Glucose target: 1‐hour BG <8 mmol/L |
Macrosomia |
| Zhang (2013)64 (Cohort study) | China (Zhejiang, Ningbo) [2008–2012] |
Age: [I] 28.4 ± 2.1 [C] 27.9 ± 2.1 BMI: [I] 22.4 ± 2.7 [C] 22.7 ± 2.2 Week gestation: [I] 24.6 ± 0.5 [C] 24.4 ± 0.7 |
[I] 123 [C] 75 |
Macronutrients composition: CHO: 50–60%, fat: 25–30% and protein: 15–20% (since diagnosis) | Review appointment till delivery | FG, 2‐hour BG, caesarean section, macrosomia and preterm birth |
| Zhang (2015a)65 (Cohort study) | China (Zhejiang, Ningbo) [January 2013–September 2014] |
Age: [I] 28.76 ± 2.1 [C] 28.36 ± 1.1 Week gestation: [I] 26.1 ± 2.2 [C] 25.8 ± 1.4 |
[I] 226 [C] 122 |
Macronutrients composition: CHO: 50–60%, fat: 25–30% and protein: 15–20% (since diagnosis) | Taught about glucose target: FG ≤5.3 mmol/L; 2‐hour BG ≤6.7 mmol/L, without specifying whether review occurred | FG, 2‐hour BG, HbA1c, caesarean section, macrosomia, respiratory distress and preterm birth |
| Comparison: Low GI diet versus individualised dietary intervention | ||||||
| Hu (2014)54 (RCT) | China (Guangdong, Guangzhou) [October 2011–April 2013] |
Between 23 and 35 week gestation Age: [I] 30.3 ± 4.9 [C] 29.7 ± 3.7 BMI: [I] 21.2 ± 2.5 [C] 20.9 ± 3.4 |
[I] 66 [C] 74 |
Replacing rice with low GI staple food and keeping others the same between groups (Day 2 to Day 5 of hospitalisation) | Diet offered in hospital with record of food consumption | FG |
| Liu (2015)66 (RCT) | China (Beijing) [January 2014–December 2014] |
Age: [I] 30.1 ± 3.3 [C] 30.3 ± 3.4 BMI: [I] 23.2 ± 3.7 [C] 23.3 ± 3.7 |
[I] 258 [C] 260 |
Choosing low GI food alternatives in main meal (since diagnosis) | Review at clinic once in every 2 weeks | FG, 2‐hour BG, pre‐eclampsia, caesarean section, macrosomia, preterm birth, respiratory distress and hypoglycaemia |
| Liu (2018)67 (RCT) | China (Gansu) [June 2012–June 2017] |
Age: [I] 25.3 ± 2.3 [C] 26.3 ± 1.6 Week gestation: [I] 25.7 ± 2.4 [C] 24.1 ± 3.7 |
[I] 33 [C] 33 |
Choosing low GI food alternatives in main meal (since diagnosis) | — | FG, 2‐hour BG, HbA1c, macrosomia, preterm birth, respiratory distress and hypoglycaemia |
| Wang (2016a)68 (Cohort study) | China (Nanchong, Sichuan) [January 2015–January 2016] |
Age: [I] 30.6 ± 5.1 [C] 31.6 ± 4.1 Week gestation: [I] 26.0 ± 3.2 [C] 25.7 ± 3.4 |
[I] 70 [C] 58 |
Choosing low GI food alternatives (since diagnosis) | Routine review appointment till delivery | FG, 2‐hour BG, HbA1c and hypoglycaemia |
| Wu (2014)69 (RCT) | China (Hubei, Wuhan) [December 2010–December 2012] | GDM diagnosis at 24–28‐week gestation, no other medical conditions and would deliver in this hospital |
[I] 86 [C] 80 |
Choosing lower GI food alternatives (4 weeks) | Telephone review once a week | FG, HbA1c, caesarean section, macrosomia, preterm birth, respiratory distress and hypoglycaemia |
| Wu (2015)70 (RCT) | China (Guangdong, Guangzhou) [January 2011–May 2013] |
GDM diagnosis at 24–25‐week gestation Age: [I] 30.5 ± 3.8 [C] 29.7 ± 4.4 |
[I] 69 [C] 79 |
Choosing low GI food alternatives (8 weeks) | — | FG, 2‐hour BG |
| Comparison: Low GL diet versus individualised dietary intervention | ||||||
| Gai (2012)71 (RCT) | China (Hebei, Shijiazhuang) [January 2008–October 2009] |
Age: 22–40 Week gestation: 36–40 |
[I] 60 [C] 60 |
Choosing lower GL alternatives with suggested cooking method (since entering into study) | — | FG, 2‐hour BG, HbA1c |
| Jiang (2016)72 (RCT) | China (Guangdong, Shenzhen) [January 2013–December 2014] |
Age: [I] 28.5 ± 4.8 [C] 28.4 ± 5.0 Week gestation: [I] 26.8 ± 1.8 [C] 26.7 ± 2.0 |
[I] 40 [C] 40 |
Choosing lower GL alternatives (since diagnosis) | Irregular clinic or telephone review | FG, 2‐hour BG, HbA1c |
| Li (2017)73 (RCT) | China (Hunan) [May 2013–April 2016] |
Age: [I] 29.1 ± 3.8 [C] 29.4 ± 3.7 |
[I] 58 [C] 56 |
Choosing lower GL alternatives (since diagnosis) | – | FG, HbA1c, caesarean section, preterm birth and respiratory distress |
| Ma (2015)57 (RCT) | China (Guangdong, Guangzhou) [June 2008–July 2009] |
Age: [I] 30.1 ± 3.8 [C] 30.0 ± 3.5 Week gestation: [I] 27.5 ± 1.1 [C] 27.9 ± 1.1 |
[I] 47 [C] 48 |
Choosing low GL alternatives (since diagnosis) | 3‐Days dietary recall to assess the compliance once every 2 weeks | FG, 2‐hour BG, HbA1c, macrosomia and preterm birth |
| Zhang (2015)74 (RCT) | China (Guangdong, Shenzhen) [January 2013–January 2014] |
GDM diagnosis in third trimester Age: [I] + [C] 28.4 ± 5.2 Week gestation: [I] + [C] 38.2 ± 1.2 |
[I] 46 [C] 46 |
Choosing lower GL alternatives (since diagnosis) | – | FG, 2‐hour BG, HbA1c |
| Comparison: Fibre‐enriched diet versus individualised dietary intervention | ||||||
| Lian (2014)75 (RCT) | China (Guangdong, Guangzhou) [June 2010–June 2012] |
Age: [I] + [C] 28.4 ± 3.2 |
[I] 76 [C] 72 |
Swapping one‐third to half of staple foods to buckwheat in four main meals daily (12 weeks) | — | FG, 2‐hour BG, HbA1c, macrosomia, respiratory distress and preterm birth |
| Luo (2016)76 (RCT) | China (Guangdong, Xingning) [June 2014–Dec 2014] |
Over 28‐week gestation Age: [I] 29.2 ± 3.5 [C] 28.8 ± 3.6 |
[I] 100 [C] 100 |
Wheat bran supplementation each time, three times daily (8 weeks) | — | FG, 2‐hour BG and HbA1c |
| Pan (2015)77 (RCT) | China (Jiangsu, Nantong) [September 2014–December 2014] |
Age: [I] 29.5 ± 7.8 [C] 28.8 ± 8.3 Week gestation: [I] 25.1 ± 3.0 [C] 25.1 ± 3.1 |
[I] 48 [C] 48 |
3.5 g wheat bran supplementation before each meal (4 weeks) | Review appointment weekly | FG, 2‐hour BG, macrosomia, preterm birth and hypoglycaemia |
