Summary
Late antibody‐mediated rejection (ABMR) is a cardinal cause of kidney allograft failure, manifesting as a continuous and, in contrast with early rejection, often clinically silent alloimmune process. While significant progress has been made towards an improved understanding of its molecular mechanisms and the definition of diagnostic criteria, there is still no approved effective treatment. In recent small randomized controlled trials, therapeutic strategies with promising results in observational studies, such as proteasome inhibitor bortezomib, anti‐C5 antibody eculizumab, or high dose intravenous immunoglobulin plus rituximab, had no significant impact in late and/or chronic ABMR. Such disappointing results reinforce a need of new innovative treatment strategies. Potential candidates may be the interference with interleukin‐6 to modulate B cell alloimmunity, or innovative compounds that specifically target antibody‐producing plasma cells, such as antibodies against CD38. Given the phenotypic heterogeneity of ABMR, the design of adequate systematic trials to assess the safety and efficiency of such therapies, however, is challenging. Several trials are currently being conducted, and new developments will hopefully provide us with effective ways to counteract the deleterious impact of antibody‐mediated graft injury. Meanwhile, the weight of evidence would suggest that, when approaching using existing treatments for established antibody‐mediated rejection, “less may be more”.
Keywords: antibody‐mediated rejection, kidney transplantation, randomized controlled trial, rejection treatment
General considerations
Late antibody‐mediated rejection (ABMR) is well‐established to be a major determinant of allograft outcome 1. Nevertheless, in contrast with early acute ABMR, there is still no treatment proven to modify its natural course 2. There is a need of new innovative therapeutic approaches, which will have to be evaluated for their safety and efficiency in adequately designed intervention trials. Our increasing understanding of the pathophysiology of ABMR, its natural course and diagnosis, including its different subphenotypes, may provide a valuable basis for a robust study design. In search of new treatment concepts, transplant medicine may learn substantially from other medical disciplines, such as rheumatology or haematology, where numerous new developments have enabled considerable success in the treatment of B cell‐ and plasma cell‐driven diseases. However, we are still lacking in our understanding of the natural history of ABMR, a point that must be remembered when evaluating any study that does not have a randomized control design.
Pathogenesis of ABMR
Key elements of ABMR pathogenesis as well as potential treatments and their targets are illustrated in Fig. 1. A major trigger of ABMR is the formation of donor‐specific antibodies (DSA) against mismatched HLA class I and, particularly in chronic rejection, HLA class II antigens 1. Upon binding to the endothelium, DSA may initiate a cascade of molecular events that result in endothelial activation and inflammation in the microcirculation, ultimately culminating in irreversible tissue injury. A driving force of HLA antibody formation is the extent of tissue incompatibility between recipient and donor, suggesting that a precise definition of immunogenic HLA mismatches may significantly contribute to alloimmune risk stratification to accurately predict the risk of de novo DSA formation 3, 4, 5. Major approaches in this context – not the primary topic of this review – may be the implementation of novel allocation strategies to improve the precision of traditional HLA antigen mismatching and/or the use of immunosuppressive regimens, such as costimulation inhibitors, that a priori prevent the formation of deleterious DSA and the subsequent development of rejection 5, 6.
Figure 1.
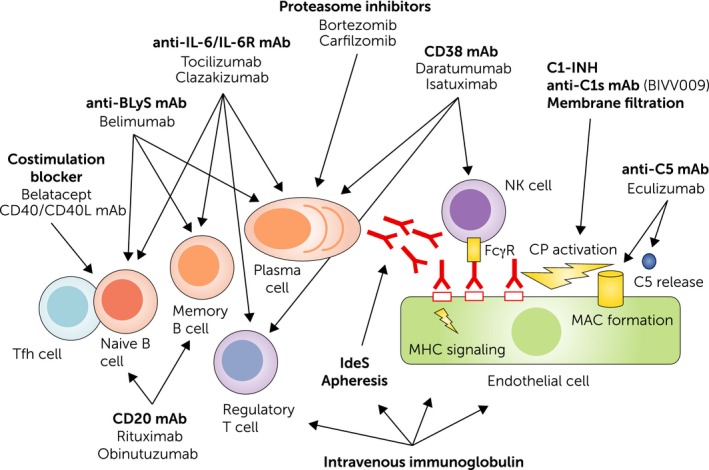
Pathogenesis of antibody‐mediated rejection and potential therapeutic targets. A primary trigger of B cell alloimmunity may be the interaction of follicular T helper cells with naive B cells. This leads to B cell proliferation and differentiation, and the generation of B memory cells and antibody‐producing plasma cells. Binding of alloantibodies to the endothelium may trigger direct signalling, induce Fc gamma receptor (FcγR) dependent cellular effects, such as natural killer (NK) cell (and macrophage) activation, and trigger complement activation via the classical pathway (CP). Costimulation blockers, monoclonal antibodies (mAb) that target the interleukin‐6 (IL‐6)/IL‐6 receptor (IL‐6R) axis, or B lymphocyte stimulator may prevent proper B cell activation/differentiation and affect the generation or integrity of plasma cells. IL‐6 antagonists may also enhance the formation of regulatory T cells. Proteasome inhibitors and CD38 mAb may deplete alloantibody‐producing plasma cells, the latter affecting also NK cells and regulatory T cells. Complement inhibitors and membrane filtration target the C1 complex, a key component of the CP, or by interference with the terminal component C5 (eculizumab), the formation of the membrane attack complex and anaphylatoxin C5a. The mode of action of intravenous immunoglobulin is multifaceted and may include interference with B and T cell activation, antibody formation and recycling, as well as complement activation.
As illustrated in Fig. 1, DSA may trigger a sequence of different events that may contribute to tissue injury, including possible direct signalling via HLA molecules (although this has only been demonstrable in in vitro systems), induction of Fc gamma receptor‐dependent cellular effects, and/or activation of the complement cascade, primarily via the classical pathway (CP) 7. In this context, also natural killer (NK) cells have recently gained attention. Studies support an involvement of transcripts related to Fc gamma receptor IIIA‐mediated NK cell activation 8, 9. In addition, morphological and molecular evidence of NK cell infiltration was associated with ABMR and inferior graft survival 10. DSA‐triggered CP activation and the subsequent release of anaphylatoxins, the recruitment of inflammatory cells with complement receptors and the formation of the membrane attack complex may contribute to tissue injury 11, 12. However, the frequent finding of C4d‐negative rejection 13 and the limited success of complement inhibitory treatment (see also below) 14, 15, 16, 17 have questioned the dominant importance of complement cascade activation in late ABMR.
ABMR diagnosis and subphenotypes
Since its first description as a separate entity, the diagnosis of ABMR has been refined in subsequent amendments of the Banff classification 18, 19, 20. Diagnostic criteria are the detection of typical morphological lesions in the microcirculation, which include glomerulitis (g), peritubular capillaritis (ptc), transplant glomerulopathy (cg), serological evidence of circulating DSA and/or the finding of C4d as a specific marker of DSA‐triggered complement activation in the microvasculature. The phenotypic presentation of ABMR is heterogenous, and, according to recent updates of the Banff scheme, not all criteria need to be fulfilled for its diagnosis. For example, ABMR is often C4d‐negative, or under certain conditions (e.g. positive C4d staining reflecting recent/current antibody interaction with vascular endothelium), this type of rejection may be diagnosed without serological DSA detection. In addition, the innovative diagnostic tool of gene expression analysis using validated platforms, such as the Molecular Microscope Diagnostic system (MMDx) 21, 22, has been in included in the Banff scheme to further increase diagnostic precision 20.
Antibody‐mediated rejection can occur at any time, but is most frequent in the late phase after transplantation 22, 23, 24. According to its timing, many authors distinguish between early and late ABMR, the latter being commonly defined by its diagnosis beyond 6 months post‐transplantation, often associated with anti‐HLA DSA, sometimes in the context of underimmunosuppression (“minimization”) or nonadherence. In addition, ABMR may present with different subphenotypes, classified according to morphological, molecular and/or serological characteristics.
