Abstract
A cortisol‐secreting adrenocortical tumour (ACT) is the cause of naturally occurring canine hypercortisolism in approximately 15% to 20% of cases. The differentiation between an adrenocortical adenoma and carcinoma is usually based on histopathology. However, histopathological parameters have never been linked to the dogs' survival. Moreover, in human medicine the inter‐observer variability of some histopathological parameters that are used for ACTs is high. The objective of this study was to establish a reliable and easy‐to‐use histopathological scoring system for cortisol‐secreting ACTs that can assess the prognosis of dogs after adrenalectomy. Cortisol‐secreting ACTs of 50 dogs, collected between 2002 and 2015, were included in this study. Twenty histopathological features were assessed by one veterinary pathologist and one resident in veterinary pathology. In addition, the Ki67 proliferation index was assessed by two observers. Only parameters with intra‐ and inter‐observer agreement scores (intra‐class correlation or Cohen's kappa coefficient) of ≥0.40 were included in survival analyses. Use of multivariate forward stepwise regression analysis with associated hazard ratios led us to a scoring system which we call the Utrecht score: the Ki67 proliferation index, +4 if more than 33% of the tumour cells have clear/vacuolated cytoplasm and + 3 if necrosis is present. Using cut‐off values of 6 and 11, we could distinguish three groups that had significantly shorter survival times with increasing Utrecht scores. We conclude that the Utrecht score can be used to assess the prognosis of dogs with cortisol‐secreting ACTs after adrenalectomy, which can help to select high‐risk dogs that might benefit from adjuvant treatment or additional monitoring.
Keywords: adrenocortical adenoma, adrenocortical carcinoma, Cushing syndrome, dogs, Ki‐67 antigen, pathology
1. INTRODUCTION
Naturally occurring hypercortisolism is one of the most common endocrine disorders in dogs. It is caused by a cortisol‐secreting adrenocortical tumour (ACT) in approximately 15% to 20% of cases, for which the treatment of choice is adrenalectomy, if no metastases can be detected.1
Without the presence of metastases, assessing the malignancy of an ACT remains challenging. The differentiation between an adrenocortical adenoma (ACA) and adrenocortical carcinoma (ACC) is usually based on histopathology. Although several studies have been conducted on the histopathological analysis of human ACTs,2, 3, 4, 5, 6, 7, 8, 9 the literature on canine ACTs is less extensive. The most recent study on histopathology of canine ACTs was published in 2004, in which the authors compared a number of histopathological criteria between ACAs and ACCs.10 The criteria that were determined to be diagnostically useful included tumour size, peripheral fibrosis, capsular invasion, trabecular growth pattern, haemorrhage, necrosis, single‐cell necrosis, haematopoiesis, fibrin thrombi, cytoplasmic vacuolation and the proliferation index by use of the Ki67 marker.10 However, whether the presence or absence of these parameters was associated with poor survival times has not been assessed. Moreover, the parameters used in this study were analysed by only one pathologist,10 whereas in the classification of human ACTs the inter‐observer variability is known to be high for some of these parameters.2, 3
In humans, the most widely used histopathological scoring system to differentiate ACCs from ACAs is the Weiss score. In this system, the presence of three or more out of nine established histopathologic criteria indicates malignant potential.6, 7 Although the Weiss score can diagnose an ACC with high sensitivity and specificity, some of the criteria included suffer from inter‐observer variation and require evaluation by an experienced endocrine pathologist.2, 3 To reduce this inter‐observer variation, other scoring systems such as the Weiss revisited score3 and the Helsinki score2 have been proposed. In addition, several studies have shown the value of immunohistochemical staining for the proliferation marker Ki67 to differentiate between ACAs and ACCs.3, 11, 12, 13, 14
In humans, the prognosis of patients with an ACC does not only differ from that of patients with an ACA, but the prognosis also varies greatly within the group of patients with an ACC.8, 15 In human ACCs, histopathological criteria that are associated with a poor prognosis include high mitotic rate, high Ki67 proliferation index (PI), and high Helsinki score.7, 8, 16, 17
The objective of this retrospective study was to establish a reliable and easy‐to‐use histopathological scoring system for cortisol‐secreting ACTs that can assess the prognosis of dogs after adrenalectomy.
2. MATERIALS AND METHODS
2.1. Case selection
Canine cortisol‐secreting ACTs were collected between 2002 and 2015. Permission to use the ACT tissue was obtained from all dog owners. The suspicion of hypercortisolism was based on the presence of clinical signs and routine laboratory findings consistent with hypercortisolism. Non‐suppressible hypercortisolism was diagnosed using the low‐dose dexamethasone suppression test, or urinary corticoid: creatinine ratios (UCCRs) combined with a high‐dose dexamethasone suppression test.18 The presence of an adrenal tumour was visualized by abdominal ultrasonography, computed tomography, or both. All dogs underwent unilateral adrenalectomy, which was performed by one of four experienced veterinary surgeons. Dogs were excluded from the study when they were euthanized or died before, during, or within 2 weeks after adrenalectomy; when the dog had bilateral adrenal tumours; when metastases were detected before or at time of surgery; when no formalin‐fixed paraffin‐embedded tissue was available; and when less than 3 months of follow‐up information was available.
2.2. Clinical parameters
The dogs' medical records were retrospectively reviewed for dog‐related parameters, clinical tumour‐related parameters and surgery‐related parameters. Dog‐related parameters included sex and neutering status, body weight, age at time of surgery and preoperative treatment for hypercortisolism. Tumour‐related parameters included the location of the tumour (left or right), whether there was evidence of venous invasion seen during diagnostic imaging or during surgery, and the tumour diameter (not including normal adrenal tissue). Measurement of the tumour diameter was performed during diagnostic imaging (ultrasound or CT) and/or after surgery. Surgery‐related parameters included the duration of the surgery, and whether the tumour capsule ruptured during surgery.