| Wu (2010)78 (Cohort study) | China (Guangdong, Guangzhou) [July 2008–June 2009] | GDM diagnosed women without insulin use |
[I] 54 [C] 51 |
Swapping one‐third of staple foods to buckwheat in four main meals daily (4 days) | — | FG, 2‐hour BG |
| Yang (2015)79 (RCT) | China (Guangdong, Guangzhou) [June 2014–December 2014] |
More than 28‐week gestation. Age: [I] 29.1 ± 3.4 [C] 28.4 ± 3.5 |
[I] 90 [C] 90 |
3.5 g wheat bran supplementation each time, twice per day (4 weeks) | — | FG, 2‐hour BG, HbA1c and macrosomia |
| Comparison: DASH diet versus individualised dietary intervention | ||||||
| Yao (2015)61 (RCT) | China (Anhui, Hefei) [March 2014–October 2014] |
Age: [I] 30.7 ± 5.6 [C] 28.3 ± 5.1 BMI: [I] 30.9 ± 4.3 [C] 29.6 ± 5.3 Week gestation: [I] 26.9 ± 1.4 [C] 25.7 ± 1.3 |
[I] 17 [C] 16 |
Macronutrients composition: 45–55% CHO, 15–20% protein and 25–30% fat. Daily intake of sodium was 2400 mg (4 weeks) | Phone review once every week during intervention. Kept under observation after intervention until delivery | FG, caesarean section and macrosomia |
| Comparison: PUFA rich diet versus individualised dietary intervention | ||||||
| Wang (2015)62 (RCT) | China (Jiangsu, Changzhou) [January 2011–January 2013] |
GDM diagnosis at 24–28 week gestation. Age: [I] 30.3 ± 4.2 [C] 29.7 ± 4.6 BMI: [I] 21.4 ± 3.0 [C] 22.2 ± 3.6 |
[I] 41 [C] 43 |
PUFA‐rich diet (6–8 weeks) | Weekly telephone follow up | FG, 2‐hour BG, macrosomia |
| Comparison: Low GL diet versus food exchange of all food groups | ||||||
| Chen (2015)80 (RCT) | China (Hainan, Wenchang) [January 2012–January 2014] |
Age: [I] 32.0 ± 3.8 [C] 31.1 ± 3.6 BMI: [I] 21.8 ± 2.9 [C] 21.5 ± 3.7 |
[I] 72 [C] 73 |
Choosing lower GL food alternatives (since diagnosis to delivery) | Clinic review once in every 2 weeks | FG, 2‐hour BG, macrosomia, preterm birth and respiratory distress |
| Huang (2015)81 (RCT) | China (Guangdong, Dongguan) [January 2012–January 2014] |
Age: [I] 28.2 ± 8.9 [C] 27.0 ± 7.9 |
[I] 40 [C] 40 |
Choosing lower GL food alternatives (since diagnosis) | Clinic review once every week | FG, 2‐hour BG, respiratory distress, jaundice and hypoglycaemia |
| Shen (2010)82 (Cohort study) | China (Shanghai) [May 2008–Mar 2009] |
GDM diagnosis at 24–28‐week gestation Age: [I] 29.5 ± 3.2 [C] 29.0 ± 2.8 |
[I] 42 [C] 38 |
Choosing lower GL food alternatives (from diagnosis till delivery) | Offer review clinic and telephone query service | FG, 2‐hour BG, HbA1c, pre‐eclampsia, macrosomia, preterm birth, respiratory distress and jaundice |
| Sun (2013)83 (RCT) |
China (Guangdong, Guangzhou) [May 2012‐ Dec 2012] |
Women diagnosed with GDM |
[I] 78 [C] 80 |
Choosing lower GL food alternatives (2 weeks) | Review appointment once in one to 2 weeks. | FG, 2 hour BG. |
| Wu (2013)84 (RCT) | China (Jiangsu, Haimen) [June 2010–December 2011] |
28‐week gestation Age: 22–43 |
[I] 30 [C] 30 |
Choosing lower GL food alternatives (since diagnosis) | Review appointment per week | BGL, 2‐hour BG |
| Zhi (2012)85 (RCT) | China (Guizhou) [June 2011–November 2011] |
30‐week gestation Age: 23–40 |
[I] 23 [C] 22 |
Choosing lower GL food alternatives (since diagnosis) | Review fortnightly | FG, 2‐hour BG, caesarean section and macrosomia |
| Comparison: Low GI diet versus low GL diet versus food exchange of all food groups | ||||||
| Wang (2016)86 (RCT) | China (Jiangsu, Nantong) [May 2013–May 2014] |
Age: [GL] 26.6 ± 4.9 [GI] 26.4 ± 4.6 [C] 26.5 ± 4.9 Week gestation: [GL] 25.6 ± 2.8 [GI] 25.7 ± 2.8 [C] 25.9 ± 2.6 |
[GL] 43 [GI] 38 [C] 32 |
Choosing lower GL or lower GI food alternatives (since diagnosis) | Review appointment once every week | FG, 2‐hour BG, HbA1c, caesarean section, macrosomia and hypoglycaemia |
Information on dietary compliance was extracted to determine whether the study design appropriately considered and evaluated participants’ adherence to assigned dietary regime. Study design was examined to determine if dietary compliance included review appointment of dietary regime and meal or supplementation given with assessment of leftovers and other food intake. Any study reported no dietary follow up was rated as high risk of bias. Studies with no report on relevant information was considered as unknown dietary compliance.
BMI, pre‐pregnancy body mass index; C, control group; CHO, carbohydrate; DASH diet, Dietary Approaches to Stop Hypertension diet; FG, fasting plasma glucose; GDM, gestational diabetes mellitus; GI diet, glycaemic index diet; GL diet, glycaemic load diet; I, intervention group; PUFA, polyunsaturated fat; RCT, randomised controlled trial; 1‐hour BG: 1‐hour plasma glucose; 2‐hour BG: 2‐hour plasma glucose.
All studies were examined for the risk of bias in different domains and are presented in Table S3. Although all studies had low or unknown detection bias and reporting bias, 7 studies57, 64, 65, 69, 73, 77, 86 rated high or critical in at least one of the forms of bias assessed. Two of these were cohort studies64, 65 using food exchange strategies in the intervention group. They had critical overall risk of bias as the review process was conducted only with the intervention group and not with the control group.64, 65 Similar bias in relation to follow up of only the intervention group was observed in an RCT examining low GI diets.69 Two other studies77, 86 had high attrition bias as they did not take into consideration the loss to follow up when comparing outcomes between groups. Pan (2015)77 and the two remaining studies57, 73 had high selection bias because of no allocation concealment.