The Banff 2017 scheme defines two major variants, that are, (i) active (formerly ‘acute active’ ABMR) and (ii) chronic active ABMR, based on the absence or presence of time‐dependent “stage” lesions: cg or the ultrastructural finding of capillary basement membrane multilayering 20. These two phenotypes may occur at any time, with active ABMR, without any chronic lesions being occasionally found even many years post‐transplantation 25. At the same time, gene expression analysis, e.g. using the innovative principle of unsupervised archetypal analysis, may allow for an alternative classification of rejection subphenotypes that differ substantially with respect to timing, intensity and prognostic impact (early stage versus fully developed versus late‐stage ABMR) 22. Late ABMR is commonly associated with de novo DSA (also referred to as type 2 rejection 26), but in this respect, transplant centres may differ substantially: series including a high proportion of high immunological risk patients subjected to desensitization at the time of transplantation have also shown an accumulation of late ABMR cases among patients with preformed DSA 25.
Of particular relevance for the design and interpretation of intervention trials is that the individual screening strategies applied to identify ABMR may critically influence rates and phenotypes detected in distinct patient cohorts (e.g. prospective protocol biopsies versus indication biopsies; longitudinal versus cross‐sectional DSA screening). For example, in a recently published cross‐sectional evaluation (BORTEJECT trial) of 741 kidney transplant recipients in outpatient care >6 months after transplantation (estimated glomerular filtration rate (eGFR) > 20 ml/min/1.73 m2), 111 DSA‐positive recipients were identified (15%), and 86 of these patients were subjected to protocol biopsies. ABMR was diagnosed in 44 recipients, at the median time of 5 years after transplantation (6% of the screened population) 25. Every second ABMR patient had a history of presensitization, which was more frequent than expected. Moreover, there was a marked heterogeneity of morphological subphenotypes, a case mix of active and chronic active (C4d‐positive or C4d‐negative) ABMR cases 25. In another cohort, prospective serial DSA monitoring in nonsensitized patients without preformed DSA revealed an initially low but steadily increasing incidence of de novo DSA, with rates of 2%, 10% and 19% after 1, 5 and 10 years, respectively 27. In this study, 76% of the recipients who underwent DSA‐triggered protocol biopsies showed active ABMR 27.
Clinical impact of ABMR
Antibody‐mediated rejection diagnosis is commonly associated with progressive deterioration of graft function and premature allograft failure. The cardinal impact of ABMR as a trigger of transplant failure may have major implications for patient survival, given the well‐documented increased risk in death following allograft loss 28. In a large cohort of 885 kidney transplant recipients who underwent biopsies for graft dysfunction, ABMR morphology was shown to be tightly associated with adverse graft survival 29. Eight‐year graft survival was 53% in C4d‐positive and 66% in C4d‐negative ABMR, as compared with 81% in patients without any rejection features. In mixed models, the calculated annual slope of eGFR among C4d‐positive recipients or patients with histomorphological evidence of ABMR was between −8 and −9 ml/min/1.73 m2 29.
In a cohort of 508 nonsensitized renal allograft recipients de novo DSA formation was associated with an eGFR slope of −3.63, compared with −0.65 ml/min/1.73 m2 per year in DSA‐negative patients 27. Among DSA‐positive subjects, those with graft dysfunction at the time of antibody detection had a steeper annual eGFR decline (mean −5.61 ml/min/1.73 m2) than subclinical cases (mean −3.15 ml/min/1.73 m2). Outcome analysis revealed a tight relationship between eGFR slope and graft survival, showing a highly significant increase (by 6%) in the risk of graft loss for each 1 ml/min/1.73 m2 decease in eGFR at 3 years postsubclinical de novo DSA onset 27.
The clinical course of ABMR may vary substantially between individuals, and may critically depend on varying characteristics, such as the extent of graft dysfunction at baseline 27, capillary C4d staining, the complement‐fixing capability of detected DSA (which may correlate mainly with antibody levels in the circulation and the amount of antibody bound in solid phase assays) 30, 31, and the presence or absence of chronic microcirculation injury (cg) 32.
Treatment of late ABMR – concepts evaluated in RCTs
Potential anti‐rejection therapies and their targets in ABMR are illustrated in Fig. 1. Numerous therapeutic concepts, amongst them apheresis (plasmapheresis, immunoadsorption), intravenous immunoglobulin (IVIG), CD20 antibody rituximab, proteasome inhibitor bortezomib, and anti‐C5 antibody eculizumab have been evaluated in the treatment of ABMR. Levels of evidence, however, have remained low 33. Only three distinct therapies – IVIG/rituximab, bortezomib and eculizumab – have now been tested systematically in the specific context of late/chronic ABMR, but results of RCTs are disappointing (Table 1) 16, 34, 35.
Table 1.
Randomized controlled trials in late and/or chronic ABMR after kidney transplantation
| Author, year | Trial design | Inclusion criteria | Treatment | Patients | Immunosuppression | Follow‐up | Major EP | Major results |
|---|---|---|---|---|---|---|---|---|
| Kulkarni, 2017 16 | Single centre nonblinded RCT | HLA‐DSA+, 20% eGFR decline upon 12 months | Eculizumab, 600 mg/week for 4 weeks; 900 mg every 2 weeks for 26 weeks |
Treatment: n = 10 Control: n = 5 |
Not specified | 1 year |
Primary EP: eGFR decline Secondary EP: acute rejection; treatment failure (death, graft loss, loss to follow‐up or withdrawal from trial); biopsies at 3, 6 and 12 months, DSA MFI and C1q fixation |
Marginal improvement of eGFR trajectory (P = 0.09); no effect on morphological and molecular biopsy results |
| Moreso, 2018 35 | Multicentre placebo‐controlled RCTa | HLA‐DSA+, chronic ABMR (cg > 0) |
IVIGx4 (0.5 g/kg) every 3 weeks RTX (375 mg/m2) 1 week after the last IVIG infusion |
Treatment: n = 13 Placebo: n = 12 |
Tac/MMF Tac C0: 5–10 ng/ml |
1 year |
Primary EP: eGFR decline Secondary EP: proteinuria, biopsies at 12 months, DSA MFI |
No effect on eGFR decline, biopsy results and DSA‐MFI; no differences in adverse events |
| Eskandary, 2018 34 | Single centre placebo‐controlled RCT | HLA‐DSA+, late ABMR after >180 days | Bortezomib (two cycles; each four injections, 1.3 mg/m2; 3‐month interval) |
Treatment: n = 21 Placebo: n = 23 |
Triple immunosuppression Tac C0: 7–10 ng/ml CyA C0: 80–120 ng/ml |
2 years |
Primary EP: eGFR slope Secondary EP: proteinuria; biopsies at 24 months, DSA MFI |
No effect on eGFR decline, biopsy results and DSA MFI Higher rate of SAEs |
ABMR, antibody‐mediated rejection; DSA, donor‐specific antibody; eGFR, estimated glomerular filtration rate; EP, endpoint; MMF, mycophenolate mofetil; MFI, mean fluorescence intensity; RCT, randomized controlled trial; SAE, severe adverse event; Tac, tacrolimus.
Planned sample size: 25 patients per group (not achieved because of budgetary constraints and slow patient recruitment).
IVIG plus rituximab
Many authors promote the use of high dose IVIG combined with rituximab as a treatment of ABMR. For late/chronic ABMR, however, treatment efficiency is still controversial.
Observational studies suggesting treatment efficiency
In a small observational study (four patients with chronic ABMR) by Fehr et al. 36, IVIG/rituximab was associated with a reduction in DSA levels over time and improved graft function. These results were supported by a series from Heidelberg (six paediatric recipients), which showed improved renal function 12 months after treatment 37. Four years later, the same group reported on an extended cohort of 20 paediatric recipients followed for 2 years 38. Again, combined treatment was associated with a decline in the mean fluorescence intensity (MFI) of detected DSA and, in parallel, improved renal function and follow‐up biopsy results (less C4d staining), whereby response rates were higher among patients without features of chronic injury (100% vs. 45% in patients with chronic lesions) 38.
