Abstract
Aim
Evidence is limited on how frailty affects the association between diabetes and adverse outcomes at the population level. The present community‐based study aimed to clarify the relative risks of death and disability in older Japanese adults with diabetes, frailty, both or neither.
Methods
The present prospective study analyzed data from 1271 Japanese residents aged ≥65 years in Kusatsu town in Gunma Prefecture, Japan, who participated in annual health checkups carried out between 2002 and 2011, and were initially free of disability. A Cox proportional hazards regression model was used to identify associations of diabetes and frailty with all‐cause mortality and incident disability.
Results
Among the 1271 participants, 176 (14%) had diabetes (mean hemoglobin A1c 7.5%, body mass index 24.2 kg/m2, 45% using diabetes medications) and 151 (12%) had frailty at baseline. Compared with non‐frail participants without diabetes, those with diabetes and frailty had higher risks of mortality (multivariable hazard ratio 5.0, 95% CI 2.4–10.3) and incident disability (hazard ratio 3.9, 95% CI 2.1–7.3). In contrast, non‐frail participants with diabetes did not have a significantly increased risk of mortality, although they had a higher tendency for the incidence of disability, as compared with non‐frail participants without diabetes.
Conclusions
At the population level, the risks of death and disability in persons with mild diabetes were strongly affected by the presence of frailty. From a community‐based perspective, diabetes‐related mortality and disability incidence might be reduced by preventing or improving frailty in conjunction with glycemic control. Geriatr Gerontol Int 2019; 19: 423–428.
Keywords: diabetes, disability, frailty, mortality, prospective cohort study
Introduction
Data from the International Diabetes Federation show that the number of adults with diabetes has been gradually increasing worldwide since the 2000s, and that the number of adults with diabetes aged 65–79 years will almost double, from 98 million in 2017 to 191 million in 2045.1 Accordingly, strategies to prevent diabetes‐related mortality and disability are required in order to prolong the healthy life expectancy of people with diabetes.
The risk of frailty has recently been acknowledged as a factor affecting the prognosis of older adults with diabetes. Older diabetes patients with frailty had more hospitalizations than did robust diabetes patients in a Chinese hospital,2 and a 3.6‐fold risk of disability at medical clinics in the American city of Saint Louis, Missouri, USA.3 In population‐based studies, diabetes and frailty had independent mutual effects on mortality in Canadian and Italian residents: in both populations, frailty had a greater effect on mortality than did diabetes itself. 4, 5 A Spanish longitudinal study showed that mortality and disability risks were associated with frailty severity among diabetes patients.6 These clinical and epidemiological studies, however, did not examine the combined effect of diabetes and frailty on death or disability, as compared with non‐diabetic adults. Additionally, no studies have reported how frailty affects the association between diabetes and adverse outcomes in a population in Asia, which, like other regions, has an increasing diabetes prevalence.1 As compared with Western countries, Asian countries have a similar or even higher prevalence of diabetes, despite lower rates of overweight and obesity.7
The aim of the present prospective, community‐based study was to investigate the relative risks of death and disability in older Japanese adults with diabetes, frailty, both or neither at the population level.
Methods
Study cohort
The study cohort comprised 1524 residents aged ≥65 years who participated in annual health checkups carried out between 2002 and 2011 in Kusatsu, a town in Gunma Prefecture, Japan. Almost all older residents who participated in annual preventive health checkups underwent a comprehensive geriatric assessment. Participants with pre‐existing disabilities (n = 71) and those with missing data (n = 182) were excluded. The data for the remaining 1271 participants (544 men and 727 women) were analyzed in the present study. Written informed consent was obtained from all participants after they were provided with a detailed explanation of the study protocol, which was approved by the ethics committee of the Tokyo Metropolitan Institute of Gerontology.