The results of meta‐analyses of each outcome in each intervention comparison are presented in Tables 4 and 5 (with forest plots presented in Figure 2), with names of studies involved and total number of women for each outcome listed in brackets after each weighted mean difference or relative risks. The quality of evidence of each outcome from two or more studies was assessed using GRADE criteria and is provided in Table S4. Funnel plot asymmetry was not used because each meta‐analysis had fewer than 10 studies and hence publication bias could not be assessed. The quality of evidence of each outcome can be found in Tables 4 and 5. Regarding maternal glycaemic control, when an individualised diet was the proxy control, the low GI, low GL and fibre‐enriched diets were significantly associated with reduction in fasting plasma glucose, 2‐hour plasma glucose and HbA1c. Similarly, the low GL diet performed significantly better in glycaemic control than food exchange education. However, the quality of evidence was low because of the high risk of bias in some studies57, 73 and substantial inconsistency in the magnitude, but not direction, of results (shown in forest plots of Figure 2).
Table 4.
A summary of glycaemic control and maternal outcomes for different intervention strategies
| Intervention strategies | Fasting plasma glucose (mmol/L) after dietary intervention [studies included; total number of participants] | 2‐Hour plasma glucose (mmol/L) after dietary intervention [studies included; total number of participants] | HbA1c (%) after dietary intervention [studies included; total number of participants] | Pre‐eclampsiaa [studies included; total number of participants] | Caesarean sectiona [studies included; total number of participants] |
|---|---|---|---|---|---|
| Compared to individualised dietary intervention | |||||
| Food exchange |
−0.94 (−1.37, −0.51) [Chen 2017; 118] (RCT) −0.61 (−0.69, −0.53)b [Zhang 2015a; 348] (Cohort study) |
−1.26 (−1.73, −0.79) [Chen 2017; 118] (RCT) −0.89 (−1.17, −0.61)b [Zhang 2015a; 348] (Cohort study) |
−0.90 (−1.29, −0.51) [Chen 2017; 118] (RCT) −0.98 (−1.12, −0.84)b [Zhang 2015a; 348] (Cohort study) |
— |
0.93 (0.79, 1.11)4 b [Zhang 2013; Zhang 2015a; 546] (Cohort studies) |
| Low GI diet |
−0.62 (−1.12, −0.13)3 [Hu 2014, Liu 2015, Liu 2018, Wu 2014, Wu 2015; 1038] (RCTs) 0.16 (−0.01, 0.33)b [Wang 2016a; 128] (Cohort study) |
−1.19 (−2.29, −0.09)3 [Liu 2015, Liu 2018, Wu 2015; 732] (RCTs) −1.44 (−2.09, −0.79)b [Wang 2016a; 128] (Cohort study) |
−1.17 (−1.85, −0.49)4 [Liu 2018, Wu 2014, Wu 2015; 380] (RCTs) −0.15 (−0.35, 0.05)b [Wang 2016a; 128] (Cohort study) |
0.72 (0.23, 2.24) [Liu 2015; 518] (RCT) |
0.70 (0.45, 1.08)3 [Liu 2015, Wu 2014; 684] (RCTs) |
| Low GL diet |
−2.00 (−3.33, −0.67)4 [Gai 2012, Jiang 2016, Li 2017, Ma 2015, Zhang 2015; 489] (RCTs) |
−3.12 (−4.91, −1.32)4 [Gai 2012, Jiang 2016, Ma 2015, Zhang 2015; 375] (RCTs) |
−1.06 (−2.01, −0.10)4 [Gai 2012, Jiang 2016, Li 2017, Ma 2015, Zhang 2015; 489] (RCTs) |
— |
0.21 (0.05, 0.95) [Li 2017; 114] (RCT) |
| Fibre‐enriched diet |
−0.42 (−0.79, −0.04)3 [Lian 2014, Luo 2016, Pan 2015, Yang 2015; 624] (RCTs) −0.90 (−1.49, −0.31)b [Wu 2010; 105] (Cohort study) |
−1.40 (−2.30, −0.50)3 [Lian 2014, Luo 2016, Pan 2015, Yang 2015; 624] (RCTs) −1.20 (−1.92, −0.48)b [Wu 2010; 105] (Cohort study) |
−0.61 (−1.07, −0.14)3 [Lian 2014, Luo 2016, Yang 2015; 528] (RCTs) |
— | — |
| DASH diet |
−0.66 (−0.80, −0.51) [Yao 2015; 33] (RCT) |
— | — | — |
0.58 (0.33, 1.01) [Yao 2015; 33] (RCT) |
| PUFA‐rich diet |
0.18 (−0.17, 0.53) [Wang 2015; 84] (RCT) |
−0.02 (−0.29, 0.25) [Wang 2015; 84] (RCT) |
— | — | — |
| Compared to food exchange intervention | |||||
| Low GI diet |
0.20 [−0.00, 0.40] [Wang 2016; 64] (RCT) |
−0.70 (−1.74, 0.34) [Wang 2016; 64] (RCT) |
−0.20 (−0.65, 0.25) [Wang 2016; 64] (RCT) |
— |
0.74 (0.40, 1.37) [Wang 2016; 64] (RCT) |
| Low GL diet |
−0.36 (−0.66, −0.07)3 [Chen 2015, Huang 2015, Sun 2013, Wang 2016, Wu 2013, Zhi 2012; 552] (RCTs) −0.10 (−0.47, 0.27)b [Shen 2010; 80] (Cohort study) |
−1.05 (−1.54, −0.56)3 [Chen 2015, Huang 2015, Sun 2013, Wang 2016, Wu 2013, Zhi 2012; 552] (RCTs) −1.10 (−1.78, −0.42)b [Shen 2010; 80] (Cohort study) |
−0.50 (−0.88, −0.12) [Wang 2016; 64] (RCT) −0.20 (−0.49, 0.09)b [Shen 2010; 80] (Cohort study) |
0.45 (0.04, 4.79)b [Shen 2010; 80] (Cohort study) |
0.68 (0.36, 1.28)4 [Wang 2016, Zhi 2012; 109] (RCTs) |
Dichotomous outcomes expressed in relative risks (95% confidence interval).
Results from cohort studies.
DASH, Dietary Approaches to Stop Hypertension; GI, glycaemic index; GL, glycaemic load; PUFA, polyunsaturated fatty acid; LGA, large for gestational age; NICU, neonatal intensive care unit admission; SGA, small for gestational age.
Continuous outcomes expressed in weighted mean differences (95% confidence interval).
The quality of evidence of each outcome from two or more studies was assessed using GRADE and was presented as number superscript after each outcome in the level of quality of evidence of: 1high, 2moderate, 3low and 4very low.
Table 5.
