Abstract
Vaccination against communicable diseases is crucial for disease prevention, but this practice poses challenges to healthcare professionals in patients with haemophilia. Poor knowledge of the vaccination requirements for these patients and safety concerns often result in vaccination delay or avoidance. In order to address this issue, a panel of 11 Italian haemophilia and immunization experts conducted a Delphi consensus process to identify the main concerns regarding the safe use of vaccines in patients with haemophilia. The consensus was based on a literature search of the available evidence, which was used by the experts to design 27 consensus statements. A group of clinicians then rated these statements using the 5‐point Likert‐type scale (1 = strongly disagree; 5 = strongly agree). The main issues identified by the expert panel included vaccination schedule for haemophilic patients; protocol and optimal route of vaccine administration; vaccination of haemophilic patients with antibodies inhibiting coagulation factor VIII (inhibitors); and vaccination and risk of inhibitor development. This manuscript discusses these controversial areas in detail supported by the available literature evidence and provides evidence‐ and consensus‐based recommendations. Overall, participants agreed on most statements, except those addressing the potential role of vaccination in inhibitor formation. Participants agreed that patients with haemophilia should receive vaccinations according to the institutional schedule for individuals without bleeding disorders; however, vaccination of patients with haemophilia requires comprehensive planning, taking into account disease severity, type and route of vaccination, and bleeding risk. Data also suggest vaccination timing does not need to take into consideration when the patient received factor VIII replacement.
Keywords: bleeding disorder, factor VIII inhibitor, haemophilia, immunization, vaccination
1. INTRODUCTION
The prevention of communicable diseases by vaccination has been a major public health success over the past century.1, 2, 3 However, vaccination of patients with severe congenital bleeding disorders remains a challenge, and clinicians are often uncertain about immunization recommendations for these patients.
Haemophilia, caused by the deficiency of coagulation factor VIII (haemophilia A) or coagulation factor IX (haemophilia B), is the most common severe congenital bleeding disorder.4 Patients with haemophilia require lifelong treatment with replacement therapy starting at an early age. While patients experiencing acute bleeding require immediate medical attention, prophylaxis with regular intravenous infusions of coagulation factors is also needed to prevent excessive bleeding and joint damage. However, about 20%‐30% of patients with severe haemophilia A and up to 5% of those with haemophilia B who are treated with replacement therapy develop antibodies (inhibitors) that neutralize factors VIII and IX,5, 6, 7, 8 thus compromising treatment outcomes.
A number of issues related to vaccination of patients with haemophilia remain controversial, including the immunogenicity and tolerability of off‐label subcutaneous administration of vaccines,9 and the risk of inhibitor formation with vaccination in patients receiving coagulation factor replacement therapy.5, 10 The risk of bleeding in patients with coagulation disorders needs to be carefully evaluated before intramuscular administration of any vaccine, and the subcutaneous route should only be used if the efficacy is similar to that of the intramuscular route.11 Most experts agree that individuals with haemophilia should be vaccinated according to the schedule for the general population, and that subcutaneous vaccine administration is preferred over the intramuscular route when feasible.12, 13 Patients with haemophilia should be vaccinated against hepatitis A virus and hepatitis B virus (HBV) infection, particularly if they are candidates for plasma‐derived products.13, 14 However, no clear guidelines are available on the vaccination of patients with haemophilia in clinical practice.
To address this information gap, a panel of Italian experts in the areas of haemophilia and immunization was convened within the Haemophilia and Vaccinations (HEVA) project to identify the key concerns regarding vaccination of patients with haemophilia, and provide evidence‐ and consensus‐based recommendations.
2. METHODS
A modified Delphi consensus15, 16, 17 was conducted between September 2017 and May 2018, consisting of the following steps: (a) establishment of a steering committee of Italian experts in haemophilia, infectious diseases and immunology, to define the topics; (b) validation of the initial statements by a separate group of specialists (hereafter mentioned as reviewers); (c) submission of the validated statements to clinicians from Italian Haemophilia Centres for consensus evaluation; (d) discussion of the results by the steering committee; (e) second‐round evaluation of those statements on which no consensus was achieved in the first round, by all first‐round participants; and (f) finalization of the consensus‐based recommendations.
The steering committee included 11 members, whose expertise was proven by reputation, attendance at national and international scientific meetings, and participation in clinical trials and expert panels. A literature search was performed before the first meeting (Milan, Italy; September 2017) to gain insight into current recommendations concerning vaccinations in patients with haemophilia and to identify controversial issues.
Once validated by external reviewers, the statements were submitted to clinicians operating at haemophilia centres across Italy using a secure website (http://www.progettoheva.it). The clinicians expressed their level of agreement/disagreement on each statement anonymously using a 5‐point Likert‐type scale (1 = strongly disagree, 2 = disagree, 3 = somewhat agree, 4 = agree and 5 = strongly agree). The number and percentage of participants who scored each item as 1 or 2 (disagreement) or as 3, 4 or 5 (agreement) were calculated. Consensus was considered to be reached when the sum for disagreement or agreement was ≥66%.
Statements without consensus were discussed by the steering committee during a second meeting (Milan, April 2018) and subjected to a second round of evaluation by the participants of the first Delphi round, using the online system. Finally, a series of practical consensus recommendations were drafted based on the results from the Delphi process.
3. RESULTS
3.1. Delphi process
A PubMed search of the peer‐reviewed literature published in English until 30 August 2017 (Table 1) identified 985 potentially relevant articles; 966 were excluded after reviewing the title and the abstract (Figure 1). The main reasons for exclusion were (publication prior to 1970) articles describing animal studies, haemophilia‐related articles unrelated to vaccination, studies lacking statistical power and articles that were poorly written. The full texts of the remaining 19 articles (plus 1 article that was published after completion of the literature search) were selected for discussion at the first meeting (Table S1).
Table 1.
Search strategy for the Delphi consensus during the study
| Search strategy used for the Delphi consensus |
|---|
| The search terms used were ((((vaccin*[Text Word]) AND (((((hemophilic*[Text Word] OR haemophilic*[Text Word] OR haemophilia*[Text Word] OR "factor VIII"[Text Word] OR "factor 8"[Text Word] OR BS[Text Word] OR "factor IX"[Text Word])) OR ("factor 9"[Text Word] OR "Christmas Disease"[Text Word])) OR ("Inherited Blood Coagulation"[Text Word] OR "Blood Coagulation disorder"[Text Word] OR "blood coagulation disorders"[Text Word]))))) OR ((((((("Hemophilia A"[Mesh]) OR "Hemophilia B"[Mesh])) OR "Factor VIII"[Mesh]) OR (("Blood Coagulation Disorders, Inherited"[Mesh]) OR "Blood Coagulation Disorders"[Mesh])) OR (("Blood Coagulation Factor Inhibitors"[Mesh]) OR "Blood Coagulation Factors"[Mesh])) AND (("Vaccination"[Mesh]) OR "Vaccines"[Mesh]))). |
Figure 1.
