Abstract
Objectives
As a noninvasive method for evaluation of cerebral hemodynamics, the correct interpretation of transcranial Doppler or transcranial imaging (TCI) data remains a major challenge. We explored how to interpret the pulsatility index (PI) derived via TCI during evaluations of cerebral hemodynamics in posthemicraniectomy patients.
Methods
We included patients who underwent invasive arterial pressure and intracranial pressure (ICP) monitoring and simultaneous TCI examinations after hemicraniectomy. We classified the PI of the middle cerebral artery (MCA) into ipsilateral (craniectomy side) and contralateral (opposite side) and analyzed both data sets. The statistical analysis was performed by the Bland‐Altman approach, by calculating intraclass correlation coefficients and Spearman correlations, and by drawing receiver operating characteristic curves. Pulsatility index probability charts were created for ICPs exceeding 20, 25, and 30 mm Hg and cerebral perfusion pressures (CPPs) lower than 70, 60, and 50 mm Hg; we thus explored defined ICP and CPP values.
Results
The ipsilateral and contralateral MCA PI data differed. Only the ipsilateral MCA PI showed a weak correlation with ICP (r = 0.378; P < .001). The receiver operating characteristic curve analysis revealed limited diagnostic utility of bilateral MCA PIs for ICP and CPP assessments. An extremely elevated MCA PI indicated that patients were at high risk of a dangerous ICP elevation or CPP reduction. However, MCA PI values within the normal range did not effectively rule out an ICP of 20 mm Hg or higher but effectively eliminated a CPP lower than 50 mm Hg.
Conclusions
In posthemicraniectomy patients, the Doppler‐based MCA PI value was ineffectively for quantitative ICP and CPP evaluations but a useful index for assessment of cerebral hemodynamics in terms of the probability of an ICP elevation or a CPP reduction.
Keywords: cerebral perfusion pressure, craniectomy, intracranial pressure, pulsatility index
Abbreviations
- AUC
area under the curve
- CPP
cerebral perfusion pressure
- CVR
cerebrovascular resistance
- ICP
intracranial pressure
- MCA
middle cerebral artery
- PI
pulsatility index
- TCI
transcranial imaging
The Doppler‐based pulsatility index (PI) is important in terms of interpretation of transcranial Doppler or transcranial imaging (TCI) data.1 Unlike flow velocity, the PI is independent of the angle of the ultrasound beam and is therefore effective for the evaluation of cerebral hemodynamics.2 Additionally, TCI can easily be performed at the bedside of neurocritical patients, particularly those with skull defects after craniectomy.3, 4 In previous studies, the PI was generally misinterpreted as an index of distal cerebral perfusion resistance and thus used for alternative noninvasive assessment of intracranial pressure (ICP).5 The fact that the relationship between the PI and ICP was unclear blurred the utility of the PI for evaluation of cerebral hemodynamics.6, 7, 8, 9 As many factors affect the PI, it should be interpreted with caution rather than ignored.10, 11 In our clinical practice, we have found that an abnormal PI of the middle cerebral artery (MCA) indicates an imbalance between systemic and cerebral hemodynamics. In other words, the PI reflected the result of an interaction between systemic and intracerebral hemodynamics. Here, we assessed the utility of the Doppler‐based PI for evaluation of ICP and cerebral perfusion pressure (CPP) in patients after hemicraniectomy.
Materials and Methods
Study Design and Participants
We performed a retrospective study in a 16‐bed intensive care unit of a hospital affiliated with the School of Medicine of Zhejiang University. Patients admitted to our unit after hemicraniectomy were screened from April 2014 to February 2016. All eligible patients had an external ventricular drain or an intraparenchymal ICP monitor (Codman & Shurtleff, Inc, Raynham, MA) in place. We routinely monitored invasive arterial blood pressure and performed TCI for evaluation of cerebral hemodynamics in neurocritical patients. We collected and analyzed ICP, arterial blood pressure, and TCI data for 72 hours after hemicraniectomy. Patients with diastolic reversed flow in their first Doppler waveforms were excluded. We classified PI data from the bilateral MCA into ipsilateral and contralateral MCA PI data by reference to the side of the craniectomy. This human study was performed in accordance with the Declaration of Helsinki and was approved by the Second Affiliated Hospital of Zhejiang University Institutional Review Board. All adult participants provided written informed consent to participate in this study.
