Abstract
Animal cells constantly receive information about and respond to environmental factors, including ionizing radiation. Although it is crucial for a cell to repair radiation‐induced DNA damage to ensure survival, cellular responses to radiation exposure during early embryonic development remain unclear. In this study, we analyzed the effects of ionizing radiation in zebrafish embryos and found that radiation‐induced γH2AX foci formation and cell cycle arrest did not occur until the gastrula stage, despite the presence of major DNA repair‐related gene transcripts, passed on as maternal factors. Interestingly, P21/WAF1 accumulation began ∼6 h post‐fertilization, although p21 mRNA was upregulated by irradiation at 2 or 4 h post‐fertilization. These results suggest that the cellular responses of zebrafish embryos at 2 or 4 h post‐fertilization to radiation failed to overcome P21 protein accumulation and further signaling. Regulation of P21/WAF1 protein stabilization appears to be a key factor in the response to genotoxins during early embryogenesis.
Keywords: early development, radiation, zebrafish
Abbreviations
- ATM
ataxia‐telangiectasia mutated kinase
- DSBs
double‐strand breaks
- hpf
hours post fertilization
- ATR
ataxia telangiectasia and Rad3‐related protein
Introduction
All organisms are surrounded by environmental stresses that affect their reproduction and development. Early‐stage embryos are often extremely sensitive to the effects of ionizing radiation (Heyer et al., 2000; Valentin, 2003). Such high sensitivity can be partly explained by the fact that transcription and adaptive responses are inoperative during early development (Pelegri, 2003). However, the precise mechanisms by which living cells respond to environmental radiation during early development remain unclear.
The most frequently reported cellular responses to ionizing radiation in somatic cells begin with recruitment of the Mre11‐Rad50‐Nbs1 complex and ataxia‐telangiectasia mutated kinase (ATM) at the site of double‐strand breaks (DSBs) in DNA, followed by autoactivation of ATM (Price and D'Andrea, 2013; Smeenk and van Attikum, 2013; Watts, 2016). Autoactivated ATM phosphorylates histone H2AX, a variant of the H2A family of proteins, and forms γH2AX foci at the DSBs. Activated ATM also phosphorylates TP53 and activates p53 signaling, leading to upregulation of p21 mRNA and P21/WAF1 protein levels (Hirao et al., 2000; Lossaint et al., 2011). Accumulation of the P21/WAF1 causes cell cycle arrest and promotes DNA repair (Gartel et al., 1996; Brugarolas et al., 1999; Wang et al., 1997; Roque et al., 2012). These signaling pathways are crucial for orchestrating DNA repair and maintaining genome stability in cells that survive irradiation (Wang et al., 1997; Hirao et al., 2000).
Cellular responses to irradiation during early development have been examined in various animal species. For example, irradiation of mice embryos before implantation either tends to be lethal or has no effects: this phenomenon is known as an all‐or none stage (Russell and Russell, 1954; De Santis et al., 2007). During mouse development, γH2AX foci formation is delayed in embryos at the one‐cell or two‐cell stage, but occurs normally in six–eight‐cell stage embryos (Adiga et al., 2007). At the one‐ or two‐cell stage, mouse embryos have the ability to undergo G2/M cell cycle arrest (Yukawa et al., 2007); however, unlike two‐cell stage embryos, one‐cell stage embryos do not show an apoptotic response to irradiation (Adiga et al., 2007). In Xenopus embryos, irradiation during early developmental stages leads to apoptosis of all embryonic cells (Anderson et al., 1997). In later stages (after mid‐blastula transition), cells develop the ability to induce cell cycle arrest, which prevents apoptosis in the embryos (Anderson et al., 1997).
The zebrafish is a powerful model organism; its transparent body provides a distinct advantage for imaging studies, particularly during early development (Driever and Fishman, 1996; Brownlie et al., 1998; Dooley and Zon, 2000; Ward and Lieschke, 2002). Recently, this teleost fish has increasingly been used to study cellular responses to chemicals and environmental stresses (Hill et al., 2005; Hwang et al., 2007; MacRae and Peterson, 2015). Unlike Xenopus, zebrafish embryos do not undergo apoptosis until the mid‐gastrulation stage (Ikegami et al., 1997; Negron and Lockshin, 2004). As the cellular machinery that responds to irradiation differs among organisms, further studies are required to reveal the underlying mechanisms that prevent radiation‐induced apoptosis in zebrafish embryos in their early developmental stage. Previous studies showed that zebrafish embryos exposed to more than 4 Gy of radiation at 1 hour post‐fertilization (hpf) are especially sensitive and unable to survive for longer than 24 hpf (McAleer et al., 2005). Increased cell death in retina and brain has been observed in zebrafish embryos irradiated with 5, 10, and 20 Gy at several timepoints in early developmental period, whereas such phenomenon can be ameliorated by a radioprotective agent, amifostine (Geiger et al., 2006). Continuous exposure to gamma irradiation to zebrafish embryos leads to their morphological abnormalities accompanied by altered gene expression profiles (Gagnaire et al., 2015; Hurem et al., 2017). It is of note here that the mRNA levels of tp 53 and sox19a, a crucial factor in embryogenesis, in zebrafish embryos irradiated at 3 hpf were reported to show continuous upregulation from 24 to 72 h after radiation exposure (Praveen Kumar et al., 2017). However, at least to our knowledge, there have been very few studies that clarify the underlying cellular mechanisms by which zebrafish embryos differentially regulate their susceptibility to ionizing irradiation during early developmental stages.
In this study, we examined the changes in cellular responses over time and investigated cells in zebrafish embryos in early developmental stages respond to ionizing radiation.
Materials and methods
Animals
This study was approved by the Institutional Animal Care and Use Committee of Hiroshima University and carried out according to the Hiroshima University Animal Experimentation Regulations. Embryos were obtained from natural spawning events of wild‐type colonies (SAT or WIK). Fish were maintained at the Research Institute for Radiation Biology and Medicine, Hiroshima University, on a 14‐h light/10‐h dark cycle at 28.5°C. Embryos were staged according to Kimmel et al. (1995) by the number of cells, somites, or hpf at 28.5°C.