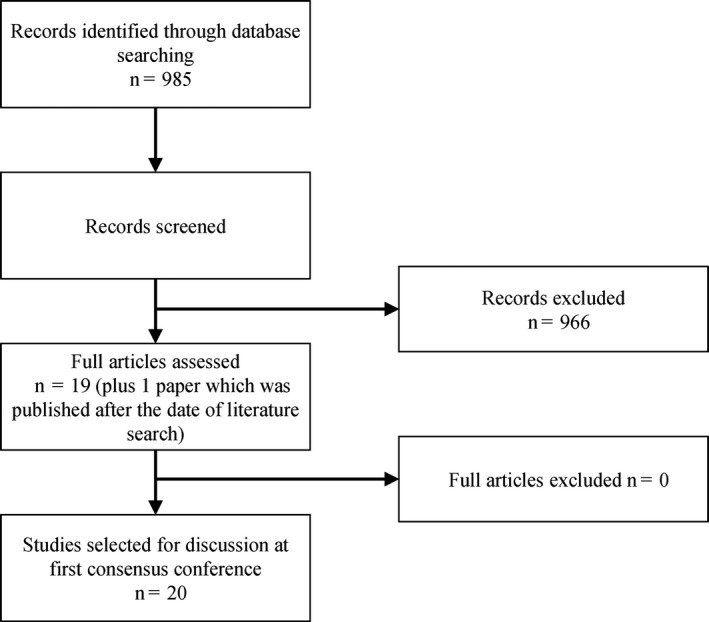
Flow diagram of the literature search and selection process
Based on the selected literature and their clinical experience, the steering committee identified five key areas of uncertainty, namely: (a) vaccination schedules; (b) vaccination protocol and optimal administration route; (c) vaccination of specific subgroups of patients; (d) the risk of inhibitor development with vaccination; and (e) vaccination of patients who have already developed inhibitors. The steering committee produced 27 statements addressing practical issues related to the five controversial areas identified (Table 2).
Table 2.
Statements and results of the Delphi consensus process
| No. | Statement | Consensus degree (%) | |
|---|---|---|---|
| First round | Second round | ||
| 1. Vaccination in patients with haemophilia | |||
| 1.1 | Children with haemophilia should receive mandatory and recommended vaccinations as per the institutional vaccination schedule, irrespective of the residual level of activity of the deficient factor | 99% agreement | ‐ |
| 1.2 | There is sufficient scientific evidence to modify the institutional vaccination schedule for paediatric patients with haemophilia | 84% disagreement | ‐ |
| 1.3 | Adults with haemophilia should receive mandatory and recommended vaccinations as per the institutional vaccination schedule, irrespective of the residual level of activity of the deficient factor | 99% agreement | ‐ |
| 2. Vaccine administration in patients with haemophilia | |||
| 2.1 | Subcutaneous administration of vaccines is preferred over intramuscular administration in patients with haemophilia, regardless of disease severity, to reduce the risk of bleeding | 83% agreement | ‐ |
| 2.2 | There is no evidence that the vaccines delivered by the intramuscular route are more effective than those administered subcutaneously | 82% agreement | ‐ |
| 2.3 | Antibody titration should not be performed before vaccination in patients with haemophilia | 71% agreement | ‐ |
| 2.4 | If intramuscular administration of vaccine is mandatory, it is advisable for patients with haemophilia to receive factor replacement prior to vaccination | No consensus | No consensus |
| 2.5 | The routine application of ice to the injection site is recommended before and after vaccine administration in patients with haemophilia | 93% agreement | ‐ |
| 2.6 | Rubbing of the injection site should be avoided in patients with haemophilia; instead, compression at the injection site is recommended | 98% agreement | ‐ |
| 2.7 | When vaccinating patients with haemophilia, use the thinnest possible needle | 94% agreement | ‐ |
| 3. Vaccinating subgroups of patients with haemophilia | |||
| 3.1 | The use of live attenuated vaccines is contraindicated in immunocompromiseda patients with haemophilia | 84% agreement | ‐ |
| 3.2 | Immunocompromiseda patients with haemophilia should be vaccinated against pneumococcus and influenza | 94% agreement | ‐ |
| 3.3 | Patients with haemophilia who are travelling to areas where yellow fever and/or typhus are endemic should be vaccinated against these diseases | 94% agreement | ‐ |
| 3.4 | Patients with haemophilia aged ≥65 years should be vaccinated against pneumococcus and influenza as per the institutional vaccination schedule | 99% agreement | ‐ |
| 3.5 | The administration of hyposensitizing therapy by subcutaneous and/or intradermal route is not contraindicated in patients with haemophilia and atopy | 92% agreement | ‐ |
| 4. Vaccination and the risk of inhibitor development in patients with haemophilia | |||
| 4.1 | There is sufficient scientific evidence supporting the association between vaccination of patients with haemophilia and development of neutralizing antibodies (inhibitor) against the deficient factor | 84% disagreement | ‐ |
| 4.2 | It is advisable to avoid vaccination on the same day as administration of factor replacement therapy to prevention inhibitor development | No consensus | 70% disagreement |
| 4.3 | When possible, the administration of prophylactic factor replacement therapy should be delayed ≥24 hours after vaccination | No consensus | 66% disagreement |
| 4.4 | When possible, the administration of prophylactic factor replacement therapy should be delayed ≥48 hours after vaccination | 75% disagreement | ‐ |
| 4.5 | When possible, the administration of prophylactic factor replacement therapy should be delayed ≥72 hours after vaccination | 77% disagreement | ‐ |
| 4.6 | The risk of inhibitor development increases if haemophilia patients are given a vaccine during a switch between different types of factor replacement therapy | 67% disagreement | ‐ |
| 4.7 | The risk of inhibitor development increases if haemophilia patients are given a vaccine concurrently with replacement therapy for trauma | No consensus | No consensus |
| 4.8 | The risk of inhibitor development increases if haemophilia patients are given a vaccine concurrently with replacement therapy for acute bleeding | No consensus | No consensus |
| 5. Vaccination of haemophilia patients with inhibitors | |||
| 5.1 | In patients with haemophilia and inhibitors, there is no evidence to that any type of vaccination should be postponed until the levels of inhibitor become undetectable | 86% agreement | ‐ |
| 5.2 | Any type of vaccination can promote the persistence of inhibitors | 77% disagreement | ‐ |
| 5.3 | There is evidence that vaccination can compromise the efficacy of immune tolerance induction therapy in patients with haemophilia and inhibitors | 77% disagreement | ‐ |
| 5.4 | All types of vaccination should be postponed in haemophilia patients with inhibitors who are undergoing immune tolerance induction therapy until there is a complete response to immune tolerance | 75% disagreement | ‐ |
Patients receiving biologic therapy; patients with HIV aged >5 years with CD4+ count <200; patients with HIV aged 1‐5 years with CD4+ count <500; patients with HIV aged <1 year with CD4+ count <750.
During the first round of consensus development, 83 participants (including the steering committee) evaluated 27 statements and reached consensus on 22, agreement on 13 and disagreement on nine (Table 2). The five statements on which no consensus was reached were concerned with the timing of vaccination in relation to the administration of coagulation factor replacement therapy (Table 2). All participating clinicians in the first round completed the survey (100% response rate).
