Abstract
Impaired classical NF‐κB pathway signaling causes reduced antibody responses to T‐independent (TI) antigens. We investigated the potential reasons for defective TI responses in mice lacking the atypical inhibitory kappa B (IκB) protein of the NF‐κB pathway, IκBNS. Analyses of the plasma cell compartment in vitro and in vivo after challenge with lipopolysaccharide (LPS) showed significant decreases in the frequencies of plasma cells in the absence of IκBNS. In vitro activation of B cells via the B cell receptor or via Toll‐like receptor 4 revealed that early activation events were unaffected in IκBNS‐deficient B cells, while proliferation was reduced compared to in similarly stimulated wildtype (wt) B cells. IκBNS‐deficient B cells also displayed impaired upregulation of the transmembrane activator and calcium modulator cyclophilin ligand interactor (TACI), which is essential for TI responses, and decreased sensitivity to TACI ligands upon stimulation. Furthermore, IκBNS‐deficient B cells, in contrast to wt B cells, displayed altered expression of IRF4, Blimp‐1 and Pax5 upon LPS‐induced differentiation, indicating impaired transcriptional regulation of plasma cell generation.
Keywords: APRIL, IκBNS, nfkbid, NF‐κB, plasma cell differentiation, TACI
This study identified alterations in B cell activation and plasma cell differentiation as underlying reasons for the impaired humoral responses against T‐independent (TI) antigens associated with IκBNS deficiency. In the absence of IκBNS, B cells exhibited reduced proliferative capacity, a loss of TACI‐mediated antibody production, and altered expression of the transcription factors Pax5, IRF4 and Blimp‐1, and fail to generate plasma cells. These findings indicate that TACI function and plasma cell differentiation are regulated by IκBNS, and may help explain mechanisms for impaired TI responses associated with defects in components of the NF‐κB signaling pathway.
Introduction
The activation and differentiation of B lymphocytes into plasma cells (PC) and their subsequent production of antibodies is pivotal for establishing efficient humoral immune responses to infections. After ligation of their B cell receptors (BCR), B cells are able to differentiate into antibody‐secreting PC or memory B cells either with or without T‐cell help, resulting in T‐dependent (TD) or T‐independent (TI) antibody responses, respectively. The TI antigens are classified according to whether they are capable of stimulating antibody production in mice with reduced BCR signaling due to Bruton's tyrosine kinase (xid/btk) deficiency (TI‐1) or not (TI‐2).1 Thus, intact BCR signaling machinery is required for responses to TI‐2 antigens. The TI‐1 antigens stimulate B cells by binding to both BCR and pathogen recognition receptors such as Toll‐like receptors (TLR), while TI‐2 antigens display repetitive determinants, usually composed of polysaccharides, which activate B cells via BCR ligation.2 The TNF superfamily ligands, B cell activating factor (BAFF/BLyS) and a proliferation inducing ligand (APRIL), have been implicated in the response to TI antigens. While BAFF and APRIL also signal through the BAFF receptor (BAFFR) and/or BCMA, it is their ligation to the transmembrane activator and calcium modulator cyclophilin ligand interactor (TACI) that is considered essential for TI antibody responses.3, 4, 5
The TI antigens are found primarily on the surface of encapsulated bacteria such as Streptococcus pneumoniae and Haemophilus influenzae. These bacteria are among the most prevalent microorganisms causing recurrent respiratory tract infections in patients with primary antibody deficiencies (PAD).6 Approximately 8–10% of patients with the most common PAD, combined variable immunodeficiency (CVID), have homo‐ or heterozygous mutations in TACI. However, there is no clear clinical phenotype associated with specific TACI mutations, suggesting that additional environmental or genetic factors contribute to TACI deficiency‐associated CVID.7, 8 BCR and TLR stimulation also induce NF‐κB activation and dysfunctional NF‐κB signaling is associated with various defects in B cell function,9 which potentially manifests as CVID.10, 11, 12, 13
The NF‐κB transcription factors, p50 (NF‐κB1), p52 (NF‐κB2), p65 (RelA), c‐Rel and RelB, regulate transcription by binding to promoters of target genes. In classical NF‐κB signaling, the NF‐κB transcription factors are rendered transcriptionally inactive through sequestering in the cytoplasm by inhibitors of κB (IκB), such as IκB‐α, IκB‐β, IκB‐ε, and the p50 precursor p105.14 In addition to the cytoplasmic IκB proteins, the atypical nuclear IκB proteins BCL‐3, IκBζ, IκBNS and IκBη were identified based on their ankyrin repeat structure through which they are able to bind NF‐κB proteins and regulate their activity.15 For example, IκBNS was found to bind nuclear p50, p52, p65, RelB and c‐Rel.16, 17 Rather than being constitutively expressed and regulated through proteasomal degradation similar to classical IκB proteins, expression of IκBNS is induced by BCR ligation or TLR stimulation.18, 19
Recent studies have revealed distinct functions of atypical IκB proteins in lymphopoiesis and immunological responses (reviewed in 20). For instance, BCL‐3 deficiency leads to increased marginal zone B (MZB) cell numbers and fewer follicular B (FOB) cells,21 whereas the phenotype of IκBNS‐deficient mice resembles other strains with classical NF‐κB pathway deficiency. Similar to p50−/− and c‐Rel−/− mice, the MZB and B‐1 cell numbers are reduced and serum IgM and IgG3 levels are decreased in IκBNS−/− mice.19 The IκBNS‐deficient bumble (bmb) strain harbors a mutation in the donor splice site of intron 4 within the nfkbid gene, which introduces a premature stop codon in the transcript and encodes for a severely truncated IκBNS protein that is not expected to retain any function.18 Similar to IκBNS knock‐out mice, the bumble mice completely lack the B‐1a cell population,18, 22 while in p50−/− mice this population is only reduced.23 Development of the B‐1a population via the neonatal transitional B‐1a (TrB‐1a) cell stage and MZB population via the transitional‐2 marginal zone precursor stage depends on IκBNS.22, 24 Furthermore, the bumble mice are unable to respond to TI antigens while heterozygous bumble mice are haploinsufficient in terms of TI antibody responses despite intact B cell development.25 These results indicated that IκBNS is required for normal antibody responses to TI antigens, in addition to its role in B cell development.
IκBNS is also required for normal function in other immune cells. In T cells, IκBNS mediates TCR‐induced cell death during negative selection in the thymus,16 governs the development of regulatory T cells through the induction of Foxp320 and is essential for cytokine production in TH17 cells.15 In the myeloid lineage, IκBNS dampens the proinflammatory response through suppression of IL‐6 and IL‐12p40 production in macrophages and regulating IL‐10 production by dendritic cells upon lipopolysaccharide (LPS) stimulation.26, 27, 28
In this study, we investigated potential reasons for the lack of TI responses in the absence of IκBNS using the bumble mouse strain.18 We found that bumble B cells displayed impaired expression of TACI, both at steady‐state and in response to stimulation, as well as reduced responses to the TACI ligands APRIL and BAFF. A comparison of LPS‐stimulated B cell cultures from bumble and wildtype (wt) mice revealed altered expression of the transcription factors Pax5, IRF4 and Blimp‐1, all of which coordinate PC differentiation. These findings demonstrate that IκBNS deficiency is associated with both impaired TACI expression and defective transcriptional regulation of PC differentiation.