2.3. Histopathological parameters
The ACTs were collected within 10 minutes after surgical removal. A representative section of the tumour was fixed in formaldehyde for histopathology and immunohistochemistry, the remaining tumour sections were used for other research purposes. The tissues were fixed in 4% buffered formaldehyde for 24 to 48 hours, embedded in paraffin, and cut into 4‐μm sections. One tissue section for each ACT was stained with haematoxylin and eosin, and one with the Gordon and Sweet's reticulin stain. Twenty histopathological parameters were assessed by one veterinary pathologist (G.C.M.G.) and one resident in veterinary pathology (K.C.): (a) reticulin fibre density; (b) growth pattern; (c) morphology of cytoplasm; the presence or absence of (d) haemorrhage, (e) extramedullary haematopoiesis, (f) intra‐tumoural fibrosis, (g) peripheral fibrosis, (h) capsular invasion, (i) venous invasion, (j) sinusoidal invasion, (k) fibrin thrombi, (l) necrosis, (m) atypical mitotic figures, (n) nucleoli and (o) nuclear chromasia; and establishment of the (p) number of cells undergoing single‐cell necrosis, (q) number of mitotic figures, (r) nuclear size, (s) cellular size, and (t) nuclear grade.
The reticulin fibre density was scored as the percentage of the ACT with decreased fibre density compared to the density of reticulin fibres in the zona fasciculata of a normal adrenal gland. The growth patterns were evaluated as being diffuse, nesting or trabecular, and the growth pattern that predominated was recorded. The reticulin fibre density and the growth patterns were evaluated in the Gordon and Sweet's reticulin stain, all other parameters in the haematoxylin and eosin stain. An estimate of the percentage of cells with clear/vacuolated cytoplasm was noted. Peripheral fibrosis was considered to be present when a multi‐layered band of fibrous tissue surrounded at least part of the ACT. Capsular invasion was considered to be present when the ACT infiltrated or perforated the peripheral capsule; small nests of adrenocortical cells that resembled cells of the zona glomerulosa within the capsule were considered to be normal, because these nodules are also often encountered in adrenal glands of healthy dogs. Necrosis was considered to be present when confluent nests of necrotic cells were visible. For the evaluation of single‐cell necrosis and the number of mitotic figures, first the sections were screened to determine where most cells undergoing single‐cell necrosis or mitosis appeared to be present, and then these parameters were evaluated in the one high power field (HPF; 400× magnification) where they seemed to be most abundant. Nuclear and cellular sizes were evaluated and given a number relative to a nucleus or cell in the zona fasciculata of a normal adrenal gland (eg, equally large: 1; twice as large: 2), the size that predominated was recorded. The nuclear grade was evaluated as described by Fuhrman et al. (1982).19 All histopathological parameters were visually estimated in the entire tissue section using a light microscope, no image analysis was performed. Both observers assessed the tissue sections twice at separate time points, and were blinded to the clinical data.
2.4. Ki67 proliferation index
For Ki67 immunohistochemistry, one tissue section per ACT was rehydrated in a series of xylene and ethanol baths. Antigen retrieval was performed with Tris‐EDTA buffer (pH 9) in a microwave, at 850 W for 7 minutes and at 450 W for 15 minutes. After the slides were cooled down they were incubated with 0.35% H202 in Tris buffered saline (TBS) for 30 minutes to block endogenous peroxidase. The slides were blocked with 10% normal goat serum with 1% bovine serum albumin (BSA) in TBS for 30 minutes. Incubation with a mouse monoclonal primary anti‐Ki67 antibody (MIB‐1 clone, M7240, Dako, Agilent, Amsterlveen, The Netherlands), diluted 1:75 in 1% BSA in TBS, took place overnight at 4°C. The following day the slides were incubated with secondary antibody (HRP‐labelled goat‐anti‐mouse, EnVision+, Dako) for 30 minutes. The slides were incubated with Dako Liquid DAB+ Substrate Chromogen System (K3468, Dako) for 10 minutes, and counterstained with haematoxylin. A series of ethanol and xylene baths were used to dehydrate the slides, after which the slides were mounted with VectaMount Mounting Medium (H‐5000, Vector Laboratories, Peterborough, UK). During intermediate steps the slides were washed in TBS. Canine colon tissue slides were used as positive control tissue, and the primary antibody was replaced by normal mouse IgG (SC3877, Santa Cruz Biotechnology, Heidelberg, Germany) for the negative control.
The Ki67 PI was assessed by the resident in veterinary pathology (K.C.) and a researcher with experience in adrenocortical tumours (K.S.) in hot spot areas, which were the areas that appeared to have the highest percentage of Ki67 positive cells. For each tissue slide five images were captured on an Olympus BX60 microscope with Leica LAS‐AF software, at 200× magnification. A minimum of 1000 nuclei were counted in total per ACT on the one or two images with the highest amount of Ki67 positive cells, for which ImageJ software20 was used to keep track of the number of counted positive and negative cells. Only nuclear staining was considered to be positive. Care was taken to only include ACT cells and not for example, cells of the stromal compartment or extramedullary haematopoiesis. The PI was calculated as the percentage of Ki67 positive nuclei relative to the total number of counted nuclei for each ACT.