Doppler‐Based PI Measurement
We routinely performed a TCI analysis of patients with skull defects after craniectomy. All examinations were performed with a portable ultrasound machine (M9; Mindray, Inc, Shenzhen, China) fitted with a low‐frequency (1–5‐MHz) phased array transducer. First, we placed the transducer on the defective bony window (deriving the ipsilateral MCA PI) and then on the temporal widow with an intact skull (deriving the contralateral MCA PI) and then adjusted the transducer angle and ultrasound depth until bone echoes from the opposite side appeared. Next, we used color Doppler imaging to detect the cerebral arteries (principally the MCA) and then acquired Doppler spectra of the M1 MCA segment via pulsed Doppler imaging. The relevant Doppler parameters, including flow velocity and PI, were automatically calculated (Figure 1). To avoid confusion by single‐vessel or segmental vascular disease, we explored different segments and the neighboring vessels. If the flow velocity was high (mean velocity > 120 cm/s or peak systolic velocity > 160 cm/s), a cerebrovascular spasm or stenosis was suspected, and a further examination scheduled.12 If the single vessel or segment of the MCA with high flow velocity was suspected of a spasm or stenosis, first, different segmental or bilateral MCA velocities were compared; second, extracerebral arteries (internal carotid arteries) were examined; and third, cerebral oxygenation monitoring was performed to differentiate a spasm from hyperperfusion. In our study, only 3 patients were excluded for an MCA with high velocity (suspected spasm), and the final result was cerebral hyperperfusion after craniectomy.
Figure 1.
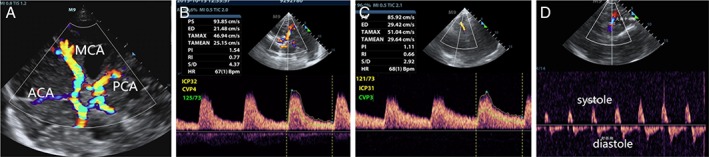
Graphs of Doppler‐based MCA PI measurement. A, Circle of wills under color Doppler imaging. B, Doppler waveforms obtained from the ipsilateral MCA and Doppler‐based parameters were autocalculated, including the ipsilateral MCA PI. C, Doppler waveforms obtained from the contralateral MCA and Doppler‐based parameters were autocalculated, including the contralateral MCA PI. D, Doppler waveform pattern of diastolic reversal flow. ACA indicates anterior cerebral artery; ED, end‐diastolic velocity; HR, heart rate; PCA, posterior cerebral artery; PI, pulsatility index; PS, peak systolic velocity; RI, resistive index; S/D, systolic‐to‐diastolic ratio; TA, time‐averaged velocity.
Data Collection
Arterial pressure was continuously monitored via a radial or femoral artery catheter. The neurosurgeon placed an invasive ICP monitor during the operation. During data recording, the extraventricular drainage was closed, and an invasive arterial blood pressure monitor was calibrated at the heart level. The CPP was the difference between the mean arterial pressure and the ICP. Two experienced physicians (Q.L. and C.W.) reviewed all TCI images and usually collected paired data on the bilateral MCA PIs from each examination for later analysis. We selected data by reference to the quality of the Doppler waveforms; we prioritized the absence of aliasing and use of the sphenoidal segment of the MCA as the vascular target.
Statistical Analysis
Categorical variables are presented as counts (percentages). We calculated means with standard deviations of normally distributed variables and medians with interquartile ranges of non‐normally distributed variables. We explored the extent of agreement between bilateral MCA PI data using the Bland‐Altman approach and by calculating interclass correlation coefficients. Spearman correlations were sought between the bilateral MCA PI data and the ICP and CPP values. Receiver operating characteristic curve analyses were performed to explore the utility of the PI value for prediction of an increased ICP (or a decreased CPP). We calculated measures of diagnostic accuracy (sensitivity, specificity, positive predictive value, and negative predictive value) and the P values of the areas under the curves (AUCs) at optimal cutoffs for different ICP thresholds (20, 25, and 30 mm Hg) and CPP thresholds (50, 60, and 70 mm Hg). Additionally, as described by Zweifel et al,6 2 sets of empirical, second‐order polynomial models were formulated to chart ICP and CPP probabilities, respectively. All statistical analyses were performed with the aid of Stata version 12.0 statistical software (StataCorp, College Station, TX). Statistical significance was set at P < .05.