Summary of neonatal outcomes among different intervention strategies
| Intervention strategies | Macrosomiaa [studies included; total number of participants] | LGAa [studies included; total number of participants] | SGAa [studies included; total number of participants] | Hypoglycaemiaa [studies included; total number of participants] | NICUa [studies included; total number of participants] | Preterm birtha [studies included; total number of participants] | Jaundicea [studies included; total number of participants] | Respiratory distressa [studies included; total number of participants] |
|---|---|---|---|---|---|---|---|---|
| Compared to individualised dietary intervention | ||||||||
| Food exchange |
0.25 (0.06, 1.11) [Ma 2011; 86] (RCT) 0.45 (0.26, 0.76)4 b [Zhang 2013, Zhang 2015a; 546] (Cohort studies) |
— | — | — | — |
0.57 (0.06, 5.58)4 b [Zhang 2013, Zhang 2015a; 546] (Cohort studies) |
— |
0.18 (0.04, 0.88)b [Zhang 2015a; 348] (Cohort study) |
| Low GI diet |
0.49 (0.32, 0.74)2 [Liu 2015, Liu 2018, Wu 2014; 750] (RCTs) |
— | — |
0.37 (0.20, 0.69)2 [Liu 2015, Liu 2018, Wu 2014; 750] (RCTs) 0.39 (0.17, 0.88)b [Wang 2016a; 128] (Cohort study) |
— |
0.45 (0.25, 0.81)2 [Liu 2015, Liu 2018, Wu 2014; 750] (RCTs) |
0.56 (0.14, 2.26) [Wu 2014; 166] (RCT) |
0.59 (0.37, 0.96)1 [Liu 2015, Liu 2018, Wu 2014; 750] (RCTs) |
| Low GL diet |
0.51 (0.05, 5.43) [Ma 2015; 83] (RCT) |
— | — | — | — |
0.45 (0.19, 1.07)4 [Li 2017, Ma 2015; 197] (RCTs) |
— |
0.12 (0.02, 0.93) [Li 2017; 114] (RCT) |
| Fibre‐enriched diet |
0.39 (0.12, 1.33)4 [Lian 2014, Pan 2015, Yang 2015; 424] (RCTs) |
— | — |
0.09 (0.01, 1.60) [Pan 2015; 96] (RCT) |
— |
0.19 (0.07, 0.54)3 [Lian 2014, Pan 2015; 244] (RCTs) |
— |
0.05 (0.00, 0.76) [Lian 2014; 148] (RCT) |
| DASH diet |
0.16 (0.02, 1.16) [Yao 2015; 33] (RCT) |
— | — | — | — | — | — | — |
| PUFA‐rich diet |
0.26 (0.03, 2.25) [Wang 2015; 84] (RCT) |
— | — | — | — | — | — | — |
| Compared to food exchange intervention | ||||||||
| Low GI diet |
0.38 (0.13, 1.07) [Wang 2016; 64] (RCT) |
— | — |
0.47 (0.04, 4.92) [Wang 2016; 64] (RCT) |
— | — | — | — |
| Low GL diet |
0.31 (0.12, 0.84)3 [Chen 2015, Wang 2016, Zhi 2012; 254] (RCTs) 0.18 (0.02, 1.48)b [Shen 2010; 80] (Cohort study) |
— | — |
0.19 (0.02, 1.62)3 [Huang 2015, Wang 2016; 144] (RCTs) |
— |
0.71 (0.29, 1.76) [Chen 2015; 145] (RCT) 0.68 (0.16, 2.84)b [Shen 2010; 80] (Cohort study) |
0.40 (0.08, 1.94) [Huang 2015; 80] (RCT) 0.60 (0.11, 3.42)b [Shen 2010; 80] (Cohort study) |
0.89 (0.28, 2.84)3 [Chen 2015, Huang 2015; 225] (RCTs) 1.36 (0.24, 7.69)b [Shen 2010; 80] (Cohort study) |
Dichotomous outcomes expressed in relative risks (95% confidence interval).
Results from cohort studies.
DASH, Dietary Approaches to Stop Hypertension; GI, glycaemic index: GL, glycaemic load; LGA, large for gestational age; NICU, neonatal intensive care unit admission; PUFA, polyunsaturated fatty acid; SGA, small for gestational age.
The quality of evidence of each outcome from two or more studies was assessed using GRADE and was presented as number superscript after each outcome in the level of quality of evidence of: 1high, 2moderate, 3low and 4very low.
Figure 2.
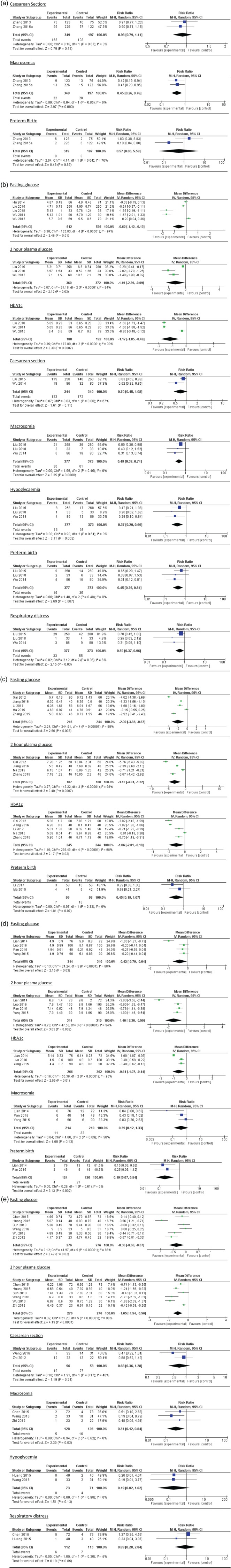
Forest plots of each outcome of dietary intervention strategies comparison: (a) Food exchange diet compared to individualised dietary intervention. (b) Low glycaemic index diet compared to individualised dietary intervention. (c) Low glycaemic load (GL) diet compared to individualised dietary intervention. (d) Fibre‐enriched diet compared to individualised dietary intervention. (e) Low GL diet compared to food exchange intervention.
Regarding maternal outcomes, low GL diets were significantly associated with reduced caesarean section risk (odds ratio: 0.21; CI: 0.05, 0.95) compared with individualised diets, where there was low quality of evidence because of the limited number of studies available.
Regarding neonatal outcomes, moderate quality of evidence showed that food exchange education was associated with a reduction in macrosomia risk in cohort studies (odds ratio: 0.45; CI: 0.26, 0.76) compared with individualised dietary advice. However, when macrosomia risk was compared between the food exchange education and low GL diets, low GL diets were significantly associated with reduced macrosomia risk (odds ratio: 0.31; CI: 0.12, 0.84). Even with a limited number of studies reporting on neonatal outcomes, low GI diets were significantly associated with reduction of risk of macrosomia (odds ratio: 0.49; CI: 0.32, 0.74), hypoglycaemia (odds ratio: 0.37; CI: 0.20, 0.69), preterm birth (odds ratio: 0.45; CI: 0.25, 0.81) and respiratory distress (odds ratio: 0.59; CI: 0.37, 0.96). Low GL and fibre‐enriched diets were both significantly associated with reduced risk of respiratory distress. In addition, fibre‐enriched diets were significantly associated with reduced risk of preterm birth (odds ratio: 0.19; CI: 0.07, 0.54). The quality of evidence of these neonatal outcomes ranged from low to high levels of evidence, depending on whether there was high risk of bias of studies or small sample size.
The only RCT86 which compared low GI, low GL and food exchange education intervention, showed that the low GL diet was associated with greater reductions in caesarean section, neonatal hypoglycaemia and foetal distress risk than low GI diets. However, high attrition bias was noted as mentioned above.