In a recent single‐centre observational study of the Wisconsin group, Parajuli et al. 39 evaluated outcomes among 78 kidney transplant recipients with late acute or chronic active ABMR subjected to high dose steroids and IVIG or steroids and IVIG/rituximab (one dose of 375 mg/m2). Treatment was associated with a significant decline in DSA‐MFI and microvascular inflammation in follow‐up biopsies performed within 6 weeks. The authors reported that patients who received rituximab less often experienced graft failure (15% graft loss at 1 year vs. 32% in patients who did not receive rituximab, P = 0.02). This outcome effect was independent in multivariate analysis. In contrast with the results of the Heidelberg study, the extent of chronic injury had no significant clinical impact 39.
Finally, Redfield et al. 40 reported on a large observational series of 123 consecutive kidney transplant recipients diagnosed with chronic ABMR. Ninety‐three percent of the patients received anti‐rejection treatment, including steroids (93%), steroids/IVIG (87%), rituximab (30%), plasmapheresis (13%) and anti‐thymocyte globulin (ATG) (10%). Overall, the median graft survival after diagnosis of rejection was only 1.9 years. Retrospective analysis revealed that the use of steroids/IVIG, as compared with no treatment, was associated with a reduced risk of graft loss. Patients treated with additional rituximab or ATG showed superior survival rates, however, observed differences did not achieve statistical significance. Discussing their results, the authors pointed out that the ability to detect differences was limited by an inherent selection bias and a small number of cases 40.
Observational studies suggesting no treatment efficiency
Bachelet et al. 41 reported on a series of 21 patients with transplant glomerulopathy who received four doses of IVIG and two doses of rituximab. Results were compared with those obtained in a control group of 10 patients. 24‐month graft survival was similar in both groups, and there was only a marginal difference in DSA‐MFI. The number of adverse events, however, was higher in the treatment group 41. More recently, Pineiro et al. 42 evaluated a cohort of 62 patients with chronic active ABMR, of whom 23 received treatment with IVIG/rituximab together with plasmapheresis (PP). The other 39 recipients (control group) did not receive any therapy. Combined treatment had no effect on eGFR decline and graft survival, but was associated with significantly higher infection rates 42.
TRITON trial
This multicentre (six transplant units in Spain) randomized controlled double‐blinded trial was designed to evaluate the effect of IVIG plus rituximab in patients with chronic ABMR 35. The study included subjects with transplant glomerulopathy (cg score > 0) and anti‐HLA DSA (predominance of HLA class II antibodies), but stable graft function between the index biopsy and trial inclusion. Patients with an eGFR below 20 ml/min/1.73 m2 and severe IFTA were excluded. Treatment consisted of four doses of 0.5 g/kg IVIG and rituximab at 375 mg/m2. The study was powered to detect a 10 ± 10 ml/min/1.73 m2 inter‐group difference in eGFR decline over 1 year. The calculated sample size was 25 subjects per group, but because of financial constraints and a low case number, patient recruitment was prematurely stopped, and only 25 subjects were enrolled. One major result was that there was no significant difference in the eGFR decline within the first year (−4.2 ± 14.4 vs. −6.6 ± 12.0 ml/min/1.73 m2 in the treatment versus placebo group). Moreover, treatment did not affect renal lesions in follow‐up biopsies, the course of proteinuria, or DSA‐MFI. Graft loss rates were low, with one loss in each group. Treatment was well‐tolerated, and there were no differences regarding adverse events and hospitalization rates, respectively. Although underpowered to provide definitive answers, this trial may argue against a relevant therapeutic effect of IVIG/rituximab 35.
Proteasome inhibition
Inhibition of the 26S proteasome in myeloma is well‐established to prevent the proper degradation of misfolded proteins, a trigger of endoplasmatic reticulum stress and a proapoptotic condition promoting the death of malignant plasma cells. In the last decade, the use of proteasome inhibitors to affect the integrity of nonmalignant plasma cells has gained increasing interest in transplant medicine 43, 44.
Uncontrolled studies
In early studies, treatment of kidney transplant recipients with the first‐generation proteasome inhibitor bortezomib was shown to trigger apoptosis of alloantibody‐producing bone marrow plasma cells 45. Since then, this agent has been broadly used for recipient pretransplant desensitization or ABMR treatment, commonly in combination with other therapies 46, 47, 48, 49, 50. A large number of case series was thereby supportive of a treatment effect, even though interpretation of results was complicated by considerable therapeutic polypragmasia (i.e. administering of multiple drugs) and the lack of robust RCTs. In an observational cohort study, Walsh et al. 48 reported therapeutic efficiency of bortezomib in combination with PP, IVIG and rituximab in early ABMR. This was supported by a marked improvement of eGFR and a considerable morphological response. However, in the same study, patients with late ABMR were less responsive, suggesting limited treatment efficacy in this indication. As in many other observational studies, the concomitant use of “standard of care” treatment (PP, IVIG), however, complicated a valid interpretation of results 48.
BORTEJECT trial
Very recently, Eskandary et al. 34 reported a double‐blinded single centre RCT evaluating bortezomib as the sole treatment of late ABMR. Forty‐four recipients with late ABMR were randomized to receive either two cycles of bortezomib or placebo. The primary endpoint was the course of eGFR over 2 years as a surrogate endpoint predicting long‐term graft survival, whereby, anticipating an eGFR slope of about −9 ml/min/1.73 m2 per year, the study was powered to detect an annual 5 ml/min/1.73 m2 difference in the eGFR slope. A key finding in this study was that groups did not differ with respect to kidney function, both showing an eGFR slope of approximately −5 ml/min/1.73 m2 per year. Notably, the eGFR decline in the control arm was less pronounced than expected, and the study was not powered to detect smaller changes in kidney function. There was, however, no effect of bortezomib on other secondary outcomes, including the course of DSA‐MFI and the morphological and molecular results of 24‐month follow‐up biopsies. Bortezomib, however, was associated with a trend towards more severe adverse events, in particular, gastrointestinal side effects and haematological toxicity 34. A recent nonhuman primate model has provided possible explanations for the disappointing results of the BORTEJECT trial 51. In this study, animals were sensitized by incompatible skin allografts. Treatment with bortezomib led to a transient decrease in antibody‐secreting cells, however, was followed by an immediate increase in germinal centre T cells and reconstitution of B cells as well as antibody secreting cells 51.
New concepts
Experimental data suggest that the rebound of humoral alloimmunity may be overcome by the combination of bortezomib with costimulation blockers. In a study by Burghuber et al. 52, in a sensitized nonhuman primate transplant model, such combined treatment was found to effectively prevent sensitization and transplant rejection. However, in this experimental study considerable rates of severe infections and deaths were reported 52. As shown in Table 2, there are currently two ongoing registered trials where bortezomib (two cycles) is applied as an add‐on to other treatments, such as PP, steroids, rituximab and/or IVIG 53, 54. Finally, a potentially interesting concept may also be the use of alternative proteasome inhibitors, such as carfilzomib, which in contrast with bortezomib is an irreversible proteasome inhibitor with a favourable toxicity profile. This compound was recently evaluated in an observational study including 14 lung transplant recipients believed to have acute ABMR 55. In this study, carfilzomib was used in addition to PP and IVIG. The authors reported on 10 responders in whom treatment led to a decrease in DSA levels and C1q fixation, which was associated with less chronic graft dysfunction and ABMR progression 55.
Table 2.
