Abstract
Background
Remote monitoring of implantable cardioverter‐defibrillators has been associated with reduced rates of all‐cause rehospitalizations and mortality among device recipients, but long‐term economic benefits have not been studied.
Methods and Results
An economic model was developed using the PREDICT RM database comparing outcomes with and without remote monitoring. The database included patients ages 65 to 89 who received a Boston Scientific device from 2006 to 2010. Parametric survival equations were derived for rehospitalization and mortality to predict outcomes over a maximum time horizon of 25 years. The analysis assessed rehospitalization, mortality, and the cost‐effectiveness (expressed as the incremental cost per quality‐adjusted life year) of remote monitoring versus no remote monitoring. Remote monitoring was associated with reduced mortality; average life expectancy and average quality‐adjusted life years increased by 0.77 years and 0.64, respectively (6.85 life years and 5.65 quality‐adjusted life years). When expressed per patient‐year, remote monitoring patients had fewer subsequent rehospitalizations (by 0.08 per patient‐year) and lower hospitalization costs (by $554 per patient year). With longer life expectancies, remote monitoring patients experienced an average of 0.64 additional subsequent rehospitalizations with increased average lifetime hospitalization costs of $2784. Total costs of outpatient and physician claims were higher with remote monitoring ($47 515 vs $42 792), but average per patient‐year costs were lower ($6232 vs $6244). The base‐case incremental cost‐effectiveness ratio was $10 752 per quality‐adjusted life year, making remote monitoring high‐value care.
Conclusion
Remote monitoring is a cost‐effective approach for the lifetime management of patients with implantable cardioverter‐defibrillators.
Keywords: cost‐effectiveness, implantable cardioverter‐defibrillators, remote monitoring
1. INTRODUCTION
The survival of patients at high risk of sudden cardiac arrest can be improved with the use of implantable cardioverter‐defibrillators (ICDs).1 The long‐term mortality and morbidity of patients who receive ICDs remain substantial, however. In addition to the physician visits needed to manage disease‐related morbidity, current guidelines recommend that patients with ICDs should be evaluated every 3 to 6 months to assess device function.2 This regimen can impose a considerable burden on both patients and physicians if patients must be evaluated in the office. As a consequence, device follow‐up is not reliable in routine clinical practice, with nearly one‐quarter of patients not seen in‐person within a year of device implantation.3
Remote patient monitoring (RPM) has been promoted as a strategy to reduce this burden. It can improve the efficiency of care delivery by replacing at least some in‐office visits with remote monitoring transmissions4, 5, 6, 7 without compromising safety.7, 8, 9 Remote monitoring may also improve patient satisfaction and quality of life as it entails less travel time, time off work, and interruption of patient activities. Data suggests that clinically actionable events are detected sooner with remote monitoring than with standard in‐office follow‐up,10 potentially allowing clinicians to act on these issues before they cause increased morbidity, hospitalizations, and costs. RPM also provides a convenient means for regular assessment of device‐related parameters, such as lead impedance and battery status, which may allow early detection of a device and lead malfunction.11, 12, 13, 14, 15 RPM can, therefore, enhance device safety and potentially improve clinical outcomes.10, 16, 17, 18, 19, 20
RPM was associated with lower hospitalization rates and reduced mortality in the large, real‐world PREDICT RM study,21 and its routine use has been endorsed by professional societies.22 However, while RPM is widely available, it is still not universally utilized by clinicians. In a recent U.S. study, fewer than half of ICD recipients enrolled in and activated RPM,21 and utilization is significantly lower in Europe. To determine whether RPM has economic benefits in addition to the associated clinical benefits and to determine the magnitude of the health and economic incentives for increased use of RPM, we developed an economic model to conduct an analysis of the clinical outcomes and costs of RPM versus no RPM from a Medicare perspective. Previous studies done over limited time horizons have shown RPM to be relatively cost‐effective,23, 24, 25, 26, 27 but this has not been evaluated over a lifelong time horizon.
2. METHODS
2.1. The PREDICT RM database
This study represents a collaborative effort between Boston Scientific Corporation, the American College of Cardiology Foundation (ACCF) and the Yale/New Haven Hospital Center for Outcomes Research and Evaluation. Use of the ALTITUDE database was approved by Boston Scientific Corporation. Use of the ACCF National Cardiovascular Data Registry (NCDR) ICD Registry was approved by the ICD Registry Research and Publications Committee. Institutional Review Board approval was obtained and data set linkage and analysis was approved by the Yale University School of Medicine Human Investigation Committee.
The PREDICT RM database was constructed by linking various data sources22 and applying a set of inclusion criteria that identified patients with an RPM‐capable device and Medicare fee‐for‐service claims. This allowed us to estimate the effects of RPM on the risks and costs of rehospitalizations and outpatient care.
The PREDICT RM database was constructed by linking four data sources: (1) the American College of Cardiology (ACC) National Cardiovascular Data Registry (NCDR) ICD Registry, (2) the Boston Scientific Corporation ALTITUDE database, (3) the Social Security Death Master File, and (4) Medicare administrative claims data.22, 28, 29, 30 The data set from the NCDR ICD Registry was limited to only those patients who had previously been linked to the Death Master File using direct identifiers (including Social Security Number) to determine vital status. Patients were included if they received an RPM‐capable device with first‐time device implantation between January 2006 and March 2010. The indirect identifiers age, sex, date of implant, and facility Medicare Provider Number were used to link the ICD Registry data to a comparably limited data set from the ALTITUDE database. Risk of rehospitalization was determined by linking the study cohort with corresponding Medicare fee‐for‐service administrative claims data for beneficiaries who were 65 or older.
2.2. Patient population
The patient population for this study was composed of Medicare patients with RPM‐capable devices (N = 15 254; control = 9906; RPM = 5348) taken from the population studied in the PREDICT RM database. Simulated individual‐patient profiles were created based on the categorical distributions of patient characteristics in the PREDICT RM database (Table 1). To reflect the heterogeneity of the real patient population and to better preserve correlations among patient characteristics, the patient population was stratified into subgroups based on the predicted times to rehospitalization and death—the key outcomes of interest. Details of the risk stratification (eTable 1) and patient characteristics for the risk‐stratified subgroups (eTable 2) can be found in the Appendix (“Patient Characteristics by Risk Strata”).
Table 1.