Measurement of baseline variables
The study protocol was previously described in detail.8, 9 Briefly, blood was drawn from an arm, regardless of fasting status, and immediately centrifuged to separate the serum. Total cholesterol, albumin, glucose, creatinine, hemoglobin and hemoglobin A1c (HbA1c) were measured with standardized methods. Estimated glomerular filtration rate was calculated by using the standardized formula of the Japan Society of Nephrology Chronic Kidney Disease Initiative: glomerular filtration rate (mL/min/1.73 m2) = 194 × (serum creatinine [enzyme method])−1.094 × (age)−0.287 × (0.739 for women).10 HbA1c values were originally measured using the method of the Japan Diabetes Society (JDS), but were converted to values used by the National Glycohemoglobin Standardization Program (NGSP) by using the following formula: HbA1c (NGSP) (%) = 1.02 × HbA1c (Japan Diabetes Society) (%) + 0.25%.11
The participants’ height was measured standing in stocking feet and weight was measured while they were wearing light clothing, and body mass index (BMI) was calculated as weight (kg) / height (m)2. Systolic and fifth‐phase diastolic blood pressures were measured on the right arm by trained nurses using an automatic blood pressure monitoring device using the oscillometric method after a 5‐min rest. We carried out interviews to ascertain smoking status, illness history (hypertension, hyperlipidemia, diabetes mellitus, stroke, heart diseases, cancer) and use of medications for chronic diseases, including hypertension, dyslipidemia and diabetes mellitus.
The comprehensive geriatric assessment included grip strength, gait speed, the Mini‐Mental State Examination,12, the short form of the Geriatric Depression Scale,13 and a brief questionnaire for frailty screening. Grip strength (kg) was measured twice in the dominant hand, with the participant squeezing a standard hydraulic handgrip dynamometer as hard as possible; the higher of the two measures was used in the analysis. Gait speed was measured over a straight 11‐m walkway marked with tape at 3 and 8 m. Well‐trained observers gauged the time required to walk 5 m at a usual speed and calculated usual gait speed (m/s).
Diabetes and frailty
Diabetes was defined in accordance with the diagnostic criteria of the American Diabetes Association 14 as a fasting (≥8 h) glucose concentration ≥ 7.0 mmol/L (126 mg/dL), a non‐fasting glucose concentration ≥ 11.1 mmol/L (200 mg/dL), an HbA1c ≥6.5%, or current use of antidiabetic medication. Frailty was defined as the presence of three or more of the following five modified components from Fried's phenotype:15 for weight loss, an answer of “yes” to the question, “Have you lost 2–3 kg or more in the past 6 months?”; for weakness, a grip strength <26 kg for men or <18 kg for women; for exhaustion, an answer of “no” to the question, “Do you feel full of energy?” on the Geriatric Depression Scale; for slowness, a usual gait speed <1.0 m/s; for low physical activity, an answer of “less than once a week” to the question, “How often do you usually go outdoors?”.16 Prefrailty was defined as the presence of one or two components, whereas non‐frailty was defined as the absence of all components.
Follow up and ascertainment of death and disability
The participants were followed until December 2015 to ascertain death and incident disability. During a mean follow‐up period of 8.1 years (maximum 13.4 years), 129 (10.1%) participants who moved out of the community were censored at the moving date. We ascertained all‐cause deaths by checking local registries and linking them with Japanese National Vital Statistics. Disability was considered present when participants were certified as needing care because of physical or cognitive disability by the Japanese Long‐term Care Insurance system.17 In the present study, incident disability was defined as new certification by the Long‐term Care Insurance system, and the date of Long‐term Care Insurance application was defined as the incident date of disability.
Statistical analysis
Analysis using a cumulative logistic regression model or analysis of covariance was used to test for differences in age‐ and sex‐adjusted means, and proportions of baseline characteristics according to frailty category and stratified by diabetes status. A Cox proportional hazards regression model was used to examine age‐ and sex‐adjusted cumulative survival and disability‐free rates; differences among non‐frail, prefrail and frail persons were stratified by diabetes status. Hazard ratios (HR) and 95% confidence intervals (CI) for all‐cause death and incident disability were calculated for prefrail and frail groups, and the non‐frail group was used as reference. Person‐years were calculated as the sum of individual follow‐up durations until the occurrence of death or disability, or emigration from the community, whichever occurred first. To reduce the effect of reverse causality, we calculated cumulative survival, disability‐free rates and HR for all‐cause mortality or disability for 1214 participants, after excluding those with a follow‐up period <1 year.