Results
Baseline Characteristics
A total of 127 patients were potentially eligible for the TCI data analysis after hemicraniectomy. Only 56 patients (44%) underwent invasive ICP monitoring, and 6 were excluded because they had diastolic reversed flow waveforms. Of the 50 enrolled patients, 86% (43 of 50) had traumatic brain injury, and 14% (7 of 50) had hemorrhagic stroke. Baseline characteristics of the enrolled patients are presented in Table 1. Finally, 89 sets of TCI measurements were collected from the 50 posthemicraniectomy patients.
Table 1.
Baseline Characteristics of the Included Patients (n = 50)
| Characteristic | Value |
|---|---|
| Age, y | 51 ± 14 |
| Male | 32 (64) |
| Cause of surgery | |
| Traumatic brain injury | 43 (86) |
| Hemorrhagic stroke | 7 (14) |
| Initial Glasgow Coma Scale score (GCS) | |
| 3–5 | 21 (42) |
| 6–8 | 11(22) |
| >8 | 18(36) |
| Initial pupil reaction | |
| Both reactive | 23 (46) |
| One reactive | 17 (34) |
| None reactive | 10 (20) |
| Initial Helsinki computed tomographic score | 6 ± 3 |
| Sequential organ failure assessment score | 8 ± 3 |
| Duration of intensive care unit stay, d | 8 ± 5 |
| Location of craniectomy (right) | 24 (48) |
| Glasgow Outcome Scale score at 6 mo | |
| 1–2 | 13 (26) |
| 3 | 13 (26) |
| 4–5 | 24 (48) |
Data are presented as mean ± SD and number (percent).
Disagreements Between Bilateral MCA PI Measurements
Of the 89 TCI measurements, 5 ipsilateral and 13 contralateral MCA PI data sets were missing or excluded because the acoustic windows were poor or a vascular spasm was suspected. Ultimately, 71 paired bilateral MCA PI measurements were analyzed. The Bland‐Altman analysis showed that the mean difference between bilateral MCA PI values was 0.03. The limits of agreement were –0.75 and 0.85; 4.2% (3 of 71) of plots exceeded these limits (Figure 2). The interclass correlation coefficient analysis revealed that the coefficient between ipsilateral and contralateral MCA PIs was 0.492 (95% confidence interval, 0.264–0.650) and thus less than 0.9, indicating that the MCA PIs were not in agreement.
Figure 2.
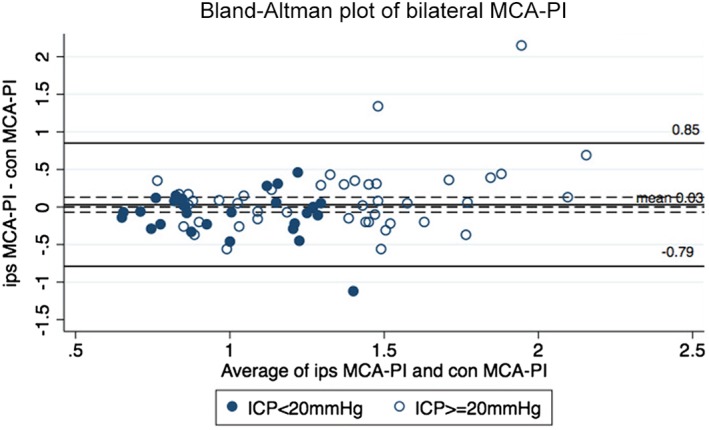
Bland‐Altman plot for bilateral MCA PIs. The plot displays the disagreement of the ipsilateral (ips) and contralateral (con) MCA PIs. The solid black line indicates the mean of the difference between ipsilateral and contralateral PIs (0.03), and the dashed black lines indicate upper and lower limits of agreement (–0.79, 0.85). In total, 4.2% (3 of 71) of the points were out of the limits of agreement.