2.5. Analyses
The intra‐ and inter‐observer agreement scores for histopathological parameters and the Ki67 PI were quantified using the intra‐class correlation coefficient (ICCC) for continuous variables, and Cohen's kappa coefficient for categorical variables. The strength of agreement was interpreted as follows: <0.40, poor; 0.40‐0.59, modere; 0.60‐0.79, good; 0.80‐1.00, excellent.21 Only parameters with an agreement score of more than 0.40 in all areas (ie, intra‐observer agreement of both observers, and inter‐observer agreement) were included in survival analyses. In case of continuous variables, the results of all four observations (ie, two observations per observer) were averaged for further analyses. In case of categorical variables, the result that was most prevalent was noted. If this could not be established, the parameter was scored again for these slides and the final outcome was noted.
Dogs were considered to have died as a result of the ACT when they were euthanized because of metastases or comorbidities related to recurrence of hypercortisolism. Recurrence of hypercortisolism was confirmed by the presence of hypercortisolism‐related clinical signs and elevated UCCRs, which could be either because of regrowth of the ACT or metastases. For each dog, survival time was calculated from the time of surgery to euthanasia because of recurrence. If a dog died from unrelated causes, was lost to follow‐up, or was still alive at the end of the study, then the dog was censored and the last known date that the dog was still alive was used as censoring date.
Univariate analyses were performed with the Cox proportional hazards model. All variables that had a P value of <0.15 in the univariate analyses were subsequently included in multivariate stepwise regression with forward selection. Optimal cut‐off values were calculated with receiver operating characteristic curves. The value with the highest Youden index (sensitivity + specificity − 1) was selected as the optimal cut‐off value. Survival times were calculated using the Kaplan‐Meier product‐limit method. The log‐rank method was used to calculate if differences between groups were significant.
P values of <0.05 were considered significant. All statistical analyses were performed with SPSS Statistics for Windows (Version 24.0, IBM Corp, Armonk, New York).
3. RESULTS
3.1. Cases
A total of 50 dogs were included in the study. The most represented dog breeds were Labrador Retriever (5), Dachshund (5), Jack Russel Terrier (4), Schnauzer (2), Maltese (2), Fox Terrier (2) and White Shepherd (2). Of the remaining dogs, 11 were mixed‐breed dogs and 17 were of breeds that were represented once. Information on additional dog‐related parameters is included in Table 1.
Table 1.
Effects of clinical parameters on survival
| Parameter | Distribution | Total | Hazard ratio (95%CI) | P value |
|---|---|---|---|---|
| Sex | 25 male, 25 female | 50 | 1.046 (0.420‐2.607) | 0.921 |
| Neutered | 22 no, 28 yes | 50 | 1.483 (0.576‐3.814) | 0.414 |
| Body weight | 14.2 (3.6‐74.7) kg | 50 | 1.011 (0.979‐1.045) | 0.506 |
| Age | 10.0 (2.1‐12.9) y | 50 | 1.099 (0.844‐1.431) | 0.483 |
| Treated before surgery | 36 no, 9 yes | 45 | 1.060 (0.346‐3.250) | 0.918 |
| Location | 23 left, 25 right | 48 | 1.263 (0.502‐3.176) | 0.620 |
| Venous invasion (macro) | 27 no, 16 yes | 43 | 0.722 (0.277‐1.882) | 0.505 |
| Tumour diameter | 2.5 (1.0‐10.0) cm | 46 | 1.424 (1.110‐1.827) | 0.005* |
| Surgery duration | 156 (42‐290) min | 43 | 1.002 (0.993‐1.012) | 0.630 |
| Capsule rupture | 31 no, 16 yes | 47 | 1.339 (0.525‐3.415) | 0.541 |
Univariate analyses performed with the Cox proportional hazards model. Distribution is indicated in categories for categorical variables, and in median (with the range in parentheses) for continuous variables. Total indicates the total number of dogs for which this parameter was known. Hazard ratio (with the 95% confidence interval in parentheses) indicates the hazard of the second category compared to the first in case of categorical parameters, and the hazard per stated unit in case of continuous variables. Significant P values are indicated in italic with an asterisk.
The estimated median survival time after adrenalectomy for all 50 dogs as calculated by the Kaplan‐Meier method was 54.7 months (95% CI 47.1‐62.2 months) (Figure 1A). Of the 50 dogs, 19 were known to have had recurrence of hypercortisolism. The median survival time of these 19 dogs with recurrence was 16.9 months (95% CI 10.8‐49.3 months). All 31 dogs that had no recorded recurrence were censored in the survival analyses so no estimated median survival was reached using the Kaplan‐Meier method, but the median follow‐up time was 27.1 months (95% CI 15.4‐42.0 months).
Figure 1.
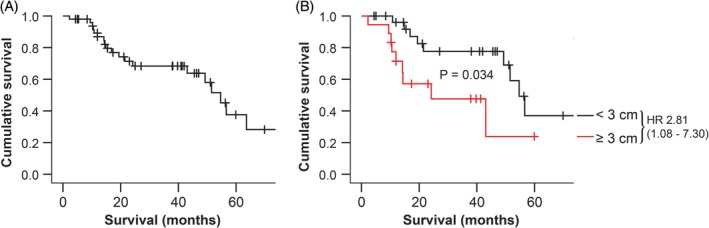
A, Overall survival of all dogs included in the study (n = 50) and (B) survival stratified according to tumour diameter (n = 46) using Kaplan‐Meier analysis. Survival times were calculated from the time of surgery to euthanasia due to recurrence. Censored dogs are indicated as tick marks. B, Dogs were classified as having an adrenocortical tumour diameter < 3 cm (black line, n = 28), or ≥ 3 cm (red line, n = 18). HR indicates estimated hazard ratio (with 95% confidence interval designated in parentheses). P value indicates the significance of the difference between the groups as calculated with the log‐rank test
3.2. Clinical parameters
Of all clinical parameters that were analysed, only the tumour diameter was significantly associated with survival (univariate analysis, Table 1). The optimal cut‐off value was approximately 3 cm, which resulted in a significant (P = 0.034) difference in survival times between dogs with an ACT ≥3 cm (n = 18, estimated median survival time 24.2 months, 95% CI 1.3‐47.1 months) and dogs with an ACT <3 cm (n = 28, estimated median survival time 54.7 months, 95% CI 48.2‐61.1 months) (Figure 1B). Cut‐off values of 2 or 5 cm as previously suggested in literature10, 22 did not result in significant differences in survival times (P = 0.255 and P = 0.172, respectively).
