Abstract
The aim of this study was to develop an observational metric that could be used to assess the performance of a practitioner in completing an acute surgical wound‐dressing procedure using aseptic non‐touch technique (ANTT). A team of clinicians, academics, and researchers came together to develop an observational metric using an iterative six‐stage process, culminating in a Delphi panel meeting. A scoping review of the literature provided a background empirical perspective relating to wound‐dressing procedure performance. Video recordings of acute surgical wound‐dressing procedures performed by nurses in clinical (n = 11) and simulated (n = 3) settings were viewed repeatedly and were iteratively deconstructed by the metric development group. This facilitated the identification of the discrete component steps, potential errors, and sentinel (serious) errors, which characterise a wound dressing procedure and formed part of the observational metric. The ANTT wound‐dressing observational metric was stress tested for clarity, the ability to be scored, and interrater reliability, calculated during a further phase of video analysis. The metric was then subjected to a process of cyclical evaluation by a Delphi panel (n = 21) to obtain face and content validity of the metric. The Delphi panel deliberation verified the face and content validity of the metric. The final metric has three phases, 31 individual steps, 18 errors, and 27 sentinel errors. The metric is a tool that identifies the standard to be attained in the performance of acute surgical wound dressings. It can be used as both an adjunct to an educational programme and as a tool to assess a practitioner's performance of a wound‐dressing procedure in both simulated and clinical practice contexts.
Keywords: aseptic non‐touch technique (ANTT), metric development, proficiency‐based progression training, surgical wound‐dressing procedure
1. INTRODUCTION
Every day, approximately 81 000 people acquire a health care‐associated infection (HCAI) in hospitals across Europe.1 Surgical site infections (SSIs) are one of the most common types of HCAI, representing up to 20% of this patient group. Health care professionals caring for patients with surgical wounds need specialist expertise, knowledge, and skills to ensure optimum evidence‐based wound care.2 However, standardised education for health care professionals performing surgical wound dressings is lacking.3
The association between microorganisms in the clinical environment and HCAIs is well accepted.4 A substantial number of surgical site infections are caused by microorganism contamination during and after invasive clinical procedures through surgically initiated breaks in the skin.5 It is estimated that nearly 50% of surgical site infections are preventable by following evidence‐based guidelines.6, 7 SSIs are burdensome on individuals, their families, and on the health services.8, 9, 10 .Each SSI is associated with approximately 7 to 11 additional postoperative hospital days, and patients with SSI have a 2 to 11 times higher risk of death compared with operative patients without an SSI.11
Given that SSIs are acutely problematic and preventable, it is important that health care professionals caring for wounds exercise strict asepsis in order to minimise their incidence.2 Indeed, the use of aseptic technique for changing or removing wound dressings is one of the most important components of wound management given that a break in aseptic non‐touch technique (ANTT) potentially causes the introduction of exogenous microbes, thereby contaminating the wound.12
1.1. Aseptic non‐touch technique
“Aseptic non‐touch technique” is a term applied to a set of specific practices used to ensure asepsis and prevent the transfer of potential pathogens to a susceptible site on the body (eg, an open wound or insertion site for an invasive medical device) or to sterile equipment/devices.13 Non‐touch (of key parts and key sites) is an integral component of ANTT and is the cornerstone of recommended best practices during surgical procedures.12 The National Institute Clinical Effectiveness (NICE) recommends the use of ANTT for changing or removing surgical wound dressings and the use of sterile saline for wound cleansing up to 48 hours after surgery. The National evidence‐based wound management guidelines, 2009 recommend using an aseptic wound technique when the individual is immuno‐compromised or if the wound is located in a sterile body cavity, for example, nephrostomy or central venous line,14 whilst the Australian National Safety and Quality Health Service Standards recommend that health care professionals be trained in ANTT, that compliance with ANTT is audited, and that actions are taken to increase compliance with ANTT.15 Practical evidence‐based recommendations published to assist acute care hospitals in preventing SSIs include the recommendation to “evaluate wound care practices”.11 In relation to the type of dressings used, a Cochrane review found no clear evidence that one dressing type was better than any other or that covering wounds with any dressing at all reduced the risk of SSIs.16 However, the review authors noted that the number of studies was very small and that the level of evidence was low or very low and recommended that decisions relating to wound dressings be based on patient and clinician preferences and dressing costs.
Despite substantial focus being placed on the importance of the correct performance of aseptic technique,5, 12 it appears that achieving the correct technique is problematic in clinical practice.5, 17 Lack of provision of feedback to health care professionals regarding HCAI prevention strategies, such as ANTT, may be a contributory factor.3 It may be that the procedural elements of ANTT are unclear and would benefit from closer analysis and examination to ensure that health care professionals are cognisant of the exact steps to be taken in the use of the non‐touch technique.