PREDICT RM patient population characteristics
| Patient characteristic | Category | Control | RPM | Total | |||
|---|---|---|---|---|---|---|---|
| n = 9906 | n = 5348 | n = 15 254 | |||||
| RPM enrolled | 2664 | 26.9% | 5348 | 100.0% | 8012 | 52.5% | |
| Age | 65‐74 | 4524 | 45.7% | 2580 | 48.2% | 7104 | 46.6% |
| ≥75 | 5382 | 54.3% | 2768 | 51.8% | 8150 | 53.4% | |
| NYHA class | I/II | 3348 | 33.8% | 1651 | 30.9% | 4999 | 32.8% |
| III/IV | 6552 | 66.1% | 3690 | 69.0% | 10 242 | 67.1% | |
| Sex | Male | 7104 | 71.7% | 3846 | 71.9% | 10 950 | 71.8% |
| Female | 2802 | 28.3% | 1502 | 28.1% | 4304 | 28.2% | |
| Race | White, non‐Hispanic | 8270 | 83.5% | 4789 | 89.5% | 13 059 | 85.6% |
| Black, non‐Hispanic | 786 | 7.9% | 323 | 6.0% | 1109 | 7.3% | |
| Hispanic | 486 | 4.9% | 111 | 2.1% | 597 | 3.9% | |
| Other | 352 | 3.6% | 120 | 2.2% | 472 | 3.1% | |
| Admission reason | Admitted for this procedure | 5839 | 58.9% | 3518 | 65.8% | 9357 | 61.3% |
| Hospitalized, cardiac | 1571 | 15.9% | 656 | 12.3% | 2227 | 14.6% | |
| Hospitalized, noncardiac | 2118 | 21.4% | 1054 | 19.7% | 3172 | 20.8% | |
| Hospitalized, unknown | 361 | 3.6% | 117 | 2.2% | 478 | 3.1% | |
| CHF duration | No | 1426 | 14.4% | 762 | 14.2% | 2188 | 14.3% |
| <9 mo | 2543 | 25.7% | 1386 | 25.9% | 3929 | 25.8% | |
| >9 mo | 5932 | 59.9% | 3190 | 59.6% | 9122 | 59.8% | |
| CHF hospitalization | Not hospitalized | 5141 | 51.9% | 3022 | 56.5% | 8163 | 53.5% |
| <6 mo ago | 2856 | 28.8% | 1334 | 24.9% | 4190 | 27.5% | |
| >6 mo ago | 1898 | 19.2% | 977 | 18.3% | 2875 | 18.8% | |
| Atrial fibrillation/ atrial flutter | 4025 | 40.6% | 2066 | 38.6% | 6091 | 39.9% | |
| Nonischemic dilated cardiomyopathy | No | 7070 | 71.4% | 3668 | 68.6% | 10 738 | 70.4% |
| <9 mo ago | 977 | 9.9% | 555 | 10.4% | 1532 | 10.0% | |
| >9 mo ago | 1854 | 18.7% | 1122 | 21.0% | 2976 | 19.5% | |
| Previous CABG/PCI | 4350 | 43.9% | 2292 | 42.9% | 6642 | 43.5% | |
| Pacemaker insertion | 1535 | 15.5% | 786 | 14.7% | 2321 | 15.2% | |
| Cerebrovascular disease | 1683 | 17.0% | 854 | 16.0% | 2537 | 16.6% | |
| Chronic lung disease | 2589 | 26.1% | 1325 | 24.8% | 3914 | 25.7% | |
| Diabetes | 4015 | 40.5% | 1958 | 36.6% | 5973 | 39.2% | |
| Hypertension | 8054 | 81.3% | 4312 | 80.6% | 12 366 | 81.1% | |
| Renal failure (dialysis) | 425 | 4.3% | 131 | 2.4% | 556 | 3.6% | |
| QRS duration (msec) | ≤120 | 3483 | 35.2% | 1705 | 31.9% | 5188 | 34.0% |
| >120 | 6423 | 64.8% | 3643 | 68.1% | 10 066 | 66.0% | |
| Intraventricular conduction | Normal | 2789 | 28.2% | 1380 | 25.8% | 4169 | 27.3% |
| Abnormal (LBBB) | 3625 | 36.6% | 2179 | 40.7% | 5804 | 38.0% | |
| Abnormal (RBBB) | 880 | 8.9% | 434 | 8.1% | 1314 | 8.6% | |
| Paced | 1047 | 10.6% | 539 | 10.1% | 1586 | 10.4% | |
| Other | 1551 | 15.7% | 810 | 15.1% | 2361 | 15.5% | |
| Creatinine level (mg/dL) | ≤1.5 | 7449 | 75.2% | 4117 | 77.0% | 11 566 | 75.8% |
| 1.5–2.5 | 1887 | 19.0% | 1013 | 18.9% | 2 900 | 19.0% | |
| >2.5 | 555 | 5.6% | 208 | 3.9% | 763 | 5.0% | |
| BUN level (mg/dL) | ≤20 | 3889 | 39.3% | 2226 | 41.6% | 6 115 | 40.1% |
| 20–40 | 4618 | 46.6% | 2465 | 46.1% | 7083 | 46.4% | |
| >40 | 1379 | 13.9% | 645 | 12.1% | 2024 | 13.3% | |
| Sodium level (mEq/L) | ≤135 | 1687 | 17.0% | 826 | 15.4% | 2513 | 16.5% |
| 135–145 | 8070 | 81.5% | 4459 | 83.4% | 12 529 | 82.1% | |
| >145 | 123 | 1.2% | 51 | 1.0% | 174 | 1.1% | |
| Systolic BP (mm Hg) | ≤100 | 635 | 6.4% | 326 | 6.1% | 961 | 6.3% |
| 100–130 | 4393 | 44.3% | 2217 | 41.5% | 6610 | 43.3% | |
| >130 | 4826 | 48.7% | 2786 | 52.1% | 7612 | 49.9% | |
| ICD type | Single chamber | 1102 | 11.1% | 420 | 7.9% | 1522 | 10.0% |
| Dual chamber | 2737 | 27.6% | 1334 | 24.9% | 4071 | 26.7% | |
| Biventricular | 6054 | 61.1% | 3590 | 67.1% | 9644 | 63.2% | |
| Teaching status | COTH | 277 | 2.8% | 208 | 3.9% | 485 | 3.2% |
| Teaching | 2782 | 28.1% | 1412 | 26.4% | 4194 | 27.5% | |
| Other | 2466 | 24.9% | 1298 | 24.3% | 3764 | 24.7% | |
| Pop. density (per sq. mile) | ≤3000 | 8747 | 88.3% | 4972 | 93.0% | 13 719 | 89.9% |
| >3000 | 1122 | 11.3% | 356 | 6.7% | 1478 | 9.7% | |
Abbreviations: BP, blood pressure; BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; CHF, coronary heart failure; COTH, Council of Teaching Hospitals and Health Systems; ICD, implantable cardioverter‐defibrillator; LBBB, left bundle branch block; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; RBBB, right bundle branch block; RPM, remote patient monitoring.
2.3. Economic model
An economic model was developed using Microsoft Excel to simulate individual patients using a time‐to‐event approach to evaluate the clinical outcomes and costs of RPM from a United States (US) Medicare perspective (Figure 1).
Figure 1.