3.3. Histopathological parameters and Ki67 PI
In assessing intra‐ and inter‐observer reliability, four histopathological parameters had agreement scores of more than 0.4 in all areas (ie, intra‐observer agreement scores for both observers, and inter‐observer agreement score): decreased reticulin fibre density (Figure 2A, lowest agreement score 0.56, P < 0.001), extramedullary haematopoiesis (Figure 2B, lowest agreement score 0.59, P < 0.001), clear/vacuolated cytoplasm (Figure 2C and D, lowest agreement score 0.67, P < 0.001) and necrosis (Figure 2E, lowest agreement score 0.51, P < 0.001). No intra‐observer agreement scores could be calculated for the Ki67 PI (Figure 2F) because both observers assessed all ACTs once, but the inter‐observer agreement score was excellent (0.96, P < 0.001). All intra‐ and inter‐observer agreement scores are provided in Supporting Information Table S1.
Figure 2.
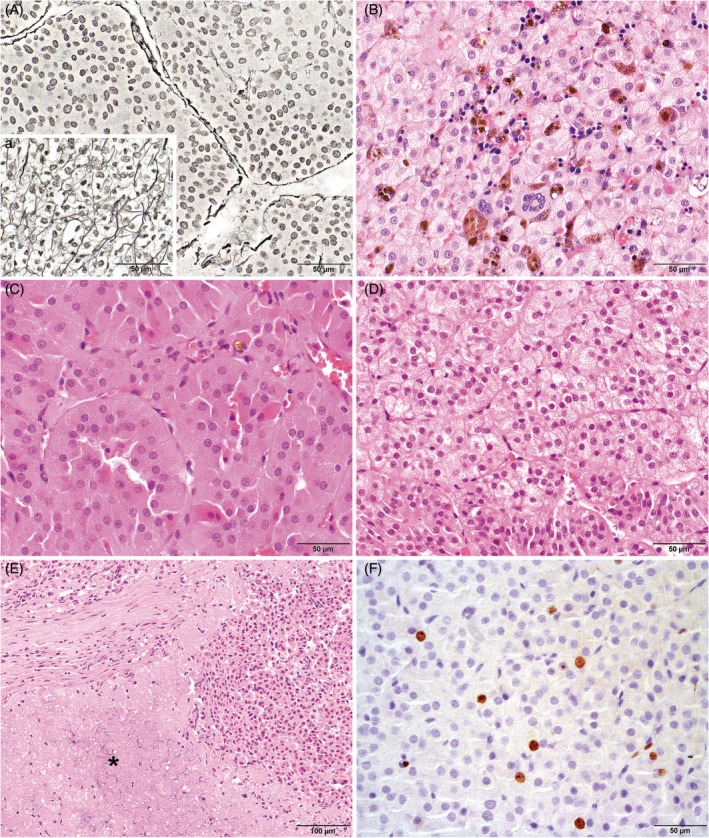
Histopathological parameters in canine cortisol‐secreting adrenocortical tumours with intra‐ and inter‐observer agreement scores of >0.4. A, Example of tumour area with decreased reticulin fibre density compared to (a) the zona fasciculata of a normal adrenal gland. Gordon and Sweet's reticulin stain, bar represents 50 μm. B, Example of tumour with extramedullary haematopoiesis, including megakaryocytes. Haematoxylin and eosin stain, bar represents 50 μm. C, Example of adrenocortical tumour cells with eosinophilic/granular cytoplasm. Although some tumour cells do contain cytoplasm with a small number of vacuoles, the cytoplasm of the cells in this image was generally characterized as eosinophilic/granular. Haematoxylin and eosin stain, bar represents 50 μm. D, Example of adrenocortical tumour cells with clear/vacuolated cytoplasm. The majority of cells in this image are characterized by cytoplasm with numerous small vacuoles. The cells in the bottom part of the image contain cytoplasm with a more eosinophilic/granular aspect. Haematoxylin and eosin stain, bar represents 50 μm. E, Example of an adrenocortical tumour in which part of the cells are necrotic (asterisk). Haematoxylin and eosin stain, bar represents 100 μm. F, Example of an adrenocortical tumour with Ki67 positive staining (brown) within the nuclei of a subset of tumour cells. Anti‐Ki67 immunohistochemical staining with haematoxylin counterstaining, bar represents 50 μm
In assessing the potential of the histopathological parameters as prognostic indicators, three parameters were significantly associated with survival according to univariate analyses (Table 2): the percentage of clear/vacuolated cytoplasm, the presence of necrosis, and the Ki67 PI. For the continuous variables that were significantly associated with survival, we calculated optimal cut‐off values, for which dogs that had recurrence and died within 30 months after surgery were included in the positive group (n = 13), and dogs that had no recorded recurrence and lived for at least 30 months were included in the negative group (n = 15). For the percentage of clear/vacuolated cytoplasm, the optimal cut‐off value was approximately 33%. For the Ki67 PI, the optimal cut‐off value was 4.6%.