Correlation Between MCA PI and ICP and CPP Values
The bilateral MCA PIs were non‐normally distributed; the median (interquartile range) of the ipsilateral MCA PI was 1.17 (0.88–1.5), and that of the contralateral MCA PI was 1.16 (0.89–1.46). The Spearman correlations between MCA PI and ICP and CPP data were analyzed. Only the ipsilateral MCA PI showed a weak correlation with ICP (r = 0.378; 95% confidence interval, 0.178–0.548; P < .001); no significant correlation was evident between the ipsilateral MCA PI and the CPP or the contralateral MCA PI and the ICP or CPP (Figure 3).
Figure 3.
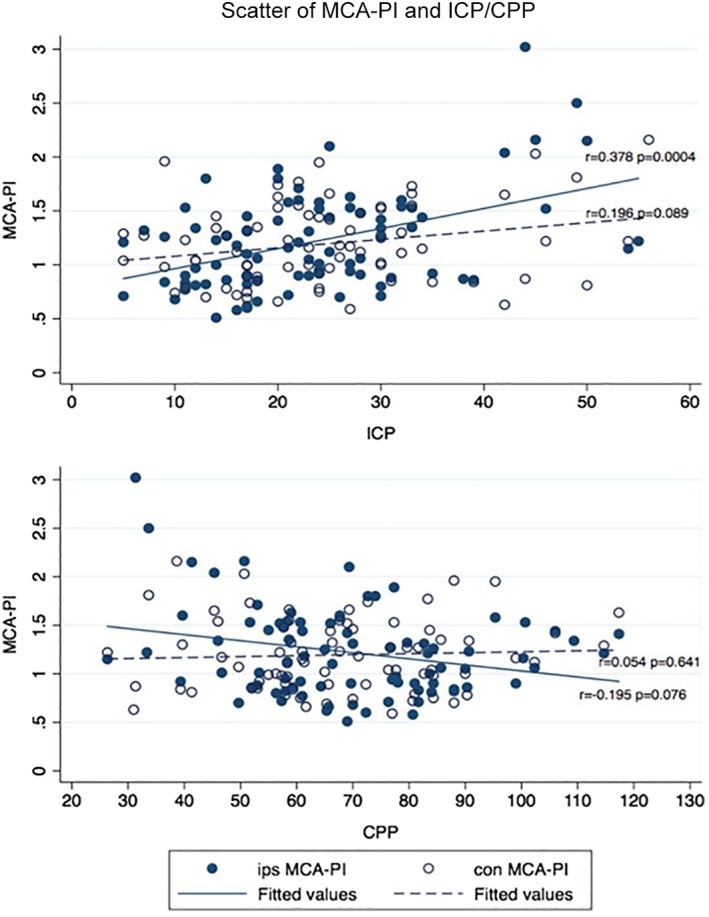
Scatter diagrams of bilateral MCA PIs with ICP and CPP. The diagrams display the ipsilateral (ips) and contralateral (con) MCA PIs. The Spearman correlation coefficient was significant at the .05 level.
Middle Cerebral Artery PI in Terms of Evaluations of Increased ICP and Decreased CPP
The results of the receiver operating characteristic curve analysis are presented in Table 2. For the ICP, the AUC of the ipsilateral MCA PI ranged from 0.669 (ICP ≥30 mm Hg) to 0.751 (ICP ≥20 mm Hg); for the CPP, the AUC of the ipsilateral MCA PI ranged from 0.616 (CPP <70 mm Hg) to 0.742 (CPP <50 mm Hg). The AUCs of the contralateral MCA PI were less than 0.7 for all ICP and CPP thresholds. The optimal cutoff for ipsilateral MCA PI detection at an ICP of 20 mm Hg or higher was 1.34, with sensitivity of 50% and specificity of 90.6%. The optimal cutoff for ipsilateral MCA PI detection at a CPP lower than 50 mm Hg was 1.58, with sensitivity of 50% and specificity of 89.2%. Additionally, we drew probability charts of the effects of the ipsilateral MCA PI on ICP increases and CPP decreases (Figure 4). The charts can be used to assess the probabilities that the ICP and CPP values were above or below the measured MCA PI. If the measured ipsilateral PI was 1.5, the probability was approximately 80% that the ICP exceeded 20 mm Hg but less than 35% that the ICP exceeded 30 mm Hg. Similarly, for a PI of 1.5, the probability was about 55% that the CPP was lower than 70 mm Hg but less than 15% that the CPP was lower than 50 mm Hg.