Three of the five statements (2.4, 4.2 and 4.3) underwent a second round of evaluation due to availability of a relevant article published after 30 August 2017.18 Overall, 134 clinicians were contacted during the second round, and 74 (55%) responded. The second round resulted in consensus on two statements (4.2 and 4.3).
All participating clinicians responded to all statements in the two consensus rounds (100% response rate for each statement).
The final consensus statements are discussed in detail below, along with the evidence supporting these decisions.
3.2. Consensus statements
1. Vaccination schedules in patients with haemophilia
1.1 Children with haemophilia should receive mandatory and recommended vaccinations as per the institutional vaccination schedule, irrespective of the residual level of activity of the deficient factor (99% consensus).
1.2 There is insufficient scientific evidence to modify the institutional vaccination schedule for paediatric patients with haemophilia (84% consensus).
1.3 Adults with haemophilia should receive mandatory and recommended vaccinations as per the institutional vaccination schedule, irrespective of the residual level of activity of the deficient factor (99% consensus).
The near‐complete consensus on the need for paediatric and adult patients with haemophilia to be immunized reflects the universal acceptance of vaccinations as the most effective way to prevent infectious diseases.3, 19 While special attention is required in patients with congenital bleeding disorders, the benefits of vaccination clearly outweigh the risks. The recommended vaccinations and their schedules may vary between countries; those issued by the Italian Ministry of Health are summarized in Table S2.
2. Vaccine administration in patients with haemophilia
2.1 Subcutaneous administration of vaccines is preferred over intramuscular administration in patients with haemophilia, regardless of disease severity, to reduce the risk of bleeding (83% consensus).
2.2 There is no evidence that vaccines delivered by the intramuscular route are more effective than those administered subcutaneously (82% consensus).
2.3 There is no need to measure antibody titre prior to administration of booster doses of vaccine in patients with haemophilia; patients with haemophilia should receive booster doses by the standard schedule, without reference to antibody coverage (71% consensus).
2.5 The routine application of ice to the injection site is recommended before and after vaccine administration in patients with haemophilia (93% consensus).
2.6 Compression of the injection site is recommended after vaccination of patients with haemophilia; rubbing the injection site should be avoided (98% consensus).
2.7 When vaccinating patients with haemophilia, a needle with the smallest possible gauge should be used (94% consensus).
The group agreed (>90% consensus) with the currently recommended protocol for administering vaccines intramuscularly to individuals with bleeding disorders, which involves applying an ice pack to the injection site before and after vaccination, using the smallest gauge needle available, and applying firm pressure to the injection site without rubbing, for ≥5 minutes after vaccination.20
Some discrepancies exist in the recommendations regarding the route of vaccination in patients with bleeding disorders,20, 21 with concerns that the subcutaneous route may not provide the same level of immunogenicity as intramuscular administration.20, 22, 23 Studies have shown that subcutaneous injections were associated with vaccine failure due to lower rates of seroconversion, the effect being more pronounced in elderly patients.24, 25 A recent retrospective analysis found no significant difference in the immunogenicity of HBV vaccine in children (n = 767) with bleeding disorders who received the vaccine subcutaneously compared with those who received it intramuscularly, but there was a higher incidence of local haematoma formation in those receiving intramuscular vaccination.21 A pilot study investigating the immunogenicity of 3‐4 doses of subcutaneous diphtheria and tetanus vaccines in children with haemophilia (aged <6 years; n = 8) reported the development of a positive antibody titre to both antigens, thus confirming the feasibility of the subcutaneous route for diphtheria and tetanus vaccines in this population.9
Choosing the subcutaneous route is not always possible because some vaccines are only suitable for intramuscular use. Evidence supporting the prophylactic administration of coagulation factor concentrate before intramuscular vaccine administration is lacking. During the first consensus round, the participants were not in agreement about this practice, with 54% of the participants agreeing on the need to administer a clotting factor concentrate before intramuscular vaccine administration to minimize the risk of muscle haematoma (statement 2.4, Table 2). The lack of consensus persisted after the second round, when 50% agreed and 50% disagreed.
It was previously thought that administering factor VIII at the same time as a vaccine increased the risk of inhibitor development in patients with severe haemophilia. However, this is no longer considered to be true, and the Medical and Scientific Advisory Council of the US National Hemophilia Foundation recommends that patients may be given prophylactic factor replacement within 1 day after intramuscular vaccination to decrease the risk of injection site haematoma.20
3. Vaccination for subgroups of patients with haemophilia
Immunocompromised individuals
3.1 The use of live attenuated vaccines is contraindicated in immunocompromised patients with haemophilia (84% consensus).
3.2 Immunocompromised patients with haemophilia should be vaccinated against pneumococcus and influenza (94% consensus).
A substantial number of adults with haemophilia are human immunodeficiency virus (HIV)‐positive due to blood products received before routine screening of donated blood began.13 In this vulnerable population, vaccination against preventable disease is fundamental to avoid co‐infections that may have particularly detrimental effects. Currently, no data are available on vaccination of HIV‐infected patients with bleeding disorders. However, vaccination in the general HIV‐positive population is considered safe. Early studies reported transient increases in plasma viral load following vaccination in HIV‐positive patients26, 27; more recent studies did not confirm these results.28, 29, 30, 31, 32 International guidelines consistently recommend vaccination of HIV‐positive individuals using inactivated vaccines. Live vaccines are not recommended in adults with a CD4 count <200 cells/mm3, but are feasible and safe when the CD4 count is ≥200 cells/mm3.33, 34, 35, 36
For the present consensus, immunocompromised individuals were defined as patients receiving biologic therapy, and patients with HIV infections and low CD4+ T lymphocyte counts (<200 cells/mm3 if aged >5 years). The HEVA participants believed that evidence on vaccination from the general HIV population can be extrapolated to HIV‐infected haemophilia patients. There was good agreement that HIV‐infected patients with haemophilia and severe immunodeficiency (<200 CD4+ cells/mm3) should not be given live attenuated vaccines and almost full agreement that these patients should be vaccinated against pneumococcus and influenza virus.
Travellers
3.3 Patients with haemophilia who are travelling to areas where yellow fever and/or typhus are endemic should be vaccinated against these diseases (94% consensus).
The HEVA group agreed that patients with haemophilia who travel to countries where specific infectious diseases are endemic should be vaccinated. Depending on the country of destination, proof of vaccination for certain diseases including yellow fever or polio may be required, and other vaccinations are strongly recommended; patients with haemophilia should receive vaccinations as recommended for their travel destinations.
Elderly patients
3.4 Patients with haemophilia aged ≥65 years should be vaccinated against pneumococcus and influenza as per the institutional vaccination schedule (99% consensus).