Optimal performance of the aseptic technique relies on effective staff training in infection control, safe environments, and equipment that is fit for purpose.18 Given that ANTT is used in the performance of most acute surgical wound dressings in hospital wards, more research is required to develop a valid metric, which can be used to train practitioners to become proficient in performing ANTT and to audit their practice.
1.2. Proficiency‐based progression training
There have been public concerns in recent years regarding the skills of some health care professionals, with many high‐profile cases linked to the suboptimal performance of practitioners.19, 20 In addition, there has been public discourse around the fact that trainees in some disciplines are graduating with considerably less clinical experience than might have been the case historically. This has prompted a change in the pedagogical approach taken to the teaching of high‐volume and high‐risk technical and non‐technical skills. It appears that providing trainees with precise feedback on their performance and with specific recommendations for improvement proximate to their performance is the optimal training style.21, 22 This approach to training is referred to as proficiency‐based progression training (PBPT).23 It recommends that trainees be provided with a quantitative performance benchmark to work towards, and this benchmark should be a valid representation of the procedure.22 PBPT requires learners to practice until a performance benchmark is unambiguously demonstrated. Feedback using metrics that clearly define performance criteria and delineate deviation from optimal performance is a key element of PBPT. This approach relies on a comprehensive and quantitative characterisation of a skill to be learned for a certain procedure. In the present study, the procedure is acute surgical wound dressings. The performance characteristics, or “metrics”, and their “operational definitions” offer very specific goals and guidance, which can be used as part of a training curriculum for trainee health care professionals.
Many health care professionals perform aseptic techniques, but, with time, their actions may become automated, and it is difficult for trainees to pick up the level of intricate detail needed to become “proficient” in their performance. It is challenging to identify the key features that characterise optimum performance given that health care professionals rarely think about performing the aseptic technique in micro detail. The units of performance that constitute a skill can be quantified with a “task analysis” or breakdown of the key steps and actions imperative for the procedure. The steps then need to be operationally defined and not simply described. The definition or “metric” for a specific procedure provides a standard of measurement that can be used to objectively assess performance. Thus, this study set about objectively developing such a metric for ANTT wound‐dressing procedures.
The primary aim of the present study was to develop an observational metric to assess the performance of a practitioner in completing an acute surgical wound‐dressing procedure using ANTT. The secondary aim was to validate the appropriateness of the metric steps, errors, and sentinel errors identified within the metric.
2. MATERIALS AND METHODS
2.1. Study Design
A team of clinicians, academics, and researchers came together to develop an observational metric using an iterative six‐stage process, culminating in a Delphi panel meeting. The developmental process was underpinned by PBPT principles (Figure 1).
Figure 1.
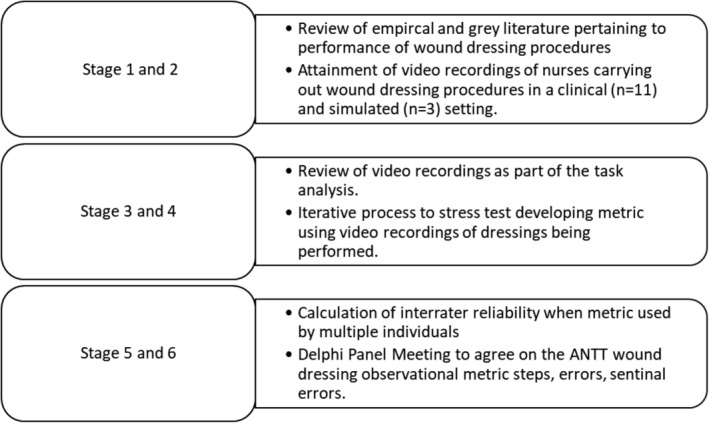
The study followed a number of distinct stages as outlined in this graphic
2.2. Scoping review of literature
EBSCO (CINAHL with full text and Medline) and Cochrane were searched using a combination of search terms divided into two main concepts: Aseptic non‐touch technique (aseptic, “aseptic technique”, “aseptic non‐touch technique,” ANTT, dressing, “clean technique”, “sterile technique”, sterile) AND wound (wound, “wound care”, “wound infection”, “wound management”, “surgical wound infection”, “surgical site infection”, “surgical wound care”, incision, drain, suture, staple). The search was extended to include literature pertaining to central venous catheters as well (“venous catheter”, “central line”, “peripherally inserted central catheter”, PICC, Hickman). Each concept was searched as a group and then as a combination with the Boolean terms “OR” and “AND”, respectively. Reference lists of included papers were reviewed.