Table 2.
Receiver Operating Characteristic Curve Analyses of Bilateral MCA PIs for Increased ICP and Decreased CPP at Different Thresholds
| Parameter | Cutoff | AUC | 95% CI | Sens/Spec, % | PPV/NPV, % | P |
|---|---|---|---|---|---|---|
| ICP ≥20 mm Hg | ||||||
| Ipsilateral MCA PI | 1.34 | 0.751 | 0.645–0.839 | 50.0/90.6 | 89.6/52.7 | <.0001 |
| Contralateral MCA PI | 1.35 | 0.668 | 0.550–0.772 | 40.8/92.6 | 89.4/50.5 | .008 |
| ICP ≥25 mm Hg | ||||||
| Ipsilateral MCA PI | 1.32 | 0.671 | 0.560–0.770 | 55.9/76.0 | 61.3/71.7 | .005 |
| Contralateral MCA PI | 1.04 | 0.599 | 0.480–0.710 | 69.7/53.5 | 53.5/69.7 | .136 |
| ICP ≥30 mm Hg | ||||||
| Ipsilateral MCA PI | 1.32 | 0.669 | 0.558–0.768 | 60.9/72.1 | 45.2/83.0 | .013 |
| Contralateral MCA PI | 1.48 | 0.596 | 0.477–0.707 | 39.1/84.9 | 53.0/76.2 | .197 |
| CPP <50 mm Hg | ||||||
| Ipsilateral MCA PI | 1.58 | 0.742 | 0.635–0.831 | 50.0/89.2 | 38.5/93.0 | .005 |
| Contralateral MCA PI | 1.53 | 0.545 | 0.427–0.660 | 36.4/83.1 | 26.8/88.5 | .723 |
| CPP <60 mm Hg | ||||||
| Ipsilateral MCA PI | 1.32 | 0.628 | 0.516–0.731 | 53.3/72.2 | 51.6/73.6 | .051 |
| Contralateral MCA PI | 0.99 | 0.500 | 0.383–0.617 | 43.3/71.7 | 45.0/66.0 | .996 |
| CPP <70 mm Hg | ||||||
| Ipsilateral MCA PI | 1.34 | 0.616 | 0.503–0.720 | 47.8/81.6 | 75.9/56.3 | .062 |
| Contralateral MCA PI | 0.79 | 0.492 | 0.375–0.609 | 88.6/21.9 | 60.9/58.3 | .905 |
CI indicates confidence interval; NPV, negative predictive value; PPV, positive predictive value; Sens, sensitivity; and Spec, specificity.
Figure 4.