Improved care and access to safe factor replacement products have substantially increased the life expectancy of patients with haemophilia,37, 38 resulting in the need to manage age‐related diseases in this population. Current guidelines recommend immunizing people aged ≥65 years against pneumonia, influenza and herpes zoster. These vaccinations can also be given to elderly patients with haemophilia based on the available evidence showing that older adults vaccinated against influenza or pneumococcal disease have a lower risk of developing these illnesses compared with unvaccinated age‐matched individuals.39, 40
Atopic patients
3.5 The use of hyposensitizing therapy administered by the subcutaneous or intradermal route is not contraindicated in atopic patients with haemophilia (92% consensus).
4. Vaccination and the risk of inhibitor development in patients with haemophilia
4.1 There is insufficient scientific evidence supporting the association between vaccination of patients with haemophilia and development of neutralizing antibodies (inhibitor) against the deficient factor (84% consensus).
4.2 There is no need to avoid vaccination in association with the administration of the replacement therapy with the deficient factor (on the same day) in patients with haemophilia to prevent inhibitor development (70% consensus).
4.3 There is no need to delay administration of prophylactic therapy with the deficient factor by at least 24 hours after vaccination in patients with haemophilia (66% consensus).
4.4 There is no need to delay administration of prophylactic therapy with the deficient factor by at least 48 hours after vaccination in patients with haemophilia (75% consensus).
4.5 There is no need to delay administration of prophylactic therapy with the deficient factor by at least 72 hours after vaccination in patients with haemophilia (77% consensus).
4.6 The risk of inhibitor development does not appear to be increased if haemophilia patients undergo vaccination during a switch between different factor replacement therapies (67% consensus).
Some authors have suggested that vaccination may lead to the development of inhibitors in patients receiving factor replacement therapy, but this association remains speculative. Most HEVA participants agreed that there is currently insufficient evidence supporting an association between vaccination and inhibitor formation in haemophilia patients. At the end of the first round of evaluation, no consensus was reached on whether to avoid concurrent vaccination and factor replacement therapy to prevent inhibitor formation (statement 4.2; 37% agreement), or if the administration of factor replacement therapy should be postponed by ≥24 hours (statement 4.3; 43% agreement). After the second round of evaluation, only 30% of participants agreed on statement 4.2 (postpone by ≥24 hours) and 34% agreed with statement 4.3 (postpone by ≥48 hours). Therefore, the final consensus was that there is no need to postpone the administration of replacement therapy by 24‐72 hours following vaccination.
Few studies have investigated the potential effect of vaccination on inhibitor formation, and the generalizability of these studies is limited by small sample size and short follow‐up duration.41 A pilot study comparing an early versus standard prophylaxis regimen in previously untreated severe haemophilia A patients (n = 26) reported a significantly lower risk of inhibitor formation with early versus standard prophylaxis.41
The timing of vaccination relative to factor VIII infusion may be relevant, but data from the PedNet Registry suggested otherwise.18 This study compared the risk of inhibitor development between previously untreated patients with severe haemophilia (n = 375) who did and did not receive vaccinations within 24, 72 or 120 hours of factor VIII infusion,18 and found that vaccination administered close to factor VIII exposure did not increase the risk of inhibitor formation.18
5. Vaccination of patients who have already developed inhibitors
5.1 In patients with haemophilia and inhibitors, there is no evidence that any type of vaccination should be postponed until the levels of inhibitor become undetectable (86% consensus).
5.2 Vaccination does not promote the persistence of inhibitors in patients with haemophilia who have developed inhibitors (77% consensus).
5.3 There is no evidence that vaccination can compromise the efficacy of immune tolerance (77% consensus).
5.4 Vaccination does not need to be postponed in haemophilia patients with inhibitors who are undergoing immune tolerance induction therapy (75% consensus).
The HEVA participants agreed that, based on current evidence, inhibitor antibodies do not need to be eradicated before vaccination, and vaccination does not induce inhibitor persistence. Furthermore, the current evidence indicates that vaccination does not have a negative impact on immune tolerance induction with daily high‐dose coagulation factor in haemophilia patients who have inhibitors, and vaccination does not need to be postponed until the achievement of a complete response to immune tolerance therapy.
4. CONCLUSIONS AND PERSPECTIVES
This article describes issues that may be of interest not only to haemophilia experts, but also to general practitioners, paediatricians and healthcare professionals at vaccination centres in Italy, and provides consensus‐based recommendations in key areas of uncertainty (Table 3). Participants agreed on most statements, except those addressing the potential role of vaccination in inhibitor formation. Available data showed no association between vaccination and the risk of inhibitor development, and the final consensus statements of HEVA study reflect these data.
Table 3.
Finalized consensus statements and results of the Delphi consensus process
| No. | Statement | Consensus degree (%) |
|---|---|---|
| 1. Vaccination schedules in patients with haemophilia | ||
| 1.1 | Children with haemophilia should receive mandatory and recommended vaccinations as per the institutional vaccination schedule, irrespective of the residual level of activity of the deficient factor | 99 |
| 1.2 | There is insufficient scientific evidence to modify the institutional vaccination schedule for paediatric patients with haemophilia | 84 |
| 1.3 | Adults with haemophilia should receive mandatory and recommended vaccinations as per the institutional vaccination schedule, irrespective of the residual level of activity of the deficient factor | 99 |
| 2. Vaccine administration in patients with haemophilia | ||
| 2.1 | Subcutaneous administration of vaccines is preferred over intramuscular administration in patients with haemophilia, regardless of disease severity, to reduce the risk of bleeding | 83 |
| 2.2 | There is no evidence that vaccines delivered by the intramuscular route are more effective than those administered subcutaneously | 82 |
| 2.3 | There is no need to measure antibody titre prior to administration of booster doses of vaccine in patients with haemophilia; patients with haemophilia should receive booster doses by the standard schedule, without reference to antibody coverage | 71 |
| 2.5 | The routine application of ice to the injection site is recommended before and after vaccine administration in patients with haemophilia | 93 |
| 2.6 | Compression of the injection site is recommended after vaccination of patients with haemophilia; rubbing the injection site should be avoided | 98 |
| 2.7 | When vaccinating patients with haemophilia, a needle with the smallest possible gauge should be used | 94 |
| 3. Vaccination for subgroups of patients with haemophilia | ||
| 3.1 | The use of live attenuated vaccines is contraindicated in immunocompromiseda patients with haemophilia | 84 |
| 3.2 | Immunocompromiseda patients with haemophilia should be vaccinated against pneumococcus and influenza | 94 |
| 3.3 | Patients with haemophilia who are travelling to areas where yellow fever and/or typhus are endemic should be vaccinated against these diseases | 94 |
| 3.4 | Patients with haemophilia aged ≥ 65 years should be vaccinated against pneumococcus and influenza as per the institutional vaccination schedule | 99 |
| 3.5 | The use of hyposensitizing therapy administered by the subcutaneous or intradermal route is not contraindicated in atopic patients with haemophilia | 92 |
| 4. Vaccination and the risk of inhibitor development in patients with haemophilia | ||
| 4.1 | There is insufficient scientific evidence supporting the association between vaccination of patients with haemophilia and development of neutralizing antibodies (inhibitor) against the deficient factor | 84 |
| 4.2 | There is no need to avoid vaccination in association with the administration of the replacement therapy with the deficient factor (on the same day) in patients with haemophilia to prevent inhibitor development | 70 |
| 4.3 | There is no need to delay administration of prophylactic therapy with the deficient factor by at least 24 hours after vaccination in patients with haemophilia | 66 |
| 4.4 | There is no need to delay administration of prophylactic therapy with the deficient factor by at least 48 hours after vaccination in patients with haemophilia | 75 |
| 4.5 | There is no need to delay administration of prophylactic therapy with the deficient factor by at least 72 hours after vaccination in patients with haemophilia | 77 |
| 4.6 | The risk of inhibitor development does not appear to be increased if haemophilia patients undergo vaccination during a switch between different factor replacement therapies | 67 |
| 5. Vaccination of patients who have already developed inhibitors | ||
| 5.1 | In patients with haemophilia and inhibitors, there is no evidence that any type of vaccination should be postponed until the levels of inhibitor become undetectable | 86 |
| 5.2 | Vaccination does not promote the persistence of inhibitors in patients with haemophilia who have developed inhibitors | 77 |
| 5.3 | There is no evidence that vaccination can compromise the efficacy of immune tolerance | 77 |
| 5.4 | Vaccination does not need to be postponed in haemophilia patients with inhibitors who are undergoing immune tolerance induction therapy | 75 |
Patients receiving biologic therapy; patients with HIV aged >5 years with CD4+ count <200; patients with HIV aged 1‐5 years with CD4+ count <500; patients with HIV aged <1 year with CD4+ count <750.