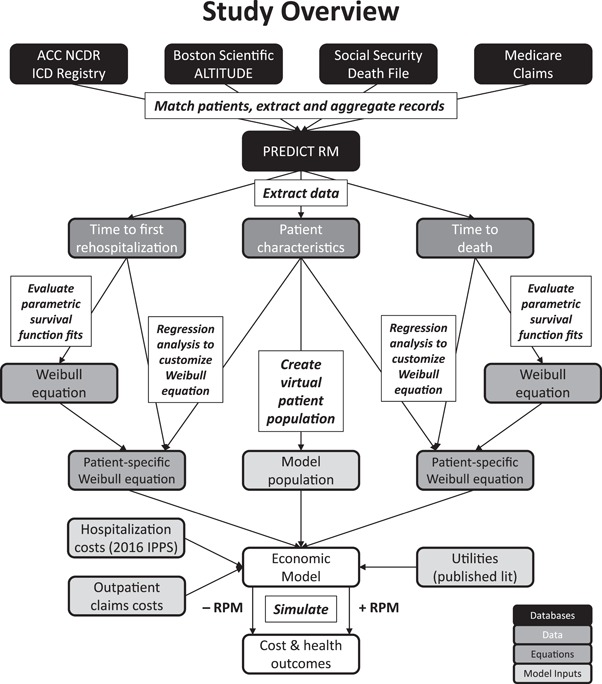
Overview of the model development process (outpatient claims not shown). The figure illustrates the data sources and inputs used in the economic model, and how the final inputs were derived. Boxes next to or over arrows describe the process completed to map PREDICT RM data to model inputs
The model simulates hospitalization and all‐cause death events during the follow‐up period of PREDICT RM and projects those risks beyond the study period to assess the different clinical, quality of life, and cost implications of RPM versus no RPM. The model assesses the number of hospitalizations, outpatient claims, cardiovascular deaths, life years (LYs), quality‐adjusted life years (QALYs), and costs with and without RPM. Patients were followed over their lifetimes, assuming a maximum time horizon of 25 years. Costs and benefits were discounted at 3% per year.
2.3.1. Model structure
The model creates simulated patients based on patient characteristic distributions from PREDICT RM. Simulated patients are then cloned and assigned to a treatment (RPM or no RPM). Patients are at risk of two major events—rehospitalization (all hospitalizations after initial device implantation) and death—while experiencing regular outpatient care.
A patient's utility is a function of baseline and accumulated comorbidities from hospitalizations in addition to the patient being hospitalized. When a patient has a rehospitalization event, comorbidity and length of stay are assigned. These factors are used to estimate a patient's quality of life.
Rehospitalization and outpatient visit rates are initially assigned based on patient characteristics and risk stratification categories, respectively. After the first rehospitalization, future rehospitalization and outpatient visit rates are assigned based solely on treatment arm.
Simulated patients continuously accumulate costs and health outcomes for rehospitalization events, outpatient claims, and accumulated comorbidities until death or the end of the time horizon. Both cost and outcomes were discounted at 3.0%.31
To efficiently simulate RPM in ICD patients, the model used Discretely Integrated Condition Event (DICE) simulation.32 The DICE modeling technique conceptualizes the decision‐analytic problem in terms of conditions (aspects of patients or the management of their health that persist over time) and events (things that happen at points in time). Conditions in the model included patient demographics, risk factors, and comorbidities; events included rehospitalizations, death, and outpatient claims. The model simulated individual patients by tracking how their conditions evolved over time and as events occur. The evolving conditions, in turn, influenced the risk of a patient experiencing future events.
2.3.2. Model inputs: Clinical
Parametric survival equations
Kaplan‐Meier survival curves were generated for first rehospitalization and death; extrapolation beyond the observation period of the database study was achieved using parametric survival fits to the Kaplan‐Meier curves. Construction of the survival curves and the time‐to‐rehospitalization and time‐to‐death analyses are described in the Appendix (“Survival Curves for Time‐to‐rehospitalization and Time‐to‐death Analysis”).
Regression analysis of predictors
The survival fitting yielded distributions for each time‐to‐event curve for the overall population; the fits were then adjusted to account for individual patient characteristics. Times to first rehospitalization and death were modified based on patient characteristics and, for mortality, history of the first rehospitalization. Model building with predictors is described in detail in the Appendix (“Model Building with Predictors”).
Rates of subsequent rehospitalizations
The model accounts for rehospitalization events subsequent to a patient's first rehospitalization using a constant rate. Counts of second and additional rehospitalizations were divided by the duration of follow‐up (counting from the time of the first rehospitalization) to give subsequent rehospitalization rates by treatment arm (RPM vs no RPM).
Rates of outpatient claims
The model accounts for three classes of outpatient claims: hospital outpatient claims, ambulatory surgical center (ASC) claims, and physician claims. ASC claims constituted less than 0.6% of the total, however, and so were combined with hospital outpatient claims.
For each type of outpatient claim, rates were calculated separately before and after the first rehospitalization as the number of unique claims per patient divided by the appropriate average time—time to first rehospitalization or time from the first rehospitalization until death.
2.3.3. Model inputs: Economic
Costs
Each hospitalization event was assigned an average cost based on the distribution of diagnosis‐related groups (DRGs) codes in the observed hospitalization events and the associated DRG costs. Outpatient claim costs were specific to the type of claim, the treatment arm, and whether the claim occurred before or after the first rehospitalization. Additional details regarding the calculation of hospitalization and outpatient claim costs can be found in the Appendix (eTable 3).
Utilities
Utility values for the patients are dependent on a patient's baseline characteristics, comorbidities, and rehospitalization.30 Patients were assumed to have a utility of 0 for their assigned length of stay during rehospitalization events. The effects of both patient characteristics (eTable 4) and comorbidities (eTable 5) were included in the estimation of patient utility.
2.3.4. Model validation
To verify that the model would reproduce the observed results upon which the model inputs were based, we simulated two cohorts of patients, one with RPM and one without, and compared the model outputs to the observed data from the PREDICT RM database.
2.3.5. Sensitivity and scenario analyses
With other studies failing to find an improvement in mortality due to use of RPM, complementary scenario analyses assessed the effects of patient characteristics and the importance of RPM effects on mortality as well as first rehospitalization. One‐way deterministic sensitivity analyses were conducted to investigate the sensitivity of model results to uncertainty in the values of input parameters. Additional analyses focused on the results at shorter time horizons, RPM effects on outpatient claim rates and costs, and the effects of assumptions regarding utilities.
3. RESULTS
3.1. Validation of time‐to‐event model equations
The fitting of parametric survival functions to the Kaplan‐Meier curves resulted in the selection of Weibull functions for both time to first rehospitalization and time to death. Weibull fits were selected based on a review of the Akaike and Bayesian information criteria and visual inspection of how well the fits matched the observed data (eTable 6). Extrapolations of the Weibull fits beyond the observed data were evaluated by visual inspection and judged to be clinically plausible (eFigure 1). To verify that the survival curves were properly implemented in the economic model, we compared the survival predicted by the economic model to the survival curves described by the regression equations (eFigure 2).