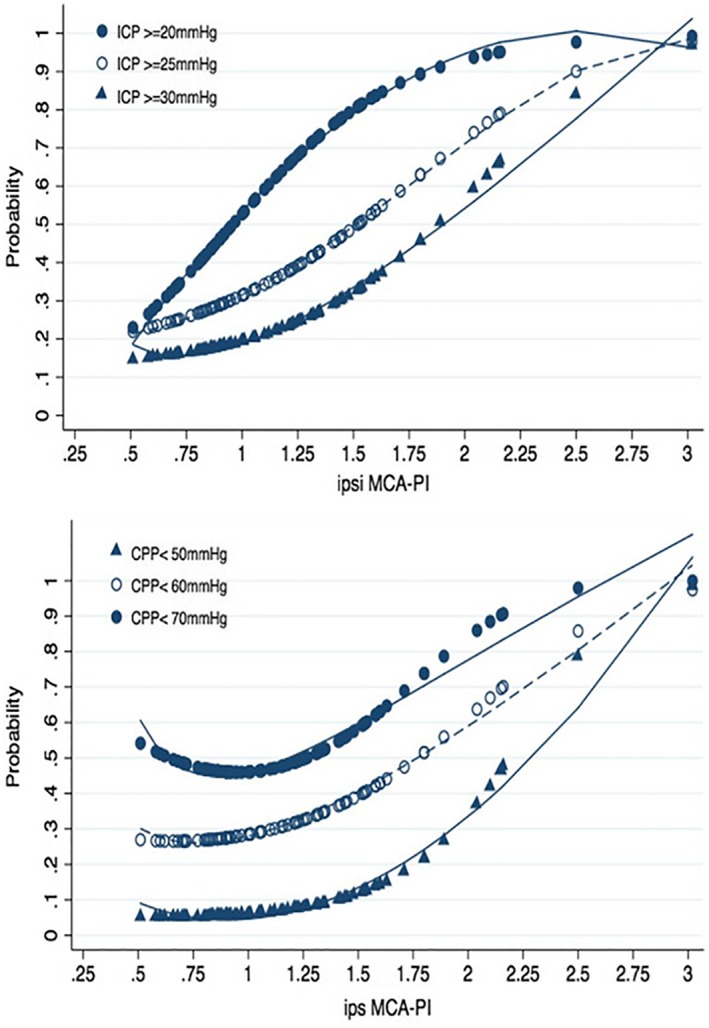
Probability charts of the ipsilateral (ipsi and ips) MCA PI for ICP and CPP. Probability charts were created for the ipsilateral MCA PI to assess the possibility to be above a particular ICP value (20/25/30 mm Hg) and below a particular CPP value (70/60/50 mm Hg).
Discussion
Intracranial pressure monitoring can help improve the prognosis after craniectomy, but not all patients undergo invasive ICP monitoring given the economic costs and the risk of invasive procedures.13, 14 In our study, only 44% of postcraniectomy patients underwent invasive ICP monitoring in terms of ICP‐ or CPP‐guided cerebral protective management. Middle cerebral artery PI values derived via TCI were always associated with ICP elevation, CPP reduction, or poor neurologic outcomes in previous studies.5, 6, 15, 16 However, unlike traditional transcranial Doppler imaging, which requires dedicated equipment, clinical staff can perform TCI on patients with brain injuries using multipurpose ultrasound machines.3, 4 Therefore, we assessed the utility of the MCA PI principally for ICP and CPP evaluations in posthemicraniectomy patients.
Notably, we divided MCA PI data into ipsilateral and contralateral sets by reference to the side of craniectomy (rather than right‐ and left‐side data or an average). We unexpectedly found that the Bland‐Altman and interclass correlation coefficient analyses indicated poor agreement between ipsilateral and contralateral MCA PI values, possibly because hemicraniectomy patients had focal brain injuries, and the sides of injury triggered higher PI values. The ultimate cause may be an imbalance in or dysfunction of the cerebral circulation induced by the primary brain injury or craniectomy per se.17, 18 Therefore, we analyzed the bilateral MCA PIs in terms of the ICP and CPP.
The MCA PI was very commonly misinterpreted as an alternative index of cerebrovascular resistance (CVR) in previous studies.19 By reference to the ICP, CVR increased, followed by an MCA PI increase, as the ICP varied. However, we found that only the ipsilateral MCA PI showed a weak correlation with the ICP (r = 0.378; P < .001), and the MCA PI was of limited diagnostic utility for certain ICP values (the AUCs ranged from 0.669 to 0.751). By reference to the literature, we advance 2 tentative explanations for the weak correlation between the PI and ICP. First, apart from an ICP elevation, many factors affect MCA PI‐induced CVR changes. For example, Ghorbani et al20 showed that the MCA PI was associated with cerebral small‐vessel disease. Stretti et al11 found that body temperature affected cerebral hemodynamics; an elevated temperature induced cerebrovascular relaxation associated with a reduced CVR but an increased ICP attributable to cerebral hyperemia. Also, the MCA PI cannot serve as a direct indicator of the CVR under all circumstances. For example, one animal experiment revealed a 2‐way correlation between the PI and CVR under different conditions.21 Moreover, we had little knowledge about the effect of craniectomy (rather than a craniectomy‐induced ICP reduction) or cranioplasty on the MCA PI, and the surgery (craniectomy or cranioplasty) may alter cerebral hemodynamics.19, 22, 23 Additionally, we found no significant correlation between the MCA PI and the CPP (P > .05), and the MCA PI poorly predicted the CPP (AUC <0.75). Therefore, care must be exercised when using MCA PI data derived via TCI as alternative noninvasive assessments of the cerebral hemodynamic status because of the poor relationships between the MCA PI and ICP and CPP.