Inclusion criteria were papers that: were published within the timeframe of January 2006 to January 2017, were written in English, and presented primary empirical data or were structured reviews. Papers were included if they presented an analysis that (a) focused on the processes for preventing contamination of wounds during a wound‐dressing procedure in adult patients in the acute care setting, (b) examined the clinical effectiveness (harm/benefit) of ANTT, (c) evaluated the effectiveness of an ANTT educational programme in the context of dressing a wound or central line, and (d) empirically derived the steps involved in performing an acute surgical wound dressing. The team also reviewed manufacturer guidelines for various wound‐dressing products.
Retrieved search hits from EBSCO (n = 5993) and from Cochrane (n = 82) were exported to EndNote (version 7) where duplicates (n = 1893) were identified and removed. All remaining papers from both searches (n = 4182) were uploaded to “Covidence Online.” Title and abstract screening identified 442 papers for full‐text screening, with 7 papers being deemed eligible for inclusion. Selected papers underwent a full‐text assessment (JH, DD, VH), and eligibility issues were resolved with a third author (SC).
2.3. Procedure for obtaining videos of wound dressings being performed
Nurses working in areas where they routinely performed wound dressings self‐selected to partake in the study. A GoPro camera was mounted on the forehead of nurses while performing an acute surgical wound‐dressing procedure. The nurses were given directions to start the procedure from the very beginning, progressing through the procedure to the disposal of waste and performance of hand hygiene as per their normal nursing daily routine. The nurses were not interrupted or guided during the procedure. Video recordings were stored on a secure university server that was only accessible to select members of the research team (n = 3). A further three Additional recordings were attained using mannequins in the simulated context from three experienced nurses with specialist expertise in performing wound dressings. Attaining recordings from nurses with a variety of experiences in performing wound dressings ensured variation in the level of experience of those performing the procedures and enhanced the researchers’ understanding of the nuanced variation in the performance of the procedure.
2.4. Task analysis and interrater reliability
Key terms associated with the development of the metric are identified in Table 1. Video recordings of acute surgical wound‐dressing procedures performed by nurses in clinical (n = 11) and simulated (n = 3) settings were viewed repeatedly and iteratively. Team members conducted a “task analysis”, deconstructing the procedure into a series of procedural “steps.” Procedural potential “errors” and “sentinel errors” were also identified. During the process of iterative reviewing of the videos, each metric step was identified, and the step was defined to include both a beginning and endpoint. Such a precise definition of each step allowed for unambiguous scoring of the step as having either occurred or not occurred, with a high degree of reliability. The observational metric included both the specific operative steps and the general order in which the steps should be accomplished. Procedural phases were specified for groups of related steps. The task analysis and initial stress testing of the developed metric (stages 3 and 4 [Figure 1)] took 2.5 days for the team to complete (20 hours). After the primary members of the metric development group were satisfied that the entirety of the procedure had been characterised, the metric was then stress tested so that inter‐rater reliability of the metric could be scored. Two members of the team watched videos in silence a maximum of three times before calculating the level of agreement between raters when scoring a nurse's performance when doing a wound dressing procedure. The process of independent viewing, scoring, and revising the step and error metrics was continued until the metrics group was satisfied that the metric accurately characterised the procedural steps of an acute surgical wound‐dressing procedure with a consistent inter‐rater reliability ≥0.85. Once the team was satisfied with the metric, it was brought to a Delphi panel meeting (Stage 6, Figure 1).
Table 1.