Table 2 shows the results of the regression analysis, including equation coefficients for all predictors that remained significant in the final analysis. RPM was found to have a coefficient of 0.0968 for time to the first rehospitalization, corresponding to a roughly 10% extension in time to rehospitalization with RPM. The RPM coefficient for time to death was 0.1666, corresponding to an approximately 18% extension in survival, all other characteristics (including rehospitalization) being equal. The large, negative coefficient (−1.545) for rehospitalization in the mortality equation is significant given that virtually all death events occurred after the first rehospitalization. RPM has, therefore, both a direct effect on mortality and an indirect effect on mortality by delaying the time to rehospitalization.
Table 2.
Coefficients for time‐to‐event regression equations
| Time to first rehospitalization | Time to death | ||||||
|---|---|---|---|---|---|---|---|
| Term | Value | Coefficient | SE | P | Coefficient | SE | P |
| Scale | 1.2024 | 0.0128 | 0.9875 | 0.0146 | |||
| Weibull shape | 0.8316 | 0.0088 | 1.0127 | 0.015 | |||
| Intercept | 7.5446 | 0.0583 | <0.0001 | 9.976 | 0.1219 | <0.0001 | |
| RPM | 0.0968 | 0.0307 | 0.0016 | 0.1666 | 0.0346 | <0.0001 | |
| Age | ≥75 | −0.1822 | 0.0305 | <0.0001 | −0.3385 | 0.0339 | <0.0001 |
| NYHA class | III/IV | −0.1446 | 0.0355 | <0.0001 | −0.2279 | 0.0438 | <0.0001 |
| Sex | Female | −0.1257 | 0.0342 | 0.0002 | 0.1613 | 0.0382 | <0.0001 |
| Hospitalization during follow‐up | −1.5451 | 0.0652 | <0.0001 | ||||
| Race | Black non‐Hispanic | −0.2122 | 0.0596 | 0.0004 | −0.1957 | 0.0597 | 0.001 |
| Hispanic | −0.1508 | 0.0762 | 0.0478 | 0.1268 | 0.0839 | 0.1307 | |
| Other | 0.0143 | 0.085 | 0.8663 | −0.0258 | 0.0896 | 0.7733 | |
| Admission reason | Hospitalized, cardiac | −0.2144 | 0.0454 | <0.0001 | −0.2046 | 0.0433 | <0.0001 |
| Hospitalized, noncardiac | −0.2108 | 0.0392 | <0.0001 | −0.1265 | 0.0419 | 0.0026 | |
| Hospitalized, unknown | −0.2999 | 0.0913 | 0.001 | −0.255 | 0.0812 | 0.0017 | |
| CHF duration | <9 mo | 0.0171 | 0.0535 | 0.7494 | – | – | – |
| >9 mo | −0.1339 | 0.0485 | 0.0057 | – | – | – | |
| Prior CHF hospitalization | Yes, within 0–6 mo | – | – | – | −0.2303 | 0.0385 | <0.0001 |
| Yes, >6 mo ago | – | – | – | −0.1616 | 0.043 | 0.0002 | |
| Flutter | −0.183 | 0.0312 | <0.0001 | −0.1895 | 0.033 | <0.0001 | |
| Nonischemic dilated cardiomyopathy | Yes, within past 9 mo | 0.1899 | 0.057 | 0.0009 | 0.1951 | 0.0651 | 0.0027 |
| Yes, >9 mo | 0.1076 | 0.043 | 0.0123 | −0.0625 | 0.047 | 0.1834 | |
| CABG/PCI | −0.0885 | 0.034 | 0.0093 | −0.1427 | 0.0369 | 0.0001 | |
| Permanent pacemaker | −0.1441 | 0.0543 | 0.008 | 0.1533 | 0.0587 | 0.009 | |
| CV disease | −0.127 | 0.0392 | 0.0012 | −0.155 | 0.0399 | 0.0001 | |
| Lung disease | −0.3403 | 0.0335 | <0.0001 | −0.2539 | 0.0344 | <0.0001 | |
| Diabetes | −0.1414 | 0.031 | <0.0001 | −0.1304 | 0.0334 | <0.0001 | |
| Hypertension | 0.0784 | 0.0418 | 0.0605 | ||||
| Dialysis | −0.2753 | 0.0921 | 0.0028 | −0.1688 | 0.0783 | 0.0312 | |
| QRS duration | >120 msec | 0.1117 | 0.0447 | 0.0124 | |||
| Intraventricular conductance | Abnormal‐LBBB | 0.1806 | 0.0399 | <0.0001 | −0.0131 | 0.0536 | 0.8066 |
| Abnormal‐RBBB | −0.0226 | 0.0576 | 0.6947 | −0.1856 | 0.066 | 0.0049 | |
| Paced | 0.1374 | 0.0685 | 0.045 | −0.0706 | 0.0789 | 0.3706 | |
| Other | −0.0421 | 0.0473 | 0.3726 | −0.1724 | 0.054 | 0.0014 | |
| Creatinine | 1.5‐2.5 mg/dL | −0.2105 | 0.0431 | <0.0001 | −0.1641 | 0.043 | 0.0001 |
| >2.5 mg/dL | −0.392 | 0.0879 | <0.0001 | −0.5601 | 0.075 | <0.0001 | |
| BUN | 20‐40 mg/dL | −0.1701 | 0.0337 | <0.0001 | −0.3104 | 0.0402 | <0.0001 |
| >40 mg/dL | −0.4382 | 0.0588 | <0.0001 | −0.5614 | 0.0582 | <0.0001 | |
| Sodium | ≤135 mEq/L | −0.1765 | 0.0406 | <0.0001 | −0.289 | 0.0393 | <0.0001 |
| >145 mEq/L | 0.0376 | 0.1516 | 0.8043 | −0.2652 | 0.1375 | 0.0538 | |
| Systolic BP | 100–130 mm Hg | – | – | – | 0.1389 | 0.0607 | 0.0221 |
| >130 mm Hg | – | – | – | 0.2658 | 0.0617 | <0.0001 | |
| ICD type | Dual chamber | – | – | – | 0.1859 | 0.0605 | 0.0021 |
| Biventricular | – | – | – | 0.2123 | 0.0617 | 0.0006 | |
| Teaching status | Teaching | – | – | – | −0.0483 | 0.045 | 0.2833 |
| Other | – | – | – | −0.13 | 0.0385 | 0.0007 | |
| Population density | >3000/sq mi | −0.1302 | 0.0501 | 0.0094 | – | – | – |
Abbreviations: BP, blood pressure; BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; CHF, coronary heart failure; CV, cardiovascular; ICD, implantable cardioverter‐defibrillator; LBBB, left bundle branch block; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; RBBB, right bundle branch block; RPM, remote patient monitoring; SE, standard error.
3.2. Event rates
Event rates were calculated for rehospitalizations following the first rehospitalization and for all types of outpatient claims. The rate of subsequent rehospitalizations was different in the RPM (1.65 per year) and no‐RPM arms (1.79 per year). As with first rehospitalizations, RPM showed a benefit compared to no RPM.