Results
PC generation in response to the T‐independent antigen LPS requires functional IκBNS
We previously reported a requirement for IκBNS for intact antibody responses to TI antigens.18, 22, 25 TI antigens stimulate rapid extrafollicular plasmablast and PC responses.29 bumble mice displayed impaired antibody responses to immunization with the TI‐1 antigen 2,4,6‐trinitrophenyl (TNP)‐LPS and the TI‐2 antigens NP (4‐hydroxy‐3‐nitrophenylacetic)‐Ficoll and Pneumococcal polysaccharides (Pneumovax).22 To investigate the role of IκBNS for antibody induction, we assessed PC generation in response to the TLR4 ligand LPS, which provides a TI‐1 antigen stimulus. We first examined the splenic plasmablast and PC compartments in vivo after injection with 5 μg LPS i.v. We found that the frequencies of both B220+ CD138+ plasmablasts and B220− CD138+ PC were reduced significantly in bumble mice compared to in wt mice (Figure 1a). We also examined PC generation in vitro. B cells committing to PC fate upregulate CD138 after approximately four division cycles.30 Therefore, we labeled isolated splenic B cells with the cell trace violet (CTV) dye, cultured them with LPS for 84 h (3.5 days), and then stained the cells for CD138 expression. LPS stimulation resulted in efficient generation of CD138+ cells in wt, but not in bumble B cell cultures (Figure 1b). The reduction in CD138+ cell frequencies in bumble B cell cultures was accompanied by reduced secretion of IgM and IgG3 into the culture supernatant (Figure 1c). In addition, we stimulated sorted FOB cells (purity approximately 99%, Supplementary figure 1) to exclude the possibility that the reduction in CD138+ cells was influenced by the decreased MZB compartment in bumble mice.18, 24 Similar to the cultures from total splenic B cells, the frequencies of CD138+ cells from sorted FOB cells were reduced in bumble cultures compared to in wt cultures (Figure 1d). Thus, IκBNS is required for intact generation of plasmablast and PC responses to the TI‐1 antigen LPS.
Figure 1.
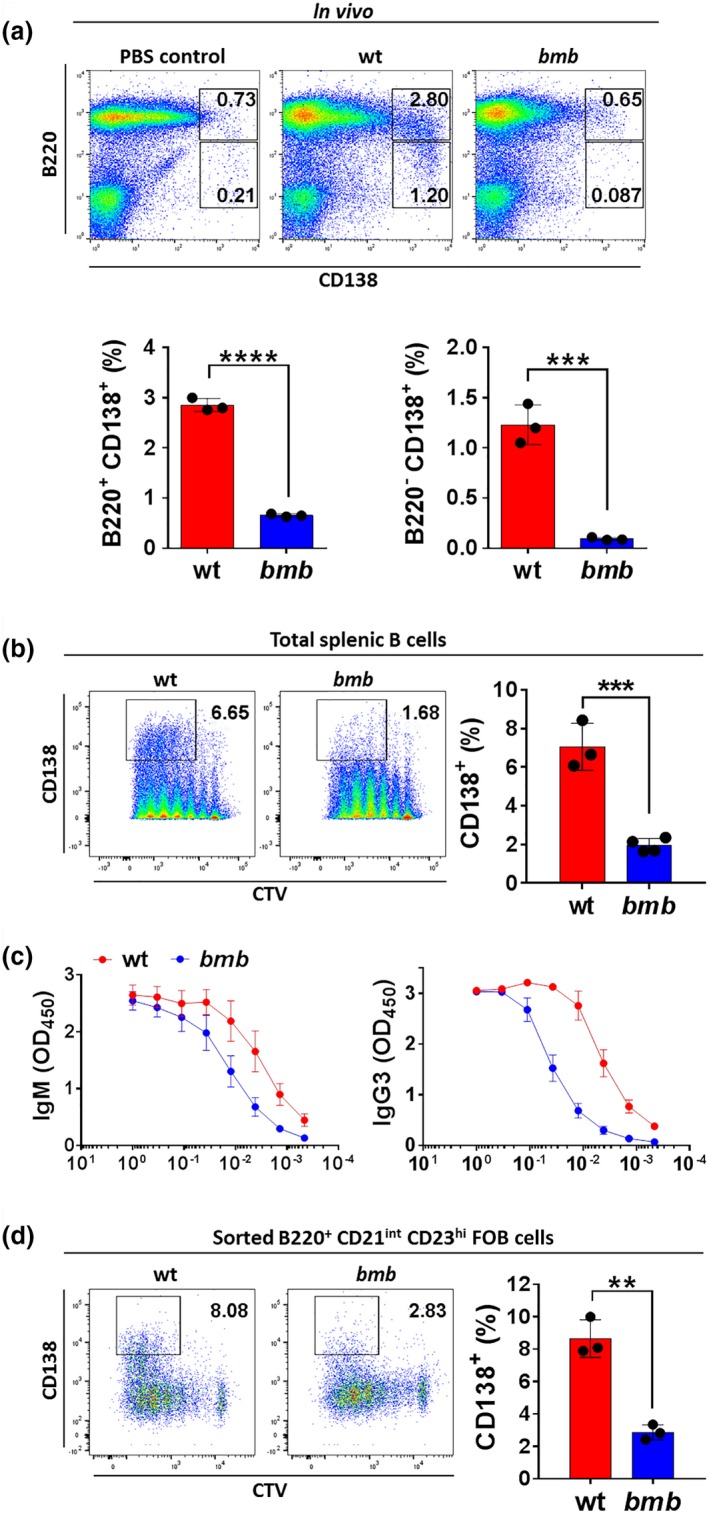
Plasma cell generation in response to the T‐independent antigen LPS requires functional IκBNS. LPS‐induced PC generation in wt mice and IκBNS‐deficient bumble (bmb) mice. (a) Wt and bumble mice were injected with 5 μg S. minnesota LPS intravenously and plasmablast and plasma cell frequencies were determined at day 3. Representative plots show the B220+ CD138+ plasmablast and the B220− CD138+ plasma cell populations (upper panel). Frequencies of plasmablasts and plasma cells in LPS‐immunized wt and bumble mice (lower panel). Graph bars and error bars indicate mean ± s.d. Differences between groups were determined using an unpaired Student's t‐test with *** and **** indicating P ≤ 0.001 and P ≤ 0.0001, respectively. Data are representative of two independent experiments with 3 wt and 3 bumble mice. (b) Isolated splenic B cells from wt and bumble mice were CTV‐labeled, stimulated with 10 μg mL−1 LPS, and stained for CD138 expression at 84 h. Representative plots show the gate for identifying cells undergoing plasma cell differentiation (left panel). Frequencies of CD138+ cells in wt and bumble B cell cultures (right panel). Graph bars and error bars indicate mean ± s.d. Differences between groups were determined using an unpaired Student's t‐test with *** indicating P ≤ 0.001. Data are representative of three independent experiments with 3–5 mice in each group. (c) Isolated splenic B cells from wt and bumble mice were stimulated with 10 μg mL−1 LPS for 6 days. Levels of IgM and IgG3 were determined by ELISA from the supernatant of wt and bumble B cell cultures. Each line and error bar indicate mean ± s.d. Data are representative of five independent experiments with 3–5 mice in each group. (d) Sorted B220+ CD21int CD23hi FOB cells from wt and bumble mice were CTV‐labeled and stimulated with 10 μg mL−1 LPS, and stained for CD138 expression at 84 h (left panel). Graph bars and error bars indicate mean ± s.d. Statistical differences were determined using an unpaired Student's t‐test with ** indicating P ≤ 0.01. Data are representative of two independent experiments with 3 wt and 3 bumble mice.
Early activation events are modestly altered in IκBNS‐deficient B cells
To investigate further the role of IκBNS in TI responses, we assessed whether initial signaling events downstream of BCR ligation were intact in bumble mice. To induce BCR signaling, we used anti‐IgM F(ab’)2 fragments for BCR ligation to model TI‐2 antigen stimulation. Activated B cells increase in size allowing identification of blasted cells by the forward and side scatter profiles. After activation via BCR or TLR4 ligation, the frequencies of blasted B cells in wt and bumble cultures were comparable at 24 h (Supplementary figure 2a). We also observed similar surface expression of the co‐stimulatory protein CD86 on bumble and wt B cells (Supplementary figure 2b). As the NF‐κB transcription factors p50/p65 are bound and sequestered by IκB proteins, activation of the classical pathway depends on IκBα phosphorylation and degradation upon BCR signaling. Mice with impaired BCR signaling, for example, because of mutations in btk, 31 or genes encoding classical NF‐κB pathway proteins such as NEMO or IKKβ,32, 33, 34 fail to induce IκBα phosphorylation. To assess whether induction of BCR signaling was intact, we measured IκBα degradation in wt and bumble B cells. IκBα was degraded normally after 90 min of stimulation with anti‐IgM, PMA and ionomycin or after treatment with the protein phosphatase inhibitor, calyculin A (Supplementary figure 2c). Since differentiation of activated B cells into PC requires several cellular divisions,30, 35 we next examined proliferation in wt and bumble B cells in response to anti‐IgM or LPS. In response to anti‐IgM, only a few cells reached division cycles 4–6 in both wt and bumble B cell cultures at 72 h. However, we observed clear decreases in the frequencies of B cells reaching division cycle 4 or beyond in bumble compared to in wt B cell cultures at 72 h in response to LPS (Figure 2 and Supplementary figure 3). Thus, while the initial activation of B cells was unaffected in terms of blasting, CD86 upregulation, and IκBα degradation, subsequent proliferation events were impaired in bumble B cells.
