Dear Editor, Higher rates of conjunctivitis have been reported in patients with atopic dermatitis (AD) treated with dupilumab, an anti‐interleukin (IL)‐4Rα antibody inhibiting IL‐4 and IL‐13, vs. patients treated with placebo.1 However, the exact pathomechanism has not been clarified. Given the necessity for optimal treatment and risk management, the aim of this study was to describe the histopathological characteristics of conjunctivitis during dupilumab treatment in patients with AD.
Participants, selected from the BioDay registry, consisted of 74 patients with moderate‐to‐severe AD treated with dupilumab for at least 16 weeks. Of these, 23% developed ophthalmologist‐confirmed conjunctivitis requiring anti‐inflammatory treatment. We sequentially included six patients [three male; median age 39 years, interquartile range (IQR) 29–54] in whom a diagnostic conjunctival biopsy of the inferior fornix was performed before initiation of ocular anti‐inflammatory treatment. Biopsies were fixed, paraffin‐embedded and stained with haematoxylin and eosin for histological assessment, and additionally with CD3/CD4 [T helper (Th) cells] and Alcian blue [mucus‐containing goblet cells (GCs)]. Conjunctival biopsies of two healthy controls were included. Biopsies were assessed by two independent experienced pathologists. This study did not fall under the scope of the Medical Research Involving Human Subjects Act, confirmed by the local Medical Research Ethics Committee (METC 18/537).
The most prominent histopathological feature in conjunctival biopsies from patients with AD developing conjunctivitis during dupilumab treatment was a scarcity of intraepithelial GCs. Median GC density was 3·3 cells mm−1 (IQR 1·1–4·9)(Fig. 1a, b) in patients with AD with conjunctivitis vs. 28·3 and 36·3 cells mm−1 in the two control samples. Five patients showed a multicellular immune‐cell stromal infiltrate, consisting mainly of T cells (CD3+/CD4+) and eosinophils (Fig. 1c), partially migrating into the conjunctival epithelium.
Figure 1.
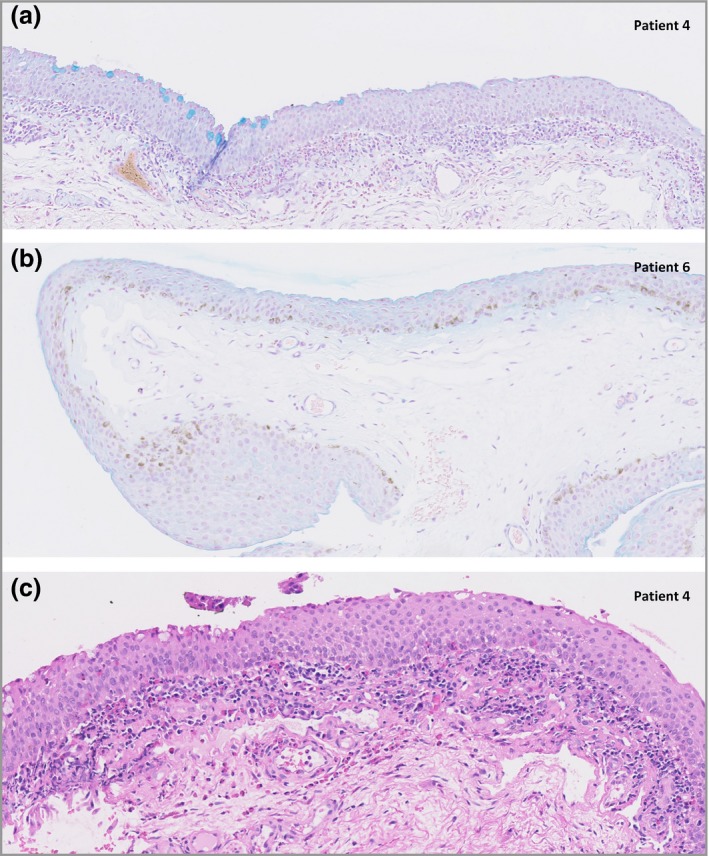
Alcian blue‐stained histological sections of the inferior bulbar conjunctiva under light microscopy shows the presence of decreased goblet‐cell density in patients with AD treated with dupilumab (original magnification × 40). (a) Regions with no goblet cells (GCs) interspersed with smaller regions of normal GC density. (b) In patient 6 no GC was found in the conjunctival biopsy. (c) Haematoxylin and eosin stained histological sections of the inferior bulbar conjunctiva under light microscopy show the presence of a superficial inflammatory multicellular infiltrate in the conjunctival stroma consisting of mainly T cells and eosinophils, partially migrating into the conjunctival epithelium.
Conjunctival GCs are specialized mucus‐secreting cells, vital for ocular surface function.2 In healthy individuals lower forniceal GC counts vary between 8·8 and 30 cells mm−1.3 All patients included in our study had a decreased GC count (median 3·3 cells mm−1) vs. controls (mean 32·3 cells mm−1).