We adjusted for age and sex in the initial model for calculating HR, and further adjusted for other potential confounding variables, namely hypertension (systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg or antihypertensive medication use), high total cholesterol (≥5.69 mmol/L or use of cholesterol‐lowering medication), low total cholesterol (<4.65 mmol/L), low estimated glomerular filtration rate (<60 mL/min/1.73 m2), overweight (BMI ≥25.0 kg/m2), low BMI (BMI ≤20.0 kg/m2), anemia (hemoglobin <13.0 g/dL in men and <12.0 g/dL in women), hypoalbuminemia (serum albumin ≤3.8 g/dL), low Mini‐Mental State Examination score (≤23), history of stroke and current smoking. We assessed effect modification by the presence of diabetes by using an interaction term generated by multiplying the ordinal values for each frailty category (i.e. non‐frail = 1, prefrail = 2, frail = 3) by diabetes status (absence = 0, presence = 1). Probability values for statistical tests were two‐tailed. A P‐value of <0.05 was considered to show statistical significance. All analyses were carried out with IBM spss Statistics version 23 (IBM Corporation, Armonk, NY, USA).
Results
Among the 1271 participants, 176 (14%) had diabetes (mean HbA1c 7.5%, BMI 24.2 kg/m2, 45% used medications for diabetes), and 151 (12%) had frailty at baseline. Among participants using antidiabetic medications, 10% were insulin users. Frail participants with and without diabetes were more likely to be older and female (Table 1). Furthermore, frailty was positively associated with the use of antihypertensive medication, low total cholesterol, low Mini‐Mental State Examination, history of stroke in participants without diabetes and hypoalbuminemia in those with diabetes. Mean HbA1c was 7.4–7.6% and mean BMI was 24.0–24.5 kg/m2 in participants with diabetes, and approximately 5.5% and 23.0 kg/m2, respectively, in those without diabetes, regardless of degree of frailty.
Table 1.
Age‐ and sex‐adjusted risk characteristics at baseline, stratified by diabetes status and frailty category (Kusatsu Study, 2002–2011)
| Without diabetes | With diabetes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk characteristic | Overall | Non‐frail | Prefrail | Frail | P‐value | Non‐frail | Prefrail | Frail | P‐value | |
| n | 1271 | 361 | 601 | 128 | 52 | 106 | 23 | |||
| Women (%) | 57.2 | 49.9 | 62.6 | 76.6 | <0.001 | 17.3 | 42.5 | 82.6 | <0.001 | |
| Age (years) | 71.0 (5.6) | 69.1 | 71.1 | 76.1 | <0.001 | 69.6 | 71.0 | 72.5 | 0.047 | |
| HbA1c (%) | 5.78 (1.0) | 5.50 | 5.49 | 5.48 | 0.885 | 7.39 | 7.58 | 7.47 | 0.792 | |
| Antidiabetic medication use (%) | 6.4 | 0 | 0 | 0 | ‐ | 51.0 | 44.5 | 32.0 | 0.395 | |
| Hypertension (%) | 58.9 | 55.0 | 58.6 | 54.3 | 0.453 | 68.7 | 73.3 | 67.6 | 0.768 | |
| Antihypertensive medication (%) | 32.9 | 26.9 | 34.7 | 34.2 | 0.042 | 36.0 | 39.5 | 36.7 | 0.909 | |
| Total cholesterol (mmol/L) | 5.31 (0.91) | 5.39 | 5.30 | 5.21 | 0.129 | 5.07 | 5.23 | 5.52 | 0.192 | |
| High total cholesterol (%) | 41.5 | 43.6 | 42.5 | 40.8 | 0.858 | 30.6 | 33.4 | 51.0 | 0.244 | |
| Low total cholesterol (%) | 20.2 | 15.2 | 19.7 | 28.7 | 0.006 | 28.5 | 24.9 | 25.0 | 0.893 | |
| Low eGFR (%) | 26.6 | 25.8 | 27.0 | 32.5 | 0.385 | 21.2 | 19.6 | 41.5 | 0.088 | |
| Current smoker (%) | 18.9 | 17.0 | 19.3 | 16.8 | 0.585 | 21.2 | 19.5 | 40.3 | 0.113 | |
| Body mass index (kg/m2) | 23.2 (3.2) | 23.2 | 23.0 | 22.9 | 0.548 | 24.5 | 24.1 | 24.0 | 0.831 | |
| Overweight (%) | 25.8 | 25.3 | 23.0 | 25.9 | 0.631 | 38.7 | 34.0 | 38.2 | 0.826 | |
| Low body mass index (%) | 15.6 | 14.3 | 17.2 | 18.6 | 0.435 | 2.0 | 12.4 | 20.8 | 0.058 | |
| Anemia (%) | 8.4 | 9.7 | 7.8 | 10.4 | 0.469 | 8.6 | 3.6 | 16.0 | 0.085 | |
| Hypoalbuminemia (%) | 4.7 | 2.8 | 4.6 | 8.1 | 0.063 | 5.9 | 4.0 | 20.7 | 0.020 | |
| Low MMSE score (%) | 8.7 | 6.2 | 8.9 | 14.9 | 0.015 | 4.0 | 10.3 | 13.1 | 0.379 | |
| History of stroke (%) | 5.7 | 3.1 | 5.3 | 13.4 | <0.001 | 6.9 | 6.5 | 6.5 | 0.997 | |
Data are proportions for categorical variables or means (standard deviation) for continuous variables.