Irradiation
Embryos at 2, 4, or 6 hpf were irradiated using a GammaCell 40 Exactor (137Cs; Best Theratronics, Inc., Ontario, Canada). The dose rate was ∼0.8 Gy/min. Embryos were placed in 25‐cm2 flasks with ∼10 mL embryo medium. To examine time‐dependent effects, 12–15 embryos per flask at 2, 4, or 6 hpf were irradiated with a total 1, 5, or 10 Gy. Embryos were irradiated at 1 Gy in experiments to examine the effect on cell cycle checkpoint, γH2AX foci formation, and p21 mRNA and P21/WAF1 protein expression. For cell cycle checkpoint and γH2AX detection, 60–80 embryos per flask of 2, 4, or 6 hpf and 20–30 embryos per flask for p21 real‐time PCR were irradiated each time. All experiments were conducted in three biological replicates.
Immunohistochemistry
For γH2AX detection, embryos were fixed at intervals of 15 min after irradiation for 2 h and at intervals of 30 min for cell cycle checkpoint analysis.
Whole‐mount immunostaining was performed as previously reported with slight modifications (Honjo et al., 2008). For anti‐γH2AX antibody staining, embryos were fixed with methanol at −20°C and were incubated in acetone at −20°C for 7 min, and then incubated in blocking solution (2% normal goat serum, 1% bovine serum albumin, 1% Triton‐X100, 1% dimethyl sulfoxide in phosphate‐buffered saline [PBS]) after brief washing with PBSTx (1% Triton‐X100 in PBS). For anti‐phospho‐HH3 antibody staining, embryos were fixed with 4% paraformaldehyde and were washed briefly in PBSTx, rinsed with water, and then washed again in PBSTx, after which they were incubated in blocking solution. For both antibody staining, the embryos were incubated overnight in primary antibody solution at 4°C after blocking. The embryos were then incubated in secondary antibody solution at room temperature (RT) for 4–5 h. An anti‐γH2AX antibody (Gentex, Zeeland, MI, USA, GTX127342) was used at a dilution of 1:200, anti‐phospho‐HH3 antibody (Millipore, Billerica, MA, USA, #06‐570) was used at a dilution of 1:100, and either Alexa Fluor 488‐ or 596‐conjugated goat anti‐rabbit polyclonal antibodies were used as secondary antibodies. Nuclei were stained with Hoechst 33342 (Sigma, St. Louis, MO, USA). Fluorescence was visualized with an Opera Phenix system (PerkinElmer) and analyzed using Hermony image analysis software (PerkinElmer). Positive foci number (γH2AX) or cell number (pHH3) per cell were calculated as the total antibody‐positive number among the total Hoechst positive number. The median number of total Hoechst‐positive cells in average of nine embryos at each time point were 18,853 (range, 2,118–42,029) for γH2AX and 5,387.5 (range, 3,328–9,267) for pHH3 staining.
Real‐time PCR
A total of 10 irradiated or normal embryos were pooled for each time point and all experiments were repeated three times. Total RNA was extracted using TRIzol reagent (Molecular Research Center, Inc., Cincinnati, OH, USA). cDNA was generated using the PrimeScript RT reagent kit (Takara). Each reaction contained 500 ng RNA in a total volume of 10 μL. cDNA was used as a template for real‐time PCR using a SYBR Premix Ex Taq II kit (Takara) with a 7500 Real‐time PCR system (Bio‐Rad). The reaction involved an initial holding step at 95°C (30 s), followed by 40 cycles at 95°C (5 s) and 60°C (34 s). We quantified mRNA expression levels of DNA repair genes relevant for the following mechanisms: (i) homologous recombination (rad51b, rad51c, rad 54, braca2, and mre11a); (ii) non‐homologous end joining (ku70, ku80, xrcc4, and ligase 4); (iii) base excision repair (xrcc1 and ligase 3); (iv) DNA mismatch repair (msh2 and msh6); (v) nucleotide excision repair (xpc, ccnh, and xab2). Also evaluated were the levels of mRNA expression of genes relevant for the p53 signaling pathway:tp53, p21/waf1, ATM, and ataxia telangiectasia and Rad3‐related protein (ATR). The primers used are listed in Table 1. Real‐time PCR results were analyzed using the comparative ΔΔCt method and normalized against 18S ribosomal RNA as a reference gene (McCurley and Callard, 2008). As a negative control, real‐time PCR was carried out without a template. For repair‐related gene expression, the expression of each gene was normalized to the reference gene expression level at each embryo stage. p21 gene expression was normalized to the expression levels of control embryos at each timepoint after normalization to reference gene expression.
Table 1.
Primer sequences used for real‐time qPCR
| Gene name | Forward primer | Reverse primer |
|---|---|---|
| rad51B | TCTTCAACCTTCCTCAGGCT | TGAGCGTTTAGCTGACATCC |
| rad51C | TTCAACACTGCACCCTCCTA | GGTAAACCTCCGCTAACAGC |
| rad54 | GGTGTCGTCTTGATGGACAG | GGTTTAGTCCCACTCCTCCA |
| brca2 | GGCCTATCAGGCTTCTACTTTC | CTATTGGGCTTCTTCTCCATCC |
| mre11a | TGTGACCTTCGACGAGATTATG | TCTTGCGGGACGGTTTATT |
| Ku70 | AAGGATGAGGTGGATGAGATAAAG | TGAAGAGAGCAGGTCGTAAATG |
| Ku80 | GGCTGAGATTACAGAGGGAAAC | GCACGCTTGATCGAAAGAAAC |
| xrcc4 | GATGGAGCGTGATCGGTATG | GGTGTCAGCTGGAAGGTAAA |
| lig4 | TCGCATTGGATCAGGCTATAC | GGCTGCGGGAGGATTATTT |
| xrcc1 | GAGGATAAACCCATCCCTGAAC | CGATGATGTAGCGCAGAAGTAG |
| lig3 | GGAATCACAGGAGCAGAGTTT | CGTCTTCCAGTCCTTGTCATC |
| msh2 | ACGGTGCGAGTGTTTGATAG | CTGTTCCCTGAACCCAGATTT |
| msh6 | GGGAGGCAAATCCACTCTAATG | GACTCGATCCACAGGAGTAAGA |
| xpc | TGTTGCCCATAGGATGTGTT | TCACTGCTAAAGCGCAGTCT |
| ccnh | CCCATTGTTAGAGAACCCAGAG | GCAATCTGAGATGGAGAGAAGAG |
| xab2 | GACGATGCTCGCACTATCTT | CTCAGCTCCATCTCTCCATATTC |
| tp53 | GTACAAGTCCCTCCTGGAAATC | GGCAAATGCGTGTAAACAGTAA |
| p21 | CCAGAGACGACACCGTTTATT | GGAAGACTGAGGAATGGATCTTT |
| atm | CAGTGGAGGAGTGAGAAATGAG | CCACGTCTCCCAGAAGAATATC |
| atr | GCTGTGTCAAAGTCCTCCTATC | GTCTGTTGGCATCTCCGATAAA |
Generation of anti‐P21/WAF1 antibody
Rabbit polyclonal antibody against zebrafish P21/WAF1 peptides was generated by Cosmo Bio, Inc. P21 peptides (pos:140–154; CQAKKRLVATPRKSGQ) were also designed and generated by Cosmo Bio, Inc. based on the cDNA sequence of zebrafish cdkn1a. Immunization and purification of the anti‐P21/WAF1 rabbit serum was conducted by Cosmo Bio, Inc.