The rates of hospital outpatient/ASC claims and physician claims were consistently higher in the RPM arm than they were in the no‐RPM arm. This was true before and after the first rehospitalization, and it was true for the overall population as well as for most of the risk‐stratified bins of patients (eTable 7).
We also investigated whether the number of outpatient claims varied by patient risk. We assigned a risk rank to each risk stratum based on the combination of baseline risks of rehospitalization and death (see Appendix (eTable 1)). Plotting the mean outpatient claim rates by risk ranking showed a clear trend toward lower outpatient claim rates in the lower‐risk groups (Figure 2).
Figure 2.
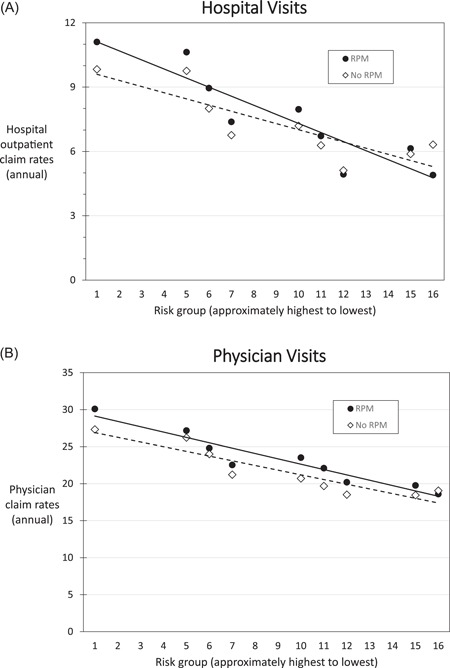
Annual outpatient claim rates by risk group. Rates for groups of fewer than 100 patients have been omitted. Lines have been added to show trends. Risk groups created by combinations of categorical rehospitalization and mortality risks are listed sequentially from highest to lowest. A linear trendline was added to illustrate observed trends in the data. For both hospital outpatient and physician claim rates, a similar trend is observed with higher rates for RPM vs no RPM, RPM, remote patient monitoring
3.3. Cost‐effectiveness analysis: Base case
Base‐case results are presented in Table 3. The results show clinical benefits for RPM relative to no RPM associated with a moderate increase in costs. Over the cohort's lifetime (maximum 25 years), no RPM resulted in an average of 6.85 LYs, 5.65 QALYs, and total costs of $99 815 per patient (all values discounted). No‐RPM patients had an average of 10.4 rehospitalization events and 262 outpatient unique claims throughout their follow‐up, which translated to 1.26 rehospitalization events and 32 outpatient unique claims per patient‐year (PPY). RPM resulted an increase in both LYs (7.62) and QALYs (6.29) with higher total costs ($106 729). Patients receiving RPM had both higher rehospitalization counts (11.0) and outpatient unique claims (317); however, the greater number of rehospitalizations was due to RPM patients living longer and thus having a longer time at risk to incur these events. With RPM, patients experienced only 1.18 rehospitalization events PPY, a reduction of 0.08 rehospitalization events PPY from the no‐RPM case.
Table 3.
Base‐case results
| Outcome | No RPM | RPM | Difference |
|---|---|---|---|
| LYs | 6.85 | 7.62 | 0.77 |
| QALYs | 5.65 | 6.29 | 0.64 |
| Rehospitalizations | 10.4 | 11.0 | 0.6 |
| PPY | 1.26 | 1.18 | −0.08 |
| Hospital outpatient/ASC claims | 68.1 | 84.6 | 16.4 |
| Physician claims | 194 | 233 | 39 |
| Total outpatient claims | 262 | 317 | 55 |
| PPY | 31.7 | 34.0 | 2.2 |
| Rehospitalization costs | $57 023 | $59 214 | $2191 |
| PPY | $8321 | $7767 | ‐$554 |
| Nonhospital cost | $42 792 | $47 515 | $4723 |
| PPY | $6244 | $6232 | ‐$12 |
| Total costs | $99 815 | $106 729 | $6914 |
| PPY | $14 566 | $13 999 | ‐$566 |
| Incremental cost per LY gained | $8966 | ||
| Incremental cost per QALY gained | $10 752 |
Abbreviations: ASC, ambulatory surgical center; LY, life‐year; PPY, per patient‐year; QALY, quality‐adjusted life‐year; RPM, remote patient monitoring
When RPM is compared with no RPM, RPM was associated with an incremental gain of 0.64 QALYs and an increase in costs of $6914, resulting in an incremental cost‐effectiveness ratio (ICER) of $10,752/QALY.
3.4. Cost‐effectiveness analysis: Scenario and sensitivity analyses
Key scenarios in the analysis were those that removed specific RPM effects from the RPM‐arm projections (Table 4). When the RPM effect on survival was removed, the RPM arm dominated the no‐RPM arm (RPM was less costly and more effective). RPM still reduced the number of rehospitalizations, which indirectly reduced mortality. However, without the additional direct RPM effect on mortality, RPM and non‐RPM patients enjoyed similar life expectancies, and the additional costs associated with increased longevity were not accrued. When the RPM effect on the risk of the first rehospitalization was removed, the ICER increased 29%. Patients were still living longer with RPM but without the benefit of reduced rehospitalization rates. Results were also examined for each of the different risk strata and key baseline characteristics of interest. The most‐severe risk strata (high projected risks of both rehospitalization and death) had much shorter life expectancies than did patients in other risk strata, which resulted in lower incremental QALYs and a higher ICER than in the base case.
Table 4.