Planned and ongoing, trials evaluating new therapies in late and/or chronic ABMR after kidney transplantation
| Treatment | Participating centres | Design; interventions | ClinicalTrials.gov |
|---|---|---|---|
| Anti‐IL‐6 antibody | Los Angeles, CA (USA) 70 | Single arm trial (n = 10): clazakizumab (6 months) | NCT03380377 |
| Anti‐IL‐6 antibody | Vienna (Austria); Berlin (Germany) 68, 69 | RCT (n = 20): clazakizumab vs. placebo (part A month 0–3); clazakizumab (open‐label part B: month 4–12) | NCT03444103 |
| Anti‐IL‐6 antibody | USA, Europe, Australia 71 (IMAGINE trial) | RCT (IMAGINE trial; n = 350): clazakizumab versus placebo (260 weeks) | NCT03744910 |
| DFPP | Genoble (France) 83 | Open‐label RCT (n = 16): PP + RTX vs. DFPP + RTX (11 days treatment course; 1 year follow‐up) | NCT03436134 |
| Bortezomib – combined regimen | France 53 (TRIBUTE trial) | RCT (n = 100): bortezomib + PP + IVIG + dexamethasone vs. PP + IVIG + dexamethasone | NCT02201576 |
| Bortezomib – combined regimen | Tehran (Iran) 54 | RCT (n = 20): bortezomib + PP + IVIG + RTX vs. PP + IVIG + RTX | NCT03737136 |
| Corticotropin | Birmingham, Alabama (USA), Baltimore, Maryland (USA) 89 | Single‐arm trial (n = 20): corticotropin (24 weeks) in addition to centre‐specific standard therapy | NCT02546492 |
| Mesenchymal stem cells | Ljubljana (Slovenia) 90 | Single‐arm (n = 10): mesenchymal stem cells + PP, IVIG and steroids | NCT03585855 |
DFPP, double filtration plasmapheresis; IL‐6, interleukin‐6; IVIG, intravenous immunoglobulin; PP, plasmapheresis; RCT, randomized controlled trial; RTX, rituximab.
Complement blockade
Anti‐C5 antibody eculizumab
In a study from the Mayo clinic, C5 blockade using the monoclonal antibody was suggested to prevent acute ABMR in flow crossmatch‐positive kidney transplant recipients (comparator: historical control group), even though long‐term treatment failed to counteract the development of chronic ABMR 14, 15, 56. In a subsequent (still unpublished) RCT, eculizumab has failed to prevent acute ABMR in sensitized live donor kidney transplant recipients 57. In the setting of chronic ABMR there is one small nonblinded RCT available 16. In this study, 15 patients were included to receive either eculizumab (n = 10) or no such treatment (n = 5). There was no effect on gene expression patterns in follow‐up biopsies. However, the authors noted a marginal effect on the course of allograft function (trajectories of eGFR). The study was limited by its small sample size, which complicated interpretation of results 16.
Anti‐C1s antibody BIVV009
There is evidence of limited efficacy of C5 blockade in late ABMR, and one may argue that terminal complement inhibition does not preclude earlier key steps of DSA‐triggered complement activation, such as C3 cleavage and the release of the anaphylatoxin C3a. In this respect, early blockade of complement at the level of key component C1 may be of interest. One compound of interest is the C1s monoclonal antibody BIVV009 (former term TNT009) which allows for a selective blockade of the CP. In a phase 1 study, this antibody was shown to abrogate CP activity in healthy volunteers 58, and in a subsequent uncontrolled pilot trial, the antibody was tested in patients with late ABMR associated with features of complement activation (C4d staining and/or complement fixation to microbeads) 17. Patients received four doses of BIVV009, which led to complete CP blockade in peripheral blood for at least 5 weeks. A major finding was that in follow‐up biopsies C4d staining was markedly reduced, suggesting that CP was also effectively inhibited at tissue level. Nevertheless, treatment failed to affect gene expression patterns in biopsies performed after 5 weeks. In parallel, there was no significant effect on features of microcirculation inflammation (glomerulitis or peritubular capillaritis scores) 17.
C1 esterase inhibitor
Another approach is the use of purified C1 esterase inhibitor (C1‐INH), which might have a variety of relevant pharmacological actions beyond the dissociation/inactivation of C1, including interference with the lectin pathway, the alternative pathway, coagulation and the kallikrein‐kinin system 59. In a randomized controlled two‐centre study by Montgomery et al. 60, C1‐INH was suggested to prevent the development of chronic injury. However, no significant effect was seen in early follow‐up biopsies (the primary endpoint), but in a subset of patients with late biopsies, there was less development of cg. At the same time, the authors reported a trend towards an improved graft function. These data may be in line with an uncontrolled study evaluating patients with refractory ABMR 61. Comparison with a historical control group revealed some benefit, including improved kidney function and regression of ABMR features in follow‐up biopsies 61. Currently, a large multicentre study evaluating C1‐INH as add‐on to standard treatment in acute ABMR, which plans to enroll 90 patients, is underway 62.
Treatment of late ABMR – concepts in the pipeline
IL‐6/IL‐6R interference
IL‐6 is a pleotropic cytokine involved in many facets of innate and adaptive immunity. Blockade of the Il‐6/IL‐6R axis, a concept well‐established for the treatment of rheumatoid arthritis 63, was discussed to slow ABMR progression because of its effects on B cell immunity, including the generation and integrity of antibody‐producing plasma cells 64. Moreover, a beneficial mode of action may be an altered balance between effector and regulatory T cells 64.
Recently, Choi et al. 65 reported on a series of 36 paediatric and adult kidney transplant recipients with chronic ABMR refractory to IVIG/rituximab plus/minus PP, who all underwent monthly treatment with anti‐IL‐6R monoclonal antibody tocilizumab. The authors reported an acceptable safety profile, favourable graft survival rates (80% at 6 years, a significant reduction in DSA levels over time, stabilization of renal function, and a reduction in microcirculation inflammation and C4d staining in follow‐up biopsies 65. These data support a therapeutic impact of tocilizumab in ABMR. However, interpreting these data, concerns about the varying natural history of rejection need to be considered, and the true safety and efficacy of IL‐6(IL‐6R) antagonists will need to be clarified in a systematic trial with rigorous controls.
An interesting approach in this respect may be the use of clazakizumab, a genetically engineered anti‐IL6 monoclonal antibody that has earlier been shown to be highly effective in rheumatoid arthritis and psoriasis arthritis 66, 67. As shown in Table 2, two pilot trials evaluating clazakizumab in late/chronic ABMR are currently underway 68, 69, 70, and, very recently, a large multicentre trial has been registered in the NIH database 71. One concern about these biologicals is what toxicities will be incurred in fully immunosuppressed kidney transplant recipients: it is likely to be higher than the toxicity in rheumatoid patients, who are not on full immunosuppression.
Targeting B‐lymphocyte stimulator
Another interesting strategy to modulate B cell alloimmunity may be targeting B‐lymphocyte stimulator (BLyS), a cytokine that enhances B cell survival and proliferation and significantly contributes to the plasma cell niche 72. Belimumab, a humanized anti‐BLyS antibody was shown to be effective in the treatment of systemic lupus erythematosus 73 and has now entered clinical research in transplantation. In a phase 2 randomized double‐blind trial, belimumab was evaluated as an induction treatment in 28 kidney transplant recipients 74. There was no significant effect on the primary endpoint, naïve B cell counts in peripheral blood from baseline to week 24. However, the IL‐10/IL‐6 ratio of the B cell profile was skewed towards a more regulatory profile, and activated memory B cells and plasmablasts were significantly reduced. In parallel, tissue‐specific antibodies in serum were lowered. Gene expression analysis in peripheral blood suggested attenuation of genes coding for immunoglobulin G (IgG), and at the same time markers of T cell proliferation were reduced 74. Of course, the study was too small to be powered for detection of clinical outcome differences, but some of the preliminary results of this trial may be of interest for the context of ABMR treatment. Currently, there is a study underway, where belimumab – combined with bortezomib, PP and rituximab – is evaluated as a pretransplant desensitization therapy 75 (Table 2).