Discussion
Ethnic Chinese women are an increasingly prevalent immigrant population with a high risk of GDM, and a ninefold increased risk of type 2 diabetes within 8 years postpartum.36, 37 Clinicians will need good evidence to determine optimal treatment options. Hence, this review focussed on determining which dietary intervention strategies were found to be effective among ethnic Chinese women living with GDM, which remains unclear in the existing literature.13, 14, 26, 27, 35 Overall, low GI, low GL and fibre‐enriched diets were found to be associated with improved outcomes compared to individualised dietary interventions as described in this review.
This review demonstrates that a low GI diet improves glycaemic control and pregnancy outcomes in ethnic Chinese women with GDM, and confirms previous evidence about the association between insulin resistance and a lower GI diet.38 Replacing high GI foods with lower GI alternatives in a low GI diet improves insulin sensitivity and provides greater satiety.38 In diabetes, lower GI is associated with lowering of HbA1c and improvements in glycaemia and weight, whether pregnant or not.13, 39, 40 It is well known that mechanistically, postprandial glucose excursion is associated with adverse pregnancy outcomes,41, 42 and insulin resistance is the greatest in the third trimester during pregnancy.39 A low GI diet blunts the pregnancy‐associated rise in insulin resistance, and therefore provides improved glycaemic stability and control and in turn, pregnancy outcomes.39, 41 Some countries have already incorporated GI‐based GDM education to optimise GDM management of ethnically diverse populations.10 This review supports the clinical relevance of low GI diets in improving specific pregnancy outcomes among ethnic Chinese women with GDM.
Similarly, this review found that a low GL diet was associated with improved glycaemic control and reduced certain adverse maternal and neonatal outcomes in these Chinese populations (acknowledging that GL is an extended concept of GI, with a focus on the amount, as well as type of carbohydrate content of meals).43 Because a high GL diet is known to increase the risk of type 2 diabetes,44 and a stepwise rise in GL provides a predictable increase in glycaemic and insulinemic effects,45 a low GL diet is thought to be beneficial in GDM, similar to a low GI diet. Inclusion of the carbohydrate amount and composition of the food might provide more accurate glycaemic potency in dietary information and thus assist women with GDM in making decisions about food consumption. This review further suggests that among ethnic Chinese women with GDM, a low GL diet appeared to enable better glycaemic control compared to a low GI diet, although direct comparison was not possible in this review.
In this review, fibre‐enriched diets were also found to be associated with better glycaemic control, and reduced preterm birth and respiratory distress risk among Chinese GDM patients. The interrelationship between low GI, low GL and fibre‐enriched diets might explain the observed benefits of commencing fibre‐enriched diets. Compared with the high GI diet, the low GI diet has lower GL values, dependent on food composition and quantity.46, 47, 48, 49, 50 The shift from ingestion of high GI foods to lower GI options might introduce more fibre into diets. The fibre‐enriched diet in this review refers to either replacing some portion of refined rice with buckwheat or including wheat bran supplementation. Buckwheat is a lower GI and GL food compared to refined rice.38 Similarly, wheat bran is a commonly used fibre supplement in research and is found to significantly reduce the postprandial glucose response in subjects with impaired fasting glucose51 and provide better postprandial glycaemic control.52 By commencing a fibre‐enriched diet, overall GI of the diet is lowered and hence provides better glycaemic control and pregnancy outcomes.
Although this systematic review clearly indicates a paucity of research on ethnic Chinese women with GDM who are migrants, it demonstrates the clinical relevance of incorporating GI and GL concepts in GDM education, particularly in populations which are predominantly ethnic Chinese.22, 53, 54 Over the last few decades there has been some degree of westernisation of Chinese diets in their country of birth. This has been referred to as the modern Chinese dietary pattern.53 Coarse grain has become a smaller contributor to overall energy intake, and sugar‐sweetened cakes and beverages are newer additions.53 This suggests a wide societal shift in food consumption from lower to higher GI options. With migration, bi‐directional food acculturation in Australia55 suggests a combination of both western and modern Chinese dietary patterns among Chinese migrants. However, traditional Chinese cultural practices appear maintained to some extent, irrespective of the country in which Chinese women currently live.15, 20, 23, 24, 25 Notwithstanding the documented cultural and regional variations in diet between provinces of China, and between countries with Chinese inhabitants, differences and changes in food availability, family structure and agricultural characteristics19, 22, 56 have meant that staple foods are either high GI refined rice or wheat‐based foods.54
Furthermore, ethnic Chinese people generally consume more refined rice or wheat‐based staple foods than other Asian ethnic groups such as Malays and Indians.21 A study of dietary intake of women in China during their third trimester found that the median quantity of carbohydrate they consumed was double the recommendation provided in the Chinese Dietary Reference Intakes Handbook.22 Hence, health professionals need to be aware of the nature of high GL Chinese dishes and Chinese dietary habits. For instance, more than half the typical traditional cuisine in southern China is of high GL value,57 while half is of low or medium GI value.57 Even though education regarding the choice of lower GI alternatives might improve blood glucose levels by slowing glucose release from food,58 GL provides a more accurate prediction of peak blood glucose following a meal by taking into account both GI and the quantity of carbohydrates in the food consumed.59 In addition, westernised diets which include increased sugary drink intake and decreased lentils and greens consumption,55 might increase dietary GL of an already high GL Chinese diet during pregnancy (with a high GL diet during pregnancy being a predictor of poor diet quality).59 Therefore, provision of advice to Chinese migrant women diagnosed with GDM regarding lower GL diets may not only help with improving glycaemic control, but may assist with improved long‐term diet quality.
Pregnancy is also life stage heavily patterned by cultural rituals and taboos often resulting in different dietary practices across cultures.15 In this context, culturally acceptable dietary interventions are viewed as critical to optimal management of GDM.9, 10, 11, 12 Participants’ adherence to assigned dietary regimens might provide some clues on cultural acceptability of suggested diets. However, insufficient information is available to understand cultural acceptability of dietary intervention strategies in this population group. To our knowledge no published research has focussed specifically on examining the dietary practices of ethnic Chinese women with GDM. Future research needs to not only determine which of a range of dietary strategies provide clinically desirable outcomes, but also which are culturally acceptable and thus have the potential to enhance adherence to prescribed dietary regimens. Such research will also need to examine the role of migration in relation to ethnic Chinese women with GDM.
Heterogeneity in the meta‐analyses shown in Figure 2 and Table S4 is an acknowledged limitation and is because of the use of different GDM diagnostic criteria; differences in clinical practice or guidelines between hospitals; different study designs in terms of study duration and frequency of follow up appointments; variations in patients’ characteristics including unreported gestational weight gain, week of gestation and pre‐pregnancy BMI; as well as factors that influence women's experiences such as interaction with family members and previous pregnancy experience. Variations between studies in the composition of individualised dietary intervention (in terms of recommended macronutrient composition), or food exchange education (in relation to serving sizes calculation) as the comparator diets might have further increased heterogeneity of the meta‐analysis. Therefore, future direct comparison of different dietary intervention strategies in an RCT is recommended where such variations are not an issue.