| Glossary | Definition |
|---|---|
| Step (S) | A component task or defined unit of behaviour, the series aggregate of which constitutes the completion of a specific procedure |
| Error (E) | A deviation from optimal performance |
| SE | Is an event or occurrence involving a serious deviation from optimal performance defined by events that, by themselves, could lead to any of the following: contamination of the wound; surgical site infection; risk of health care professional exposure to bodily fluid, or broader risk for hospital acquired infection |
| Wound | The wound in this context is the surgical wound. The wound boundaries are defined as the area encompassing the staple or suture insertion points, incision line of wound and/or perimeter of wound drain |
| Aim of aseptic technique | Asepsis: is the prevention of the transfer of pathogenic microorganisms to the wound. In the context of the wound dressing this is the set of procedures performed which seek to stop the direct transfer of microorganisms to the wound through contact with other surfaces, equipment or persons (health care worker or patient). |
| Aseptic field | A designated aseptic working area that contains the equipment needed for the procedure and the external surface of the sterile gloves |
| Violation of aseptic non‐touch technique | The asepsis of “key‐parts” and “key‐sites” were not fully protected during the wound dressing as they were contaminated through clear and explicit contact with contaminated surface(s), equipment or persons (patient or health care professional). Non‐touch technique also involves the avoidance of touching key parts and key sites with gloved hand |
| Key site | Is the wound incision/site |
| Key part | Are the surfaces that come in direct contact with the wound |
| Violation of aseptic dressing field |
That is a clear and explicit contamination of either the upward facing surface of the opened dressing pack or external surface of sterile gloves or forceps if used to touch a key part or key site |
| Contamination | Occurs by contact with other contaminated surfaces, equipment or persons (patient or health care professional) |
2.5. Delphi panel meeting
The development of the Delphi method has been attributed to Dalkey and Helmer,24 although the conceptual roots can be traced further back in time.25 The pivotal components of this type of research are the identification of a panel of experts and use of a multi‐stage iterative approach, with each stage building on the results of the previous, coming closer to the desired result as the number of iterations increase.22, 25 In the case of the ANTT wound‐dressing observational metric, the desired result was consensus on the appropriateness of the metric in its definition of steps, errors, and sentinel errors. This consensus was acquired by means of repeated cycles of questioning; deliberation; modification of the metric; and ultimate voting on the definitions, steps, and errors. Twenty‐one members of the Delphi panel attended the formal meeting. The meeting began with a presentation that introduced the Delphi panel to the metric and the objectives and goals of the meeting and an overview of ANTT, acute surgical wound‐dressing procedures, PBPT, and any other definitions and components important to understanding the metric (Table 1).
An affirmative vote by a Delphi panel member indicated that the metric steps presented were accurate and acceptable as written but not necessarily that it was the manner in which that particular panellist might have chosen to complete the steps. This was in recognition that slightly different approaches to acute surgical wound‐dressing procedures are taken in different settings. “Consensus” meant that all Delphi panel members agreed with the definition of phases, steps, and that they were “not wrong” (pg.1434)21 while recognising that individuals may have personal preferences for the sequencing of particular steps.
Each of the phases were broken down into their steps and errors and evaluated individually by the panel. The panel had a copy of the metric and were facilitated to read through each step as it was discussed. If consensus could not be reached or there was disagreement, the metric was modified according to feedback using an onscreen version of the metric, and a new vote on the acceptability of the modified metric was carried out. This meant that, throughout the presentation of the steps, any modifications that were suggested could be altered immediately, and so, the final modifications could be seen, saved, and then voted on by all attendees. This process was repeated until each phase had been voted on.
2.6. Ethics
Ethical approval was received from the local Clinical Research Ethics Committee, and approval was given to enter the hospitals. Potential participants were given full information about the study and assured of anonymity. Written consent was obtained from both nurses and patients in the case of clinically based observations.
2.7. Statistical analysis
The inter‐rater reliability was expressed by Cohen's Kappa (κ) for each item (step, error, and sentinel error) separately.26 Reliability κ scores ≥0.85 were considered appropriate for this study.
The developed metric was scored for (a) number of steps observed/total number of steps, (b) number of errors observed/total number of potential errors, and (c) number of errors observed/total number of potential sentinel errors.
3. RESULTS
3.1. Review of literature
A number of relevant papers were sourced (n = 13).3, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 The team also reviewed manufacturer guidelines (n = 3) for various wound‐dressing products and the Royal Marsden Procedural Manuel.18 This information described how the development process should begin and what areas of the procedure the metric should focus on but did not supply the team with a tool that could be adapted.
The papers provided background perspectives on the potential effectiveness of ANTT in the provision of care to surgical patients. However, there was a lack of high‐quality empirical evidence on the actual measured benefits or harms of using ANTT generally or in the performance of acute surgical wound‐dressing procedures (ie, in reducing mortality, hospital acquired infection rates, and surgical site infections) in the reviewed literature. The papers reviewed shared a common theme, which is the lack of empirically derived and tested education and training initiatives surrounding the implementation of ANTT for acute surgical wound‐dressing procedures in the clinical settings. In addition, the wound‐dressing requirements for acute surgical wounds were not differentiated according to patient risk categories, types of surgery (elective, emergency), operative time, size of wound, presence/absence of drains, or surgery type involving entry to larger body cavities (eg, abdominal and thoracic cavities). The focus within the reviewed papers was predominantly on the uncomplicated clean surgical wound healing by primary intention.