Targeting CD38
Another target of potential interest may be the transmembrane protein CD38, which is expressed at high levels in plasma cells. Daratumumab, a humanized monoclonal anti‐CD38 antibody, has now been approved for the treatment of relapsed or refractory myeloma 76, 77. Its mode of action includes the induction of complement‐dependent cytotoxicity and apoptotic signalling in CD38‐expressing cells. One may argue that this antibody also depletes alloantibody‐producing plasma cells, perhaps being an effective way to counteract alloantibody production. In addition, an interesting mode of action may be the induction of fratricide of CD38‐expressing NK cells 78. Considering the discussed pathogenetic role of NK cells in rejection, one may argue that this effect could be beneficial in ABMR treatment. In support of effective interference with humoral alloimmunity, Chapuy et al. 79 reported on the successful use of daratumumab for red cell repletion in a case of ABO‐incompatible allogeneic stem‐cell transplantation, presumably the result of a marked effect on ABO antibody production. One concern using this antibody in transplant patients may be the earlier shown reduction of CD38‐expressing regulatory T cells, an effect that may be advantageous in the context of cancer treatment, as it promotes host‐antitumor immune responses 77. To our knowledge there are no published studies that have evaluated the use of daratumumab or other CD38 antibodies, such as isatuximab 80 in organ transplant rejection. Nevertheless, the unique mode of action may be of interest for the prevention and treatment of ABMR.
Apheresis
Apheresis (PP, immunoadsorption) to deplete circulating alloantibodies is broadly used in the treatment and prevention of acute ABMR. Even in this setting, however, evidence levels are low, and only few small RCTs are available 33, 81. Some authors have promoted the use of apheresis, combined with other treatments, for use in late or chronic ABMR 42. However, given the lack of randomized studies, the true efficiency of extracorporeal antibody depletion in this specific context remains unclear. Different techniques may vary considerably in their treatment efficacy. This may include the depletion of DSA as well as other components that potentially contribute to injury, such as complement proteins. For example, the combined use of a porous membrane filter and conventional immunoadsorption, the latter to eliminate IgG, was recently shown to markedly enhance the depletion of macromolecules, including IgM and CP key component C1q 82. Currently, a controlled trial is underway, where double filtration plasmapheresis to selectively remove macromolecular plasma components versus conventional PP, both in combination with rituximab, are evaluated in chronic ABMR 83.
Immunoglobulin G–degrading enzyme of Streptococcus pyogenes
There is now increasing interest in the use of immunoglobulin G–degrading enzyme of Streptococcus pyogenes (IdeS) for enzymatic degradation of alloantibodies 84. Recent studies have shown that, upon virtually complete elimination of intact IgG, IdeS creates a window of opportunity allowing for transplantation across major HLA antibody barriers 85, 86. A caveat is its short half‐life and a rapid neutralizing anti‐IdeS antibody response that impedes repeated administration 87. Moreover, transient cleavage of the IgG type of B cell receptor resulting in a profound inhibition of receptor signalling and memory B cell activation 88 may not be able to considerably affect rebound antibody responses: first studies on the use of IdeS as a pretransplant desensitization treatment have shown that, despite effective IgG elimination, a significant proportion of treated patients developed rejection, which was attributed to a rapid rebound of antibody 85, 86. It was suggested that the type of adjunctive immunomodulatory treatment might be decisive in preventing this phenomenon. In this respect, however, a major challenge is that IdeS also inactivates therapeutic antibodies (e.g. IVIG, rituximab or rabbit ATG). IdeS has to our best knowledge so far not been tested in late (chronic) ABMR. Given its short half‐life and the transient effect on IgG integrity, respectively, one may argue that IdeS would have limited therapeutic efficiency in this specific context.
Alternative strategies studied in ongoing trials
Other new strategies tested in ongoing registered trials are corticotropin 89 and the use of mesenchymal stem cell transplantation 90, both as an add‐on to centre‐specific standard treatment, in an effort to modulate alloimmunity in chronic rejection.
The challenge of trial design
There is a need for robust interventional trials to clarify the efficiency of innovative treatment concepts in ABMR. However, the design of a high standard trial in late ABMR, given the variation in natural history, must use the gold‐standard of a randomized placebo‐controlled design, which represents a unique challenge. Several important aspects, such as adequate case selection, stratification for stage and the choice of an appropriate primary endpoint that precisely predicts long‐term allograft survival, need to be considered. This also includes the definition of a proper sample size to meet the goal of detecting meaningful outcome differences.
The use of hard clinical endpoints, in particular, graft survival, would require the recruitment of hundreds of patients and, given rather low overall event rates, prolonged observation periods. Early alternative outcome parameters that are able to reliably predict the long‐term fate of allografts may be an effective strategy to substantially reduce sample size requirements. One useful surrogate endpoint may be the slope of eGFR, which in the context of de novo DSA was shown to be tightly associated with long‐term renal allograft survival 27. However, to detect meaningful slope differences, still a considerable number of patients need to be included, which supports the choice of a multicentric approach. In this respect, much has been learned from the BORTEJECT trial, where a power analysis based on registry data (average eGFR decline and its variation in an Austrian transplant population) revealed the need of a comparatively small sample size (44 patients) to establish an annual slope difference of 5 ml/min/1.73 m2 between study groups 91. This difference was chosen on the basis of retrospective data that suggested an eGFR slope of −8 ml/min/1.73 m2 per year for patients with late C4d‐positive ABMR, and the assumption that bortezomib would approximate the course of eGFR to that of nonrejecting patients. At the end, deterioration of graft function in the BORTEJECT trial, however, was far less pronounced than expected, likely because many cases were subclinical at ABMR diagnosis. Placebo (and bortezomib) patients had an annual eGFR decline of approximately −5 ml/min/m2. Thus, reaching the endpoint would have required a slope reduction to zero in patients receiving treatment, which is unrealistic and highlights the importance of a careful sample size calculation adjusted to the studied patient population, and of course the critical importance of controls 34.
Another (invasive) surrogate endpoint may be the read‐out of systematic follow‐up biopsies. Lesions reflecting microcirculation inflammation are well‐known to be associated with the development of chronic lesions, such as transplant glomerulopathy or basement membrane multilayering in peritubular capillaries 92, and chronic microcirculation injury has shown to be a strong predictor of graft survival 32. In addition, one may argue that detecting patterns of transcript expression that specifically reflect the extent of rejection and injury may help to dissect treatment responsiveness (or nonresponsiveness) early after initiation of a given intervention. Gene expression analysis using the MMDx platform has been used as a secondary endpoint in two prospective intervention trials conducted in late ABMR, the BORTEJECT study and a trial evaluating C1s inhibitor BIVV009 17, 34. The result of absent changes in gene expression patterns thereby paralleled the lack of any relevant treatment effect on transplant outcomes 17, 34.
Again, a major point is that enhanced immunosuppression to counteract rejection confers a risk of increased toxicity. Besides substance‐specific patterns of toxicity, compounds that effectively target critical key steps of B cell immunity and/or affect plasma cell integrity, can be expected to confer a substantial risk of overimmunosuppression and associated adverse events, in particular, infections. This may be of particular relevance for the transplant setting, where most treated patients are already on multi‐compound baseline immunosuppression, with a history of prior anti‐rejection treatments in many cases. In this specific context, the choice of appropriate medication dosage, paired with careful patient monitoring and adjustment of baseline immunosuppression, needs to be considered.
Planning a systematic evaluation of new therapeutic concepts, one attractive strategy may be the design of one or more (controlled or uncontrolled) pilot trials conducted in advance of a larger RCT adequately powered to detect relevant outcome differences. Pilot studies may provide first insights on the safety and efficacy of a given treatment, supporting the appropriate design of a subsequent pivotal trial. A representative example is the current multi‐step approach for the evaluation of IL‐6 monoclonal antibody clazakizumab in late ABMR. This compound, which has been successfully tested in patients with autoimmune disease, is now being evaluated in the context of late and/or chronic ABMR. Two small independent investigator‐initiated short‐term pilot trials are currently underway, primarily to explore the safety and tolerability of clazakizumab in patients on standard immunosuppression 68, 69, 70. Moreover, in both studies an array of efficacy endpoints including protocol biopsies will be evaluated. Preliminary results can be expected to provide a valuable foundation for the design of a large multicentre RCT (IMAGINE trial) which is planned to include more than 300 recipients with an extended follow‐up of 5 years to detect meaningful differences in eGFR slope and graft survival, respectively 71.