To further explore the utility of the MCA PI for assessment of cerebral hemodynamics, we drew probability charts of the associations between the ipsilateral MCA PI and ICP and CPP for ICPs exceeding 20, 25, and 30 mm Hg and CPPs lower than than 70, 60, and 50 mm Hg by various MCA PI values. Although the optimal CPP of neurocritical patients remains unclear, CPPs lower than 50 mm Hg should be avoided.22 As the charts show, an extremely high MCA PI indicated a dangerous ICP elevation or CPP reduction. This may explain why the MCA PI elevation was associated with poor outcomes in previous studies.16, 23 However, an MCA PI within the normal range did not effectively rule out an ICP of 20 mm Hg or higher but did eliminate a CPP lower than 50 mm Hg. If the ipsilateral MCA PI was less than 1.1, the patient was unlikely to have a CPP lower than 50 mm Hg. On the contrary, if the MCA PI was greater than 2, the patient likely had a CPP lower than 50 mm Hg. Therefore, the MCA PI is a useful warning of an increased ICP and a decreased CPP. Consistent with the data of Zweifel et al,6 we found that the MCA PI could be used to screen for high risks of an ICP elevation and a CPP reduction. However, unlike what the cited authors concluded, our MCA PI measurements differed by side, and even a normal MCA PI could not rule out an ICP elevation but possibly eliminated a CPP reduction.
In conclusion, in posthemicraniectomy patients, the MCA PI derived via TCI is ineffective when used for quantitative ICP and CPP evaluations. Moreover, the receiver operating characteristic curve analysis revealed that the MCA PI was of limited diagnostic utility for certain critical ICP and CPP values (AUC <0.8). However, the use of the MCA PI to evaluate postcraniectomy patients assisted assessments of cerebral hemodynamics; the MCA PI was associated with the probabilities that the ICP exceeded 20, 25, or 30 mm Hg and the CPP was lower than 70, 60, or 50 mm Hg.
We thank the professional editors, both native speakers of English, who assisted with manuscript preparation. The data sets used and analyzed in this study are available from the corresponding author by request.
References
- 1. Nedelmann M, Stolz E, Gerriets T, et al. Consensus recommendations for transcranial color‐coded duplex sonography for the assessment of intracranial arteries in clinical trials on acute stroke. Stroke 2009; 40:3238–3244. [DOI] [PubMed] [Google Scholar]
- 2. Wakerley BR, Sharma VK. Transcranial Doppler derived pulsatility index in the assessment of intracranial pressure: the trend is your friend. Neurosurgery 2013; 72:E319–E320. [DOI] [PubMed] [Google Scholar]
- 3. Caricato A, Mignani V, Bocci MG, et al. Usefulness of transcranial echography in patients with decompressive craniectomy. Crit Care Med 2012; 40:1745–1752. [DOI] [PubMed] [Google Scholar]
- 4. Krejza J, Swiat M, Pawlak MA, et al. Suitability of temporal bone acoustic window: conventional TCD versus transcranial color‐coded duplex sonography. J Neuroimaging 2007; 17:311–314. [DOI] [PubMed] [Google Scholar]
- 5. Cardim D, Robba C, Bohdanowicz M, et al. Non‐invasive monitoring of intracranial pressure using transcranial Doppler ultrasonography: is it possible? Neurocrit Care 2016; 25:473–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zweifel C, Czosnyka M, Carrera E, de Riva N, Pickard JD, Smielewski P. Reliability of the blood flow velocity pulsatility index for assessment of intracranial and cerebral perfusion pressures in head‐injured patients. Neurosurgery 2012; 71:853–861. [DOI] [PubMed] [Google Scholar]