Results of scenario analyses
| Scenario | Incremental QALYs | Incremental costs | ICER |
|---|---|---|---|
| Base case | 0.64 | $6914 | $10 752 |
| Each risk‐stratified subpopulation individually | |||
| D1‐R1 | 0.33 | $4998 | $15 275 |
| D2‐R1 | 0.47 | $5452 | $11 492 |
| D3‐R1 | 0.71 | $5829 | $8262 |
| D1‐R2 | 0.43 | $15 044 | $35 198 |
| D2‐R2 | 0.59 | $7803 | $13 241 |
| D3‐R2 | 0.69 | $8525 | $12 275 |
| D4‐R2 | 0.71 | $17 109 | $24 263 |
| D2‐R3 | 0.65 | $6730 | $10 303 |
| D3‐R3 | 0.71 | $6783 | $9493 |
| D4‐R3 | 0.72 | $4419 | $6170 |
| D2‐R4 | 0.66 | $14 442 | $21 772 |
| D3‐R4 | 0.72 | $2354 | $3270 |
| D4‐R4 | 0.72 | −$4066 | Dominant |
| Patient characteristics | |||
| AF = 100% | 0.60 | $7211 | $11 967 |
| Cerebrovascular disease = 100% | 0.57 | $7210 | $12 539 |
| Chronic lung disease = 100% | 0.57 | $6756 | $11 901 |
| Diabetes = 100% | 0.61 | $7123 | $11 760 |
| Hypertension = 100% | 0.64 | $6926 | $10 853 |
| RPM coeff = 0, rehospitalization | 0.55 | $7650 | $13 914 |
| RPM coeff = 0, death | 0.12 | −$3734 | Dominant |
| RPM coeff = 0, rehospitalization and death | 0.02 | −$3303 | Dominant |
| Time horizon | |||
| Time horizon = 2 | 0.03 | −$768 | Dominant |
| Time horizon = 5 | 0.13 | −$635 | Dominant |
| Time horizon = 10 | 0.30 | $1058 | $3573 |
| Costs and resource use | |||
| Death cost = DRG 283 (MI Death) cost | 0.64 | $6619 | $10 294 |
| LoS equivalent (no RPM value), first rehospitalization and subsequent rehospitalization | 0.64 | $6829 | $10 729 |
| Equal hospitalization outpatient/ASC costs (no RPM value), before and after rehospitalization | 0.64 | $10 003 | $15 555 |
| Equal physician visit costs (no RPM value), before and after rehospitalization | 0.64 | $6884 | $10 705 |
| Equal hospitalization outpatient/ASC rates (no RPM value), before and after rehospitalization | 0.64 | $4470 | $6951 |
| Equal physician visit rates (no RPM value), before and after rehospitalization | 0.64 | $5849 | $9094 |
| Equal subsequent rehospitalization rates (no RPM value) | 0.63 | $11 447 | $18 102 |
| Utility | |||
| No utility of zero during hospitalization | 0.64 | $6914 | $10 790 |
| No disutility following hospitalization | 0.66 | $6914 | $10 467 |
| Two lines above combined | 0.66 | $6914 | $10 504 |
Abbreviations: AF, atrial fibrillation; ASC, ambulatory surgical center; DRG, diagnosis‐related group; ICER, incremental cost‐effectiveness ratio; LoS, length of stay; MI, myocardial infarction; QALY, quality‐adjusted life‐year; RPM, remote patient monitoring
Changes that occurred in hospital/ASC outpatient claims before a patient's first rehospitalization had a greater effect than proportionally similar changes in hospital/ASC outpatient claims after rehospitalization or any physician claims. The main driver of results—rehospitalizations—had generally proportional effects on results when varied by 20% (eTable 8). Details of these and other scenarios and sensitivity analyses are described in the Appendix (“Scenario and Sensitivity Analysis”).
4. DISCUSSION
The clinical utility of remote monitoring in ICD patients is well established and endorsed in the Heart Rhythm Society/European Heart Rhythm Association (HRS/EHRA) Expert Consensus Statement.33 However, the cost‐effectiveness of remote monitoring is less well established and may contribute to the underutilization of RPM. This study utilizes an economic model to analyze the cost‐effectiveness of remote monitoring of ICDs over a lifelong time horizon based on Medicare claims data. The following are the main findings.
-
1)
RPM is associated with improved survival, with an increase in discounted life expectancy of 9.3 months (0.77 LYs) and in discounted quality‐adjusted life expectancy of 7.7 months (0.64 QALYs).
-
2)
Although total costs and the number of rehospitalizations are increased due to improved survival, the number of rehospitalizations and overall costs PPY decrease with RPM.
-
3)
With only a direct effect of RPM on the hospitalization rate, RPM becomes a cost‐saving strategy that still provides health benefits above that of no‐RPM.
-
4)
The incremental cost‐effectiveness ratio for remote monitoring was $10 752 per QALY gained, making RPM “high‐value” care by the ACC/American Heart Association (AHA) criterion (<$50 000/QALY).34
-
5)
These results were robust to various sensitivity and scenario analyses.
Previous cost‐effectiveness analyses from small randomized studies have shown RPM to be cost saving or neutral compared with conventional in‐office follow‐up.23, 27, 35, 36 Nonhospital costs were generally lower with RPM due to fewer scheduled office visits in the RPM arm (as defined by the protocol). In these studies, the number of unscheduled visits was higher with RPM possibly related to increased detection of arrhythmias and device malfunctions. However, the total number of scheduled plus unscheduled visits was still reduced. In our study, RPM was cost‐effective despite an increase in the rate of outpatient claims. In addition to lower visit rates in these previous studies, inpatient costs were also reduced due to fewer hospitalizations and shorter lengths of stays.35 In addition, device cost savings were seen in the ECOST trial37 due to improved battery longevity. Had such cost savings been included in our analysis, RPM would have been even more cost‐effective.
Clinical equipoise remains regarding the effect of remote monitoring on mortality. Retrospective analysis of two large independent databases19, 38 both showed an association between RPM utilization and improved survival, with a graded benefit related to the level of adherence to RPM.38 Results from prospective randomized trials, however, have been mixed. The IN‐TIME trial39 demonstrated a substantial reduction in mortality in the RPM group; however, the rigorous protocol of daily RPM transmissions also resulted in increased direct patient contact. The REM‐HF trial40 did not demonstrate a survival benefit for RPM; however, both arms actually utilized remote monitoring to some extent, as usual care in the control arm could have included remote follow‐up every 6 months. Similarly, no benefit was seen in the MORE‐CARE trial,9 which replaced in‐office visits with RPM; however, this trial was not powered to detect mortality differences. As PREDICT RM is a nonrandomized database, it is possible that the observed beneficial effects on hospitalization and mortality could be secondary to confounding factors. Sensitivity analysis was, therefore, performed to evaluate the cost‐effectiveness of RPM in the absence of any survival benefit or reduction in hospitalization rate. When the beneficial effect on rehospitalization was removed, the ICER was still well within the realm of high‐value care. When the effect on survival was removed, RPM became a dominant strategy. It continued to have beneficial effects on hospitalizations and was now also cost‐saving since there was no increase in survival time during which additional costs would accrue. The cost‐effectiveness of RPM was robust across a wide range of sensitivity analyses, with ICERs well below the “high‐value care” threshold of $50,000/QALY in every risk group and in every sensitivity and scenario analysis examined.
4.1. Limitations of study
A key limitation of this study is that it draws primarily from an observational data source. Although this provided a large sample from which to estimate parameters, observational studies may have unobserved confounding factors that cannot be controlled for. However, estimates for all parameters in this study were controlled for a large set of patient and provider characteristics.
This study did not differentiate Medicare costs for hospitalization or outpatient claims due to cardiac conditions from those associated with other medical conditions. Therefore, the costs associated with RPM include many outpatient claims and hospitalizations unrelated to the intervention. However, as the increased costs leading to an ICER of $10 752/QALY for RPM are due to costs accrued during the extra 9.3 months of survival, the ICER would have been even more favorable if those noncardiac expenses were excluded.
In calculating the costs of hospitalizations, hospital outpatient claims, and physician claims, we used average costs. Although these averages differentiated between RPM and no RPM, it is possible that a more detailed analysis of cost differences in the two groups would lead to different and more informative cost estimates.
Patients were included in the RPM group if only a single transmission was received, but it has been shown that there is a dose‐response relationship with RPM.38 Including patients with only sporadic RPM may underestimate the value of regular remote monitoring.