P‐values were calculated for difference among frailty categories.
eGFR, estimated glomerular filtration rate; MMSE, Mini‐Mental State Examination.
During follow up, we identified 275 deaths, 372 disabilities and 475 deaths or disabilities, including 172 persons who died after the disability occurred. Cumulative survival and disability‐free rates worsened greatly as frailty level increased in participants without diabetes (Fig. 1). In the diabetic group, the downward trend was similar but stronger in frail participants. In non‐diabetic participants, the risks of each adverse event, after multivariable adjustment, were 1.6‐ and approximately 2.0‐fold higher in prefrail and frail participants, respectively (Table 2). Among diabetic participants, the risks of all‐cause mortality and disability were also higher in frail persons. A significant interaction between the presence of diabetes and frailty category was noted for incident disability, but not for mortality.
Figure 1.
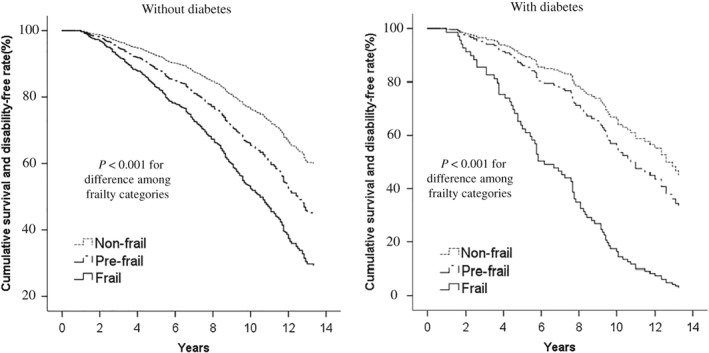
Cumulative survival and disability‐free curves, according to frailty category, in persons without and with diabetes (Kusatsu Study, 2002–2011, followed until 2015). A Cox proportional hazards model was used to adjust cumulative rates for age and sex according to frailty category.P‐values were calculated by using a Cox proportional hazards regression model in which ordinal numbers were assigned to each frailty category (non‐frail = 1, prefrail = 2, frail = 3).
Table 2.