- 7. O'Brien NF, Maa T, Reuter‐Rice K. Noninvasive screening for intracranial hypertension in children with acute, severe traumatic brain injury. J Neurosurg Pediatr 2015; 16:420–425. [DOI] [PubMed] [Google Scholar]
- 8. Morgalla MH, Magunia H. Noninvasive measurement of intracranial pressure via the pulsatility index on transcranial doppler sonography: is improvement possible? J Clin Ultrasound 2016; 44:40–45. [DOI] [PubMed] [Google Scholar]
- 9. Robba C, Donnelly J, Bertuetti R, et al. Doppler non‐invasive monitoring of ICP in an animal model of acute intracranial hypertension. Neurocrit Care 2015; 23:419–426. [DOI] [PubMed] [Google Scholar]
- 10. de Riva N, Budohoski KP, Smielewski P, et al. Transcranial Doppler pulsatility index: what it is and what it isn't. Neurocrit Care 2012; 17:58–66. [DOI] [PubMed] [Google Scholar]
- 11. Stretti F, Gotti M, Pifferi S, Brandi G, Annoni F, Stocchetti N. Body temperature affects cerebral hemodynamics in acutely brain injured patients: an observational transcranial color‐coded duplex sonography study. Crit Care 2014; 18:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swiat M, Weigele J, Hurst RW, et al. Middle cerebral artery vasospasm: transcranial color‐coded duplex sonography versus conventional nonimaging transcranial Doppler sonography. Crit Care Med 2009; 37:963–968. [DOI] [PubMed] [Google Scholar]
- 13. Olson DM, Batjer HH, Abdulkadir K, Hall CE. Measuring and monitoring ICP in neurocritical care: results from a national practice survey. Neurocrit Care 2014; 20:15–20. [DOI] [PubMed] [Google Scholar]
- 14. Huang Y, Ou C. Prognostic impact of intracranial pressure monitoring after primary decompressive craniectomy for traumatic brain injury. World Neurosurg 2016; 88:59–63. [DOI] [PubMed] [Google Scholar]
- 15. Varsos GV, Kolias AG, Smielewski P, et al. A noninvasive estimation of cerebral perfusion pressure using critical closing pressure. J Neurosurg 2015; 123:638–648. [DOI] [PubMed] [Google Scholar]
- 16. Bouzat P, Oddo M, Payen J. Transcranial Doppler after traumatic brain injury. Curr Opin Crit Care 2014; 20:153–160. [DOI] [PubMed] [Google Scholar]
- 17. Kenney K, Amyot F, Haber M, et al. Cerebral vascular injury in traumatic brain injury. Exp Neurol 2016; 275:353–366. [DOI] [PubMed] [Google Scholar]
- 18. Smith M. Monitoring intracranial pressure in traumatic brain injury. Anesth Analg 2008; 106:240–248. [DOI] [PubMed] [Google Scholar]
- 19. Donnelly J, Czosnyka M, Harland S, et al. Cerebral haemodynamics during experimental intracranial hypertension. J Cereb Blood Flow Metab 2017; 37:694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghorbani A, Ahmadi M, Shemshaki H. The value of transcranial Doppler derived pulsatility index for diagnosing cerebral small‐vessel disease. Adv Biomed Res 2015; 4:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Czosnyka M, Richards HK, Whitehouse HE, Pickard JD. Relationship between transcranial Doppler‐determined pulsatility index and cerebrovascular resistance: an experimental study. J Neurosurg 1996; 84:79–84. [DOI] [PubMed] [Google Scholar]
- 22. Paredes I, Castaño AM, Cepeda S, et al. The effect of cranioplasty on cerebral hemodynamics as measured by perfusion computed tomography and Doppler ultrasonography. J Neurotrauma 2016; 33:1586–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Daboussi A, Minville V, Leclerc‐Foucras S, et al. Cerebral hemodynamic changes in severe head injury patients undergoing decompressive craniectomy. J Neurosurg Anesthesiol 2009; 21:339–345. [DOI] [PubMed] [Google Scholar]