Western blotting
Irradiated or control embryos at 2, 4, and 6 hpf were collected in SDS sample buffer after 1 or 2 h of irradiation. Samples were separated on SuperSep Ace 5–20% gels (Wako Pure Chemicals, Osaka, Japan) at 20 mA and transferred onto polyvinylidene fluoride membranes (Bio‐Rad). Blotted membranes were blocked with blocking solution (4% skim milk, 1% Tween 20 in PBS) for 1 h, followed by incubation with the primary antibody in blocking solution at RT for 1 h or at 4°C overnight. The membranes were then washed three times with 1% Tween 20 in PBS (PBST) for 5 min, followed by incubation in secondary antibody solution (horseradish peroxidase‐conjugated goat anti‐rabbit IgG antibody at a dilution of 1:3,000; Zymed Laboratories, South San Francisco, CA, USA, 81‐6120 in blocking solution) for 30 min. After washing three times for 5 min with PBST, the membranes were developed using ECL Prime Western blotting detection reagent (Amersham Biosciences, Amersham, UK). Anti‐gamma tubulin (at a dilution of 1:3,000; GeneTex, GTX113286) and anti‐P21 (at a dilution of 1:2,000) were used as primary antibodies. Images were acquired using an Image Quant LAS 4000mini (GE Healthcare Japan Inc.).
Morpholino antisense oligo knockdown
The sequence of the p21 Morpholino antisense oligo was used as described by Sidi et al. (2008). Morpholino oligo (MO) was purchased from Gene Tools, Inc. (Philomath, OR, USA). The p21 morpholino oligo was used at 0.01 M and approximately 5 nL of MO was injected into the embryos. Injected embryos were collected for western blot analysis at 6 hpf.
Statistical analysis
Statistical data were analyzed with Prism 6 software (GraphPad, Inc., Chicago, IL, USA). Data shown are presented as the mean ± SD. * denotes a significant p value of < 0.05, ** denotes P‐value of <0.01, *** denotes P‐value of <0.001. The P values were determined by a two‐tailed unpaired student's t‐test with Welch's correction or one‐way analysis of variance (ANOVA) with Tukey's post‐test where appropriate. The range of expression levels in qPCR was determined from six independent experiments with three biological replicates by calculating the standard deviation of the ΔCt (Pfaffl, 2001).
Results
Effects of radiation on embryos differed according to developmental stages
First, to examine time‐dependent changes induced by irradiation of early‐stage embryos, we irradiated zebrafish embryos at 2 (64‐cell stage), 4 (blastula stage), and 6 (gastrula stage) hpf with 1, 5, and 10 Gy, respectively. At 24 hpf, the phenotypes of embryos irradiated at different times were variable. The most sensitive time‐point was found to be 2 hpf, as previously reported (Figure 1, Table 2; Supplementary materials, Figure S1 and Table S1) (McAleer et al., 2005; Geiger et al., 2006). When exposed to 10 Gy of radiation at 2 hpf, no embryos survived until 24 hpf (P < 0.001), whereas more than 84% of embryos survived in control. At an exposure level of 5 Gy of radiation, the survival rate was more than 51.8 %, although most embryos exhibited the shorter axis phenotype compare to control (P <0.001). With 1 Gy exposures, no morphological abnormalities were detected, except that the brain exhibited a gray color, which is the possibility of indicator of TUNEL positive cell death as was previously demonstrated (Bladen et al., 2005, 2007; Geiger et al., 2006). The severity of malformation in irradiated embryos was milder when the embryos were irradiated at later stages of development. For example, approximately 94% embryos exposed to 10 Gy of radiation at 4 or 6 hpf survived until 24 hpf (Praveen Kumar et al., 2017). These results suggest that the ability of cells to respond and survive following radiation exposure differs and changes quickly according to the developmental stage in zebrafish embryos.
Figure 1.
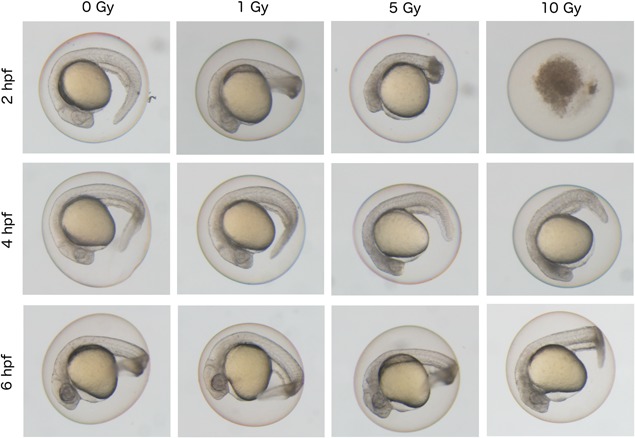
Differential effect of ionizing radiation on phenotypes of zebrafish embryos according to irradiated time‐points during early developmental stages. Zebrafish embryos at 24 hpf after irradiation at 2, 4, or 6 hpf with doses of 0, 1, 5, and 10 Gy of radiation. Non‐irradiated control embryos were shown in the left end panels (0 Gy). Whole embryos irradiated at 2 hpf (64‐cell stage) showed the most severe phenotype. At a dose of 10 Gy, embryos irradiated at 2 hpf were dead, whereas those at 4 hpf (blastula stage) or 6 hpf (gastrula stage) showed normal body shape.