Conclusion
Currently, there is no treatment proven to be effective in late and/or chronic ABMR. For sole treatment with bortezomib, and the combined use of high dose IVIG plus rituximab, double‐blind RCTs have failed to demonstrate a meaningful short‐term effect on ABMR progression. Complement inhibitors may have limited efficacy, as small studies – including one controlled pilot trial – revealed no or only marginal clinical effects. However, there are several promising new treatment concepts in the pipeline, which require careful evaluation in robust intervention trials before they can be introduced into broad clinical practice.
Authorship
G.A.B., F.E., D.E. and P.F.H. wrote and reviewed the manuscript.
Funding
The authors have declared no funding.
Conflict of interest
The authors have declared no conflicts of interest.
References
- 1. Lefaucheur C, Loupy A. Antibody‐mediated rejection of solid‐organ allografts. N Engl J Med 2018; 379: 2580. [DOI] [PubMed] [Google Scholar]
- 2. Budde K, Dürr M. Any progress in the treatment of antibody‐mediated rejection? J Am Soc Nephrol 2018; 29: 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wiebe C, Rush DN, Nevins TE, et al Class II eplet mismatch modulates tacrolimus trough levels required to prevent donor‐specific antibody development. J Am Soc Nephrol 2017; 28: 3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lachmann N, Niemann M, Reinke P, et al Donor‐recipient matching based on predicted indirectly recognizable HLA epitopes independently predicts the incidence of de novo donor‐specific HLA antibodies following renal transplantation. Am J Transplant 2017; 17: 3076. [DOI] [PubMed] [Google Scholar]
- 5. Wiebe C, Kosmoliaptsis V, Pochinco D, et al HLA‐DR/DQ molecular mismatch: a prognostic biomarker for primary alloimmunity. Am J Transplant 2018; 10.1111/ajt.15177. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Everly MJ, Roberts M, Townsend R, Bray RA, Gebel HM. Comparison of de novo IgM and IgG anti‐HLA DSAs between belatacept‐ and calcineurin‐treated patients: an analysis of the BENEFIT and BENEFIT‐EXT trial cohorts. Am J Transplant 2018; 18: 2305. [DOI] [PubMed] [Google Scholar]
- 7. Thomas KA, Valenzuela NM, Reed EF. The perfect storm: HLA antibodies, complement, FcgammaRs, and endothelium in transplant rejection. Trends Mol Med 2015; 21: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Venner JM, Hidalgo LG, Famulski KS, Chang J, Halloran PF. The molecular landscape of antibody‐mediated kidney transplant rejection: evidence for NK involvement through CD16a Fc receptors. Am J Transplant 2015; 15: 1336. [DOI] [PubMed] [Google Scholar]
- 9. Parkes MD, Halloran PF, Hidalgo LG. Evidence for CD16a‐mediated NK cell stimulation in antibody‐mediated kidney transplant rejection. Transplantation 2017; 101: e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yazdani S, Callemeyn J, Gazut S, et al Natural killer cell infiltration is discriminative for antibody‐mediated rejection and predicts outcome after kidney transplantation. Kidney Int 2019; 95: 188. [DOI] [PubMed] [Google Scholar]
- 11. Biglarnia AR, Huber‐Lang M, Mohlin C, Ekdahl KN, Nilsson B. The multifaceted role of complement in kidney transplantation. Nat Rev Nephrol 2018; 14: 767. [DOI] [PubMed] [Google Scholar]
- 12. Böhmig GA, Wahrmann M, Eskandary F, Rostaing L. Novel approaches to block complement. Transplantation 2018; 102: 1837. [DOI] [PubMed] [Google Scholar]
- 13. Haas M. Pathology of C4d‐negative antibody‐mediated rejection in renal allografts. Curr Opin Organ Transplant 2013; 18: 319. [DOI] [PubMed] [Google Scholar]
- 14. Cornell LD, Schinstock CA, Gandhi MJ, Kremers WK, Stegall MD. Positive crossmatch kidney transplant recipients treated with eculizumab: outcomes beyond 1 year. Am J Transplant 2015; 15: 1293. [DOI] [PubMed] [Google Scholar]
- 15. Schinstock CA, Bentall AJ, Smith BH, et al Long‐term outcomes of eculizumab‐treated positive crossmatch recipients: allograft survival, histologic findings, and natural history of the donor‐specific antibodies. Am J Transplant 2018; 10.1111/ajt.15175. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kulkarni S, Kirkiles‐Smith NC, Deng YH, et al Eculizumab therapy for chronic antibody‐mediated injury in kidney transplant recipients: a pilot randomized controlled trial. Am J Transplant 2017; 17: 682. [DOI] [PubMed] [Google Scholar]
- 17. Eskandary F, Jilma B, Mühlbacher J, et al Anti‐C1s monoclonal antibody BIVV009 in late antibody‐mediated kidney allograft rejection‐results from a first‐in‐patient phase 1 trial. Am J Transplant 2017; 18: 916. [DOI] [PubMed] [Google Scholar]
- 18. Racusen LC, Colvin RB, Solez K, et al Antibody‐mediated rejection criteria – an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant 2003; 3: 708. [DOI] [PubMed] [Google Scholar]
- 19. Haas M, Sis B, Racusen LC, et al Banff 2013 meeting report: inclusion of c4d‐negative antibody‐mediated rejection and antibody‐associated arterial lesions. Am J Transplant 2014; 14: 272. [DOI] [PubMed] [Google Scholar]
- 20. Haas M, Loupy A, Lefaucheur C, et al The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell‐mediated rejection, antibody‐mediated rejection, and prospects for integrative endpoints for next‐generation clinical trials. Am J Transplant 2018; 18: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Halloran PF, Reeve J, Akalin E, et al Real time central assessment of kidney transplant indication biopsies by microarrays: the INTERCOMEX study. Am J Transplant 2017; 17: 2851. [DOI] [PubMed] [Google Scholar]
- 22. Reeve J, Böhmig GA, Eskandary F, et al Assessing rejection‐related disease in kidney transplant biopsies based on archetypal analysis of molecular phenotypes. JCI Insight 2017; 2: pii: 94197. 10.1172/jci.insight.94197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sellares J, de Freitas DG, Mengel M, et al Understanding the causes of kidney transplant failure: the dominant role of antibody‐mediated rejection and nonadherence. Am J Transplant 2012; 12: 388. [DOI] [PubMed] [Google Scholar]
- 24. Halloran PF, Merino Lopez M, Barreto Pereira A. Identifying subphenotypes of antibody‐mediated rejection in kidney transplants. Am J Transplant 2016; 16: 908. [DOI] [PubMed] [Google Scholar]
- 25. Eskandary F, Bond G, Kozakowski N, et al Diagnostic contribution of donor‐specific antibody characteristics to uncover late silent antibody‐mediated rejection‐results of a cross‐sectional screening study. Transplantation 2017; 101: 631. [DOI] [PubMed] [Google Scholar]
- 26. Aubert O, Loupy A, Hidalgo L, et al Antibody‐mediated rejection due to preexisting versus de novo donor‐specific antibodies in kidney allograft recipients. J Am Soc Nephrol 2017; 28: 1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wiebe C, Gibson IW, Blydt‐Hansen TD, et al Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor‐specific antibody. Am J Transplant 2015; 15: 2921. [DOI] [PubMed] [Google Scholar]
- 28. Kaplan B, Meier‐Kriesche HU. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant 2002; 2: 970. [DOI] [PubMed] [Google Scholar]
- 29. Kikic Z, Kainz A, Kozakowski N, et al Capillary C4d and kidney allograft outcome in relation to morphologic lesions suggestive of antibody‐mediated rejection. Clin J Am Soc Nephrol 2015; 10: 1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sapir‐Pichhadze R, Curra SP, John R, et al A systematic review of the role of C4d in the diagnosis of acute antibody‐mediated rejection. Kidney Int 2014; 87: 182. [DOI] [PubMed] [Google Scholar]