Table 2.
Effects of histopathological parameters and the Ki67 PI on survival: univariate analyses
| Parameter | Distribution | Total | Hazard ratio (95%CI) | P value |
|---|---|---|---|---|
| Decreased reticulin fibre density | 85 (19‐100) % | 50 | 1.018 (0.987‐1.051) | 0.257 |
| Clear/vacuolated cytoplasm | 48 (5‐96) % | 50 | 1.023 (1.004‐1.043) | 0.020* |
| Extramedullary haematopoiesis | 37 no, 13 yes | 50 | 0.882 (0.284‐2.737) | 0.828 |
| Necrosis | 30 no, 20 yes | 50 | 3.495 (1.336‐9.141) | 0.011* |
| Ki67 PI | 3 (0‐22) % | 50 | 1.174 (1.065‐1.295) | 0.001* |
Abbreviation: PI, proliferation index.
Univariate analyses performed with the Cox proportional hazards model. Distribution is indicated in categories for categorical variables, and in median (with the range in parentheses) for continuous variables. Total indicates the total number of dogs for which this parameter was known. Hazard ratio (with the 95% confidence interval in parentheses) indicates the hazard of the second category compared to the first in case of categorical parameters, and the hazard per stated unit in case of continuous variables. Significant P values are indicated in italic with an asterisk.
3.4. The Utrecht score
To determine which parameters were independent predictors of poor survival, we used multivariate logistic regression with forward selection. This indicated that the Ki67 PI, clear/vacuolated cytoplasm in at least 33% of the tumour cells, and the presence of necrosis were all independent predictors of survival. Based on their hazard ratios in the multivariate analysis (Table 3), this led to the scoring system: 1.12 × Ki67 PI, + 4.35 if clear/vacuolated cytoplasm was present in at least 33% of the tumour cells, + 3.18 if necrosis was present. We simplified this system to: the Ki67 PI, + 4 if ≥33% of tumour cells have clear/vacuolated cytoplasm, + 3 if necrosis is present, which we call the Utrecht score (Figure 3).
Table 3.
Independent predictors of survival: multivariate analysis
| Parameter | Distribution | Hazard ratio (95%CI) | P value |
|---|---|---|---|
| Ki67 PI | 3.1 (0.0‐22.2) % | 1.124 (1.018‐1.242) | 0.021* |
| Clear/vacuolated cytoplasm in ≥33% | 18 no, 32 yes | 4.350 (1.224‐15.455) | 0.023* |
| Presence of necrosis | 30 no, 20 yes | 3.181 (1.103‐9.173) | 0.032* |
Abbreviation: PI, proliferation index.
Multivariate stepwise regression performed with the Cox proportional hazards model with forward selection. Distribution is indicated in categories for categorical variables, and in median (with the range in parentheses) for continuous variables. Hazard ratio (with the 95% confidence interval in parentheses) indicates the hazard of the second category compared to the first in case of categorical parameters, and the hazard per stated unit in case of continuous variables. Significant P values are indicated in italic with an asterisk.
Figure 3.
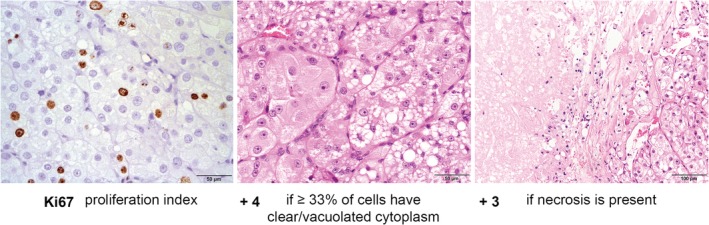
The Utrecht score in canine adrenocortical tumours: Ki67 proliferation index, + 4 if ≥33% of tumour cells have clear/vacuolated cytoplasm, + 3 if necrosis is present. The images of the parameters shown here are from the adrenocortical tumour of one patient, which had the highest Utrecht score of our dataset. The adrenocortical tumour in this example had a Ki67 proliferation index of 22.2%, clear/vacuolated cytoplasm (small to very large vacuoles) in approximately 66% of tumour cells (so more than 33%), and fields of necrosis were present. This resulted in a total score of 22.2 + 4 + 3 = 29.2
The median Utrecht score was 7.1 (range, 0.4‐29.2), and the optimal cut‐off value was approximately 6. To further divide the ACTs with scores of 6 or higher into two groups with different survival times, we used a cut‐off value of 11, which was calculated as the approximate optimal cut‐off value in the subset of patients with scores of more than 6. We then categorized the ACTs according to their Utrecht score in three groups: (1) Utrecht score < 6 (n = 17), (2) Utrecht score ≥ 6 to <11 (n = 22) and (3) Utrecht score ≥ 11 (n = 11). As illustrated in Figure 4, the estimated median survival time was not reached for group 1, was 51.5 months (95% CI 43.7‐59.3) for group 2 (significantly different from group 1, P = 0.012), and 14.4 months (95% CI 10.5‐18.2) for group 3 (significantly different from group 2, P = 0.005).
Figure 4.