Some included studies shown in Table S3 had a study design of unknown random allocation concealment and intervention compliance. The quality of future intervention studies could be improved by enhancing transparency of reporting, comparing the adherence to cultural dietary patterns before and after intervention, having review appointments during the intervention period and asking participants for feedback about maintenance of lifestyle after the intervention period. Given the unknown generalisability of our results to Chinese migrants in western countries as previously mentioned, future research examining ethnic Chinese migrants with GDM is recommended. Notwithstanding the small number of studies included in this review, a key strength of our research was accessing databases in two languages which provided greater breadth of insights regarding the need for and direction of future research.
In conclusion, low GI, low GL and fibre‐enriched diets all appear to be associated with better maternal glycaemic control in Chinese women with GDM with moderate to very low quality of evidence because of the heterogeneity and small number of included studies. Low GI diets were associated with a reduced risk of macrosomia, neonatal hypoglycaemia, preterm birth and respiratory distress. Low GL diets were associated with reduced risk of caesarean section, macrosomia and respiratory distress. Fibre‐enriched diets were associated with a reduced risk of preterm birth and respiratory distress. Low GL dietary education, with inclusion of the concept of GI, seems useful in optimising glycaemic control. Given the heterogeneity of results of the meta‐analysis, direct comparisons of different dietary intervention strategies using RCT designs would provide clearer evidence for future clinical practice. Future research targeting ethnic Chinese migrants in the countries to which they have migrated is recommended to identify optimal dietary intervention strategies for the best possible GDM management in those settings.
Funding source
CSW was supported by Research Training Program from the Australian Government. We thank senior systematic review researcher Dr Marie Misso for organising a systematic review training program, librarian Ms Lorena Romero for assistance with finalising the search strategies and statistician Mr Sanjeeva Ranasinha for confirming the statistical method and data interpretation. Their expertise facilitated the completion of this review.
Conflict of interest
Authors have no conflicts of interest to declare regarding the contents of the manuscript nor has it been submitted for consideration elsewhere.
Authorship
All authors equally contributed to the conception and design of the research. CSW conducted the literature search and data extraction. CSW and RA contributed to the critical appraisal and data analysis. All authors contributed to the interpretation of the data and CSW drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Supporting information
Table S1 Full search strategy for Ovid Medline search.
Table S2 Types of comparisons of included studies.
Table S3 (a) Summary of risk of bias judgements about each risk of bias item for randomised control trials. (b) Summary of risk of bias judgements about each risk of bias item for cohort studies.
Table S4 (a) Quality of evidence using the GRADE approach for outcomes which used individualised dietary intervention in comparison group. (b) Quality of evidence using the GRADE approach for outcomes which used food exchange intervention in control group to compare Low GL diet.
C.S. Wan, MNutr&Diet, APD, PhD Candidate
A. Nankervis, MBBS, MD, FRACP, Clinical Head of Departments of Diabetes and Endocrinology
H. Teede, MBBS, PhD, FRACP, Director of Monash Centre for Health Research and Implementation
R. Aroni, PhD, Senior Lecturer in Health Science
References
- 1. Nankervis A, Conn J. Gestational diabetes mellitus: negotiating the confusion. Aust Fam Physician 2013; 42: 528. [PubMed] [Google Scholar]
- 2. O'Reilly SL. Prevention of diabetes after gestational diabetes: better translation of nutrition and lifestyle messages needed. Healthcare 2014; 2: 468–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moses RG, Wong VC, Lambert K, Morris GJ, San GF. The prevalence of hyperglycaemia in pregnancy in Australia. Aust N Z J Obstet Gynaecol 2016; 56: 341–5. [DOI] [PubMed] [Google Scholar]
- 4. Wong VW, Lin A, Russell H. Adopting the new World Health Organization diagnostic criteria for gestational diabetes: how the prevalence changes in a high‐risk region in Australia. Diabetes Res Clin Pract 2017; 129: 148–53. [DOI] [PubMed] [Google Scholar]
- 5. Templeton M, Pieris‐Caldwell I. Gestational Diabetes Mellitus in Australia, 2005–06. Canberra: Australian Institute of Health and Welfare, 2008. (Available from: https://www.aihw.gov.au/getmedia/25af6300-a546-4324-816d-d0d8539e838d/gdmia05-06.pdf.aspx?inline=true, accessed 15 August 2018). [Google Scholar]
- 6. Moses RG, Morris GJ, Petocz P, San Gil F, Garg D. The impact of potential new diagnostic criteria on the prevalence of gestational diabetes mellitus in Australia. Med J Aust 2011; 194: 338. [DOI] [PubMed] [Google Scholar]
- 7. Virjee S, Robinson S, Johnston D. Screening for diabetes in pregnancy. J R Soc Med 2001; 94: 502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McMahon M, Ananth C, Liston R. Gestational diabetes mellitus. Risk factors, obstetric complications and infant outcomes. J Reprod Med 1998; 43: 372–8. [PubMed] [Google Scholar]
- 9. Metzger BE, Buchanan TA, Coustan DR et al Summary and recommendations of the fifth international workshop—Conference on gestational diabetes mellitus. Diabetes Care 2007; 30: S251–S60. [DOI] [PubMed] [Google Scholar]
- 10.Queensland Clinical Guidelines. Gestational diabetes mellitus. Queensland: Department of Health, 2015. (Available from: https://www.health.qld.gov.au/qcg/publications, accessed 15 August 2018).