3.2. Task analysis and inter‐rater reliability
The videos of nurses performing wound dressings were reviewed iteratively. The majority of the video recordings showed dressing changes of wounds that were relatively large (>13 cm), often included a port entry for a drain, and involved the care of patients with complex comorbidities.
A group of experts from academic (n = 8) and clinical (n = 4) fields reviewed past literature, the evidence pertaining to the steps involved in and performance of an acute surgical wound dressing procedure, and the videos of practitioners performing the wound dressings.
The created metric was broken down into three phases, and each phase contained a series of related, explicitly defined, and observable steps. Each step had a clearly marked beginning point and endpoint. All steps, errors, and sentinel errors that occur throughout the metric are outlined in Tables 2 and 3.
Table 2.
Three phases of acute surgical wound dressing and abridged summary of the steps within the procedure post‐Delphi process
| Phase I | |
| 1. | Disinfect top shelf of dressing trolley with alcohol wipe(s) |
| 2. | Disinfect bottom shelf of dressing trolley with alcohol wipe(s) |
| 3. | Disinfect all the legs of trolley in turn with alcohol wipe(s) |
| 4. | Dispose of all used alcohol wipe(s) |
| 5. | Gather basic equipment/products for wound dressing |
| 6. | Check expiry dates of solutions |
| 7. | Performs hand hygiene (before touching patient) |
| Phase II | |
| 8. | Don apron (before exposure of wound) |
| 9. | Expose dressing area |
| 10. | Performs hand hygiene (before clean aseptic procedure) |
| 11. | Open the dressing pack and lay it flat to create a critical aseptic field on the top shelf of trolley |
| 12. | Pick up non‐clinical waste bag |
| 13. | Pick up clinical waste bag |
| 14. | Pour pertinent solutions into liquid compartments of dressing tray |
| 15. | Open equipment items using non‐touch technique and place items of equipment needed for dressing on critical aseptic field |
| 16. | Remove the soiled dressing |
| 17. | Place soiled dressing in clinical waste bag |
| 18. | Performs hand hygiene (after body fluid exposure risk and before dressing procedure) |
| 19. | Don sterile gloves using non‐touch technique |
| 20. | Dispose of sterile glove wrapper |
| 21. | Place sterile drape adjacent to/under wound |
| 22. | Clean wound (if required) |
| 23. | Dispose of each used swab into the clinical waste bag |
| 24. | Apply new sterile dressing |
| Phase III | |
| 25. | Remove gloves at the bedside and dispose |
| 26. | Perform hand hygiene immediately after removing gloves |
| 27. | Remove apron and dispose in waste bag |
| 27. | Dispose of non‐clinical waste |
| 29. | Dispose of clinical waste |
| 30. | Perform hand hygiene (after touching patient/patient surroundings and exposure to bodily fluid) |
| 31. | Decontaminate and clean trolley |
Table 3.
Summary of metric errors (E) and sentinel errors (SE)
| 1. | Failure to use stainless steel trolley with two shelves. (E) |
| 2. | Failure to use visibly clean trolley. (E) |
| 3. | Did not gather equipment in advance of procedure (E) |
| 4. | Failure to perform hand hygiene. (SE) |
| 5. | Incorrect hand hygiene. (E) |
| 6. | Apron not put on. (E) |
| 7. | Violation of aseptic non‐touch technique. (SE) |
| 8. | Violation of aseptic dressing field. (E) |
| 9. | Used bare hands to remove dressing. (SE) |
| 10. | Failure to remain within the boundaries of the wound when cleaning wound. (SE) |
| 11. | Swab used for more than one cleaning wipe/motion. (SE) |
| 12. | If cleaning peri‐wound area, violation of aseptic non‐touch technique observed. (SE) |
| 13. | Crossed over the critical aseptic field whilst holding a used swab. (E) |
| 14. | Failure to drop the used swab into the clinical waste bag. (E) |
| 15. | At any point during phase II of the procedure the practitioner leaves the patient. (E) |
| 16. | Wound is exposed for any period (over and above procedural requirements) prior to the application a new dressing (e.g. ward round facilitated during dressing procedure, awaiting review by another health care professional, equipment not available). (E) |
| 17. | Failure to remove the gloves at the bedside. (E) |
| 18. | Failure to remove the apron at the bedside. (E) |
| 19. | Steps (glove removal, hand hygiene, apron removal) to be completed in the order stipulated. (E) |
| 20. | Failure to dispose of non‐clinical waste materials into a non‐clinical waste bag. (E) |
| 21. | Failure to place contaminated materials in the clinical waste bag at bedside. (SE) |
3.3. Delphi panel meeting
During the modified Delphi panel, all phases of the procedure were accepted. The alterations that occurred during the panel meeting were documented and applied to the metric. Changes included: replacement of the word “clean” trolley with “disinfect” trolley surfaces; clarifications regarding the term “critical aseptic field”; the application of the WHO five moments for hand hygiene;39 and the sequence of removing an apron, gloves at the bed side. After the Delphi panel meeting, consensus was reached for three phases, 31 steps, 18 errors (13 unique errors), and 27 sentinel errors (7 unique sentinel errors) within the observational metric. Some errors could be observed repeatedly, for example, incorrect hand hygiene technique (Table 2 and 3).