Table 2.
Summary of effects of different doses of ionizing radiation on zebrafish embryos at 24 hpf after exposure to radiation at 2, 4, or 6 hpf
| Irradiated stages | Doses (Gy) | Survival at 24 hpf (%) | Body shape abnormality at 24 hpf (%) |
|---|---|---|---|
| 2 hpf (minimum no. of embryos = 12) | 0 | 84.9 | 12.5 |
| 1 | 89.7 | 12.2 | |
| 5 | 51.8 | 100*** (P = 0.0005) | |
| 10 | 0*** (P = 0.0008) | NC | |
| 4 hpf (minimum no. of embryos = 9) | 0 | 100 | 0 |
| 1 | 96.9 | 3.3 | |
| 5 | 88.8 | 31.6 | |
| 10 | 94.8 | 100*** (P = 0.0005) | |
| 6 hpf (minimum no. of embryos = 11) | 0 | 90.4 | 3.3 |
| 1 | 79.1 | 13.8 | |
| 5 | 95.5 | 2.7 | |
| 10 | 93.5 | 57.2* (P = 0.0498) |
Maternal transcripts include abundant varieties and quantities of DNA repair genes
Early embryos use mRNAs derived from the oocyte rather than producing mRNA through zygotic transcription (Lee et al., 2014; Paranjpe and Veenstra, 2015; Svoboda, 2018). A few hours after fertilization, these maternal mRNAs, also known as maternal factors, begin to degrade and the transcription of zygotic genes is initiated. This maternal–zygotic transition occurs at approximately 3 to 4 hpf in zebrafish. DNA repair may not function if DNA repair‐related genes are not present in the maternal transcripts. To evaluate whether DNA repair gene transcripts are found in zebrafish maternal factors, we examined the mRNA levels of various repair‐related genes at 0, 2, 4 hpf and 6 hpf compared them to reference gene. For double strand break repair mechanisms, we examined rad51b, rad51c, rad 54, braca2, and mre11a for homologous recombination and ku70, ku80, xrcc4, and ligase 4 for non‐homologous end joining. For single‐strand break repair mechanisms, we examined xrcc1 and ligase 3 for base excision repair, msh2 and msh6 for DNA mismatch repair, xpc, ccnh, and xab2 for nucleotide excision repair and tp53, p21/waf1, ATM, and ataxia telangiectasia and Rad3‐related protein (ATR) for the p53 signaling pathway.
Nearly all repair‐related genes, including that of ATM or ATR, were abundant at all stages in the developing embryos (Figure 2; for ATM, also see Imamura and Kishi, 2005). The only exceptions were p21/waf1 and p21, which were expressed at 4 and 6 hpf, although their mRNAs did not appear to be included in the maternal factors.
Figure 2.
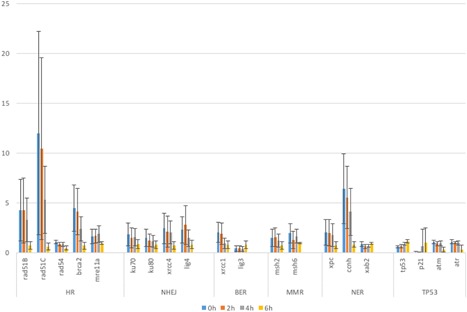
Expression levels of DNA repair‐related genes in zebrafish embryos during early developmental stages. mRNA expression levels of various DNA repair genes in zebrafish embryos were quantified by real‐time RT‐PCR and their relative‐fold expression at 0 hpf (blue bars), 2 hpf (orange bars), 4 hpf (gray bars), and 6 hpf (bright yellow bars) are shown in Y‐axis compared with the expression level of reference gene. Types of genes denoted in X‐axis are categorized as below: homologous recombination (HR)‐related genes (rad51B, rad51C, rad54, brca2, mre11a), non‐homologous end‐joining (NHEJ) repair‐related genes (ku70, ku80, xrcc4, lig4)base excision repair (BER)‐related genes (xrcc1, lig3), nucleotide excision repair (NER)‐related genes (xpc, ccnh, xab2), and p53 signaling (TP53)‐related genes (tp53, p21, atm, atr). The columns and error bars represent the mean and SD, respectively.
These results indicate that DNA repair‐related gene transcripts are present in zebrafish maternal factors.
Detection of double‐strand breaks in DNA using anti‐H2AX antibodies in very early‐stage embryos is not effective
The formation of γH2AX foci are among the very early changes observed in cells following DSBs in DNA. The number of γH2AX foci reflects the number of DSBs in the nuclei, which decrease as damage is repaired. When zebrafish embryos were irradiated at 6 hpf, the number of γH2AX foci peaked approximately at 30 min after irradiation and quickly decreased to background levels (Figure 3). In contrast, the number of γH2AX foci never exceeded 1 focus/nucleus when zebrafish embryos were irradiated at earlier timepoints (2 and 4 hpf). These observations suggest that the machinery for detecting damaged DNA may not be very efficient until developmental stages later than 4 hpf.
Figure 3.
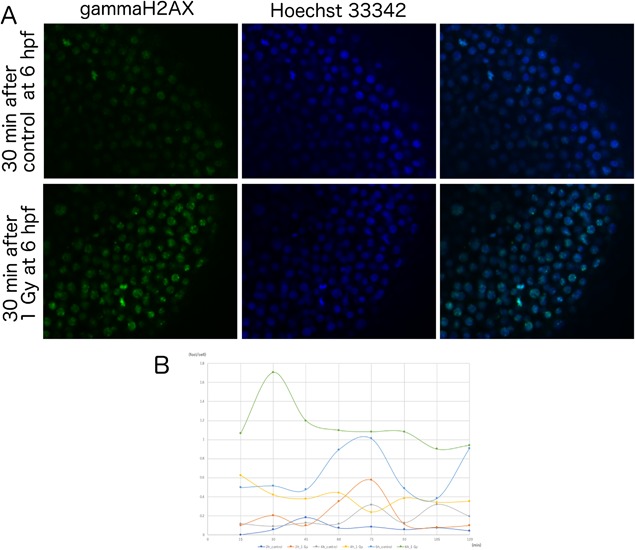
Formation of gamma‐H2AX foci in zebrafish embryos after irradiation at early developmental stages. (A) Fluorescein immunostaining images of anti‐γH2AX antibody (green, left panels), Hoechst 33342 (blue, middle panels) and merged images (right panels) in control embryos (upper panels) and 1 Gy‐irradiated embryos (lower panels) at 6 hpf. Irradiated samples showed γH2AX foci within nuclei, whereas controls have almost no γH2AX foci. (B) Embryos irradiated with 1 Gy at 2, 4, or 6 hpf were fixed at 15‐min intervals and stained with an anti‐γH2AX antibody. The numbers of foci per nuclei at each timepoint were plotted as: 2 hpf control (light blue) and 1 Gy irradiated (orange), 4 hpf control (yellow), and 1 Gy irradiated (blue) or 6 hpf control (dark blue) and 1 Gy irradiated (brown). Only the 6 hpf embryos irradiated at 1 Gy showed γH2AX foci formation. Foci numbers peaked at 30 min after irradiation.