- 11. American Diabetes Association . Standards of medical care in diabetes—2016 abridged for primary care providers. Clin Diabetes 2016; 34: 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Institute for Health and Care Excellence . Diabetes in Pregnancy: Management from Preconception to the Postnatal Period. London: NICE, 2015. [PubMed] [Google Scholar]
- 13. Han S, Crowther CA, Middleton P, Heatley E. Different types of dietary advice for women with gestational diabetes mellitus. Cochrane Database Syst Rev 2017: CD009275. [DOI] [PubMed] [Google Scholar]
- 14. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta‐analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care 2014; 37: 3345–55. [DOI] [PubMed] [Google Scholar]
- 15. Lee DT, Ngai IS, Ng MM, Lok IH, Yip AS, Chung TK. Antenatal taboos among Chinese women in Hong Kong. Midwifery 2009; 25: 104–13. [DOI] [PubMed] [Google Scholar]
- 16. Kreuter MW, Lukwago SN, Bucholtz DC, Clark EM, Sanders‐Thompson V. Achieving cultural appropriateness in health promotion programs: targeted and tailored approaches. Health Educ Behav 2003; 30: 133–46. [DOI] [PubMed] [Google Scholar]
- 17. Australian Bureau of Statistics . 3412.0—Migration, Australia, 2014–15. Canberra: Australian Bureau of Statistics, 2016. [Google Scholar]
- 18. Hooper K, Batalova J. Chinese Immigrants in the United States. Washington, DC: Migration Policy Institute, 2015. [Google Scholar]
- 19. Chang X, DeFries RS, Liu L, Davis K. Understanding dietary and staple food transitions in China from multiple scales. PLoS One 2018; 13: e0195775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao H, Stiller CK, Scherbaum V et al Dietary intake and food habits of pregnant women residing in urban and rural areas of Deyang City, Sichuan Province, China. Nutrients 2013; 5: 2933–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen LW, Low YL, Fok D et al Dietary changes during pregnancy and the postpartum period in Singaporean Chinese, Malay and Indian women: the GUSTO birth cohort study. Public Health Nutr 2014; 17: 1930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu FL, Zhang YM, Parés GV et al Nutrient intakes of pregnant women and their associated factors in eight cities of China: a cross‐sectional study. Chin Med J (Engl) 2015; 128: 1778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chee CY, Lee DT, Chong Y, Tan L, Ng T, Fones CS. Confinement and other psychosocial factors in perinatal depression: a transcultural study in Singapore. J Affect Disord 2005; 89: 157–66. [DOI] [PubMed] [Google Scholar]
- 24. Cheung NF. Chinese zuo yuezi (sitting in for the first month of the postnatal period) in Scotland. Midwifery 1997; 13: 55–65. [DOI] [PubMed] [Google Scholar]
- 25. Lee DT, Chan SS, Sahota DS, Yip AS, Tsui M, Chung TK. A prevalence study of antenatal depression among Chinese women. J Affect Disord 2004; 82: 93–9. [DOI] [PubMed] [Google Scholar]
- 26. Wang XB, Yang WL, Wang Y, Yu GW. Effect of nutrition interventions on pregnancy outcomes of gestational diabetes mellitus patients: a systematic review. J Evid Based Med 2016; 16: 362–9. [Google Scholar]
- 27. Xu T, He Y, Dainelli L et al Healthcare interventions for the prevention and control of gestational diabetes mellitus in China: a scoping review. BMC Pregnancy Childbirth 2017; 17: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. West Sussex, England: The Cochrane Collaboration, 2011. [Google Scholar]
- 29. Coleman K, Norris S, Weston A et al NHMRC Additional Levels of Evidence and Grades for Recommendations for Developers of Guidelines. Canberra: NHMRC, 2005. [Google Scholar]
- 30. Feig DS, Corcoy R, Jensen DM et al Diabetes in pregnancy outcomes: a systematic review and proposed codification of definitions. Diabetes Metab Res Rev 2015; 31: 680–90. [DOI] [PubMed] [Google Scholar]
- 31. Higgins JP, Altman DG, Gøtzsche PC et al The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sterne JA, Hernán MA, Reeves BC et al ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cochrane Collaboration , Review Manager (RevMan) [Computer program], Version 5.3. Copenhagen: The Nordic Cochrane Centre, 2014.
- 34. Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. Hamilton, Canada: The GRADE Working Group, 2013. [Google Scholar]
- 35. Yan J, Chen X, Zhang S, Lu ST, Wu CQ. Intervention effect of diet education based on blood glucose load on gestational diabetes mellitus patients: a meta‐analysis. Chin Nurs Res 2017; 31: 3144–8. [Google Scholar]
- 36. Mukerji G, Chiu M, Shah B. Impact of gestational diabetes on the risk of diabetes following pregnancy among Chinese and south Asian women. Diabetologia 2012; 55: 2148–53. [DOI] [PubMed] [Google Scholar]
- 37. Devsam BU, Bogossian FE, Peacock AS. An interpretive review of women's experiences of gestational diabetes mellitus: proposing a framework to enhance midwifery assessment. Women Birth 2013; 26: e69–76. [DOI] [PubMed] [Google Scholar]
- 38. Foster‐Powell K, Holt SH, Brand‐Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 2002; 76: 5–56. [DOI] [PubMed] [Google Scholar]
- 39. Cheung NW. The management of gestational diabetes. Vasc Health Risk Manag 2009; 5: 153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thomas D, Elliott E. The use of low‐glycaemic index diets in diabetes control. Br J Nutr 2010; 104: 797–802. [DOI] [PubMed] [Google Scholar]
- 41. Louie JC, Brand‐Miller JC, Markovic TP, Ross GP, Moses RG. Glycemic index and pregnancy: a systematic literature review. J Nutr Metab 2011; 2010: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jovanovic L. The role of continuous glucose monitoring in gestational diabetes mellitus. Diabetes Technol Ther 2000; 2: 67–71. [DOI] [PubMed] [Google Scholar]
- 43. Monro JA, Shaw M. Glycemic impact, glycemic glucose equivalents, glycemic index, and glycemic load: definitions, distinctions, and implications. Am J Clin Nutr 2008; 87: 237S–43S. [DOI] [PubMed] [Google Scholar]
- 44. Barclay AW, Petocz P, McMillan‐Price J et al Glycemic index, glycemic load, and chronic disease risk—a meta‐analysis of observational studies. Am J Clin Nutr 2008; 87: 627–37. [DOI] [PubMed] [Google Scholar]
- 45. Brand‐Miller J, Thomas M, Swan V, Ahmad Z, Petocz P, Colagiuri S. Physiological validation of the concept of glycemic load in lean young adults. J Nutr 2003; 133: 2728–32. [DOI] [PubMed] [Google Scholar]
- 46. Rodrigues SC, Dutra OJE, de Souza RA, Silva HC. Effect of a rice bran fiber diet on serum glucose levels of diabetic patients in Brazil. Arch Latinoam Nutr 2005; 55: 23–7. [PubMed] [Google Scholar]
- 47. Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med 2000; 342: 1392–8. [DOI] [PubMed] [Google Scholar]
- 48. Chutkan R, Fahey G, Wright WL, McRorie J. Viscous versus nonviscous soluble fiber supplements: mechanisms and evidence for fiber‐specific health benefits. J Am Acad Nurse Pract 2012; 24: 476–87. [DOI] [PubMed] [Google Scholar]
- 49. Brennan CS. Dietary fibre, glycaemic response, and diabetes. Mol Nutr Food Res 2005; 49: 560–70. [DOI] [PubMed] [Google Scholar]
- 50. Jimenez‐Cruz A, Bacardi‐Gascon M, Turnbull WH, Rosales‐Garay P, Severino‐Lugo I. A flexible, low‐glycemic index Mexican‐style diet in overweight and obese subjects with type 2 diabetes improves metabolic parameters during a 6‐week treatment period. Diabetes Care 2003; 26: 1967–70. [DOI] [PubMed] [Google Scholar]