Mice studies have demonstrated that ocular IL‐13 expression normally stimulates GC proliferation and mucus secretion.4 By blocking IL‐13, dupilumab treatment may lead to GC hypoplasia, as IL‐4Rα is expressed on conjunctival epithelium. This might result in decreased mucin production, subsequent tear film instability and mucosal epithelial barrier dysfunction, leading to conjunctival inflammation in a subpopulation of (predisposed) patients with AD. Clinically, the loss of GC‐produced factors may result in dry eyes, as was reported by all patients, and subsequently irritative conjunctivitis. As in this study biopsies were performed after initiation of dupilumab, GC scarcity might already be present before dupilumab treatment, although patients did not experience ocular symptoms at start of treatment.
Our histopathological findings do not correspond with the histopathology of atopic keratoconjunctivitis and allergic conjunctivitis, which is associated with an increased GC density and increased mucus production, probably due to IL‐13 overexpression.5, 6 Dupilumab treatment might theoretically be beneficial in these typical Th2‐mediated ocular surface diseases.
It has been proposed that dupilumab treatment could increase Demodex numbers in hair follicles, causing ocular rosacea‐like disease.7 Ocular rosacea is a Th17‐driven disease characterized by an inflammatory cell infiltrate, mainly consisting of CD4+ T cells, but not eosinophils.8 The unique combination of low conjunctival GC numbers accompanied by numerous lymphocytes and eosinophils found in this study may imply a new entity of conjunctivitis in dupilumab‐treated patients with AD.
Only patients with new onset of conjunctivitis symptoms or worsened symptoms in cases of pre‐existing conjunctivitis were included in this study; these probably do not represent all conjunctivitis cases during dupilumab treatment. In daily practice, we experience some patients reporting improvement of conjunctivitis symptoms during dupilumab treatment, underlining the heterogeneity of the conjunctivitis.
Limitations of this study are small sample size, and collection of conjunctival biopsies at one single time point. Therefore, dynamic differences in histopathological features before and during dupilumab treatment could not be studied. Nevertheless, the histopathological features and findings were very consistent, and constitute a first clue in the underlying pathomechanism of dupilumab‐associated conjunctivitis. However, the exact pathomechanism of this new entity of conjunctivitis could not be fully elucidated.
In conclusion, this study found a remarkable scarcity of conjunctival GCs accompanied by an inflammatory T‐cell‐ and eosinophilic infiltrate in patients with AD with conjunctivitis during dupilumab treatment. We hypothesize that the IL‐13 blocking effect of dupilumab might lead to reduction of GCs and mucin production in a subpopulation of patients with AD, which may potentially result in irritative conjunctivitis. A prospective study further characterizing conjunctivitis in patients with AD before and during dupilumab treatment will start soon.
Funding sources: none for the study; the BioDay register is financially supported through a grant held by Sanofi Genzyme/Regeneron.
Conflicts of interest: M. de B.‐W. is principal investigator, advisory board member and consultant for Regeneron Pharmaceuticals, Inc.; principal investigator and advisory board member for Sanofi Genzyme; and principal investigator for AbbVie, Pfizer and LEO Pharma. M.L.A.S. received consultancy fees from Sanofi Genzyme. All other authors declare no conflicts of interest.
D.S.B., L.F.M.A., M.R.v.D. and M.S. de B.‐W. contributed equally to this work.
References
- 1. de Bruin‐Weller M, Thaçi D, Smith CH et al Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo‐controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol 2018; 178:1083–101. [DOI] [PubMed] [Google Scholar]
- 2. Gipson IK. Goblet cells of the conjunctiva: a review of recent findings. Prog Retin Eye Res 2016; 54:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vujković V, Mikac G, Kozomara R. Distribution and density of conjunctival goblet cells. Med Pregl 2002; 55:195–200. [DOI] [PubMed] [Google Scholar]
- 4. Tukler Henriksson J, Coursey TG, Corry DB et al IL‐13 stimulates proliferation and expression of mucin and immunomodulatory genes in cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci 2015; 56:4186–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roat MI, Ohji M, Hunt LE, Thoft RA. Conjunctival epithelial cell hypermitosis and goblet cell hyperplasia in atopic keratoconjunctivitis. Am J Ophthalmol 1993; 116:456–63. [DOI] [PubMed] [Google Scholar]
- 6. Gandhi NA, Bennett BL, Graham NM et al Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov 2016; 15:35–50. [DOI] [PubMed] [Google Scholar]
- 7. Thyssen JP. Could conjunctivitis in patients with atopic dermatitis treated with dupilumab be caused by colonization with Demodex and increased interleukin‐17 levels? Br J Dermatol 2018; 178:1220. [DOI] [PubMed] [Google Scholar]
- 8. Hoang‐Xuan T, Rodriguez A, Zaltas MM et al Ocular rosacea A histologic and immunopathologic study Ophthalmology 1990; 97:1468–75. [DOI] [PubMed] [Google Scholar]