Cell cycle arrest does not occur in early zebrafish embryos
Irradiation triggers cell cycle arrest to promote successful DNA repair in normal cells. We counted cells in M‐phase at 0, 1, 2, and 3 h after irradiation using anti‐phosphorylated histone H3 antibodies. Staining for phosphorylated histone H3 revealed that embryos irradiated at 6 hpf, but not at 4 hpf, show a reduction in M‐phase cells (Figure 4).
Figure 4.
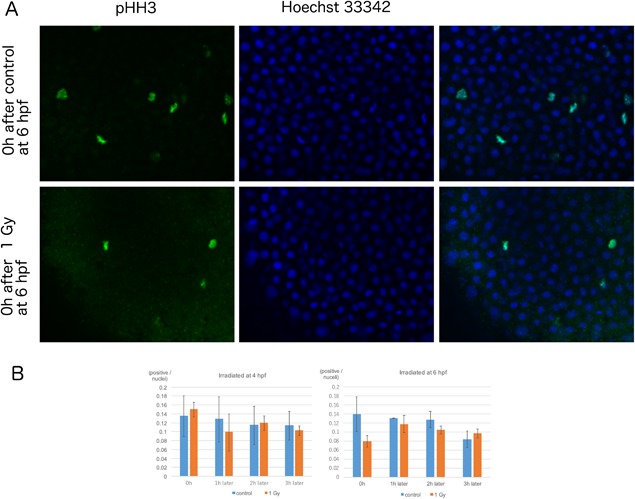
Effect of radiation exposure on M‐phase cells in zebrafish embryos at early developmental stages. (A) Immunostaining images of anti‐pHH3 antibody (green, left panels), Hoechst 33342 (blue, middle panels) and merged images (right panels) from embryos of 0 h after control (upper panels) or 1 Gy irradiation (lower panels) at 6 hpf. (B) Irradiated embryos were fixed at 1‐h intervals. The M‐phase marker, phospho‐histone H3 (phospho‐HH3) was detected by immunostaining; the ratio of positive cells/nuclei at each timepoint was calculated. In embryos irradiated at 4 hpf, the number of cells in M‐phase did not change compared to those in the control embryos (blue bars). However, cells irradiated at 6 hpf (orange bars) showed a reduction in M‐phase cells immediately after irradiation (P = 0.06). The columns and error bars represent the mean and SD, respectively.
This result, taken together with the temporal patterns of γH2AX foci formation, indicates that normal cellular responses to irradiation and DNA repair occur in zebrafish embryos at developmental timepoints of 6 hpf and later.
Early zebrafish embryos exhibit p21 mRNA responses to irradiation
Collectively, our results raised the question of whether cells in early zebrafish embryos (2 or 4 hpf) contain the molecular machinery necessary for inducing adaptive responses to irradiation. P21/WAF1 is a key downstream target of the proto‐oncogene product TP53, and upregulation of P21/WAF1 expression is a hallmark of activation of the p53 signaling pathway in response to irradiation (Langheinrich et al., 2002).
Unexpectedly, p21 mRNA levels were upregulated when the embryos were irradiated at 2 hpf compared to those in embryos irradiated at 4 or 6 hpf (Figure 5). In embryonic stem cells, it has been reported that p21 mRNA is upregulated when the expression levels of P21/WAF1 fail to increase after TP53 activation (Filion et al., 2009; Solozobova et al., 2009; Lee et al., 2010; Itahana et al., 2016). We first raised antibodies against the P21/WAF1 protein. This antibody recognized a protein of approximately 21 kD and the level of this protein was decreased in P21/WAF1 morpholino antisense oligo (P21 MO)‐injected embryos (Figure 6A), suggesting that this antibody recognized the zebrafish P21/WAF1 protein. We examined the levels of P21 protein expression in zebrafish embryos irradiated at 2, 4, and 6 hpf at timepoints of 1 and 2 h after irradiation using this antibody. Although these embryos constantly expressed P21/WAF1, the levels of this protein remained unaffected after irradiation at the 2 hpf developmental stage. In contrast, embryos irradiated at later developmental stages exhibited upregulated expression levels of P21/WAF1 (Figure 6B).
Figure 5.
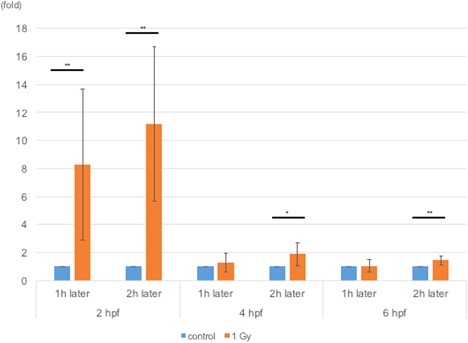
Levels of p21 mRNA expression in irradiated zebrafish embryos. RNA samples were extracted 1 or 2 h after irradiating the embryos at 2, 4, or 6 hpf. Levels of p21 mRNA were measured via qPCR and normalized to those in control embryos at each timepoint. At all timepoints, the levels of p21 mRNA were upregulated in irradiated embryos. At all timepoints, 1 and 2 h after 2 hpf (P = 0.008 and P = 0.001), 2 h after 4 hpf (P = 0.02), and 2 h after 6 hpf (P = 0.005) were significantly different compared to the control at each timepoint. Note that upregulation of p21 mRNA levels in embryos irradiated at 2 hpf were much higher than those irradiated at other time points. The columns and error bars represent the mean and SD, respectively.