The model used extrapolation of first rehospitalization and mortality events to assess a time horizon spanning the patient population's lifetime, which is beyond that of the PREDICT‐RM follow‐up. Although model results were robust to changes in the model time horizon, much of the health benefit is accumulated during the extrapolated period. Therefore, the conclusions regarding the benefits of RPM may not change, but the magnitude is uncertain. One potential cost saving with RPM involves improvement in battery longevity and management of the patient as the device approaches elective replacement. Without RPM, additional visits may be needed under these circumstances. As the median PREDICT RM follow‐up was only 2.5 years (interquartile range = 1.7‐3.3 years) years, very few patients reached the point of elective replacement. This effect, if modeled, would likely reduce RPM costs and improve cost‐effectiveness.
The model was based on data derived from a Medicare population and thus no patients under age 65 were included, with more than half of the cohort over 75. Thus the findings of this study may not apply to younger, healthier patients with ICDs. In addition, analyses in this manuscript were restricted to patients receiving Boston Scientific devices. Although nearly all currently used ICDs have the capacity for RPM, the technology and interfaces differ across manufacturers, and the extent to which our findings are generalizable to other devices is not known.
5. CONCLUSIONS
Despite its universal endorsement by professional societies, the low RPM participation rate seen in some recent clinical trials suggests that only a fraction of the potential health and economic benefits of RPM are being realized. This is likely due to a combination of factors, including the lack of long‐term cost‐effectiveness data for RPM. Using an economic model based on Medicare claims from the PREDICT RM cohort, this is the first study to assess the cost‐effectiveness of RPM over a lifetime time horizon. RPM was associated with improved survival, reduced hospitalization rates, and decreased healthcare costs PPY when compared with conventional care. Even when RPM does not have a direct effect on mortality, RPM is the preferred strategy, dominating no RPM. The ICER of $10 752/QALY gained clearly makes RPM high‐value care and underscores the importance of increasing utilization of RPM among ICD recipients.
AUTHOR CONTRIBUTIONS
JPH, JPC, and JGA contributed to design of the study; analysis and interpretation of the data; and writing of the manuscript. HB and LSO contributed to design and conduct of study; management, analysis, and interpretation, of the data, and review of the manuscript. RJL, KAD, ARK, and SS contributed to design and conduct of study; the development of the economic model; management, analysis, and interpretation of the data; and writing and review of the manuscript. SLA, PWJ and KS contributed to the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and the review and approval of the article.
FUNDING INFORMATION
This study was sponsored by Boston Scientific Corporation.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The views expressed in this presentation represent those of the author(s), and do not necessarily represent the official views of the NCDR or its associated professional societies identified at http://CVQuality.ACC.org/NCDR.
For more information, go to CVQuality.ACC.org/NCDR or email ncdrresearch@acc.org.
Hummel JP, Leipold RJ, Amorosi SL, et al. Outcomes and costs of remote patient monitoring among patients with implanted cardiac defibrillators: An economic model based on the PREDICT RM database. J Cardiovasc Electrophysiol. 2019;30:1066‐1077. 10.1111/jce.13934
Disclosures: Stacey Amorosi, Paul Jones, and Kenneth Stein are paid employees of Boston Scientific Corporation. Robert Leipold, Kirsten Deger, Anuraag Kansal, and Sean Stern are currently, or were at the time of the study, employees of Evidera, a paid consultant to Boston Scientific. James Hummel, Haikun Bao, Lesli Ott, Jeptha Curtis, and Joseph Akar have no disclosures.
References
REFERENCES
- 1. Goldenberg I, Gillespie J, Moss AJ, et al. Long‐term benefit of primary prevention with an implantable cardioverter‐defibrillator: an extended 8‐year follow‐up study of the Multicenter Automatic Defibrillator Implantation Trial II. Circulation. 2010;122(13):1265‐1271. [DOI] [PubMed] [Google Scholar]
- 2. Wilkoff BL, Auricchio A, Brugada J, et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. 2008;5(6):907‐925. [DOI] [PubMed] [Google Scholar]
- 3. Al‐Khatib SM, Mi X, Wilkoff BL, et al. Follow‐up of patients with new cardiovascular implantable electronic devices: are experts' recommendations implemented in routine clinical practice? Circulation Arrhythmia and electrophysiology. 2013;6(1):108‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bikou O, Licka M, Kathoefer S, Katus HA, Bauer A. Cost savings and safety of ICD remote control by telephone: a prospective, observational study. J Telemed Telecare. 2010;16(7):403‐408. [DOI] [PubMed] [Google Scholar]
- 5. Heidbuchel H, Lioen P, Foulon S, et al. Potential role of remote monitoring for scheduled and unscheduled evaluations of patients with an implantable defibrillator. Europace. 2008;10(3):351‐357. [DOI] [PubMed] [Google Scholar]
- 6. Raatikainen MJ, Uusimaa P, van Ginneken MM, Janssen JP, Linnaluoto M. Remote monitoring of implantable cardioverter defibrillator patients: a safe, time‐saving, and cost‐effective means for follow‐up. Europace. 2008;10(10):1145‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Varma N, Epstein AE, Irimpen A, Schweikert R, Love C, Investigators T. Efficacy and safety of automatic remote monitoring for implantable cardioverter‐defibrillator follow‐up: the Lumos‐T Safely Reduces Routine Office Device Follow‐up (TRUST) trial. Circulation. 2010;122(4):325‐332. [DOI] [PubMed] [Google Scholar]
- 8. Hindricks G, Elsner C, Piorkowski C, et al. Quarterly vs. yearly clinical follow‐up of remotely monitored recipients of prophylactic implantable cardioverter‐defibrillators: results of the REFORM trial. Eur Heart J. 2014;35(2):98‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boriani G, Da Costa A, Ricci RP, et al. The MOnitoring Resynchronization dEvices and CARdiac patiEnts (MORE‐CARE) randomized controlled trial: phase 1 results on dynamics of early intervention with remote monitoring. J Med Internet Res. 2013;15(8):e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH, Investigators C. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57(10):1181‐1189. [DOI] [PubMed] [Google Scholar]
- 11. Abdelhadi RH, Saba SF, Ellis CR, et al. Independent multicenter study of Riata and Riata ST implantable cardioverter‐defibrillator leads. Heart Rhythm. 2013;10(3):361‐365. [DOI] [PubMed] [Google Scholar]