Figure 2.
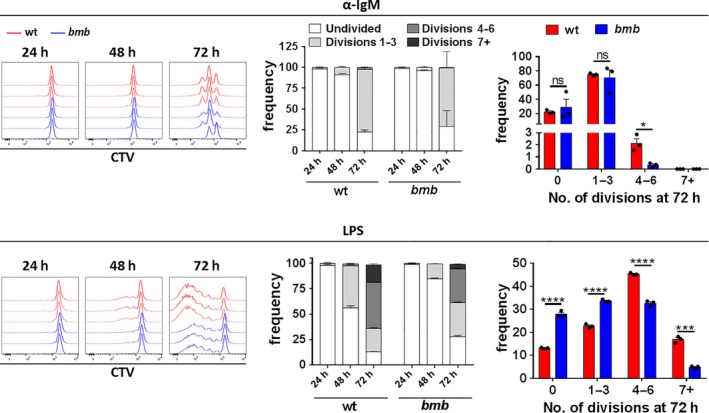
bumble B cells display reduced proliferation in response to BCR or TLR4 stimulation. Isolated B cells from spleen of wt and bumble mice were labeled with CTV, and stimulated with 10 μg mL−1 anti‐IgM F(ab’)2 fragments (upper panel) or 10 μg mL−1 LPS (lower panel) for 24, 48 and 72 h. Proliferation in response to anti‐IgM or LPS stimulation is indicated by CTV dilution. Representative histograms show proliferation profiles from 3 wt and 3 bumble samples (left panels). Frequencies of wt and bumble B cells at various phases of cellular division are summarized for 24, 48 and 72 h (middle panels). Bars and error bars indicate mean ± s.d. Frequencies of wt and bumble B cells at 72 h (right panels). Graph bars and error bars indicate mean ± s.d. Data are representative of four independent experiments with 3 wt and 3 bumble mice. Differences between groups was determined using an unpaired Student's t‐test with ns, *, *** and **** indicating P > 0.05, P ≤ 0.05, P ≤ 0.001 and P ≤ 0.0001, respectively.
IκBNS is essential for intact TACI upregulation in response to BCR or TLR stimulation
Mice lacking TACI fail to respond to TI‐1 and TI‐2 antigens.3, 5 In btk‐deficient mice, the defect in TI‐2 responses was associated with defective TACI expression and function.36 In wt mice, steady‐state TACI levels are higher on MZB compared to FOB cells,37 which we also observed in our experiments. Notably, bumble MZB and FOB cells displayed no detectable surface TACI levels at steady‐state (Figure 3a). Since the MZB population in bumble is reduced in young mice and gradually increases upon aging, we performed these experiments in 6 to 7 months old wt and bumble mice.24 For some of the experiments, we used age‐matched TACI−/− mice as controls.5 We also found that TACI expression was reduced on the transitional 1 B cell subset in the spleen (Supplementary figure 4a) and the mature B cell population in the bone marrow (Supplementary figure 4b). BCR and TLR stimulation is known to upregulate TACI expression on B cells.37 When examining cell surface TACI levels on bumble compared to wt B cells upon anti‐IgM stimulation (Figure 3b, upper panel), we found that bumble B cells were severely defective in this response. LPS stimulation resulted in upregulation of TACI on bumble B cells, although the frequencies of B cells expressing high TACI levels were lower in bumble B cell cultures compared to in wt B cell cultures (Figure 3b, lower panel). In contrast, upregulation of BAFFR and BCMA upon anti‐IgM or LPS stimulation was normal on bumble B cells (Supplementary figure 5). We also observed impaired TACI upregulation in sorted FOB cells, excluding the possibility that impaired TACI expression was due to the reduced MZB compartment in bumble mice (Figure 3c). Furthermore, impaired TACI upregulation was cell‐intrinsic, since in 1:1 mixed cultures of bumble and wt B cells, TACI upregulation was induced only on wt B cells (Figure 3d). In addition, TACI mRNA transcript levels were reduced in bumble compared to wt B cells upon both anti‐IgM and LPS stimulation (Figure 3e). TACI can be cleaved from the surface by the metalloproteinase ADAM10 to release soluble TACI (sTACI).38 We therefore investigated whether proteolytic cleavage caused low TACI expression on bumble B cells by measuring sTACI levels in the serum. We found significantly lower sTACI serum levels in bumble mice compared to in wt mice (Figure 3f), indicating that low TACI expression on bumble B cells was not the result of increased TACI cleavage. Similar to TLR4 stimulation, TLR9 ligation with CpG in vitro partially upregulated TACI on bumble B cells. Furthermore, CpG administration in vivo to bumble mice induced TACI expression on MZB cells (Supplementary figure 6a). It was previously demonstrated that CpG stimulation induced TACI expression and improved the response to TI‐2 antigens in btk‐deficient mice.36, 39 We found that in vivo co‐administration of CpG with NP‐Ficoll only minimally boosted NP‐specific antibody responses in bumble mice (Supplementary figure 6b). Taken together, bumble B cells lacked TACI expression at steady‐state and TACI upregulation was reduced compared to wt B cells upon BCR or TLR stimulation. However, bumble B cells were not completely unable to upregulate TACI, since stimulation via TLR4 or TLR9 resulted in a partial upregulation of TACI expression on these cells.
Figure 3.
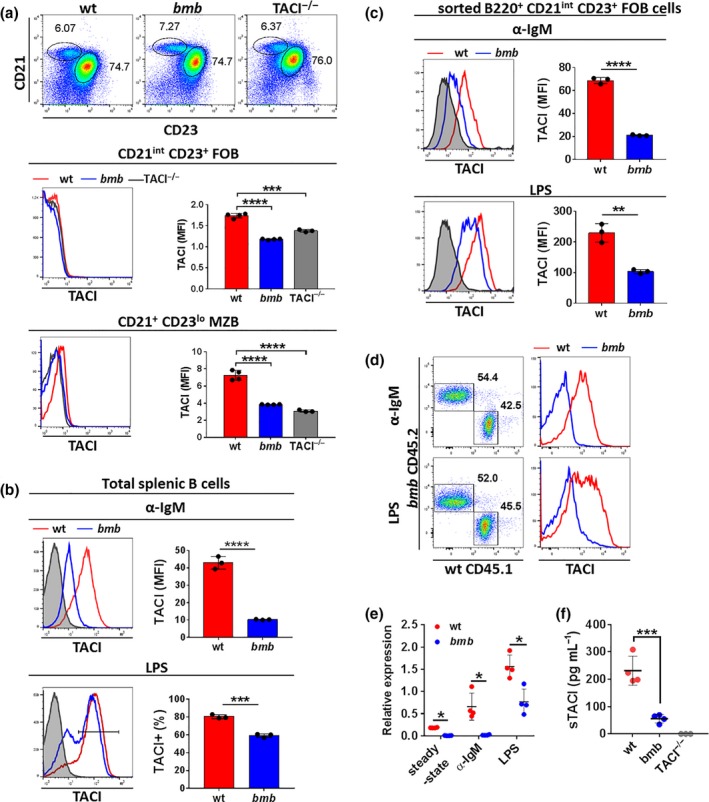
TACI upregulation upon BCR or TLR4 stimulation is impaired in IκBNS‐deficient B cells. (a) Follicular B (FOB, B220+ CD23+ CD21int) and marginal zone B (MZB, B220+ CD23− CD21hi) cells from 6 to 7 months old wt, bumble and TACI −/− mice were stained for TACI by flow cytometry. Representative plots showing the FOB and MZB compartment (upper panel). Numbers adjacent to the gates indicate frequencies. TACI expression on FOB (middle panel), and on MZB cells (lower panel). Data are representative of two independent experiments with 3 or 4 mice in each group. Graph bars and error bars indicate mean ± s.d. Statistical significance was calculated using an unpaired Student's t‐test with *** and **** indicating P ≤ 0.001 and P ≤ 0.0001, respectively. (b) Isolated B cells from spleen of wt or bumble mice were stimulated with 10 μg mL−1 anti‐IgM F(ab’)2 fragments (upper panel) or 10 μg mL−1 LPS (lower panel) for 48 h. Graph bars and error bars indicate mean ± s.d. Data are representative of four independent experiments with three mice in each group. Statistical significance was calculated using an unpaired Student's t‐test with *** and **** indicating P ≤ 0.001 and P ≤ 0.0001, respectively. (c) Sorted FOB cells from wt and bumble mice were stimulated with 10 μg mL−1 anti‐IgM or 10 μg mL−1 LPS for 48 h and stained for TACI expression. Graph bars and error bars indicate mean ± s.d. Data are representative of two independent experiments with three mice in each group. Statistical significance was calculated using an unpaired Student's t‐test with ** and **** indicating P ≤ 0.01 and P ≤ 0.0001, respectively. (d) Mixed cultures of CD45.1 wt and CD45.2 bumble isolated splenic B cells were stimulated with 10 μg mL−1 anti‐IgM or 10 μg mL−1 LPS for 24 h and TACI levels were determined on wt versus bumble derived cells. Numbers adjacent to gates indicate frequencies. Data are shown from one experiment with 2 wt and 2 bumble mice. (e) TACI mRNA levels were determined by quantitative RT‐PCR. Expression of TACI mRNA transcripts is shown relative to Polr2a mRNA transcripts at steady‐state and 48 h after 10 μg mL−1 anti‐IgM or LPS stimulation of wt and bumble B cells. Data are representative of one experiment with 4 or 5 mice in each group. Statistical significance was calculated using the Mann–Whitney U‐test with * indicating P ≤ 0.05. (f) Soluble TACI was measured in serum of wt, bumble and TACI −/− mice. Data are representative of two independent experiments with 3 or 4 mice in each group. Error bars indicate mean ± s.d. Statistical significance was calculated using an unpaired Student's t‐test with *** indicating P ≤ 0.001. MFI, median fluorescence intensity.