Notably, other procedural requirements relating to the preparation of the patient, consent, health care professional, environment and assessment of a wound, and matching the wound requirements with appropriate dressings are part of a broader educational programme and are difficult to observe objectively within an observational metric.
4. DISCUSSION
The aim of this study was to develop an observational metric to assess the performance of a practitioner in completing an acute surgical wound‐dressing procedure using ANTT and to validate the appropriateness of the metric steps, errors, and sentinel errors identified within the metric.
Results showed that an acute surgical wound dressing can be deconstructed into the essential procedural phases and steps; the potential errors related to the procedure can be identified and characterised; and face and content validity for the resulting step and error metrics can be obtained through use of the modified Delphi panel technique.
Clare and Rowley40 demonstrated that standardising aseptic technique for invasive IV procedures using the ANTT—Clinical Practice Framework (CPF), increased staff mean compliance with hand hygiene, glove use, key‐part protection, use of a non‐touch technique, key‐part cleaning, and aseptic field management (P ≤ 0.001).40 Thus, having validated metrics are essential in defining optimum standards of performance.41 The methodology used in this study provides a framework for the development of such metrics and standards.22 Metrics, thus, not only define how training should be characterised and procedures performed by the student or practitioner but also afford the opportunity for meaningful assessment of performance and progress.41
The metric provides a step‐by‐step guide underpinning the procedure of an acute surgical wound dressing whether carried out by a novice or expert. The metric is a tool that: reflects the standard to be attained in the performance of acute surgical wound dressings; supports future educational programmes relating to wound‐dressing procedures; assesses the impact of educational programmes relating to wound‐dressing procedures; and can be used to audit procedure performance in clinical practice. However, there is a need for training in the use of a metric to ensure consistency in its application.
Key challenges encountered while doing this research included: technical difficulties with the Go Pro camera and keeping the camera charged while in use. There were many variations in the performance of acute surgical wound dressings observed relating to the use/non‐use of non‐sterile gloves, use of a clean and dirty hand technique, and double gloving. Some variations in clinical practice were attributable to local preferences for dressings after different types of surgeries, differences in the content, and arrangement of dressing packs. Challenges encountered by practitioners while performing wound dressings included: patients touching wounds, limited space while doing dressings, concurrent cleaning of the environment, and interruptions (eg, consultant rounds, attendance to other clinical needs).
Notably, wound‐dressing procedural recommendations originating from the empirical literature has been structured around standard non‐complicated wounds and day care patients, whereas the reality of patients in a hospital context is much different.
4.1. Strengths and limitations of the study
The use of videos for guiding the development of the metric and a Delphi panel process lends credibility to the use of this metric for future educational programmes. This study focused on acute surgical wound healing by primary intention, post‐major surgeries, as these represented the patients staying in hospital for longer periods. The research was conducted in only one region in Ireland. Further testing of the metric in the clinical context would further enhance the validity of the metric.
5. CONCLUSION AND RECOMMENDATIONS
The development of robust tools, such as this ANTT wound‐dressing observational metric, helps remove some of the subjectivity involved in the learning and assessment of these procedures. When existing wound‐dressing metrics were studied by the team, they lacked indicative detail to make objective observations a practical reality. We believe that these metrics can be used for the assessment of the performance of wound dressings and to assist learners to self‐assess their own or a peer's performance.
A rigorous process underpinned the development of this ANTT wound‐dressing observational metric, following the steps involved in PBPT. Routine performance of practices, such as wound dressings, can benefit from closer and perhaps even microscopic analysis so that we truly understand optimum performance and can benchmark what is needed to deem a health care professional proficient in this procedure.
CONFLICTS OF INTEREST
All the authors declare that they have no conflicts of interest.
ACKNOWLEDGEMENTS
This research was funded by the School of Nursing and Midwifery, University College Cork, Ireland. The authors thank all those who assisted with this project, particularly the nurses, patients, and Delphi panel members who gave of their time to be part of this study. We thank Diane Duarte, Tony Archer, Cathal Brennan, and Jennifer Walshe for their assistance.