The overall results of the HEVA consensus suggest that patients with haemophilia should receive vaccinations according to the institutional schedule for individuals without bleeding disorders. The only difference is that vaccination of patients with haemophilia requires comprehensive planning, taking into account disease severity, type and route of vaccination, and bleeding risk. The available data also suggest vaccination timing does not need to take into consideration when the patient received factor VIII replacement.
However, a number of questions still remain unanswered and prospective studies are needed to (a) compare the immunogenicity, safety and efficacy of vaccines administered by the subcutaneous versus the intramuscular route, especially when the subcutaneous route is off‐label; and (b) elucidate the impact of vaccination on inhibitor formation in these patients. It should also be noted that these recommendations were designed by Italian experts using the Delphi consensus method, which might affect their generalizability to a broader patient population.
It is hoped that these evidence‐ and consensus‐based recommendations will provide valuable guidance for clinicians and healthcare professionals involved in the vaccination of patients with haemophilia.
DISCLOSURES
Elena Santagostino has acted as a member of advisory boards and/or speaker bureaus for Bayer, Shire, CSL Behring, Sobi, Bioverativ, Roche, Octapharma, Grifols, Kedrion, Novo Nordisk and Pfizer. Agostino Riva has received speaker's honoraria from Gilead, ViiV Health Care, Merck Sharp & Dohme, Novartis, Sobi, Sanofi and Roche. Simone Cesaro has received a fee for participation to advisory boards from Sobi. Davide Matino has received unrestricted educational grants and research funding from Pfizer, Bayer, Sobi and BIOviiix, and has also received honoraria for participating in educational events and advisory boards funded by Bayer, Sobi, Pfizer and BIOviiix. Renata Ilde Mazzucchelli is an employee of Swedish Orphan Biovitrum srl (Sobi). Giovanni Di Minno has received honoraria for scientific activities from Bayer, Novo Nordisk, Pfizer, Kedrion, CSL Behring and Boehringer Ingelheim. Susanna Esposito, Angelo Claudio Molinari, Rosamaria Mura, Lucia Dora Notarangelo, Annarita Tagliaferri, and Mario Clerici declare no conflicts of interest related to the present work.
AUTHOR CONTRIBUTIONS
The authors were the members of the HEVA steering committee. They contributed to literature search, participated in the two meetings, identified the statements to be evaluated in the Delphi process, discussed the Delphi results and drafted the final recommendations. They critically revised the various drafts of the manuscript and approved the final version before submission.
Supporting information
ACKNOWLEDGEMENTS
The HEVA project was funded by Swedish Orphan Biovitrum s.r.l. (Sobi), Milan, Italy. Alessandro Lico of Ethos SrL was the methodologist in charge of the Delphi process and of the analysis of the results. Editorial assistance in the preparation of the manuscript was provided by Catherine Rees of Springer Healthcare Communications, and by Lorenza Lanini, an independent medical writer, on behalf of Springer Healthcare Communications. This editorial assistance was funded by Sobi.
APPENDIX 1.
Members of the HEVA Study Group who participated in the Delphi process
Chiara Ambaglio, Fondazione IRCCS Policlinico S. Matteo, Centro per l'Emofilia e Coagulopatie Congenite, Pavia.
Anna Brigida Aru, SC Oncoematologia Pediatrica e Patologia della Coagulazione, Ospedale Pediatrico Microcitemico, Cagliari.
Erminia Baldacci, Centro di Riferimento e Coordinamento Regionale per le Malattie Emorragiche Congenite, Policlinico Universitario “Umberto I”, Rome.
Giovanni Barillari, Azienda Sanitaria Universitaria Integrata di Udine, Presidio Ospedaliero Santa Maria della Misericordia, SOS Malattie Emorragiche e Trombotiche, Dipartimento di Area Vasta di Medicina Trasfusionale, Udine.
Maria Basso, Fondazione Policlinico Universitario “Agostino Gemelli”, Servizio Malattie Emorragiche e Trombotiche, Polo di Scienze Oncologiche ed Ematologiche, Rome.
Sayla Bernasconi, Oncologia pediatrica Ospedale Santa Chiara, Pisa.
Marta Bertamino, Centro regionale di Riferimento per le Malattie Emorragiche, Istituto Giannina Gaslini, Genoa.
Elisa Bertoni, Unità Operativa di Oncoematologia Pediatrica, Spedali Civili, Brescia.
Chiara Biasoli, Ospedale “Maurizio Bufalini”, Centro Emofilia di Cesena, UOC Medicina Trasfusionale, Dipartimento di Patologia Clinica, Cesena.
Eugenia Federica Biguzzi, Centro Emofilia e Trombosi “Angelo Bianchi Bonomi” ‐ IRCCS Fondazione Ca' Granda Ospedale Maggiore Policlinico, Milan.
Elisa Bonetti, Oncoematologia Pediatrica, Azienda Ospedaliera Universitaria Integrata, Verona.
Alessandra Borchiellini, AOU Città della Scienza e della Salute di Torino, Centro di Riferimento Regionale per le Malattie Trombotiche ed Emorragiche dell'adulto, Struttura Complessa Ematologia, Turin.
Simona Bulgarelli, UO Medicina Trasfusionale, Ospedale “Maurizio Bufalini”, Cesena.
Sergio Cabibbo, UO Immunoematologia e Medicina Trasfusionale, Ospedale Civile di Ragusa.
Isabella Cantori, Centro di riferimento regionale per i difetti ereditari della coagulazione, Ospedale di Macerata.
Giancarlo Castaman, Azienda Ospedaliero‐Universitaria Careggi, SODc Malattie Emorragiche e della Coagulazione, Florence.