- 12. Hauser RG, Kallinen L. Deaths associated with implantable cardioverter defibrillator failure and deactivation reported in the United States Food and Drug Administration Manufacturer and User Facility Device Experience Database. Heart Rhythm. 2004;1(4):399‐405. [DOI] [PubMed] [Google Scholar]
- 13. Hauser RG, Kallinen LM, Almquist AK, Gornick CC, Katsiyiannis WT. Early failure of a small‐diameter high‐voltage implantable cardioverter‐defibrillator lead. Heart Rhythm. 2007;4(7):892‐896. [DOI] [PubMed] [Google Scholar]
- 14. Hauser RG, Maron BJ. Lessons from the failure and recall of an implantable cardioverter‐defibrillator. Circulation. 2005;112(13):2040‐2042. [DOI] [PubMed] [Google Scholar]
- 15. Maisel WH. Semper fidelis‐‐consumer protection for patients with implanted medical devices. N Engl J Med. 2008;358(10):985‐987. [DOI] [PubMed] [Google Scholar]
- 16. Klersy C, De Silvestri A, Gabutti G, et al. Economic impact of remote patient monitoring: an integrated economic model derived from a meta‐analysis of randomized controlled trials in heart failure. Eur J Heart Fail. 2011;13(4):450‐459. [DOI] [PubMed] [Google Scholar]
- 17. Klersy C, De Silvestri A, Gabutti G, Regoli F, Auricchio A. A meta‐analysis of remote monitoring of heart failure patients. J Am Coll Cardiol. 2009;54(18):1683‐1694. [DOI] [PubMed] [Google Scholar]
- 18. Landolina M, Perego GB, Lunati M, et al. Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: the evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation. 2012;125(24):2985‐2992. [DOI] [PubMed] [Google Scholar]
- 19. Saxon LA, Hayes DL, Gilliam FR, et al. Long‐term outcome after ICD and CRT implantation and influence of remote device follow‐up: the ALTITUDE survival study. Circulation. 2010;122(23):2359‐2367. [DOI] [PubMed] [Google Scholar]
- 20. Varma N, Michalski J, Epstein AE, Schweikert R. Automatic remote monitoring of implantable cardioverter‐defibrillator lead and generator performance: the Lumos‐T Safely RedUceS RouTine Office Device Follow‐Up (TRUST) trial. Circulation Arrhythmia and electrophysiology. 2010;3(5):428‐436. [DOI] [PubMed] [Google Scholar]
- 21. Akar JG, Bao H, Jones P, et al. Use of remote monitoring of newly implanted cardioverter‐defibrillators: insights from the patient related determinants of ICD remote monitoring (PREDICT RM) study. Circulation. 2013;128(22):2372‐2383. [DOI] [PubMed] [Google Scholar]
- 22. Akar JG, Bao H, Jones PW, et al. Use of remote monitoring is associated with lower risk of adverse outcomes among patients with implanted cardiac defibrillators. Circulation Arrhythmia and electrophysiology. 2015;8(5):1173‐1180. [DOI] [PubMed] [Google Scholar]
- 23. Guedon‐Moreau L, Lacroix D, Sadoul N, et al. Costs of remote monitoring vs. ambulatory follow‐ups of implanted cardioverter defibrillators in the randomized ECOST study. Europace. 2014;16(8):1181‐1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perl S, Stiegler P, Rotman B, et al. Socio‐economic effects and cost saving potential of remote patient monitoring (SAVE‐HM trial). Int J Cardiol. 2013;169(6):402‐407. [DOI] [PubMed] [Google Scholar]
- 25. Piccini JP, Mittal S, Snell J, Prillinger JB, Dalal N, Varma N. Impact of remote monitoring on clinical events and associated health care utilization: A nationwide assessment. Heart Rhythm. 2016;13(12):2279‐2286. [DOI] [PubMed] [Google Scholar]
- 26. Ricci RP, Vicentini A, D'Onofrio A, et al. Economic analysis of remote monitoring of cardiac implantable electronic devices: Results of the Health Economics Evaluation Registry for Remote Follow‐up (TARIFF) study. Heart Rhythm. 2017;14(1):50‐57. [DOI] [PubMed] [Google Scholar]
- 27. Zanaboni P, Landolina M, Marzegalli M, et al. Cost‐utility analysis of the EVOLVO study on remote monitoring for heart failure patients with implantable defibrillators: randomized controlled trial. J Med Internet Res. 2013;15(5):e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Centers for Medicare & Medicaid Services (CMS) . Physician Fee Schedule FY 2016, National Payment Amount by HCPCS code. Non‐Facility Fee. 2016; https://www.cms.gov. Accessed December 12, 2016.
- 29. Ghatnekar O, Bondesson A, Persson U, Eriksson T. Health economic evaluation of the Lund Integrated Medicines Management Model (LIMM) in elderly patients admitted to hospital. BMJ Open. 2013;3(1):e001563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sullivan PW, Ghushchyan V. Preference‐Based EQ‐5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost‐effectiveness analyses: Second panel on cost‐effectiveness in health and medicine. JAMA. 2016;316(10):1093‐1103. [DOI] [PubMed] [Google Scholar]
- 32. Caro JJ. Discretely integrated condition event (DICE) simulation for pharmacoeconomics. Pharmacoeconomics. 2016;34(7):665‐672. [DOI] [PubMed] [Google Scholar]
- 33. Heart Rhythm Society . 2015. HRS Expert Consensus Statement on Remote Interrogation and Monitoring for Cardiovascular Electronic Implantable Devices. 2015; http://www.hrsonline.org/Policy-Payment/Clinical-Guidelines-Documents/Expert-Consensus-on-the-Monitoring-of-Cardiovascular-Implantable-Electronic-Devices/2015-Expert-Consensus-Statement-on-Remote-Interrogation-and-Monitoring-for-CIEDs. Accessed March 30, 2017. [DOI] [PubMed]
- 34. Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(21):2304‐2322. [DOI] [PubMed] [Google Scholar]
- 35. Heidbuchel H, Hindricks G, Broadhurst P, et al. EuroEco (European Health Economic Trial on Home Monitoring in ICD Patients): a provider perspective in five European countries on costs and net financial impact of follow‐up with or without remote monitoring. Eur Heart J. 2015;36(3):158‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Al‐Khatib SM, Piccini JP, Knight D, Stewart M, Clapp‐Channing N, Sanders GD. Remote monitoring of implantable cardioverter defibrillators versus quarterly device interrogations in clinic: results from a randomized pilot clinical trial. J Cardiovasc Electrophysiol. 2010;21(5):545‐550. [DOI] [PubMed] [Google Scholar]
- 37. Guedon‐Moreau L, Lacroix D, Sadoul N, et al. A randomized study of remote follow‐up of implantable cardioverter defibrillators: safety and efficacy report of the ECOST trial. Eur Heart J. 2013;34(8):605‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Varma N, Piccini JP, Snell J, Fischer A, Dalal N, Mittal S. The relationship between level of adherence to automatic wireless remote monitoring and survival in pacemaker and defibrillator patients. J Am Coll Cardiol. 2015;65(24):2601‐2610. [DOI] [PubMed] [Google Scholar]
- 39. Hindricks G, Taborsky M, Glikson M, et al. Implant‐based multiparameter telemonitoring of patients with heart failure (IN‐TIME): a randomised controlled trial. Lancet. 2014;384(9943):583‐590. [DOI] [PubMed] [Google Scholar]
- 40. Morgan JM, Kitt S, Gill J, et al. Remote management of heart failure using implantable electronic devices. Eur Heart J. 2017;38(30):2352‐2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