B cells require IκBNS for a normal response to APRIL stimulation
It was previously shown that APRIL synergizes with LPS in promoting antibody secretion and class‐switching by signaling through TACI.4 To study the effect of impaired TACI expression in IκBNS‐deficient mice in response to TACI ligands, we stimulated wt and bumble B cells in vitro with APRIL or BAFF together with the suboptimal concentration of LPS (100 ng mL−1). We determined the levels of IgM, IgG1, IgG3 and IgA at day 6. Co‐stimulation with APRIL or BAFF both enhanced secretion of IgM, IgA, IgG1 and IgG3 to the low LPS dose from wt B cells. However, bumble B cells were completely unresponsive to TACI ligation as the secretion of IgM, IgG1 and IgG3 was not detectable in the cultures with low LPS regardless of the presence of APRIL or BAFF (Figure 4a). In contrast, only the high concentration of LPS (10 μg mL−1) induced IgM, IgG1 and IgG3 secretion from bumble B cells, although at reduced levels compared to those from wt B cells. In terms of IgA secretion, a moderate increase was detected in bumble B cell cultures co‐stimulated with APRIL or BAFF, which is likely to be attributed to normal expression of BCMA.4 In wt B cell cultures, APRIL or BAFF addition to the suboptimal concentration of LPS both increased the frequencies of live (L/D far red−) cells at 84 h (3.5 days) (Supplementary figure 7a), consistent with previous reports that TACI ligation provides a survival signal.40 In bumble B cell cultures, we also observed an increase in viability (Supplementary figure 7a), but both frequencies and numbers were moderately reduced compared to wt in response to the low and high concentration of LPS (Supplementary figure 7c). Addition of APRIL or BAFF to the low concentration of LPS in both wt and bumble cultures increased the frequencies of blasted cells modestly but the difference was not significant (Supplementary figure 7b). Consistent with previous findings that APRIL enhances PC generation,4 we observed a significant increase in the total numbers of CD138+ cells in the presence of APRIL but also BAFF in wt cultures (Figure 4b). However, in bumble B cell cultures, CD138+ cells were observed only in response to the high LPS concentration (10 μg mL−1) (Figure 4b). Thus, bumble B cells did not respond to TACI ligation even when cells were stimulated with LPS, consistent with low TACI expression on the cell surface under these conditions (Figure 3b, Supplementary figure 6a).
Figure 4.
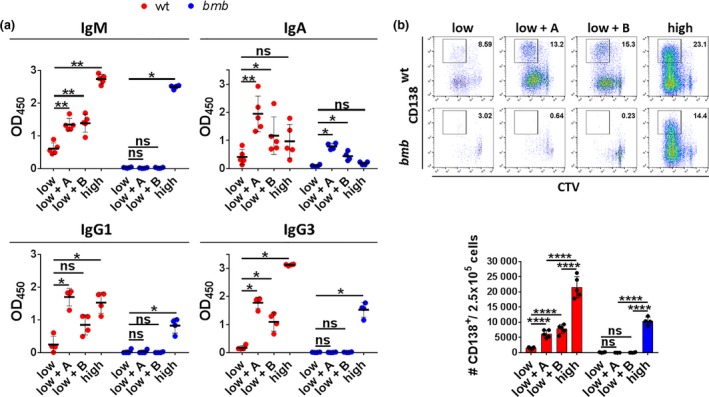
IκBNS is required for a normal response to the TACI ligands APRIL and BAFF. Isolated splenic B cells from wt and bumble mice were stimulated with a suboptimal dose of 100 ng mL−1 LPS (low), 100 ng mL−1 LPS and 1 μg mL−1 APRIL (low + A), 100 ng mL−1 LPS and 1 μg mL−1 BAFF (low + B), or 10 μg mL−1 LPS (high). (a) Levels of IgM, IgA, IgG1 and IgG3 were determined by ELISA from the supernatant of wt and bumble B cells after 6 days of stimulation. Error bars indicate mean ± s.d. Data are representative of two independent experiments with 4 or 5 mice in each group. Statistical significance was determined by the Mann–Whitney U‐test with ns, *, and ** indicating P > 0.05, P ≤ 0.01, and P ≤ 0.001, respectively. (b) Isolated splenic B cells from wt and bumble mice were labeled with CTV prior to stimulation and stained for CD138 expression at 84 h. Representative plots indicate cells undergoing PC differentiation (upper panel). Numbers adjacent to gates indicate cell frequencies. Total number of CD138+ cells per 250.000 seeded B cells (lower panel). Bars and error bars indicate mean ± s.d. Data are representative of two independent experiments with 4 or 5 mice in each group. Statistical significance was determined by an unpaired Student's t‐test with ns and **** indicating P > 0.05 and P ≤ 0.0001, respectively.