Paolo Castiglia, Dipartimento di Scienze Mediche Chirurgiche e Sperimentali, Università‐AOU di Sassari.
Antonella Coluccia, Ospedale “Veris Delli Ponti”, Centro Emofilia—UO Medicina Interna, Scorrano (LE).
Ugo Coppetelli, UO Ematologia, Ospedale “SM Goretti”, Latina.
Antonio Coppola, Regional Reference Center for Inherited Bleeding Disorders, University Hospital of Parma, Parma.
Dorina Cultrera, Centro di Riferimento Regionale per la Prevenzione, Diagnosi e Cura delle Malattie Rare della Coagulazione nel Bambino e nell'Adulto, Catania.
Erica De Candia, Fondazione Policlinico Universitario “Agostino Gemelli”, Servizio Malattie Emorragiche e Trombotiche, Polo di Scienze Oncologiche ed Ematologiche, Rome.
Grazia Delios, SIMT ASL TO4 Ospedali Riuniti del Canavese, Struttura Complessa Servizio Trasfusionale, Ivrea.
Leonardo Di Gennaro, Fondazione Policlinico Universitario “Agostino Gemelli”, Servizio Malattie Emorragiche e Trombotiche, Polo di Scienze Oncologiche ed Ematologiche, Rome.
Patrizia Di Gregorio, Servizio di Immunoematologia e medicina trasfusionale Ospedale SS Annunziata, Chieti.
Matteo Di Minno, Dipartimento Medicina Clinica—AO Universitaria “Federico II”—Policlinico, Naples.
Alfredo Dragani, Centro Emofilia e Malattie Rare del Sangue, Dipartimento di Ematologia, Medicina Trasfusionale e Biotecnologie, Pescara.
Cosimo Pietro Ettorre, Ospedale Policlinico, SSD Centro Emofilia e Trombosi, Bari.
Massimo Franchini, ASST “Carlo Poma”, Centro di Riferimento Regionale per la Diagnosi, Cura e Studio delle Malattie Emorragiche Ereditarie, Mantova.
Massimo Galli, Department of Biomedical and Clinical Sciences “L. Sacco” (DIBIC), University of Milan.
Giovanni Gallo, Azienda ULSS 2 “Marca Trevigiana”, Treviso.
Paola Giordano, Ospedale Pediatrico “Giovanni XXIII”, Centro Emofilia Pediatrico, UO Pediatria Generale e Specialistica “B. Trambusti”, Bari.
Gaetano Giuffrida, Centro di Riferimento Regionale per la Prevenzione, Diagnosi e Cura delle Malattie Rare della Coagulazione nel Bambino e nell'Adulto,Catania.
Piergiorgio Iannaccaro, Azienda Ospedaliera “Pugliese‐Ciaccio”, Struttura complessa Emofilia, Emostasi e Trombosi, Catanzaro.
Giuseppe Lassandro, Ospedale Pediatrico “Giovanni XXIII”, Centro Emofilia Pediatrico, UO Pediatria Generale e Specialistica “B. Trambusti”, Bari.
Ilaria Lazzareschi, UOC di Oncologia Pediatrica Fondazione Policlinico Universitario “Agostino Gemelli”, Rome.
Silvia Linari, Azienda Ospedaliero‐Universitaria Careggi, SODc Malattie Emorragiche e della Coagulazione, Florence.
Matteo Luciani, Ospedale Pediatrico Bambin Gesù, Centro di Riferimento Emostasi e Trombosi, Dipartimento di Ematologia, Oncologia e Medicina Trasfusionale, Rome.
Silvia Macchi, UO Centro Emofilia—Ospedale Santa Maria delle Croci, Ravenna.
Giuseppe Malcangi, Ospedale Policlinico, SSD Centro Emofilia e Trombosi, Bari.
Raniero Malizia, Servizio di Immunoematologia e medicina trasfusionale Ospedale SS Annunziata, Chieti Mariaelisa Mancuso, Centro Emofilia e Trombosi “Angelo Bianchi Bonomi”‐IRCCS Fondazione Ca' Granda Ospedale Maggiore Policlinico, Milan.
Marco Marietta, Dipartimento ad Attività Integrata di Oncologia, Ematologia e Patologie dell'apparato Respiratorio, Azienda Ospedaliero‐Universitaria di Modena, Modena.
Renato Marino, Ospedale Policlinico, SSD Centro Emofilia e Trombosi, Bari.
Michela Massoud, Ospedale Pediatrico Bambin Gesù, Centro di Riferimento Emostasi e Trombosi, Dipartimento di Ematologia, Oncologia e Medicina Trasfusionale, Rome.
Maria Gabriella Mazzucconi, Policlinico Universitario “Umberto I”—Università la Sapienza, Servizio di Emostasi e Trombosi, Centro di Riferimento e Coordinamento Regionale per le Malattie Emorragiche Congenite, Rome.
Marta Milan, Azienda Ospedaliera Universitaria di Padova, Centro Regionale multidisciplinare per la prevenzione, profilassi e trattamento avanzato dell'artropatia emofilica, Centro Emofilia, Padova.
Massimo Morfini, Centro di riferimento regionale delle malattie congenite, AO Universitaria Careggi, Florence.
Mariasanta Napolitano, Policlinico “P.Giaccone”, Centro di Riferimento Regionale per le Emocoagulopatie, UOC Ematologia, Palermo.
Samantha Pasca, Azienda Ospedaliera Universitaria di Padova, Centro Regionale multidisciplinare per la prevenzione, profilassi e trattamento avanzato dell'artropatia emofilica, Centro Emofilia, Padova
Paola Pedrazzi, Ospedale “Maurizio Bufalini”/ Centro Regionale Spoke per l'Emofilia, Cesena.
Flora A. Peyvandi, Centro Emofilia e Trombosi “Angelo Bianchi Bonomi”‐IRCCS Fondazione Ca' Granda Ospedale Maggiore Policlinico, Milan.
Lydia Piscitelli, AO U. Policlinico “S. Orsola‐Malpighi”, Bologna.
Berardino Pollio, Ospedale Infantile “Regina Margherita”, Centro di Riferimento Regionale per le Malattie Emorragiche e Trombotiche Ereditarie, SSD Medicina Trasfusionale Materno‐infantile Traumatologica, Turin.
Paola Preti, Fondazione IRCCS Policlinico S. Matteo, Centro per l'Emofilia e Coagulopatie Congenite, Pavia.
Gabriele Quintavalle, Regional Reference Center for Inherited Bleeding Disorders, University Hospital of Parma, Parma.
Paolo Radossi, UO Trasfusionale ed Immunologia—Ospedale San Giacomo Apostolo, Castelfranco Veneto Paola Ranalli, Centro Emofilia e Malattie Rare del Sangue, Dipartimento di Ematologia, Medicina Trasfusionale e Biotecnologie, Pescara.
Simona Raso, Policlinico “P. Giaccone”, Centro di Riferimento Regionale per le Emocoagulopatie, UOC Ematologia, Palermo.