- 31. Loupy A, Lefaucheur C, Vernerey D, et al Complement‐binding anti‐HLA antibodies and kidney‐allograft survival. N Engl J Med 2013; 369: 1215. [DOI] [PubMed] [Google Scholar]
- 32. Cosio FG, Gloor JM, Sethi S, Stegall MD. Transplant glomerulopathy. Am J Transplant 2008; 8: 492. [DOI] [PubMed] [Google Scholar]
- 33. Wan SS, Ying TD, Wyburn K, Roberts DM, Wyld M, Chadban SJ. The treatment of antibody‐mediated rejection in kidney transplantation: an updated systematic review and meta‐analysis. Transplantation 2018; 102: 557. [DOI] [PubMed] [Google Scholar]
- 34. Eskandary F, Regele H, Baumann L, et al A randomized trial of bortezomib in late antibody‐mediated rejection (BORTEJECT). J Am Soc Nephrol 2018; 29: 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moreso F, Crespo M, Ruiz JC, et al Treatment of chronic antibody mediated rejection with intravenous immunoglobulins and rituximab: a multicenter, prospective, randomized, double‐blind clinical trial. Am J Transplant 2018; 18: 927. [DOI] [PubMed] [Google Scholar]
- 36. Fehr T, Rusi B, Fischer A, Hopfer H, Wüthrich RP, Gaspert A. Rituximab and intravenous immunoglobulin treatment of chronic antibody‐mediated kidney allograft rejection. Transplantation 2009; 87: 1837. [DOI] [PubMed] [Google Scholar]
- 37. Billing H, Rieger S, Ovens J, et al Successful treatment of chronic antibody‐mediated rejection with IVIG and rituximab in pediatric renal transplant recipients. Transplantation 2008; 86: 1214. [DOI] [PubMed] [Google Scholar]
- 38. Billing H, Rieger S, Süsal C, et al IVIG and rituximab for treatment of chronic antibody‐mediated rejection: a prospective study in paediatric renal transplantation with a 2‐year follow‐up. Transpl Int 2012; 25: 1165. [DOI] [PubMed] [Google Scholar]
- 39. Parajuli S, Mandelbrot DA, Muth B, et al Rituximab and monitoring strategies for late antibody‐mediated rejection after kidney transplantation. Transplant Direct 2017; 3: e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Redfield RR, Ellis TM, Zhong W, et al Current outcomes of chronic active antibody mediated rejection – a large single center retrospective review using the updated BANFF 2013 criteria. Hum Immunol 2016; 77: 346. [DOI] [PubMed] [Google Scholar]
- 41. Bachelet T, Nodimar C, Taupin JL, et al Intravenous immunoglobulins and rituximab therapy for severe transplant glomerulopathy in chronic antibody‐mediated rejection: a pilot study. Clin Transplant 2015; 29: 439. [DOI] [PubMed] [Google Scholar]
- 42. Pineiro GJ, De Sousa‐Amorim E, Sole M, et al Rituximab, plasma exchange and immunoglobulins: an ineffective treatment for chronic active antibody‐mediated rejection. BMC Nephrol 2018; 19: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walsh RC, Alloway RR, Girnita AL, Woodle ES. Proteasome inhibitor‐based therapy for antibody‐mediated rejection. Kidney Int 2012; 81: 1067. [DOI] [PubMed] [Google Scholar]
- 44. Ejaz NS, Alloway RR, Halleck F, Dürr M, Budde K, Woodle ES. Review of bortezomib treatment of antibody‐mediated rejection in renal transplantation. Antioxid Redox Signal 2014; 21: 2401. [DOI] [PubMed] [Google Scholar]
- 45. Perry DK, Burns JM, Pollinger HS, et al Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant 2009; 9: 201. [DOI] [PubMed] [Google Scholar]
- 46. Everly MJ, Everly JJ, Susskind B, et al Bortezomib provides effective therapy for antibody‐ and cell‐mediated acute rejection. Transplantation 2008; 86: 1754. [DOI] [PubMed] [Google Scholar]
- 47. Trivedi HL, Terasaki PI, Feroz A, et al Abrogation of anti‐HLA antibodies via proteasome inhibition. Transplantation 2009; 87: 1555. [DOI] [PubMed] [Google Scholar]
- 48. Walsh RC, Brailey P, Girnita A, et al Early and late acute antibody‐mediated rejection differ immunologically and in response to proteasome inhibition. Transplantation 2011; 91: 1218. [DOI] [PubMed] [Google Scholar]
- 49. Waiser J, Budde K, Schutz M, et al Comparison between bortezomib and rituximab in the treatment of antibody‐mediated renal allograft rejection. Nephrol Dial Transplant 2012; 27: 1246. [DOI] [PubMed] [Google Scholar]
- 50. Woodle ES, Shields AR, Ejaz NS, et al Prospective iterative trial of proteasome inhibitor‐based desensitization. Am J Transplant 2015; 15: 101. [DOI] [PubMed] [Google Scholar]
- 51. Kwun J, Burghuber C, Manook M, et al Humoral compensation after bortezomib treatment of allosensitized recipients. J Am Soc Nephrol 2017; 28: 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Burghuber CK, Manook M, Ezekian B, et al Dual targeting: combining costimulation blockade and bortezomib to permit kidney transplantation in sensitized recipients. Am J Transplant 2019; 19: 724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. NIH U.S. National Library of Medicine . Bortezomib in Rejection of Kidney Transplants (TRIBUTE). Available from: https://clinicaltrials.gov/show/record/NCT02201576. Published July 28, 2014. Updated September 5, 2018.
- 54. NIH U.S. National Library of Medicine . Comparison Between Bortezomib and Rituximab Plus Plasmapheresis in AMR. Available from: https://clinicaltrials.gov/show/record/NCT03737136. Published November 9, 2018.
- 55. Ensor CR, Yousem SA, Marrari M, et al Proteasome inhibitor carfilzomib‐based therapy for antibody‐mediated rejection of the pulmonary allograft: use and short‐term findings. Am J Transplant 2017; 17: 1380. [DOI] [PubMed] [Google Scholar]
- 56. Stegall MD, Diwan T, Raghavaiah S, et al Terminal complement inhibition decreases antibody‐mediated rejection in sensitized renal transplant recipients. Am J Transplant 2011; 11: 2405. [DOI] [PubMed] [Google Scholar]
- 57. NIH U.S. National Library of Medicine . Safety & Efficacy of Eculizumab to Prevent AMR in Living Donor Kidney Transplant Recipients Requiring Desensitization. Available from: https://clinicaltrials.gov/show/results/NCT01399593. Published July 22, 2011. Updated October 3, 2017.
- 58. Mühlbacher J, Jilma B, Wahrmann M, et al Blockade of HLA antibody‐triggered classical complement activation in sera from subjects dosed with the anti‐C1s monoclonal antibody TNT009‐results from a randomized first‐in‐human phase 1 trial. Transplantation 2017; 101: 2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Berger M, Baldwin WM 3rd, Jordan SC. Potential roles for C1 Inhibitor in transplantation. Transplantation 2016; 100: 1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Montgomery RA, Orandi BJ, Racusen L, et al Plasma‐derived C1 esterase inhibitor for acute antibody‐mediated rejection following kidney transplantation: results of a randomized double‐blind placebo‐controlled pilot study. Am J Transplant 2016; 16: 3468. [DOI] [PubMed] [Google Scholar]
- 61. Viglietti D, Gosset C, Loupy A, et al C1 inhibitor in acute antibody‐mediated rejection nonresponsive to conventional therapy in kidney transplant recipients: a pilot study. Am J Transplant 2016; 16: 1596. [DOI] [PubMed] [Google Scholar]
- 62. NIH U.S. National Library of Medicine . Efficacy and Safety of Human Plasma‐derived C1‐esterase Inhibitor as add‐on to Standard of Care for the Treatment of Refractory Antibody Mediated Rejection (AMR) in Adult Renal Transplant Recipients. Available from: https://ClinicalTrials.gov/show/NCT03221842. Published July 19, 2017. Updated December 10, 2018.