IκBNS‐deficient B cells fail to normally regulate transcription factors involved in PC differentiation in response to LPS stimulation
As bumble mice were unable to establish intact LPS‐induced plasmablast and PC compartments, we next examined the PC differentiation program in more detail. B cell differentiation is regulated in a dose‐dependent manner by the transcription factor IRF4, which initiates PC generation when expressed at high concentrations 41, 42 by controlling Blimp‐1 expression.43 In addition, efficient terminal PC differentiation coincides with downregulation of Pax5, a key transcription factor for maintenance of the B cell lineage, through direct repression by Blimp‐1.44, 45 Activated B cells separate into a population of Pax5loIRF4hi cells, which are committed to PC differentiation, and a population of Pax5hiIRF4int cells, which preserve a germinal center B cell phenotype.46 At 84 h (3.5 days) of LPS stimulation, both the Pax5loIRF4hi and the Pax5hiIRF4int populations were present in bumble and TACI‐deficient B cell cultures similar to wt B cells (Figure 5a, left panel, Supplementary figure 8). However, bumble B cells, unlike wt and TACI −/− B cells, were unable to fully downregulate Pax5 in the IRF4hi population (Figure 5a, right panel, Supplementary figure 8). As PC differentiation is a division‐linked process, downregulation of Pax5 and upregulation of IRF4 and Blimp‐1 coincide with cellular divisions.46 Therefore, we examined Pax5, IRF4 and Blimp‐1 expression in LPS‐induced cellular divisions of wt and bumble B cells. Frequencies of Pax5lo, IRF4hi and Blimp‐1hi cells in bumble B cells were all significantly reduced in the late divisions (division 7+/8+, respectively) compared to in wt B cells (Figure 5b). Expression levels of Pax5, as measured by median fluorescent intensity (MFI), were consistently increased in the Pax5hi population in bumble B cells throughout all divisions, whereas expression of IRF4 in the IRF4hi population was unaffected (Figure 5b). Although the frequencies of Blimp‐1hi cells in bumble B cells were significantly reduced compared to in wt B cells in the late divisions, they were increased in the intermediate divisions (divisions 4–6) and displayed elevated Blimp‐1 expression in divisions 5 and 6 (Figure 5b). Thus, bumble B cells were unable to completely suppress Pax5 in the presence of Blimp‐1, and generated fewer Pax5lo, IRF4hi and Blimp‐1hi cells in the late divisions. These results demonstrated that IκBNS is required for intact transcriptional regulation during PC development.
Figure 5.
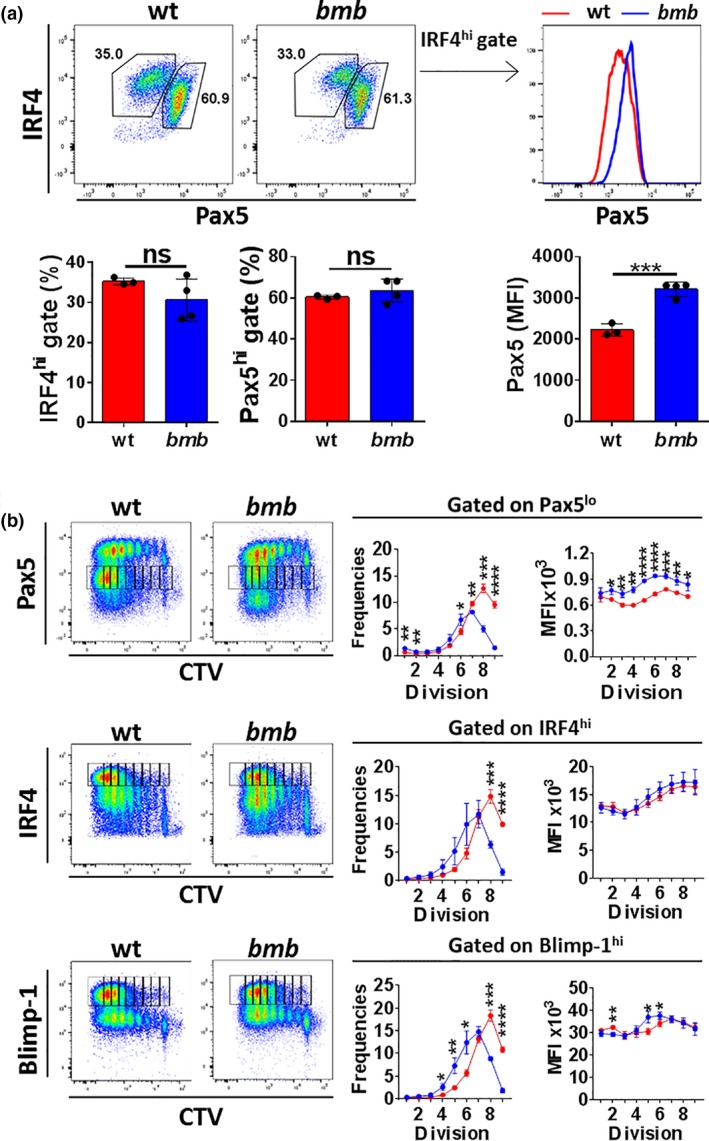
IκBNS is required for intact transcriptional regulation of PC differentiation. Isolated splenic B cells from wt and bumble mice were stimulated with 10 μg mL−1 LPS. (a) Intracellular expression of the transcription factors IRF4 and Pax5 at 84 h. Representative plots show activated B cells diverging into Pax5lo IRF4hi and Pax5hi IRF4int populations (upper left panel). Numbers adjacent to gates indicate frequencies of cells within the gate. Frequencies of the Pax5lo IRF4hi and Pax5hi IRF4int populations with error bars indicating mean ± s.d. (lower left panel). Representative histogram showing Pax5 expression within the IRF4hi population (upper right panel). Expression of Pax5 in wt and bumble cells at 84 h after LPS stimulation (lower right panel). Data are representative of four independent experiments with 3 or 4 mice in each group. Statistical significance was determined by the Mann–Whitney U‐test with ns and *** indicating P > 0.05 and P ≤ 0.001, respectively. (b) Isolated splenic B cells from wt and bumble mice were CTV‐labeled and stained for Pax5, IRF4 and Blimp‐1 at 84 h poststimulation. Representative plots are shown with gating for the Pax5lo, IRF4hi and Blimp‐1hi populations per individual division cycle (left panels). Frequencies and corresponding MFI values are shown for the Pax5lo, IRF4hi and Blimp‐1hi populations per individual division cycle as gated on in the FACS plots (middle panels and right panels). Data are representative of three independent experiments with 3 or 4 mice in each group. Error bars indicate mean ± s.d. Statistical significance was determined by unpaired t‐test with *, **, *** and **** indicating P ≤ 0.05, P ≤ 0.01, P ≤ 0.001, P ≤ 0.0001, respectively. MFI, median fluorescent intensity. Statistical test used to determine significance.
Discussion
The TI antigens are generally polysaccharides derived from bacterial capsules that engage the BCR and/or TLRs, which both activate the NF‐κB pathway. We have previously shown that antibody responses to the TI antigens NP‐Ficoll and Pneumococcal polysaccharides (Pneumovax) are impaired in bumble mice.22, 25 These mice lack expression of IκBNS, a nuclear regulator of the NF‐κB pathway. However, the role of IκBNS in the generation of TI antibody responses was not addressed in depth previously. In this study, we show that B cells lacking functional IκBNS display reduced proliferation, TACI upregulation and responsiveness to TACI ligands, and that terminal PC differentiation is impaired at the transcriptional level.
Several studies have underlined the importance of TACI signaling for TI antibody responses.3, 4, 5 B cells express surface TACI from transitional to mature stages, with MZB and B‐1 cells displaying the highest levels.37, 47, 48 TACI is upregulated upon stimulation via the BCR, TLR4 and TLR9.37, 47 We demonstrate here that IκBNS is essential in these processes, as bumble B cells lacked normal TACI expression and failed to upregulate TACI after BCR or TLR stimulation. btk‐deficient mice display impaired TACI expression, consistent with a direct role of BCR signaling for maintaining surface TACI.3 Interestingly, in contrast to btk‐deficient B cells, the early activation of IκBNS‐deficient bumble B cells, other than reduced TACI expression, was similar to that observed in wt B cells in response to anti‐IgM and LPS.18, 19 B cells lacking another component of NF‐κB signaling, Hoip‐1 of the LUBAC complex, are also activated normally upon BCR stimulation, yet fail to respond to TI‐2 antigens.49 Interestingly, LUBAC was not required for TACI upregulation upon BCR stimulation, but for TACI‐induced NF‐κB activation by APRIL.49 Impaired responses in bumble to TACI ligands could be due to altered NF‐κB regulation in the absence of IκBNS in addition to attenuated TACI expression. Enhancement of antibody responses to low doses of LPS by APRIL and BAFF is relevant in the context of physiological quantities of pathogens and antigens upon infection, emphasizing the importance of intact TACI expression and signaling. This highlights different mechanisms behind impaired response to TI‐2 antigens in various NF‐κB deficient strains, illustrating the complexity of this pathway. Moreover, as the NF‐κB1 and NF‐κB2 proteins were linked to the CVID phenotype in patients,10, 11, 12, 13 this emphasizes the possibility that defects in various components of the NF‐κB pathway could contribute to pathogenesis of immunodeficiencies in humans.