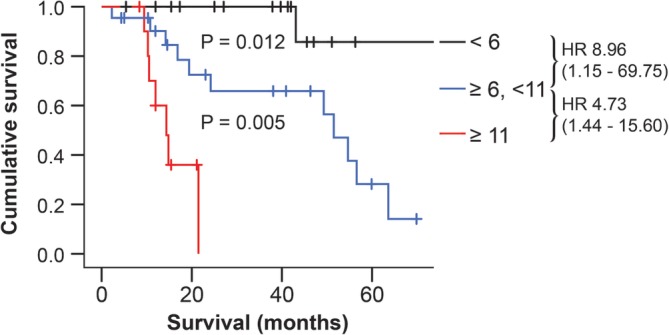
Survival stratified according to the Utrecht score using Kaplan‐Meier analysis. Survival times were calculated from the time of surgery to euthanasia because of recurrence. Censored dogs are indicated as tick marks. Dogs were classified as having a Utrecht score of <6 (black line, n = 17), of ≥6 to <11 (blue line, n = 22), or of ≥11 (red line, n = 11). HR indicates estimated hazard ratios (with 95% confidence interval designated in parentheses). P values indicate the significance of the difference between the groups as calculated with the log‐rank test
4. DISCUSSION
Here we introduce the Utrecht score: a novel histopathological scoring system that can assess the prognosis of dogs with a cortisol‐secreting ACT after adrenalectomy. With cut‐off values of 6 and 11, we could distinguish three groups that had significantly shorter survival times with increasing scores. We propose to classify ACTs with a score of <6 as having low risk of recurrence, with a score of ≥6 to <11 as having moderate risk of recurrence, and with a score of ≥11 as having high risk of recurrence.
Because most veterinary pathologists will not encounter an ACT on a daily basis, histopathological assessment of an ACT ideally should not require extensive training. In this study, 20 histopathological parameters were scored twice by both an experienced veterinary pathologist and a resident in veterinary pathology. This allowed us to include only those parameters that turned out to have low intra‐ and inter‐observer variability irrespective of the observers' experience, thereby improving the reliability of the Utrecht score.
A recent study on human ACCs reported low intra‐ and inter‐observer agreement scores for Ki67 scoring among 14 trained endocrine pathologists.23 However, in that study each observer was allowed to perform the analyses according to their own method of preference, which included visual estimation, formal manual count, and digital image analysis. In our study, the inter‐observer agreement of the Ki67 PI was excellent, which is most likely associated with the methodology used. To standardize Ki67 PI scoring as much as possible, we suggest to analyse the Ki67 PI in hot spot areas, to capture images of these areas, to count at least 1000 nuclei, and to use ImageJ20 or similar software to keep track of the number of counted positive and negative cells. A drawback of this method is that it is time‐consuming, but developments in digital microscopy‐enabled methods could facilitate future reproducible and reliable Ki67 PI assessments.23
Increased cell proliferation and tumour hypoxia are both important features of aggressive cancers,24 which explains why Ki67 PI and necrosis are important prognostic factors in canine ACTs. Why the high percentage of clear/vacuolated cytoplasm is an important factor is less clear. The appearance of clear/vacuolated cytoplasm likely indicates the presence of intra‐cytoplasmic lipid droplets, which are extracted during histologic specimen preparation unless special methods are used.25 Since cholesterol, which could be stored in these lipid droplets, functions as substrate for all steroid hormones, the percentage of cells with clear/vacuolated cytoplasm could be related to the production of cortisol or its precursors. In human ACTs, cortisol production is known to be a negative prognostic indicator.26, 27 In our study, however, only cortisol‐secreting ACTs were included so the presence or absence of hypercortisolism was not a variable, but the percentage of cells with clear/vacuolated cytoplasm could have been related to the degree of hypercortisolism. Because different methods or assays were used to establish hypercortisolism, we were unable to test this hypothesis. For future studies it would be interesting to determine whether clear/vacuolated cytoplasm is indeed related to the degree of cortisol production, since this would also indicate that non‐secreting ACTs might be less malignant overall than cortisol‐producing ACTs.
Of the clinical parameters, only the tumour diameter was significantly associated with survival. However, it did not retain its significance in the multivariate analysis, neither on a continuous scale nor when using the indicated cut‐off value. Although this means that it is not an independent predictor of survival after surgery, it is currently the only parameter that can give an assessment of prognosis before surgery. We showed that dogs with a tumour diameter of ≥3 cm had significantly worse survival times after adrenalectomy than those with a diameter of <3 cm. Although previous studies reported that cut‐off values of 2 cm10 or 5 cm22 should be used, classification based on these values did not result in significantly different survival times in our study. In some studies tumour size was not significantly associated with survival time.28, 29 For future studies it might be useful to determine whether measurement of the tumour volume has additional prognostic value compared to measurement of the tumour diameter.
In this study we assessed whether the evaluated parameters can predict long‐term survival. What we therefore did not assess was whether these parameters influence perioperative or short‐term survival. For example, although venous invasion as observed during imaging or surgery does not seem to affect long‐term survival, as also reported in other studies,30, 31 it could complicate the surgical procedure. Indeed, extensive invasion of the tumour into the caudal vena cava when the tumour thrombus extends beyond the hepatic hilus has been reported to increase perioperative mortality.31 In other studies, however, the presence of venous invasion did not affect perioperative mortality,10, 30 so this possibly depends on the extent of invasion, the experience of the surgeon, and/or whether cases with extensive venous invasion are considered to be candidates for surgery. Other factors that have been reported to increase perioperative mortality rates are acute adrenal haemorrhage and large tumours.30
When comparing the Utrecht score with scoring systems for human ACTs, it is noteworthy that it closely resembles the Helsinki score (3 × mitotic rate + 5 × necrosis + Ki67 PI).2 Because the intra‐ and inter‐observer agreement scores for the assessment of mitotic figures were inadequate in our study, we did not include the mitotic rate in our survival analyses. The low number of HPFs that were analysed for the mitotic rate is a weakness of this study, because the agreement scores for the mitotic rate could possibly be improved by counting the number of mitotic figures in more HPFs. Interestingly, in both the Weiss and Weiss revisited score, clear cytoplasm in less than 25% of the tumour is a negative prognostic factor,3, 6 similar to previously described in canine ACTs,10 whereas in the Utrecht score more than 33% tumour cells with clear/vacuolated cytoplasm is a negative prognostic factor. Clear cytoplasm was, however, reported to be the least useful criterion in the Weiss score.6 In both the human and canine previous studies, cases with an ACT were selected irrespective of their hormonal status,3, 6, 10 whereas we included only cortisol‐secreting ACTs. Whether this discrepancy in morphology of cytoplasm is related to a difference in assessment, interpretation or both, or to differences in the hormonal status of the ACT, remains to be elucidated.