Irene Ricca, Ospedale Infantile “Regina Margherita”, Centro di Riferimento Regionale per le Malattie Emorragiche e Trombotiche Ereditarie, SSD Medicina Trasfusionale Materno‐infantile Traumatologica, Turin.
Angiola Rocino, Ospedale Ascalesi, ASL Napoli 1 Centro, Centro Emofilia e Trombosi, Centro di Riferimento Regionale per le Emocoagulopatie, UOC Ematologia, Naples.
Cristina Santoro, Policlinico Universitario “Umberto I”—Università La Sapienza, Servizio di Emostasi e Trombosi, Centro di Riferimento e Coordinamento Regionale per le Malattie Emorragiche Congenite, Rome.
Rita Carlotta Santoro, Azienda Ospedaliera “Pugliese‐Ciaccio”, Struttura complessa Emofilia, Emostasi e Trombosi, Catanzaro.
Lucia Sarolo, Ambulatorio Malattie Trombotiche ed Emorragiche, Azienda Ospedaliera di Padova.
Mario Schiavoni, UO Medicina Interna e Lungodegenza,Ospedale “Veris Delli Ponti”, Scorrano (LE).
Michele Schiavulli, AORN “Santobono Pausillipon”, Centro di Riferimento Regionale per le Emocoagulopatie, Naples.
Patrizia Sciancalepore, Azienda Ospedaliera “SS. Antonio e Biagio”, Struttura Semplice Emostasi e Trombosi, Alessandria.
Maria Luisa Serino, UO Ematologia—AO Universitaria Arcispedale S. Anna, Ferrara.
Sergio Mario Siragusa, Policlinico “P.Giaccone”, Centro di Riferimento Regionale per le Emocoagulopatie, UOC Ematologia, Palermo.
Gianluca Sottilotta, Grande Ospedale Metropolitano “Melacrino Bianchi Morelli”, Centro Emofilia—Servizio Emostasi e Trombosi, Reggio Calabria.
Johanna Svahn, Centro regionale di Riferimento per le Malattie Emorragiche, Istituto Giannina Gaslini, Genoa.
Lelia Valdrè, Azienda Ospedaliero‐Universitaria Policlinico “S. Orsola‐Malpighi”, Centro Malattie Emorragiche, UO Angiologia e Malattie della Coagulazione, Bologna.
Maria Cristina Vedovati, Vascular and Emergency Medicine‐ Stroke Unit, University of Perugia.
Ezio Zanon, Azienda Ospedaliera Universitaria di Padova, Centro Regionale multidisciplinare per la prevenzione, profilassi e trattamento avanzato dell'artropatia emofilica, Centro Emofilia, Padova.
Santagostino E, Riva A, Cesaro S, et al; on behalf of the HEVA Study Group . Consensus statements on vaccination in patients with haemophilia—Results from the Italian haemophilia and vaccinations (HEVA) project. Haemophilia. 2019;25:656–667. 10.1111/hae.13756
Funding information
This manuscript was funded by Sobi, Milan, Italy.
Contributor Information
Mario Clerici, Email: mario.clerici@unimi.it.
the HEVA Study Group:
Chiara Ambaglio, Anna Brigida Aru, Erminia Baldacci, Giovanni Barillari, Maria Basso, Sayla Bernasconi, Marta Bertamino, Elisa Bertoni, Chiara Biasoli, Eugenia Federica Biguzzi, Elisa Bonetti, Alessandra Borchiellini, Simona Bulgarelli, Sergio Cabibbo, Isabella Cantori, Giancarlo Castaman, Paolo Castiglia, Antonella Coluccia, Ugo Coppetelli, Antonio Coppola, Dorina Cultrera, Erica De Candia, Grazia Delios, Leonardo Di Gennaro, Patrizia Di Gregorio, Matteo Di Minno, Alfredo Dragani, Cosimo Pietro Ettorre, Massimo Franchini, Massimo Galli, Giovanni Gallo, Paola Giordano, Gaetano Giuffrida, Piergiorgio Iannaccaro, Giuseppe Lassandro, Ilaria Lazzareschi, Silvia Linari, Matteo Luciani, Silvia Macchi, Giuseppe Malcangi, Raniero Malizia, Marco Marietta, Renato Marino, Michela Massoud, Maria Gabriella Mazzucconi, Marta Milan, Massimo Morfini, Mariasanta Napolitano, Samantha Pasca, Paola Pedrazzi, Flora A. Peyvandi, Lydia Piscitelli, Berardino Pollio, Paola Preti, Gabriele Quintavalle, Paolo Radossi, Simona Raso, Irene Ricca, Angiola Rocino, Cristina Santoro, Rita Carlotta Santoro, Lucia Sarolo, Mario Schiavoni, Michele Schiavulli, Patrizia Sciancalepore, Maria Luisa Serino, Sergio Mario Siragusa, Gianluca Sottilotta, Johanna Svahn, Lelia Valdrè, Maria Cristina Vedovati, and Ezio Zanon
REFERENCES
- 1. Greenwood B. The contribution of vaccination to global health: past, present and future. Philos Trans R Soc Lond B Biol Sci. 2014;369(1645):20130433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pandolfi F, Franza L, Todi L, et al. The importance of complying with vaccination protocols in developed countries: "anti‐vax" hysteria and the spread of severe preventable diseases. Curr Med Chem. 2018;25(42):6070‐6081. [DOI] [PubMed] [Google Scholar]
- 3. Pezzotti P, Bellino S, Prestinaci F, et al. The impact of immunization programs on 10 vaccine preventable diseases in Italy: 1900–2015. Vaccine. 2018;36(11):1435‐1443. [DOI] [PubMed] [Google Scholar]
- 4. Peyvandi F, Mannucci PM, Garagiola I, et al. A randomized trial of factor VIII and neutralizing antibodies in hemophilia A. N Engl J Med. 2016;374(21):2054‐2064. [DOI] [PubMed] [Google Scholar]
- 5. Astermark J, Altisent C, Batorova A, et al. Non‐genetic risk factors and the development of inhibitors in haemophilia: a comprehensive review and consensus report. Haemophilia. 2010;16(5):747‐766. [DOI] [PubMed] [Google Scholar]
- 6. Gouw SC, van den Berg HM. The multifactorial etiology of inhibitor development in hemophilia: genetics and environment. Semin Thromb Hemost. 2009;35(8):723‐734. [DOI] [PubMed] [Google Scholar]
- 7. Gouw Sc, van den Berg Hm, Fischer K, et al. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: the RODIN study. Blood. 2013;121(20):4046‐4055. [DOI] [PubMed] [Google Scholar]
- 8. Iorio A, Barbara Am, Makris M, et al. Natural history and clinical characteristics of inhibitors in previously treated haemophilia A patients: a case series. Haemophilia. 2017;23(2):255‐263. [DOI] [PubMed] [Google Scholar]
- 9. Schaefer BA, Gruppo RA, Mullins ES, Tarango C. Subcutaneous diphtheria and tetanus vaccines in children with haemophilia: a pilot study and review of the literature. Haemophilia. 2017;23(6):904‐909. [DOI] [PubMed] [Google Scholar]
- 10. Eckhardt Cl, van der BOM Jg, Van der naald M, Peters M, Kamphuisen Pw, Fijnvandraat K. Surgery and inhibitor development in hemophilia A: a systematic review. J Thromb Haemost. 2011;9(10):1948‐1958. [DOI] [PubMed] [Google Scholar]
- 11. Gallo G, Mel R, Ros E, Filia A. Guida alle Controindicazioni alle Vaccinazioni; 2018.