Figure 6.
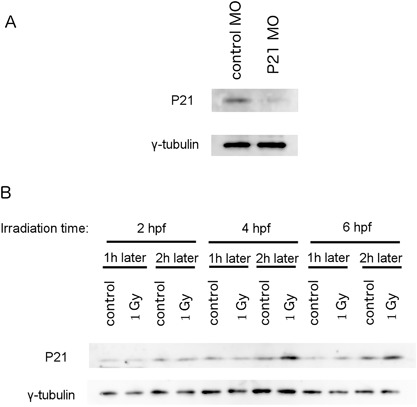
Levels of P21 protein expression in irradiated zebrafish embryos. A) Newly synthesized antibody recognized a protein between 20 and 25 kD in size. The amount of this protein decreased in the P21 antisense morpholino oligo (MO)‐injected embryos compared to embryos injected with standard control MO. B) Protein samples were obtained 1 or 2 h after irradiating embryos at 2, 4, or 6 hpf. In embryos irradiated at 6 hpf, the levels of P21/WAF1 protein were upregulated at 1 h after irradiation, whereas in embryos irradiated at 4 hpf, P21/WAF1 protein levels were upregulated only after 2 h.
Discussion
In this study, we found that cellular responses to ionizing radiation change quickly during the early periods of zebrafish development. Notably, irradiation did not induce γH2AX foci formation in embryos either at the 64‐cell stage (2 hpf) and blastula stage (4 hpf). Staining for phosphorylated histone H3 revealed that embryos irradiated at 6 hpf, but not at 4 hpf, show a reduction in M‐phase cells, consistent with previous findings that the cell number did not change significantly when zebrafish embryos at 6 hpf (after mid‐blastula transition) were treated with a DNA polymerase inhibitor (Ikegami et al., 1997).
However, the levels of p21 mRNA were upregulated in embryos irradiated at these stages, suggesting that the p53 signaling pathway was pre‐activated. In embryos exposed to radiation at the gastrula stages (6 hpf), the expression levels of P21/WAF1 protein were observed to be upregulated after irradiation. The increased expression of P21/WAF1 likely accounts for the induction of both γH2AX foci formation and cell cycle arrest at the gastrula stage. Importantly, increased P21/WAF1 expression levels were observed at only after 2 h after irradiation of the blastula. This result indicates the lack of induction of γH2AX foci formation or cell cycle arrest.
The morphological effects of radiation exposure were most prominent in embryos irradiated at 2 hpf (64‐cell stage). A likely explanation for this observation is that the activation of p53 signaling is inefficient because of the low accumulation of P21/WAF1 at this developmental stage, despite the upregulation of p21 mRNA levels. The cellular inability to accumulate P21/WAF1 compromises the cell cycle arrest pathway and can result in inefficient DNA repair. A similar phenomenon was also reported in mice and human embryonic stem cells (Filion et al., 2009; Solozobova et al., 2009; Lee et al., 2010; Itahana et al., 2016). In these cells, although p21 mRNA levels are upregulated, the synthesized P21/WAF1 protein degrades quickly, leading to insufficient accumulation of P21/WAF1, which may limit effective DNA repair responses to ionizing radiation in the early embryos of zebrafish.
Auto‐activated ATM phosphorylates H2AX and TP53 to subsequently activate both γH2AX foci formation and the p53 signaling pathway. It is not yet clear why 2 hpf embryonic cells are incapable of forming γH2AX foci despite activation of the p53 signaling pathway. Although ATM and ATR mRNA are present in cells in the early phases of embryonic development, ATM may not function properly until the gastrula stage. If this holds true, the p53 signaling pathway is likely to be activated by pathways other than those linked to ATM or ATR. Alternatively, sufficient numbers of H2AX subunits may not be present in early zebrafish embryos, although this is not the case in mice (Adiga et al., 2007; Nashun et al., 2010). However, γH2AX foci formation was delayed in one‐cell and two‐cell mouse embryos, despite the abundance of H2AX subunits (Adiga et al., 2007; Nashun et al., 2010). More detailed analyses are required to understand these phenomena.
It is known that in human or mice embryos, exposure to just 1 or 2 Gy of ionizing radiation is lethal. In contrast, zebrafish embryos are relatively resistant to radiation exposure, and the mechanisms underlying this resistance have been widely examined. Sussman (2007) has reported that the DNA repair capacity of zebrafish cells after exposure to UV radiation is much higher than that of human cells. Ku70 and Ku80 mRNAs are known to be present in the one‐cell stage in zebrafish embryos, and injections of MO for both Ku genes, together with irradiation at 6 hpf, yielded more apoptotic cells at 24 hpf than control embryos (Bladen et al., 2005, 2007). These observations suggest that non‐homologous end‐joining DNA repair plays an important role in zebrafish embryos, at least in the gastrula stage. Further studies are needed to evaluate the DSB repair capabilities of zebrafish embryos at different developmental stages.
Conclusions
Our observations in this study suggest that the cellular responses of zebrafish embryos to ionizing radiation change quickly over time in a manner dependent on early developmental stages. Regulation of P21/WAF1 protein stabilization appears to be a key factor in the response to genotoxin during early embryogenesis.
The machinery for detecting damaged DNA does not appear to function in embryos prior to 4 hpf. Increased levels of p21 mRNA in 0–2 hpf embryos after irradiation without a corresponding increase in P21 protein expression suggests the presence of other mechanisms that protect early embryos from TP53‐mediated apoptosis. Further studies are required to identify the exact molecular pathways that regulate cellular responses to radiation exposure in zebrafish embryos at early developmental stages.
Supporting information
Additional Supporting Information should be found in the online version of this article at the publisher's web‐site.
Figure S1. Images of pooled zebrafish embryos irradiated at 2, 4, or 6 hpf after irradiation with different doses (1, 5, and 10 Gy).
Figure S2. Repeated experiments of gamma‐H2AX foci measurements in embryos exposed to irradiation at 2, 4, or 6 hpf.