A weakness of this study is its retrospective nature. Not all information had been documented consistently, for example, information on the tumour diameter was missing in four cases, and measurement of the tumour diameter was not standardized. Moreover, we were not able to reliably calculate tumour volumes because the multiple dimensions of the tumour were not often documented, nor was the tumour weight, which has prognostic value in human ACTs.9 The study's retrospective nature could also have affected the reported survival times of the dogs, which were sometimes based on the owner's estimate of when their dog had died. In addition, the reason for recurrence was not investigated in every case, so we could not make a distinction between local recurrence and recurrence because of metastases.
Another remark is that although ACTs can be highly heterogeneous, all histopathological parameters and the Ki67 PI have been assessed on just one tissue section of the ACT because of the retrospective nature of this study. If the most malignant area of the tumour was not included in this tissue section, this could have resulted in an underestimation of the malignancy grade. However, even with just one tissue section the Utrecht score was able to distinguish three groups with significantly different survival times.
Although the fact that we only included parameters with intra‐ and inter‐observer agreement scores of ≥0.4 improves the reliability of the scoring system, a drawback is that we may have excluded factors that are important prognostic parameters. Moreover, the method used to create the Utrecht score has based its calculations on this specific subset of patients. To verify the validity of the score, a large prospective multi‐institutional study in other patient groups and with the participation of more veterinary pathologists is required. Additionally, in this study we only included cortisol‐secreting ACTs, and whether the score can also be a valuable prognostic tool in, for example, non‐secreting ACTs will have to be determined in future studies.
In conclusion, we introduce the Utrecht score to post‐surgically assess the prognosis of dogs with cortisol‐secreting ACTs. Having an accurate assessment of prognosis is useful to communicate to the dog's owner, but could also assist in selecting high‐risk dogs that might benefit from additional monitoring, adjuvant therapy with, for example, mitotane, or both. Moreover, a solid histopathological scoring system represents a basis for future studies on other markers of malignancy, which could facilitate the identification of potential future treatment targets.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
Supporting information
TABLE S1 Intra‐ and inter‐observer agreement scores\
Intra‐ and inter‐observer agreement scores of histopathological parameters in canine cortisol‐secreting adrenocortical tumours (n = 50). Method of score quantification indicated by a (intra‐class correlation coefficient; continuous variables) and b (Cohen's kappa coefficient; categorical variables). N/A: not available; indicates that at least one of the variables is a constant.
ACKNOWLEDGEMENTS
The authors would like to thank Jolle Kirpensteijn, Elaine Naan and Bart Sjollema for their surgical contributions to our dataset; Ellen Deelen and Adri Slob for optimization of the Ki67 staining protocol; and Tim van Olmen and the Veterinary Pathology Diagnostic Centre for technical assistance.
Sanders K, Cirkel K, Grinwis GCM, et al. The Utrecht Score: A novel histopathological scoring system to assess the prognosis of dogs with cortisol‐secreting adrenocortical tumours. Vet Comp Oncol. 2019;17:329–337. 10.1111/vco.12474
REFERENCES
- 1. Galac S, Reusch CE, Kooistra HS, Rijnberk A. Adrenals In: Rijnberk A, Kooistra HS, eds. Clinical Endocrinology of Dogs and Cats. 2nd ed. Hannover, Germany: Schlütersche; 2010:93‐154. [Google Scholar]
- 2. Pennanen M, Heiskanen I, Sane T, et al. Helsinki score ‐ a novel model for prediction of metastases in adrenocortical carcinomas. Hum Pathol. 2015;46(3):404‐410. [DOI] [PubMed] [Google Scholar]
- 3. Aubert S, Wacrenier A, Leroy X, et al. Weiss system revisited: a clinicopathologic and immunohistochemical study of 49 adrenocortical tumors. Am J Surg Pathol. 2002;26(12):1612‐1619. [DOI] [PubMed] [Google Scholar]
- 4. Blanes A, Diaz‐Cano SJ. Histologie criteria for adrenocortical proliferative lesions: value of mitotic figure variability. Am J Clin Pathol. 2007;127(3):398‐408. [DOI] [PubMed] [Google Scholar]
- 5. van Slooten H, Schaberg A, Smeenk D, Moolenaar AJ. Morphologic characteristics of benign and malignant adrenocortical tumors. Cancer. 1985;55(4):766‐773. [DOI] [PubMed] [Google Scholar]
- 6. Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol. 1984;8(3):163‐169. [DOI] [PubMed] [Google Scholar]
- 7. Weiss LM, Medeiros LJ, Vickery AL. Pathologic features of prognostic significance in adrenocortical carcinoma. Am J Surg Pathol. 1989;13(3):202‐206. [DOI] [PubMed] [Google Scholar]