- 12. Australian Haemophilia Centre Directors’ Organisation, National Blood Authority of Australia . Guidelines for the management of haemophilia in Australia. Melbourne, Vic: Australian Haemophilia Centre Directors’ Organisation; 2016. [Google Scholar]
- 13. Rocino A, Coppola A, Franchini M, et al. Principles of treatment and update of recommendations for the management of haemophilia and congenital bleeding disorders in Italy. Blood Transfus. 2014;12(4):575‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watson HG, Wilde JT, Dolan G, et al. Update to UKHCDO guidance on vaccination against hepatitis A and B viruses in patients with inherited coagulation factor deficiencies and von Willebrand disease. Haemophilia. 2013;19(3):e191‐e192. [DOI] [PubMed] [Google Scholar]
- 15. Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67(4):401‐409. [DOI] [PubMed] [Google Scholar]
- 16. Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008‐1015. [PubMed] [Google Scholar]
- 17. Powell C. The Delphi technique: myths and realities. J Adv Nurs. 2003;41(4):376‐382. [DOI] [PubMed] [Google Scholar]
- 18. Platokouki H, Fischer K, Gouw Sc, et al. Vaccinations are not associated with inhibitor development in boys with severe haemophilia A. Haemophilia. 2018;24(2):283‐290. [DOI] [PubMed] [Google Scholar]
- 19. Makris M, Conlon CP, Watson HG. Immunization of patients with bleeding disorders. Haemophilia. 2003;9(5):541‐546. [DOI] [PubMed] [Google Scholar]
- 20. National Hemophilia Foundation . MASAC recommendations on administration of vaccines to individuals with bleeding disorders. New York, NY: National Hemophilia Foundation Medical and Scientific Advisory Committee; 2013. [Google Scholar]
- 21. Carpenter SL, Soucie JM, Presley RJ, et al. Hepatitis B vaccination is effective by subcutaneous route in children with bleeding disorders: a universal data collection database analysis. Haemophilia. 2015;21(1):e39‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Hemophilia Foundation . MASAC recommendations for hepatitis A and B immunization of individuals with bleeding disorders. New York, NY: National Hemophilia Foundation Medical and Scientific Advisory Committee; 2001. [Google Scholar]
- 23. Kroger AT, Atkinson WL, Marcuse EK, Pickering LK. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2006;55(RR‐15):656‐48. [PubMed] [Google Scholar]
- 24. Poland GA, Borrud A, Jacobson RM, et al. Determination of deltoid fat pad thickness. Implications for needle length in adult immunization. JAMA. 1997;277(21):1709‐1711. [PubMed] [Google Scholar]
- 25. Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. The. BMJ. 2000;321(7271):1237‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Calmy A, Bel M, Nguyen A, et al. Strong serological responses and HIV RNA increase following AS03‐adjuvanted pandemic immunization in HIV‐infected patients. HIV Med. 2012;13(4):207‐218. [DOI] [PubMed] [Google Scholar]
- 27. Cheeseman SH, Davaro RE, Ellison RT 3rd. Hepatitis B vaccination and plasma HIV‐1 RNA. N Engl J Med. 1996;334(19):1272. [DOI] [PubMed] [Google Scholar]
- 28. Ceravolo A, Orsi A, Parodi V, Ansaldi F. Influenza vaccination in HIV‐positive subjects: latest evidence and future perspective. J Prev Med Hyg. 2013;54(1):656‐10. [PMC free article] [PubMed] [Google Scholar]
- 29. Giacomet V, Masetti M, Nannini P, et al. Humoral and cell‐mediated immune responses after a booster dose of HBV vaccine in HIV‐infected children, adolescents and young adults. PLoS One. 2018;13(2):e0192638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hung CC, Chen MY, Hsieh SM, et al. Clinical experience of the 23‐valent capsular polysaccharide pneumococcal vaccination in HIV‐1‐infected patients receiving highly active antiretroviral therapy: a prospective observational study. Vaccine. 2004;22(15–16):2006‐2012. [DOI] [PubMed] [Google Scholar]
- 31. Ibarz‐Pavon AB, French N. No changes on viral load and CD4+ T‐cell counts following immunization with 7‐valent pneumococcal conjugate vaccine among HIV‐infected adults in Malawi. Vaccine. 2018;36(19):2504‐2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rainone V, Giacomet V, Penagini F, et al. Human papilloma virus vaccination induces strong human papilloma virus specific cell‐mediated immune responses in HIV‐infected adolescents and young adults. AIDS. 2015;29(6):739‐743. [DOI] [PubMed] [Google Scholar]
- 33. Benson CA, Andersen JW, Macatangay B, et al. Safety and immunogenicity of zoster vaccine live in HIV‐infected adults with CD4+ cell counts above 200 cells/mL virologically suppressed on antiretroviral therapy. Clin Infect Dis. 2018;67:1712‐1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frésard A, Gagneux‐Brunon A, Lucht F, Botelho‐Nevers E, Launay O. Immunization of HIV‐infected adult patients ‐ French recommendations. Hum Vaccin Immunother. 2016;12(11):2729‐2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Geretti AM, Brook G, Cameron C, et al. British HIV Association guidelines for immunization of HIV‐infected adults 2008. HIV Med. 2008;9(10):795‐848. [DOI] [PubMed] [Google Scholar]
- 36. Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):309‐318. [DOI] [PubMed] [Google Scholar]
- 37. Angelini D, Konkle BA, Sood SL. Aging among persons with hemophilia: contemporary concerns. Semin Hematol. 2016;53(1):35‐39. [DOI] [PubMed] [Google Scholar]
- 38. Mauser‐bunschoten Ep, Fransen van de putte De, Schutgens R. Schutgens RE. Co‐morbidity in the ageing haemophilia patient: the down side of increased life expectancy. Haemophilia. 2009;15(4):853‐863. [DOI] [PubMed] [Google Scholar]
- 39. Demicheli V, Jefferson T, Di Pietrantonj C, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2018;2:CD004876. [DOI] [PubMed] [Google Scholar]
- 40. Falkenhorst G, Remschmidt C, Harder T, et al. Effectiveness of the 23‐valent pneumococcal polysaccharide vaccine (PPV23) against pneumococcal disease in the elderly: systematic review and meta‐analysis. PLoS One. 2017;12(1):e0169368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kurnik K, Bidlingmaier C, Engl W, Chehadeh H, Reipert B, Auerswald G. New early prophylaxis regimen that avoids immunological danger signals can reduce FVIII inhibitor development. Haemophilia. 2010;16(2):256‐262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