Hegarty J, Howson V, Wills T, et al. Acute surgical wound‐dressing procedure: Description of the steps involved in the development and validation of an observational metric. Int Wound J. 2019;16:641–648. 10.1111/iwj.13072
Funding information School of Nursing and Midwifery, University College Cork, Ireland, Grant/Award Number: SON&M/ASSERT‐ ANTT pilot project; University College Cork
REFERENCES
- 1. European Centre for Disease Prevention and Control . Point prevalence survey of healthcare associated infections and antimicrobial use in European acute care hospitals. Stockholm: ECDC; 2013. [Google Scholar]
- 2. Loveday H, Wilson J, Pratt R, et al. epic3: National Evidence‐Based Guidelines for preventing healthcare‐associated infections in NHS hospitals in England. J Hosp Infect. 2014;86:S1‐S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eskes AM, Maaskant JM, Holloway S, et al. Competencies of specialised wound care nurses: a European Delphi study. Int Wound J. 2014;11(6):665‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chemaly RF, Simmons S, Dale C Jr, et al. The role of the healthcare environment in the spread of multidrug‐resistant organisms: update on current best practices for containment. Ther Adv Infect Dis. 2014;2(3–4):79‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Megeus V, Nilsson K, Karlsson J, Eriksson B, Andersson A. Hand contamination, cross‐transmission, and risk‐associated behaviors: an observational study of team members in ORs. Asso Operating Room Nur J (AORN). 2015;102(6):645.e641‐645.e645. e612. [DOI] [PubMed] [Google Scholar]
- 6. Meeks DW, Lally KP, Carrick MM, et al. Compliance with guidelines to prevent surgical site infections: as simple as 1‐2‐3? Am J Surg. 2011;201(1):76‐83. [DOI] [PubMed] [Google Scholar]
- 7. Center for Disease Control . National and State Healthcare‐Associated Infections Progress Report. Atlanta, Georgia: CDC; 2016. [Google Scholar]
- 8. Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(Supplement_2):S82‐S89. [DOI] [PubMed] [Google Scholar]
- 9. Kilgore M, Brossette S. Cost of bloodstream infections. Am J Infect Control. 2008;36(10):S1 171‐173. [DOI] [PubMed] [Google Scholar]
- 10. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387‐397. [DOI] [PubMed] [Google Scholar]
- 11. Anderson DJ, Podgorny K, Berríos‐Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(06):605‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Institute Clinical Effectiveness . Surgical Site Infections: Prevention and Treatment Clinical Guideline [CG74]. London, UK: NICE; 2017. Published Date: October 2008 Last Updated: February 2017. [Google Scholar]
- 13. Rowley S, Clare S, Ruffell A, Beer J. High impact actions: fighting infection. Nurs Manag (Harrow). 2010;17(6):14‐19. [DOI] [PubMed] [Google Scholar]
- 14. Health Services Executive . National best practice and evidence based guidelines for wound management. Dublin, Ireland: Health Services Executive; 2009. [Google Scholar]
- 15. Australian Commission on Safety and Quality in Health Care . National safety and quality health service standards. Sydney, Australia: ACSQHC; 2011. [Google Scholar]
- 16. Dumville JC, McFarlane E, Edwards P, Lipp A, Holmes A, Liu Z. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev. 2015(4): CD003949. doi: 10.1002/14651858.CD003949.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adams J, Korniewicz D, El‐Masri M. A descriptive study exploring the principles of asepsis techniques among perioperative personnel during surgery. Can Oper Room Nurs J. 2011;29(4):6 ‐8, 14‐16, 21‐14. [PubMed] [Google Scholar]
- 18. Dougherty L, Lister S, West‐Oram A. The Royal Marsden Manual of Clinical Nursing Procedures. 9th ed. London, UK: NHS; 2015. [Google Scholar]
- 19. Government Publications . The Lourdes Hospital Inquiry: An Inquiry into peripartum hysterectomy at Our Lady of Lourdes Hospital Drogheda. Dublin, Irekland: Government Stationary Office; 2006. [Google Scholar]
- 20. Francis R. Report of the Mid Staffordshire NHS Foundation Trust Public Inquiry. London, UK: Stationary Office; 2013. [Google Scholar]