Hazard ratios for all‐cause mortality and incident disability, stratified by diabetes status and frailty category (Kusatsu Study, 2002–2011, followed until 2015)
| Without diabetes | With diabetes | P for interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non‐frail | Prefrail | Frail | P for trend | Non‐frail | Prefrail | Frail | P for trend | |||
| No. at risk | 353 | 570 | 115 | 52 | 101 | 23 | ||||
| Mortality | ||||||||||
| Person‐years | 3213.7 | 5379.2 | 1133.6 | 498.0 | 880.6 | 166.6 | ||||
| No. cases | 44 | 132 | 51 | 9 | 29 | 10 | ||||
| Crude incidence/1000 person‐years | 13.7 | 24.5 | 45.0 | <0.001 | 18.1 | 32.9 | 60.0 | 0.008 | ||
| Age‐ and sex‐adjusted HR | 1.0 | 1.5 (1.1, 2.2) | 2.2 (1.4, 3.4) | 0.001 | 1.0 | 2.2 (1.0, 4.8) | 8.9 (2.9, 27.4) | <0.001 | 0.135 | |
| Multivariable HR | 1.0 | 1.6 (1.1, 2.3) | 1.9 (1.2, 3.0) | 0.003 | 1.0 | 2.1 (0.9, 4.9) | 6.6 (2.0, 22.0) | 0.003 | 0.190 | |
| Incident disability | ||||||||||
| Person‐years | 3006.6 | 4638.6 | 797.3 | 464.8 | 790.1 | 120.3 | ||||
| No. cases | 61 | 179 | 75 | 15 | 29 | 13 | ||||
| Crude incidence/1000 person‐years | 20.3 | 38.6 | 94.1 | <0.001 | 32.3 | 36.7 | 108.1 | 0.006 | ||
| Age‐ and sex‐adjusted HR | 1.0 | 1.6 (1.2, 2.1) | 2.4 (1.7, 3.5) | <0.001 | 1.0 | 1.0 (0.5, 2.1) | 3.4 (1.3, 9.2) | 0.034 | 0.102 | |
| Multivariable HR | 1.0 | 1.6 (1.2, 2.2) | 2.1 (1.5, 3.1) | <0.001 | 1.0 | 0.9 (0.4, 2.0) | 2.9 (1.0, 8.1) | 0.059 | 0.046 | |
| Mortality or incident disability | ||||||||||
| Person‐years | 3006.6 | 4638.6 | 797.3 | 464.8 | 790.1 | 120.3 | ||||
| No. cases | 82 | 224 | 88 | 20 | 45 | 16 | ||||
| Crude incidence/1000 person‐years | 27.3 | 48.3 | 110.4 | <0.001 | 43.0 | 57.0 | 133.1 | 0.002 | ||
| Age‐ and sex‐adjusted HR | 1.0 | 1.6 (1.2, 2.0) | 2.4 (1.7, 3.3) | <0.001 | 1.0 | 1.4 (0.8, 2.5) | 4.4 (1.9, 10.2) | 0.003 | 0.251 | |
| Multivariable HR | 1.0 | 1.6 (1.2, 2.0) | 2.1 (1.5, 2.9) | <0.001 | 1.0 | 1.4 (0.7, 2.5) | 3.7 (1.6, 9.0) | 0.007 | 0.159 | |
Hazard ratios (HR) were calculated for 1214 participants after excluding those with a follow‐up period <1 year.
Multivariable HR was adjusted for age, sex, hypertension, high total cholesterol, low total cholesterol, low estimated glomerular filtration rate, overweight, low body mass index, anemia, hypoalbuminemia, low Mini‐Mental State Examination score, history of stroke and current smoking.
Compared with non‐frail participants without diabetes, diabetic participants with frailty had higher risks of mortality (multivariable HR 5.0, 95% CI 2.4–10.3), incident disability (multivariable HR 3.9, 95% CI 2.1–7.3) and mortality or incident disability (multivariable HR 4.1, 95% CI 2.4–7.2; Fig. 2). Non‐frail participants with diabetes did not have a significantly increased risk of mortality (multivariable HR 1.1, 95% CI 0.5–2.2), although they had a higher tendency for the incidence of disability (multivariable HR 1.8, 95% CI 1.0–3.2).
Figure 2.