Similar to TACI−/− mice, bumble mice displayed reduced serum IgM,18 impaired Ig secretion to APRIL stimulation, and reduced PC frequencies in response to TI antigens. However, PC differentiation in bumble B cells was more severely impaired compared to in TACI−/− B cells. PC differentiation requires a sequence of ordered events including upregulating IRF4 and downregulating Pax5, which precedes upregulation of Blimp‐1 and CD138 expression.50 TACI has been suggested to be involved in maintenance of Blimp‐1 expression during PC generation.51 Interestingly, our data showed normal IRF4 expression and Pax5 downregulation in TACI−/− B cells, indicating that PC differentiation was unaffected. Therefore, the defective TI response in TACI−/− mice could be due to a requirement for TACI‐dependent Blimp‐1 expression for PC maintenance rather than differentiation.51 A previous study showed reduced mRNA levels of IRF4 and Blimp‐1 in LPS‐activated B cells derived from IκBNS−/− mice.19 We found that B cells from bumble mice were able to express IRF4 and Blimp‐1 at the protein level in response to LPS, however, with reduced frequencies of IRF4hi and Blimp‐1hi cells at the later stages of division. The reduced proliferation caused by the loss of IκBNS likely results in fewer cells at the commitment stage for PC differentiation, which could explain the decrease in mRNA transcripts for PC markers in total B cell cultures. Additionally, despite normal expression of IRF4 and enhanced initial induction of Blimp‐1, Pax5 was not fully suppressed in bumble B cells. We found that Pax5lo, IRF4hi and Blimp‐1hi B cells were lost after division 6 which coincides with commitment to terminal PC differentiation. Hence, in bumble mice, PC generation appears to be impaired at the terminal PC differentiation phase where we observed incomplete downregulation of Pax5. Pax5 expression in B cells is regulated by an enhancer containing NF‐κB‐binding regions.52 Thus, it is possible that IκBNS modulates NF‐κB activity at this enhancer.
In conclusion, our results demonstrate that IκBNS is required for TACI upregulation, responsiveness to both APRIL and BAFF, proliferation and intact differentiation of B cells into antibody‐secreting PC, all of which are required for robust TI antibody responses (Figure 6). The results reported in this study help explain mechanisms for the lack of response to TI antigens associated with defects in the different components of NF‐κB signaling, which may have bearing on some cases of CVID.
Figure 6.
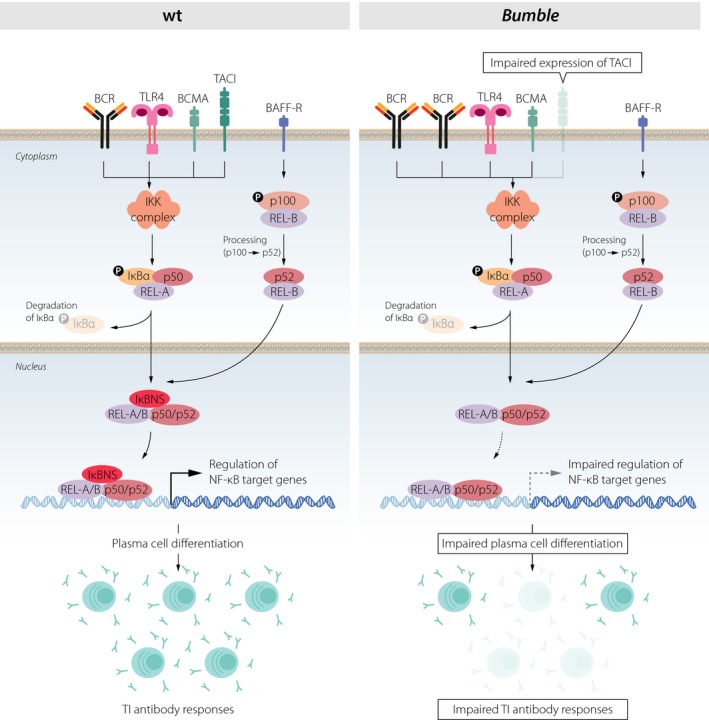
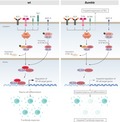
Schematic representation of how ablation of IκBNS affects B cell activation and PC differentiation. Activation of B cells in response to TI antigens is initiated through ligation of the BCR and/or Toll‐like receptors, and is enhanced through TACI. These surface receptors all trigger activation of the classical NF‐κB pathway by degrading the inhibitory IκB protein, consequently releasing the NF‐κB proteins that translocate to the nucleus where they are transcriptionally active. In bumble, the transcriptional regulation of NF‐κB target genes is altered in the absence of IκBNS, resulting in impaired TACI expression, PC differentiation and TI antibody responses.
Methods
Mice
Mice were maintained at the animal research facilities, MTC, and KM‐W, at Karolinska Institutet. Studies were performed in accordance with institutionally approved protocols and Committee for Animal Ethics (Stockholms Norra Djurförsöksetiska nämnd) approval. For bone marrow chimera experiments, C57BL/6J CD45.2 and CD45.1 mice were purchased from the Jackson laboratory. Spleens were obtained from TACI −/− mice as described previously.5 Mice harboring the bumble mutation in the gene encoding IκBNS were described previously.18, 22
Cell preparation
Splenocytes were prepared as single cell suspensions using 70 μm cell strainers in RPMI 1640 (HyClone, Logan, UT) supplemented with 2 mm l‐glutamine, penicillin (100 IU)‐streptomycin (100 μg mL−1) (Sigma‐Aldrich, St Louis, MO), β‐mercaptoethanol (0.05 mm) (Life Technologies, Waltham, MA) and 10% fetal bovine serum (HyClone) (complete RPMI medium). Splenocyte and bone marrow cell suspensions were washed once in Ca2+‐ and Mg2+‐free PBS (Sigma‐Aldrich) and treated with red blood cell lysis buffer before further processing.
In vitro B cell cultures
Mouse B cells were isolated by the EasySep Mouse B cell Negative selection Kit (STEMCELL Technologies, Vancouver) according to the manufacturer's protocol. Purity based on CD19 expression was around 95–97% as determined by flow cytometry. Cells were seeded at a cell density of 2.5 x 105 or 3 x 106 mL−1, for flow cytometry staining and ELISA, and RNA extraction, respectively. Cells were stimulated in complete RPMI with 10 μg mL−1 of unconjugated goat anti‐mouse IgM F(ab’)2 (Jackson ImmunoResearch Laboratories, West Grove, PA), 10 μg mL−1 or 100 ng mL−1 lipopolysaccharide (LPS) from E. coli 0111:B4 (Sigma‐Aldrich), 1 μg mL−1 APRIL (R&D Systems, Minneapolis, MN), 1 μg mL−1 BAFF (R&D Systems), or 1 μg mL−1 CpG oligonucleotides ODN 1826 (InvivoGen, San Diego, CA). To induce IkBα degradation, 1 x 106 cells were stimulated in 100 μL complete RPMI medium for 90 min with 10 μg mL−1 of unconjugated goat anti‐mouse IgM F(ab’)2 (Jackson ImmunoResearch Laboratories), 50 ng mL−1 PMA and 1 μm ionomycin, or 0.1 μm calyculin A, in the presence of 10 μm cyclohexamide (all from Sigma‐Aldrich).
Immunization
Mice were immunized intraperitoneally (i.p.) with 50 μg NP40‐Ficoll (Biosearch Technologies, Novato, CA) in 200 μL PBS. CpG oligonucleotides ODN 1826 (InvivoGen) were administered at a dose of 50 μg in 200 μL i.p. LPS of the Salmonella Minnesota strain (Enzo Life Sciences, Farmingdale, NY) was injected intravenously (i.v.) at a dose of 5 μg in 100 μL PBS.