- 21. Gallagher AG, O'Sullivan GC. Fundamentals of Surgical Simulation Principles and Practices. London, UK: Springer Verlag; 2011. [Google Scholar]
- 22. Gallagher AG. Metric‐based simulation training to proficiency in medical education: what it is and how to do it. Ulster Med J. 2012;81(3):107‐113. [PMC free article] [PubMed] [Google Scholar]
- 23. Angelo RL, Ryu RKN, Pedowitz RA, Gallagher AG. The Bankart performance metrics combined with a cadaveric shoulder create a precise and accurate assessment tool for measuring surgeon skill. Arthroscopy. 2015;31(9):1655‐1670. [DOI] [PubMed] [Google Scholar]
- 24. Dalkey N, Helmer O. An experimental application of the DELPHI method to the use of experts. Manag Sci. 1963;9(3):458‐467. [Google Scholar]
- 25. McKenna H. The Delphi technique: a worthwhile research approach for nursing? J Adv Med. 1994;19(6):1221‐1225. [DOI] [PubMed] [Google Scholar]
- 26. McHugh M. Interrater reliability: the kappa statistic. Biochem Med. 2012;22(3):276‐282. [PMC free article] [PubMed] [Google Scholar]
- 27. Desra AP, Breen J, Harper S, Slavin MA, Worth LJ. Aseptic technique for accessing central venous catheters: applying a standardised tool to audit ‘scrub the hub’ practices. J Vasc Access. 2016;17(3):269‐272. [DOI] [PubMed] [Google Scholar]
- 28. Dumville JC, Gray TA, Walter CJ, et al. Dressings for the prevention of surgical site infection. Cochrane Database Syst Rev. 2016;(12): CD003091. doi: 10.1002/14651858.CD003091.pub4. [DOI] [PubMed] [Google Scholar]
- 29. Flynn JM, Keogh SJ, Gavin NC. Sterile v aseptic non‐touch technique for needle‐less connector care on central venous access devices in a bone marrow transplant population: a comparative study. Eur J Oncol Nurs. 2015;19(6):694‐700. [DOI] [PubMed] [Google Scholar]
- 30. Hart S. Using an aseptic technique to reduce the risk of infection. Nurs Stand. 2007;21(47):43‐48. [DOI] [PubMed] [Google Scholar]
- 31. Ingram P, Murdoch MF. Aseptic non‐touch technique in intravenous therapy. Nurs Stand. 2009;24(8):49‐58. [DOI] [PubMed] [Google Scholar]
- 32. McMullan C, Propper G, Schuhmacher C, et al. A multidisciplinary approach to reduce central line & associated bloodstream infections. Jt Comm J Qual Patient Saf. 2013;39(2):61‐69. [DOI] [PubMed] [Google Scholar]
- 33. Nonino E, Anselmi M, Dalmas J. Quality assessment of the wound dressing procedure in patients at a university hospital. Rev Lat Am Enfermagem. 2008;16(1):57‐63. [DOI] [PubMed] [Google Scholar]
- 34. Rowley S, Clare S. ANTT: a standard approach to aseptic technique. Nurs Times. 2011;107(36):12‐14. [PubMed] [Google Scholar]
- 35. Rowley S, Clare S. Improving standards of aseptic practice through an ANTT trust‐wide implementation process: a matter of prioritisation and care. J Infect Prev. 2009;10(1_Suppl):S18‐S23. [Google Scholar]
- 36. Rowley S, Clare S, Macqueen S, Molyneux R. ANTT v2: an updated practice framework for aseptic technique. Br J Nurs. 2010;19(Sup 1):S5‐S11.20622767 [Google Scholar]
- 37. Toon C, Lusuku C, Ramamoorthy R, Davidson B, Gurusamy K. Early versus delayed dressing removal after primary closure of clean and clean‐contaminated surgical wounds. Cochrane Database Syst Rev. 2015(9): CD010259. doi: 10.1002/14651858.CD010259.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walter CJ, Dumville JC, Sharp CA, Page T. Systematic review and meta‐analysis of wound dressings in the prevention of surgical‐site infections in surgical wounds healing by primary intention. Br J Surg. 2012;99(9):1185‐1194. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization (WHO)WHO Guidelines on Hand Hygiene in Health Care, First Global Patient Safety Challenge Clean Care is Safer Care. Geneva, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- 40. Clare S, Rowley S. Implementing the aseptic non touch technique (ANTT®) clinical practice framework for aseptic technique: a pragmatic evaluation using a mixed methods approach in two London hospitals. J Infect Prev. 2018;19(1):6‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Henriksen K, Rodrick D, Grace EN, Brady P. Challenges in Health care simulation: are we learning anything new? Acad Med. 2018;93(5):705‐708. [DOI] [PubMed] [Google Scholar]