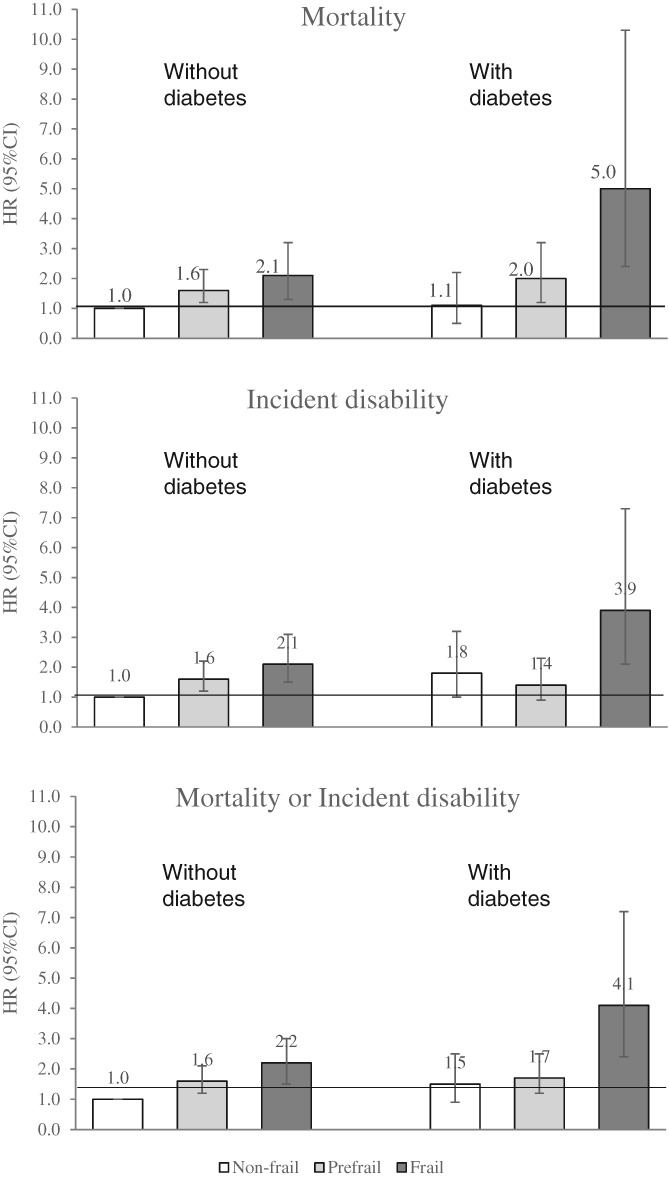
Multivariable hazard ratios (HR) and 95% confidence intervals (95% CI) for mortality and incident disability stratified by diabetes status and frailty category (Kusatsu Study, 2002–2011, followed until 2015). HR were calculated for 1214 participants after excluding those with a follow‐up period <1 year. The multivariable HR was adjusted for age, sex, hypertension, high total cholesterol, low total cholesterol, low estimated glomerular filtration rate, overweight, low body mass index, anemia, hypoalbuminemia, low Mini‐Mental State Examination score, history of stroke and current smoking.
Discussion
The present follow‐up study of 1271 community‐dwelling elderly Japanese over a mean of 8.1 years showed that the relative risks of all‐cause mortality and incident disability were significantly higher (by 5.0‐ and 3.9‐fold, respectively) in frail diabetic participants than in non‐frail participants without diabetes. In contrast, the risk of mortality in non‐frail diabetic participants was similar to those in non‐frail participants without diabetes, although the risk of incidence of disability was 1.8‐fold higher in non‐frail diabetic participants compared with those having neither diabetes nor any components of frailty.
To our knowledge, this is the first study to show the combined effect of diabetes and frailty on the incidence of death and disability at the population level. A previous follow‐up study of the effect of diabetes and frailty on outcomes for 1288 older Italians reported that the risk of all‐cause mortality increased with greater frailty in both diabetic and non‐diabetic participants.5 In addition, a follow‐up study of 1825 older Spaniards showed a positive correlation of frailty severity with risks of all‐cause mortality and disability in diabetic patients.6 These studies, however, did not report relative risks of death or disability in frail persons with diabetes compared with those without diabetes.
In the present study, diabetes patients had a mean HbA1c of 7.5%, the mean BMI was 24.2 kg/m2 and 45% used antidiabetic medications. The latter two values are lower than those in studies carried out in other countries, which reported a mean BMI among diabetes patients of 27.6–31.1 kg/m2 and that 67–96% used antidiabetic medications.4, 5, 6, 18, 19 This suggests that most of the present diabetes patients had mild diabetes. The present data therefore show that, at the population level, the risks of mortality and disability in individuals with mild diabetes are strongly affected by the presence of frailty. A large‐scale prospective study of approximately 350 000 UK adults aged 40–70 years reported a risk ratio of 2.79 for all‐cause mortality in diabetes patients with low grip strength and 1.36 in diabetes patients with high grip strength, as compared with non‐diabetic participants with high grip strength.18 Another follow‐up study of 3641 Japanese community residents reported that among participants aged >65 years, the risk of all‐cause mortality was significantly (4.2‐fold) higher for lean participants with diabetes than for participants without diabetes who had normal BMI; however, the risk was not significantly higher in participants with diabetes who had normal BMI.20 These findings support the present results.