Figure S3. Repeated western blotting analysis of P21/WAF1 protein expression in zebrafish embryos irradiated at different developmental stages.
Table S1. Summary of effects of different doses of ionizing radiation on zebrafish embryos at 4 dpf after exposure to radiation at 2, 4, or 6 hpf
Acknowledgements and funding
We thank Dr. Megumi Sasatani, Dr. Hidehiko Kawai, and Dr. Elena Karamfilova Zaharieva for useful discussions. We would also like to thank Ryoko Matsumoto, Nanae Nakaju, Sachiko Fukumoto, and Masako Ninomiya for their excellent technical and secretarial assistance. This work was supported in part by a Grant‐in‐Aid from the Ministry of Education, Culture, Sports, Science, and Technology in Japan (#15K09453, #17H04264 and #26293277 to T.I., #18K116440 to Y.H.). This work was also supported by the Program of the network‐type Joint Usage/Research Disaster Medical Science of Hiroshima University, Nagasaki University, and Fukushima Medical University (Y.H. and T.I.) and Hiroshima University Research Promotion Award for Women Scientists (Y.H.). We also thank the Low‐Dose Radiation Effects Advanced Research Program of the Research Institute for Radiation Biology and Medicine, Hiroshima University, for funding and supporting this study.
Contributor Information
Yasuko Honjo, Email: yhonjo@hiroshima-u.ac.jp.
Tatsuo Ichinohe, Email: tatsuo.ichinohe@gmail.com.
References
- Adiga SK, Toyoshima M, Shimura T, Takeda J, Uematsu N, Niwa O (2007) Delayed and stage specific phosphorylation of H2AX during preimplantation development of γ‐irradiated mouse embryos. Reproduction 133(2): 415–22. [DOI] [PubMed] [Google Scholar]
- Anderson JA, Lewellyn AL, Maller JL (1997) Ionizing radiation induces apoptosis and elevates cyclin A1‐Cdk2 activity before but not after the midblastula transition in Xenopus. Mol Biol Cell 8(7): 1195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen CL, Lam WK, Dynan WS, Kozlowski DJ (2005) DNA damage response and Ku80 function in the vertebrate embryo. Nucleic Acids Res 33(9): 3002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen CL, Navarre S, Dynan WS, Kozlowski DJ (2007) Expression of the Ku70 subunit (XRCC6) and protection from low dose ionizing radiation during zebrafish embryogenesis. Neurosci Lett 422(2): 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlie A, Donovan A, Pratt SJ, Paw BH, Oates AC, Brugnara C, Witkowska HE, Sassa S, Zon LI (1998) Positional cloning of the zebrafish sauternes gene: a model for congenital sideroblastic anaemia. Nat Genet 20(3): 244–50. [DOI] [PubMed] [Google Scholar]
- Brugarolas J, Moberg K, Boyd SD, Taya Y, Jacks T, Lees JA (1999) Inhibition of cyclin‐dependent kinase 2 by p21 is necessary for retinoblastoma protein‐mediated G1 arrest after gamma‐irradiation. Proc Natl Acad Sci USA 96(3): 1002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis M, Cesari E, Nobili E, Straface G, Cavaliere AF, Caruso A (2007) Radiation effects on development. Birth Defects Res C Embryo Today Rev 81(3): 177–82. [DOI] [PubMed] [Google Scholar]
- Dooley K, Zon LI (2000) Zebrafish: a model system for the study of human disease. Curr Opin Genet Dev 10(3): 252–6. [DOI] [PubMed] [Google Scholar]
- Driever W, Fishman MC (1996) The zebrafish: heritable disorders in transparent embryos. J Clin Invest 97(8): 1788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion TM, Qiao M, Ghule PN, Mandeville M, van Wijnen AJ, Stein JL, Lian JB, Altieri DC, Stein GS (2009) Survival responses of human embryonic stem cells to DNA damage. J Cell Physiol 220(3): 586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnaire B, Cavalié I, Pereira S, Floriani M, Dubourg N, Camilleri V, Adam‐Guillermin C (2015) External gamma irradiation‐induced effects in early‐life stages of zebrafish, Danio rerio . Aquat Toxicol 169: 69–78. [DOI] [PubMed] [Google Scholar]
- Gartel AL, Serfas MS, Tyner AL (1996) P21‐negative regulator of the cell cycle. Proc Soc Exp Biol Med 213(2): 138–49. [DOI] [PubMed] [Google Scholar]
- Geiger GA, Parker SE, Beothy AP, Tucker JA, Mullins MC, Kao GD (2006) Zebrafish as a “biosensor”? Effects of ionizing radiation and amifostine on embryonic viability and development. Cancer Res 66(16): 8172–81. [DOI] [PubMed] [Google Scholar]
- Heyer BS, MacAuley A, Behrendtsen O, Werb Z (2000) Hypersensitivity to DNA damage leads to increased apoptosis during early mouse development. Genes Dev 14(16): 2072–84. [PMC free article] [PubMed] [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE (2005) Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci 86(1): 6–19. [DOI] [PubMed] [Google Scholar]
- Hirao A, Kong Y‐Y, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW (2000) DNA damage‐induced activation of p53 by the checkpoint kinase Chk2. Science, 287(5459): 1824–7. [DOI] [PubMed] [Google Scholar]
- Honjo Y, Kniss J, Eisen JS (2008) Neuregulin‐mediated ErbB3 signaling is required for formation of zebrafish dorsal root ganglion neurons. Development (Cambridge, England) 135(15): 2615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurem S, Martín LM, Brede DA, Skjerve E, Nourizadeh‐Lillabadi R, Lind OC, Christensen T, Berg V, Teien H, Salbu B, Oughton DH, Aleström P, Lyche JL (2017) Dose‐dependent effects of gamma radiation on the early zebrafish development and gene expression. PLoS ONE 12(6): e0179259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang M, Yong C, Moretti L, Lu B (2007) Zebrafish as a Model System to Screen Radiation Modifiers. Curr Genomics 8(6): 360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami R, Rivera‐Bennetts AK, Brooker DL, Yager TD (1997) Effect of inhibitors of DNA replication on early zebrafish embryos: evidence for coordinate activation of multiple intrinsic cell‐cycle checkpoints at the mid‐blastula transition. Zygote 5(02): 153–75. [DOI] [PubMed] [Google Scholar]