- 51. Afaghi A, Omidi BR, Sarreshtehdari M et al Effect of wheat bran on postprandial glucose response in subjects with impaired fasting glucose. Curr Top Nutraceutical Res 2011; 9: 35. [Google Scholar]
- 52. Afaghi A, Ghanei L, Ziaee A. Effect of low glycemic load diet with and without wheat bran on glucose control in gestational diabetes mellitus: a randomized trial. Indian J Endocr Metab 2013; 17: 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhai F, Du S, Wang Z, Zhang J, Du W, Popkin B. Dynamics of the Chinese diet and the role of urbanicity, 1991–2011. Obes Rev 2014; 15: 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hu ZG, Tan RS, Jin D, Li W, Zhou XY. A low glycemic index staple diet reduces postprandial glucose values in Asian women with gestational diabetes mellitus. J Invest Med 2014; 62: 975–9. [DOI] [PubMed] [Google Scholar]
- 55. Wahlqvist ML. Asian migration to Australia: food and health consequences. Asia Pac J Clin Nutr 2002; 11: S562–8. [DOI] [PubMed] [Google Scholar]
- 56. Xu WT. Staple food preferences in the southern and northern part of China: how to eat staple food correctly. China Food 2017; 4: 152–5. [Google Scholar]
- 57. Chen YJ, Sun FH, Wong SHS, Huang YJ. Glycemic index and glycemic load of selected Chinese traditional foods. World J Gastroenterol 2010; 16: 1512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ma WJ, Huang ZH, Huang BX et al Intensive low‐glycaemic‐load dietary intervention for the management of glycaemia and serum lipids among women with gestational diabetes: a randomized control trial. Public Health Nutr 2015; 18: 1506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Louie JCY, Markovic TP, Ross GP, Foote D, Brand‐Miller JC. Higher glycemic load diet is associated with poorer nutrient intake in women with gestational diabetes mellitus. Nutr Res 2013; 33: 259–65. [DOI] [PubMed] [Google Scholar]
- 60. Ma XL, Li FF. Standardized dietary treatment on gestational diabetes intervention. Today Nurse 2011; 11: 44–5. [Google Scholar]
- 61. Yao J, Cong L, Zhu BL, Wang T. Effect of dietary approaches to stop hypertension diet plan on pregnancy outcome patients with gestational diabetes mellitus. Bangladesh J Pharmacol 2015; 10: 732–8. [Google Scholar]
- 62. Wang HY, Jiang HY, Yang LP, Zhang M. Impacts of dietary fat changes on pregnant women with gestational diabetes mellitus: a randomized controlled study. Asia Pac J Clin Nutr 2015; 24: 58–64. [DOI] [PubMed] [Google Scholar]
- 63. Chen Y, Zhou F, Huang Z, Chen X, Liu G. Effect of nutritional interventions based on food‐exchange part method on blood glucose of patients with gestational diabetes mellitus. Clin Med Eng 2017; 24: 1021–2. [Google Scholar]
- 64. Zhang HH. Food exchange portion in nutrition intervention of gestational diabetes mellitus. Shanghai J Prev Med 2013; 25: 229–31. [Google Scholar]
- 65. Zhang HH, Liu HY, Wang J, Chen YF, Zhao YZ. Clinical analysis of pregnancy nutrition management of gestational diabetes. Zhejiang Prev Med 2015; 27: 943–5. [Google Scholar]
- 66. Liu L, Hong ZX, Wang J, Ding BJ, Bi YX. Strengthening dietary intervention of gestational diabetes and pregnancy outcomes analysis. Asia J Health Manag 2015; 9: 413–7. [Google Scholar]
- 67. Liu H. Effect of nutritional intervention among women with gestational diabetes. Podiatr Dis 2018; 3: 123–7. [Google Scholar]
- 68. Wang XJ, Yan ZX. The application value of low glycemic index dietary in the treatment of gestational diabetes mellitus. Hebei Med 2016; 22: 1236–8. [Google Scholar]
- 69. Wu D. Influence of nursing intervention on blood glucose and pregnancy outcome of GDM patients. Chin Nurs Res 2014; 28: 4414–6. [Google Scholar]
- 70. Wu YY, Zeng J. Positive effect of using low glycemic index diet in dietary treatment of gestational diabetes. China Matern Health 2015; 26: 447–9. [Google Scholar]
- 71. Gai XL, Jiao RX. The impact of using glycemic load concept in gestational diabetes dietary intervention. China Matern Child Health 2012; 27: 993–4. [Google Scholar]
- 72. Jiang M, Wu YL, Chen QN. The influence of the food exchange portion diet intervention on the pregnant women with gestational diabetes. Pract Nurs Res 2016; 13: 15–7. [Google Scholar]
- 73. Li HY. The use of food exchange diet in glycaemic control and pregnancy outcomes among women with gestational diabetes. Huenan Med Res 2017; 26: 4591–2. [Google Scholar]
- 74. Zhang HQ, Wu YK, Wu MJ. Effect of food exchange intervention on gestational diabetes. Pract Nurs Res 2015; 12: 27–8. [Google Scholar]
- 75. Lian JF, Xia YQ, Wang T, Zeng W, Zheng XH. Retrospective analysis of curative effects of high dietary fiber intervention on gestational diabetes mellitus. Int J Lab Med 2014; 35: 2609–10. [Google Scholar]
- 76. Luo WM. Efficiency of high fiber diet control on glucose change and lipid profiles in gestational diabetes. China Med Pharm 2016; 6: 84–6, 162. [Google Scholar]
- 77. Pan F, Wan CH, Cheng XY, Wang HX. Effect of dietary fiber intervention on gestational diabetes. Guangxi Med 2015; 37: 1175–7. [Google Scholar]
- 78. Wu JH. Analysis of the effectiveness of dietary fiber intervention of patients with gestational diabetes. Pract Nurs Res 2010; 23: 978–9. [Google Scholar]
- 79. Yang JY, Zhong LR, Liu XY, Huang DL, Su FM. Study on efficacy of fiber addition on glucose change and lipid profiles in gestational diabetes. Chin J Fam Plann Gynecol 2015; 7: 48–51. [Google Scholar]
- 80. Chen YH. Comparison of glycemic control by using different medical therapy strategies among gestational diabetes patients. Anhui Med Pharm 2015; 19: 1135–7. [Google Scholar]
- 81. Huang LS, Guo L, Zhang RZ, Liu ZH, Hu JY. Dietary glycemic load value in gestational diabetic diet in education. China Med Pharm 2015; 5: 151–2, 164. [Google Scholar]
- 82. Shen CH, Shi XY, Li Q. Comparison of two kinds of food exchange lists in patients with gestational diabetes mellitus. Shanghai Nurs 2010; 10: 17–20. [Google Scholar]
- 83. Sun K, Chen MX, Liang LM. Influence of food exchange portion of dietary intervention based on glycemic load concept on pregnant women with gestational diabetes. Chin Nurs Res 2013; 27: 3862–4. [Google Scholar]
- 84. Wu LJ, Zhang XY, Shen MY. Application of food glycemic load values in patients with gestational diabetes diet education. Pract Nurs Res 2013; 10: 136–7. [Google Scholar]
- 85. Zhi W, Shen LL. The affect of different nutritional treatments on women with gestational diabetes. J Qiannan Med College Nationalities 2012; 25: 101–3. [Google Scholar]
- 86. Wang HX, Bian XY, Cheng XY, Hua YR. Impact of different food exchange method on patients with gestational diabetes. Chin J Diabetes 2016; 24: 422–5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Full search strategy for Ovid Medline search.
Table S2 Types of comparisons of included studies.
Table S3 (a) Summary of risk of bias judgements about each risk of bias item for randomised control trials. (b) Summary of risk of bias judgements about each risk of bias item for cohort studies.
Table S4 (a) Quality of evidence using the GRADE approach for outcomes which used individualised dietary intervention in comparison group. (b) Quality of evidence using the GRADE approach for outcomes which used food exchange intervention in control group to compare Low GL diet.