Real‐time PCR
RNA was isolated from 3 × 106 B cells using Trizol (Invitrogen) followed by DNase‐treatment using TURBO DNA‐free kit (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer's instructions. RNA concentration was measured on Qubit (Thermo Fisher Scientific). cDNA synthesis was performed with 100 ng of RNA using SuperScript IV (Invitrogen) according to the manufacturer's instructions. Real‐time PCR was prepared with 1 μL of cDNA and 1 μm of the forward and reverse primer in RT2 SYBR Green Master Mix (Bio‐Rad Laboratiers, Hercules, CA) in a total volume of 10 μL. Primers used for amplification were TACI‐forward 5’‐ATGGTCGTAGTACCTGCCTTG‐3’, TACI‐reverse 5’‐ATGGCATTCTGCCCCAAAGAT‐3’ (reference 4), Polr2a‐forward 5’‐CGGTTGAATCTTAGTGTGAC‐3’, and Polr2 a‐reverse 5’‐ATAGCCAACTCTTGGATCTC‐3’ (reference 53). Assays were performed in 384‐well plates on Bio‐Rad CFX384 thermal cycler under the following conditions; denaturation at 95°C for 2 min, PCR amplification at 95°C for 5 s and extension at 60°C for 20 s for 45 cycli, followed by melt‐curve analysis of 0.5°C increments per 5 s from 65 to 95°C.
ELISA
ELISA plates (Nalge Nunc, Rochester, NY) were coated with 500 ng/well of NP25 conjugated with BSA (Biosearch Technologies). To detect IgM, IgG1, IgG3 or IgA, plates were coated with unconjugated goat anti‐mouse IgM (Southern Biotech, , Birmingham, AL), IgG (Southern Biotech), or IgA (BD, Franklin Lakes, NJ), respectively. After incubation overnight (4°C), washing with PBS + 0.05% Tween20 and blocking for 1 h with PBS containing 2% dry milk, 50 μL of culture supernatant was added in a total volume of 150 μL, followed by threefold serial dilutions in blocking buffer and incubated for 2 h at room temperature (RT). Plates were washed six times, and primary antibodies, biotinylated goat anti‐mouse IgM, HRP‐coupled anti‐IgG1, HRP‐coupled anti‐IgG3 (Southern Biotech, Birmingham, AL), or biotinylated goat anti‐mouse IgA (BD Franklin Lakes, NJ), were added in 100 μL PBS/well followed by incubation for 1.5 h, at RT. After six washes, streptavidin‐HRP was added to biotinylated antibodies in 100 μL PBS/well and incubated for 1 h at RT. The assay was developed with TMB substrate (KPL), the reaction was stopped with 1 m H2SO4, and the OD was read at 450 nm using an Asys Expert 96 ELISA reader (Biochrom Ltd, Cambridge, UK). For detection of soluble TACI, the mouse TACI/TNFRSF13B DuoSet ELISA kit (R&D Systems) was used, according to the manufacturer's instructions.
Flow cytometry and cell sorts
To block nonspecific binding to Fc receptors, cells were incubated with anti‐CD16/32 antibody (BD Franklin Lakes), and then stained with different panels of fluorochrome conjugated monoclonal antibodies (Supplementary table 1) in PBS/2% FBS. For intracellular staining of transcription factors, cells were fixed, permeabilized and stained using the transcription factor buffer set (BD Franklin Lakes) according to the manufacturer's instructions. To track divisions, CellTrace Violet (Invitrogen) was used to label cells according to the manufacturer's protocol. Samples were run using a BD LSR II, BD Fortessa, BD Verse or FACSCalibur machine and data were analyzed by FlowJo software v9.6.4 (Tree Star, Ashland, OR). For follicular B cell sorts, splenocytes were stained with LIVE/DEAD Aqua dye, B220, CD21/35 and CD23 antibodies and sorted on the FACSAria II cell sorter (BD Franklin Lakes).
Statistics
Differences between groups were analyzed by a Student's t‐test or Mann–Whitney U‐test (GraphPad Prism v6.0f). Statistical significance is indicated with ns for P > 0.05, * for P ≤ 0.05, ** for P ≤ 0.01, *** for P ≤ 0.001 and **** for P ≤ 0.0001.
Conflict of Interest
The authors have no conflicting financial interests.
Supporting information
Acknowledgments
We thank Dr Bruce Beutler for kindly providing the bumble mouse strain and the personnel at the animal facility at the Department of Microbiology, Tumor and Cell Biology at the Karolinska Institutet for expert assistance. We also thank Mattias Karlén for help with the illustration shown in Figure 6. This work was supported by a Karolinska Institutet Doctoral grant to SK, fellowship grants from the Swedish Governmental Agency for Innovation Systems to EE and from David & Astrid Hageléns Stiftelse to GKP, an equipment grant from the Fondation Dormeur Vaduz, and a Distinguished Professor grant (#2017‐00968) from the Swedish Research Council to GKH.
Contributor Information
Sharesta Khoenkhoen, Email: sharesta.khoenkhoen@ki.se.
Gunilla B Karlsson Hedestam, Email: gunilla.karlsson.hedestam@ki.se.
References
- 1. Mosier DE, Mond JJ, Goldings EA. The ontogeny of thymic independent antibody responses in vitro in normal mice and mice with an X‐linked B cell defect. J Immunol 1977; 119: 1874–1878. [PubMed] [Google Scholar]
- 2. Mond JJ, Vos Q, Lees A, Snapper CM. T cell independent antigens. Curr Opin Immunol 1995; 7: 349–354. [DOI] [PubMed] [Google Scholar]
- 3. Mantchev GT, Cortesao CS, Rebrovich M, Cascalho M, Bram RJ. TACI is required for efficient plasma cell differentiation in response to T‐independent type 2 antigens. J Immunol 2007; 179: 2282–2288. [DOI] [PubMed] [Google Scholar]
- 4. Ozcan E, Garibyan L, Lee JJ, Bram RJ, Lam KP, Geha RS. Transmembrane activator, calcium modulator, and cyclophilin ligand interactor drives plasma cell differentiation in LPS‐activated B cells. J Allergy Clin Immunol. 2009;123: 1277–86e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T‐independent humoral response by TACI. Immunity 2001; 14: 573–582. [DOI] [PubMed] [Google Scholar]
- 6. Durandy A, Kracker S, Fischer A. Primary antibody deficiencies. Nat Rev Immunol 2013; 13: 519–533. [DOI] [PubMed] [Google Scholar]
- 7. Martinez‐Gallo M, Radigan L, Almejun MB, Martinez‐Pomar N, Matamoros N, Cunningham‐Rundles C. TACI mutations and impaired B‐cell function in subjects with CVID and healthy heterozygotes. J Allergy Clin Immunol 2013; 131: 468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan‐Hammarstrom Q, Salzer U, Du L, et al Reexamining the role of TACI coding variants in common variable immunodeficiency and selective IgA deficiency. Nat Genet 2007; 39: 429–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hayden MS, Ghosh S. NF‐kappaB in immunobiology. Cell Res 2011; 21: 223–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen K, Coonrod EM, Kumanovics A, et al Germline mutations in NFKB2 implicate the noncanonical NF‐kappaB pathway in the pathogenesis of common variable immunodeficiency. Am J Hum Genet 2013; 93: 812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fliegauf M, Bryant VL, Frede N, et al Haploinsufficiency of the NF‐kappaB1 Subunit p50 in Common Variable Immunodeficiency. Am J Hum Genet 2015; 97: 389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boztug H, Hirschmugl T, Holter W, et al NF‐kappaB1 haploinsufficiency causing immunodeficiency and EBV‐driven lymphoproliferation. J Clin Immunol 2016; 36: 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuehn HS, Niemela JE, Sreedhara K, et al Novel nonsense gain‐of‐function NFKB2 mutations associated with a combined immunodeficiency phenotype. Blood 2017; 130: 1553–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Q, Verma IM. NF‐kappaB regulation in the immune system. Nat Rev Immunol 2002; 2: 725–734. [DOI] [PubMed] [Google Scholar]
- 15. Annemann M, Plaza‐Sirvent C, Schuster M, et al Atypical IkappaB proteins in immune cell differentiation and function. Immunol Lett 2016; 171: 26–35. [DOI] [PubMed] [Google Scholar]
- 16. Fiorini E, Schmitz I, Marissen WE, et al Peptide‐induced negative selection of thymocytes activates transcription of an NF‐kappa B inhibitor. Mol Cell 2002; 9: 637–648. [DOI] [PubMed] [Google Scholar]