- Imamura S, Kishi S (2005) Molecular cloning and functional characterization of zebrafish ATM. Int J Biochem Cell Biol 37(5): 1105–16. [DOI] [PubMed] [Google Scholar]
- Itahana Y, Zhang J, Göke J, Vardy LA, Han R, Iwamoto K, Cukuroglu E, Robson P, Pouladi MA, Colman A, Itahana K (2016) Histone modifications and p53 binding poise the p21 promoter for activation in human embryonic stem cells. Sci Rep 6(1): 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203(3): 253–310. [DOI] [PubMed] [Google Scholar]
- Langheinrich U, Hennen E, Stott G, Vacun G (2002) Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling. Curr Biol 12(23): 2023–8. [DOI] [PubMed] [Google Scholar]
- Lee K‐H, Li M, Michalowski AM, Zhang X, Liao H, Chen L, Xu Y, Wu X, Huang J (2010) A genomewide study identifies the Wnt signaling pathway as a major target of p53 in murine embryonic stem cells. Proc Natl Acad Sci 107(1): 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MT, Bonneau AR, Giraldez AJ (2014) Zygotic genome activation during the maternal‐to‐zygotic transition. Annu Rev Cell Dev Biol 30(1): 581–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossaint G, Besnard E, Fisher D, Piette J, Dulić V (2011) Chk1 is dispensable for G2 arrest in response to sustained DNA damage when the ATM/p53/p21 pathway is functional. Oncogene 30(41): 4261–74. [DOI] [PubMed] [Google Scholar]
- MacRae CA, Peterson RT (2015) Zebrafish as tools for drug discovery. Nat Rev Drug Discov 14(10): 721–31. [DOI] [PubMed] [Google Scholar]
- McAleer MF, Davidson C, Davidson WR, Yentzer B, Farber SA, Rodeck U, Dicker AP (2005) Novel use of zebrafish as a vertebrate model to screen radiation protectors and sensitizers. Int J Radiat Oncol Biol Phys 61(1): 10–3. [DOI] [PubMed] [Google Scholar]
- Nashun B, Yukawa M, Liu H, Akiyama T, Aoki F (2010) Changes in the nuclear deposition of histone H2A variants during pre‐implantation development in mice. Development 137(22): 3785–94. [DOI] [PubMed] [Google Scholar]
- Negron JF, Lockshin RA (2004) Activation of apoptosis and caspase‐3 in zebrafish early gastrulae. Dev Dyn 231(1): 161–70. [DOI] [PubMed] [Google Scholar]
- Paranjpe SS, Veenstra GJC (2015) Establishing pluripotency in early development. Biochim Biophys Acta 1849(6): 626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegri F (2003) Maternal factors in zebrafish development. Dev Dyn 228(3): 535–54. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW (2001) A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Res 29(9): e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praveen Kumar MK, Shyama SK, Kashif S, Dubey SK, Avelyno D, Sonaye BH, Samit K, Chaubey RC (2017) Effects of gamma radiation on the early developmental stages of zebrafish (Danio rerio). Ecotoxicol Environ Saf 142(Supplement C): 95–101. [DOI] [PubMed] [Google Scholar]
- Price BD, D'Andrea AD (2013) Chromatin remodeling at DNA double‐strand breaks. Cell 152(6): 1344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque T, Haton C, Etienne O, Chicheportiche A, Rousseau L, Martin L, Mouthon M, Boussin FD (2012) Lack of a p21 waf1/cip‐dependent G1/S checkpoint in neural stem and progenitor cells after DNA damage in vivo. Stem Cells 30(3): 537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LB, Russell WL (1954) An analysis of the changing radiation response of the developing mouse embryo. J Cell Comp Physiol 43(S1): 103–49. [DOI] [PubMed] [Google Scholar]
- Sidi S, Sanda T, Kennedy RD, Hagen AT, Jette CA, Hoffmans R, Pascual J, Imamura S, Kishi S, Amatruda JF, Kanki JP, Green DR, D'Andrea AA, Look AT (2008) Chk1 suppresses a caspase‐2 apoptotic response to DNA damage that bypasses p53, Bcl‐2, and caspase‐3. Cell 133(5): 864–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeenk G, van Attikum H (2013) The chromatin response to DNA breaks: leaving a mark on genome integrity. Annu Rev Biochem 82(1): 55–80. [DOI] [PubMed] [Google Scholar]
- Solozobova V, Rolletschek A, Blattner C (2009) Nuclear accumulation and activation of p53 in embryonic stem cells after DNA damage. BMC Cell Biol 10: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman R (2007) DNA repair capacity of zebrafish. Proc Natl Acad Sci 104(33): 13379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda P (2018) Mammalian zygotic genome activation. Semin Cell Dev Biol 84: 118–26. [DOI] [PubMed] [Google Scholar]
- Valentin J (2003) Biological effects after prenatal irradiation (embryo and fetus) ICRP Publication 90 approved by the Commission in October 2002. Ann ICRP 33(1–2): 1–206. [PubMed] [Google Scholar]
- Wang YA, Elson A, Leder P (1997) Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm‐deficient mice. Proc Natl Acad Sci 94(26): 14590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AC, Lieschke GJ (2002) The zebrafish as a model system for human disease. Front Biosci 7: d827–833. [DOI] [PubMed] [Google Scholar]
- Watts F (2016) Repair of DNA double‐strand breaks in heterochromatin. Biomolecules 6(4): 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukawa M, Oda S, Mitani H, Nagata M, Aoki F (2007) Deficiency in the response to DNA double‐strand breaks in mouse early preimplantation embryos. Biochem Biophys Res Commun 358(2): 578–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information should be found in the online version of this article at the publisher's web‐site.
Figure S1. Images of pooled zebrafish embryos irradiated at 2, 4, or 6 hpf after irradiation with different doses (1, 5, and 10 Gy).
Figure S2. Repeated experiments of gamma‐H2AX foci measurements in embryos exposed to irradiation at 2, 4, or 6 hpf.
Figure S3. Repeated western blotting analysis of P21/WAF1 protein expression in zebrafish embryos irradiated at different developmental stages.
Table S1. Summary of effects of different doses of ionizing radiation on zebrafish embryos at 4 dpf after exposure to radiation at 2, 4, or 6 hpf