- 63. Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA 2018; 320: 1360. [DOI] [PubMed] [Google Scholar]
- 64. Jordan SC, Choi J, Kim I, et al Interleukin‐6, a cytokine critical to mediation of inflammation, autoimmunity and allograft rejection: therapeutic implications of IL‐6 receptor blockade. Transplantation 2017; 101: 32. [DOI] [PubMed] [Google Scholar]
- 65. Choi J, Aubert O, Vo A, et al Assessment of tocilizumab (anti‐interleukin‐6 receptor monoclonal) as a potential treatment for chronic antibody‐mediated rejection and transplant glomerulopathy in HLA‐sensitized renal allograft recipients. Am J Transplant 2017; 17: 2381. [DOI] [PubMed] [Google Scholar]
- 66. Weinblatt ME, Mease P, Mysler E, et al The efficacy and safety of subcutaneous clazakizumab in patients with moderate‐to‐severe rheumatoid arthritis and an inadequate response to methotrexate: results from a multinational, phase IIb, randomized, double‐blind, placebo/active‐controlled, dose‐ranging study. Arthritis Rheumatol 2015; 67: 2591. [DOI] [PubMed] [Google Scholar]
- 67. Mease PJ, Gottlieb AB, Berman A, et al The efficacy and safety of clazakizumab, an anti‐interleukin‐6 monoclonal antibody, in a phase IIb study of adults with active psoriatic arthritis. Arthritis Rheumatol 2016; 68: 2163. [DOI] [PubMed] [Google Scholar]
- 68. NIH U.S. National Library of Medicine . A Pilot Trial of Clazakizumab in Late ABMR. Available from: https://ClinicalTrials.gov/show/NCT03444103. Published February 23, 2018.
- 69. Eskandary F, Dürr M, Budde K, et al Clazakizumab in late antibody‐mediated rejection: study protocol of a randomized controlled pilot trial. Trials 2019; 20: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. NIH U.S. National Library of Medicine . Clazakizumab for Chronic and Active Antibody Mediated Rejection Post‐Kidney Transplant. Available from: Available from: https://ClinicalTrials.gov/show/NCT03380377. Published December 21, 2017. Updated February 28, 2018.
- 71. NIH U.S. National Library of Medicine . Interleukin 6 Blockade Modifying Antibody‐Mediated Graft Injury and Estimated Glomerular Filtration Rate (eGFR) Decline (IMAGINE). Available from: https://ClinicalTrials.gov/show/NCT03744910. Published November 19, 2018. Updated December 18, 2018.
- 72. Clatworthy MR. B‐cell regulation and its application to transplantation. Transpl Int 2014; 27: 117. [DOI] [PubMed] [Google Scholar]
- 73. Navarra SV, Guzman RM, Gallacher AE, et al Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo‐controlled, phase 3 trial. Lancet 2011; 377: 721. [DOI] [PubMed] [Google Scholar]
- 74. Banham GD, Flint SM, Torpey N, et al Belimumab in kidney transplantation: an experimental medicine, randomised, placebo‐controlled phase 2 trial. Lancet 2018; 391: 2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. NIH U.S. National Library of Medicine . Belimumab Impacting Transplant Eligibility. Available from: https://clinicaltrials.gov/show/NCT02500251. Published July 16, 2015. Updated August 8, 2018.
- 76. Palumbo A, Chanan‐Khan A, Weisel K, et al Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375: 754. [DOI] [PubMed] [Google Scholar]
- 77. van de Donk N, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood 2018; 131: 13. [DOI] [PubMed] [Google Scholar]
- 78. Wang Y, Zhang Y, Hughes T, et al Fratricide of NK cells in daratumumab therapy for multiple myeloma overcome by ex vivo‐expanded autologous NK cells. Clin Cancer Res 2018; 24: 4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chapuy CI, Kaufman RM, Alyea EP, Connors JM. Daratumumab for delayed red‐cell engraftment after allogeneic transplantation. N Engl J Med 2018; 379: 1846. [DOI] [PubMed] [Google Scholar]
- 80. Richardson PG, Attal M, Campana F, et al Isatuximab plus pomalidomide/dexamethasone versus pomalidomide/dexamethasone in relapsed/refractory multiple myeloma: ICARIA Phase III study design. Future Oncol 2018; 14: 1035. [DOI] [PubMed] [Google Scholar]
- 81. Böhmig GA, Wahrmann M, Regele H, et al Immunoadsorption in severe C4d‐positive acute kidney allograft rejection: a randomized controlled trial. Am J Transplant 2007; 7: 117. [DOI] [PubMed] [Google Scholar]
- 82. Eskandary F, Wahrmann M, Biesenbach P, et al ABO antibody and complement depletion by immunoadsorption combined with membrane filtration–a randomized, controlled, cross‐over trial. Nephrol Dial Transplant 2014; 29: 706. [DOI] [PubMed] [Google Scholar]
- 83. NIH U.S. National Library of Medicine . Treatment of Chronic Active Antibody Mediated Rejection After Kidney Transplantation by Double‐Filtration PlasmaPheresis or Plasma Exchange (DFPP). Available from: https://clinicaltrials.gov/show/NCT03436134. Published February 16, 2018.
- 84. Böhmig GA, Rostaing L. Transplantation: IdeS to desensitize organ allograft recipients. Nat Rev Nephrol 2017; 13: 666. [DOI] [PubMed] [Google Scholar]
- 85. Jordan SC, Lorant T, Choi J, et al IgG endopeptidase in highly sensitized patients undergoing transplantation. N Engl J Med 2017; 377: 442. [DOI] [PubMed] [Google Scholar]
- 86. Lonze BE, Tatapudi VS, Weldon EP, et al IdeS (Imlifidase): a novel agent that cleaves human IgG and permits successful kidney transplantation across high‐strength donor‐specific antibody. Ann Surg 2018; 268: 488. [DOI] [PubMed] [Google Scholar]
- 87. Winstedt L, Jarnum S, Nordahl EA, et al Complete removal of extracellular IgG antibodies in a randomized dose‐escalation phase i study with the bacterial enzyme IdeS–a novel therapeutic opportunity. PLoS One 2015; 10: e0132011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jarnum S, Bockermann R, Runstrom A, Winstedt L, Kjellman C. The bacterial enzyme IdeS cleaves the IgG‐type of B cell receptor (BCR), abolishes BCR‐mediated cell signaling, and inhibits memory B cell activation. J Immunol 2015; 195: 5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. NIH U.S. National Library of Medicine . Treatment of Chronic Antibody‐mediated Rejection in Kidney Transplant With Acthar. Available from: Published September 11, 2015. Updated December 10, 2018.
- 90. NIH U.S. National Library of Medicine . Mesenchymal Stem Cell Transplantation in the Treatment of Chronic ABMR. Available from: Published July 13, 2018. Updated July 13, 2018.
- 91. Eskandary F, Bond G, Schwaiger E, et al Bortezomib in late antibody‐mediated kidney transplant rejection (BORTEJECT Study): study protocol for a randomized controlled trial. Trials 2014; 15: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Haas M. The relationship between pathologic lesions of active and chronic antibody‐mediated rejection in renal allografts. Am J Transplant 2018; 18: 2849. [DOI] [PubMed] [Google Scholar]