- 17. Pedersen GK, Adori M, Karlsson Hedestam GB. NF‐kappaB signaling in B‐1 cell development. Ann N Y Acad Sci 2015; 1362: 39–47. [DOI] [PubMed] [Google Scholar]
- 18. Arnold CN, Pirie E, Dosenovic P, et al A forward genetic screen reveals roles for Nfkbid, Zeb1, and Ruvbl2 in humoral immunity. Proc Natl Acad Sci USA 2012; 109: 12286–12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Touma M, Keskin DB, Shiroki F, et al Impaired B cell development and function in the absence of IkappaBNS. J Immunol 2011; 187: 3942–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schuster M, Annemann M, Plaza‐Sirvent C, Schmitz I. Atypical IkappaB proteins ‐ nuclear modulators of NF‐kappaB signaling. Cell Commun Signal 2013; 11: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang X, Paun A, Claudio E, Wang H, Siebenlist U. The tumor promoter and NF‐kappaB modulator Bcl‐3 regulates splenic B cell development. J Immunol 2013; 191: 5984–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pedersen GK, Adori M, Khoenkhoen S, Dosenovic P, Beutler B, Karlsson Hedestam GB. B‐1a transitional cells are phenotypically distinct and are lacking in mice deficient in IkappaBNS. Proc Natl Acad Sci USA 2014; 111: E4119–E4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Montecino‐Rodriguez E, Dorshkind K. Formation of B‐1 B cells from neonatal B‐1 transitional cells exhibits NF‐kappaB redundancy. J Immunol 2011; 187: 5712–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adori M, Pedersen GK, Adori C, et al Altered marginal zone B cell selection in the absence of IkappaBNS. J Immunol 2017; 200: 775–787. [DOI] [PubMed] [Google Scholar]
- 25. Pedersen GK, Adori M, Stark JM, et al Heterozygous mutation in IkappaBNS leads to reduced levels of natural IgM antibodies and impaired responses to T‐independent type 2 antigens. Front Immunol 2016; 7: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hirotani T, Lee PY, Kuwata H, et al The nuclear IkappaB protein IkappaBNS selectively inhibits lipopolysaccharide‐induced IL‐6 production in macrophages of the colonic lamina propria. J Immunol 2005; 174: 3650–3657. [DOI] [PubMed] [Google Scholar]
- 27. Kuwata H, Matsumoto M, Atarashi K, et al IkappaBNS inhibits induction of a subset of Toll‐like receptor‐dependent genes and limits inflammation. Immunity 2006; 24: 41–51. [DOI] [PubMed] [Google Scholar]
- 28. Fujita S, Seino K, Sato K, et al Regulatory dendritic cells act as regulators of acute lethal systemic inflammatory response. Blood 2006; 107: 3656–3664. [DOI] [PubMed] [Google Scholar]
- 29. Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T‐independent blood‐borne particulate antigens. Immunity 2001; 14: 617–629. [DOI] [PubMed] [Google Scholar]
- 30. Hodgkin PD, Lee JH, Lyons AB. B cell differentiation and isotype switching is related to division cycle number. J Exp Med 1996; 184: 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Solvason N, Wu WW, Kabra N, et al Transgene expression of bcl‐xL permits anti‐immunoglobulin (Ig)‐induced proliferation in xid B cells. J Exp Med 1998; 187: 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Derudder E, Herzog S, Labi V, et al Canonical NF‐kappaB signaling is uniquely required for the long‐term persistence of functional mature B cells. Proc Natl Acad Sci USA 2016; 113: 5065–5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Newton K, Dixit VM. Mice lacking the CARD of CARMA1 exhibit defective B lymphocyte development and impaired proliferation of their B and T lymphocytes. Curr Biol 2003; 13: 1274–51 (0960‐9822 (Print)). [DOI] [PubMed] [Google Scholar]
- 34. Li ZW, Omori SF, Labuda T, Karin M, Rickert RC. IKK beta is required for peripheral B cell survival and proliferation. J Immunol. 2003; 170: 4630–4637. [DOI] [PubMed] [Google Scholar]
- 35. Barwick BG, Scharer CD, Bally APR, Boss JM. Plasma cell differentiation is coupled to division‐dependent DNA hypomethylation and gene regulation. Nat Immunol 2016; 17: 1216–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Uslu K, Coleman AS, Allman WR, et al Impaired B cell receptor signaling is responsible for reduced TACI expression and function in X‐linked immunodeficient mice. J Immunol 2014; 192: 3582–3595. [DOI] [PubMed] [Google Scholar]
- 37. Katsenelson N, Kanswal S, Puig M, Mostowski H, Verthelyi D, Akkoyunlu M. Synthetic CpG oligodeoxynucleotides augment BAFF‐ and APRIL‐mediated immunoglobulin secretion. Eur J Immunol 2007; 37: 1785–1795. [DOI] [PubMed] [Google Scholar]
- 38. Hoffmann FS, Kuhn PH, Laurent SA, et al The immunoregulator soluble TACI is released by ADAM10 and reflects B cell activation in autoimmunity. J Immunol 2015; 194: 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mond JJ, Hunter K, Kenny JJ, Finkelman F, Witherspoon K. 8‐Mercaptoguanosine‐mediated enhancement of in vivo IgG1, IgG2 and IgG3 antibody responses to polysaccharide antigens in normal and xid mice. Immunopharmacology 1989; 18: 205–212. [DOI] [PubMed] [Google Scholar]
- 40. Treml LS, Carlesso G, Hoek KL, et al TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J Immunol 2007; 178: 7531–7539. [DOI] [PubMed] [Google Scholar]
- 41. Ochiai K, Maienschein‐Cline M, Simonetti G, et al Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity 2013; 38: 918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudt LM, Singh H. Graded expression of interferon regulatory factor‐4 coordinates isotype switching with plasma cell differentiation. Immunity 2006; 25: 225–236. [DOI] [PubMed] [Google Scholar]
- 43. Shapiro‐Shelef M, Calame K. Regulation of plasma‐cell development. Nat Rev Immunol 2005; 5: 230–242. [DOI] [PubMed] [Google Scholar]
- 44. Lin KI, Angelin‐Duclos C, Kuo TC, Calame K. Blimp‐1‐dependent repression of Pax‐5 is required for differentiation of B cells to immunoglobulin M‐secreting plasma cells. Mol Cell Biol 2002; 22: 4771–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nera KP, Kohonen P, Narvi E, et al Loss of Pax5 promotes plasma cell differentiation. Immunity 2006; 24: 283–293. [DOI] [PubMed] [Google Scholar]
- 46. Lin WW, Adams WC, Nish SA, et al Asymmetric PI3K signaling driving developmental and regenerative cell fate bifurcation. Cell Rep 2015; 13: 2203–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ng LG, Ng CH, Woehl B, et al BAFF costimulation of Toll‐like receptor‐activated B‐1 cells. Eur J Immunol 2006; 36: 1837–1846. [DOI] [PubMed] [Google Scholar]
- 48. Stadanlick JE, Kaileh M, Karnell FG, et al Tonic B cell antigen receptor signals supply an NF‐kappaB substrate for prosurvival BLyS signaling. Nat Immunol 2008; 9: 1379–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sasaki Y, Sano S, Nakahara M, et al Defective immune responses in mice lacking LUBAC‐mediated linear ubiquitination in B cells. EMBO J 2013; 32: 2463–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kallies A, Hasbold J, Fairfax K, et al Initiation of plasma‐cell differentiation is independent of the transcription factor Blimp‐1. Immunity 2007; 26: 555–566. [DOI] [PubMed] [Google Scholar]
- 51. Tsuji S, Cortesao C, Bram RJ, Platt JL, Cascalho M. TACI deficiency impairs sustained Blimp‐1 expression in B cells decreasing long‐lived plasma cells in the bone marrow. Blood 2011; 118: 5832–5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Decker T, Pasca di Magliano M, McManus S, et al Stepwise activation of enhancer and promoter regions of the B cell commitment gene Pax5 in early lymphopoiesis. Immunity 2009; 30: 508–520. [DOI] [PubMed] [Google Scholar]
- 53. Farah BL, Sinha RA, Wu Y, et al Hepatic mitochondrial dysfunction is a feature of glycogen storage disease type Ia (GSDIa). Sci Rep 2017; 7: 44408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