- 8. Duregon E, Cappellesso R, Maffeis V, et al. Validation of the prognostic role of the “Helsinki score” in 225 cases of adrenocortical carcinoma. Hum Pathol. 2017;62:1‐7. [DOI] [PubMed] [Google Scholar]
- 9. Papotti M, Libè R, Duregon E, Volante M, Bertherat J, Tissier F. The Weiss score and beyond‐histopathology for adrenocortical carcinoma. Horm Cancer. 2011;2(6):333‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Labelle P, Kyles AE, Farver TB, de Cock HEV. Indicators of malignancy of canine adrenocortical tumors: histopathology and proliferation index. Vet Pathol. 2004;41(5):490‐497. [DOI] [PubMed] [Google Scholar]
- 11. Morimoto R, Satoh F, Murakami O, et al. Immunohistochemistry of a proliferation marker Ki67/MIB1 in adrenocortical carcinomas: Ki67/MIB1 labeling index is a predictor for recurrence of adrenocortical carcinomas. Endocr J. 2008;55(1):49‐55. [DOI] [PubMed] [Google Scholar]
- 12. Schmitt A, Saremaslani P, Schmid S, et al. IGFII and MIB1 immunohistochemistry is helpful for the differentiation of benign from malignant adrenocortical tumours. Histopathology. 2006;49(3):298‐307. [DOI] [PubMed] [Google Scholar]
- 13. Soon PSH, Gill AJ, Benn DE, et al. Microarray gene expression and immunohistochemistry analyses of adrenocortical tumors identify IGF2 and Ki‐67 as useful in differentiating carcinomas from adenomas. Endocr Relat Cancer. 2009;16(2):573‐583. [DOI] [PubMed] [Google Scholar]
- 14. Wachenfeld C, Beuschlein F, Zwermann O, et al. Discerning malignancy in adrenocortical tumors: are molecular markers useful? Eur J Endocrinol. 2001;145(3):335‐341. [DOI] [PubMed] [Google Scholar]
- 15. Fassnacht M, Johanssen S, Quinkler M, et al. Limited prognostic value of the 2004 international union against cancer staging classification for adrenocortical carcinoma. Cancer. 2009;115(2):243‐250. [DOI] [PubMed] [Google Scholar]
- 16. Beuschlein F, Weigel J, Saeger W, et al. Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J Clin Endocrinol Metab. 2015;100(3):841‐849. [DOI] [PubMed] [Google Scholar]
- 17. Jouinot A, Bertherat J. Adrenocortical carcinoma: differentiating the good from the poor prognosis tumors. Eur J Endocrinol. 2018;178(5):R215‐R230. [DOI] [PubMed] [Google Scholar]
- 18. Behrend EN, Kooistra HS, Nelson R, Reusch CE, Scott‐Moncrieff JC. Diagnosis of spontaneous canine hyperadrenocorticism: 2012 ACVIM consensus statement (small animal). J Vet Intern Med. 2013;27(6):1292‐1304. [DOI] [PubMed] [Google Scholar]
- 19. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6(7):655‐663. [DOI] [PubMed] [Google Scholar]
- 20. Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159‐174. [PubMed] [Google Scholar]
- 22. Massari F, Nicoli S, Romanelli G, Buracco P, Zini E. Adrenalectomy in dogs with adrenal gland tumors: 52 cases (2002–2008). J Am Vet Med Assoc. 2011;239(2):216‐221. [DOI] [PubMed] [Google Scholar]
- 23. Papathomas TG, Pucci E, Giordano TJ, et al. An international Ki67 reproducibility study in adrenal cortical carcinoma. Am J Surg Pathol. 2016;40(4):569‐576. [DOI] [PubMed] [Google Scholar]
- 24. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 25. Ovalle WK, Nahirney PD. The cell Netter's Essential Histology. Philadelphia, PA: Elsevier; 2008:1‐29. [Google Scholar]
- 26. Margonis GA, Kim Y, Tran TB, et al. Outcomes after resection of cortisol‐secreting adrenocortical carcinoma. Am J Surg. 2016;211(6):1106‐1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Else T, Williams AR, Sabolch A, Jolly S, Miller BS, Hammer GD. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2014;99(2):455‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anderson CR, Birchard SJ, Powers BE, Belandria GA, Kuntz CA, Withrow SJ. Surgical treatment of adrenocortical tumors: 21 cases (1990‐1996). J Am Anim Hosp Assoc. 2001;37:93‐97. [DOI] [PubMed] [Google Scholar]
- 29. Schwartz P, Kovak JR, Koprowski A, Ludwig LL, Monette S, Bergman PJ. Evaluation of prognostic factors in the surgical treatment of adrenal gland tumors in dogs: 41 cases (1999–2005). J Am Vet Med Assoc. 2008;232(1):77‐84. [DOI] [PubMed] [Google Scholar]
- 30. Lang JM, Schertel E, Kennedy S, Wilson D, Barnhart M, Danielson B. Elective and emergency surgical management of adrenal gland tumors: 60 cases (1999–2006). J Am Anim Hosp Assoc. 2011;47(6):428‐435. [DOI] [PubMed] [Google Scholar]
- 31. Barrera JS, Bernard F, Ehrhart EJ, Withrow SJ, Monnet E. Evaluation of risk factors for outcome associated with adrenal gland tumors with or without invasion of the caudal vena cava and treated via adrenalectomy in dogs: 86 cases (1993‐2009). J Am Vet Med Assoc. 2013;242(12):1715‐1721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Intra‐ and inter‐observer agreement scores\
Intra‐ and inter‐observer agreement scores of histopathological parameters in canine cortisol‐secreting adrenocortical tumours (n = 50). Method of score quantification indicated by a (intra‐class correlation coefficient; continuous variables) and b (Cohen's kappa coefficient; categorical variables). N/A: not available; indicates that at least one of the variables is a constant.