The present study also showed positive associations of all‐cause mortality and disability with frailty in participants without diabetes, a finding consistent with those of previous population‐based studies. Although it is difficult to compare risk ratios between previous studies because of variation in the definitions used for frailty, previous studies reported that frailty increased the risk of all‐cause mortality by 1.2‐ to 2.2‐fold15, 16, 21, 22 and the risk of disability by 1.8‐ to 3.2‐fold,15, 16, 22, 23 as shown by multivariable‐adjusted risk ratios that included diabetes as an adjustment factor.
A limitation of the present study was that the number of persons with diabetes was small, because the study population was limited to individuals who had undergone health checkups in the local community. This limitation increased the confidence intervals of risk ratios for each outcome in the analysis of frailty severity in diabetic participants. In Japan, most patients receiving treatment for diabetes do not undergo community‐based health checkups, because they visit their primary care physicians. Therefore, the effect of diabetes on outcomes in the present study might be underestimated. A larger epidemiological study would yield a more accurate risk ratio for each outcome and for the interaction between diabetes and frailty. A second limitation was the small number of deaths, which made it impossible to analyze the data in relation to cause of death. Finally, we could not adequately adjust for some confounding factors associated with outcomes, including geriatric syndromes,24 such as sarcopenia;25 falls and fractures;26 diabetic complications;27 duration of diabetes;28 and socioeconomic status.29
Despite these limitations, the present study has several strengths. The comprehensive assessment, including metabolic risk factors and geriatric factors, enabled multivariate adjustment for a wide range of potential confounding variables. Additionally, complete long‐term follow up for death and disability, using national vital statistics and the mandatory long‐term care insurance system, enhances the reliability of the present findings. Furthermore, we measured serum glucose and HbA1c for all participants. These data provide an objective ascertainment of diabetes and allow for a more robust interpretation of how diabetes severity relates to the present findings. In previous population‐based studies, diabetes was ascertained by self‐reported diagnosis by a physician or pharmacological treatment.5, 6, 18
The present findings show that preventing or improving frailty might be beneficial, in conjunction with glycemic control, for extending healthy life expectancy in persons with diabetes. Dietary and exercise interventions should be developed for different levels of frailty in diabetic persons. Although weight control is basically a preventive measure for diabetes, restrictive diets might lead to worsening anemia, hypoalbuminemia and frailty. Various dietary options should be explored for diabetic persons according to the degree of frailty. Furthermore, the present study has practical implications for frailty assessment of older adults at community‐based health checkups, regardless of diabetes status. When frailty is suspected, the underlying disease should be identified and managed in a clinical setting, and a multifactorial intervention comprising resistance exercise, protein‐rich food intake and a psychosocial program should be used to improve frailty.30
In conclusion, the present prospective study of Japanese older adults shows that the risks of death and disability in individuals with mild diabetes are strongly affected by the presence of frailty. The risks of all‐cause mortality and incident disability among frail adults with diabetes were 5.0‐ and 3.9‐fold, respectively, those of non‐frail adults without diabetes. Non‐frail participants with diabetes did not have a significantly increased risk of mortality, although they had a higher tendency for the incidence of disability, as compared with non‐frail participants without diabetes. From a community‐based perspective, diabetes‐related mortality and incident disability might be reduced by preventing or improving frailty in conjunction with glycemic control.
Disclosure statement
The authors declare no conflict of interest.
Acknowledgements
The authors thank Hideki Ito, CEO of Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology, for his valuable advice, Izumi Tanaka for help with editing figures, and other colleagues of the Research Team for Social Participation and Community Health, Tokyo Metropolitan Institute of Gerontology. We also thank the staff members of the Public Health Center and the administrative staff in Kusatsu town for their collaboration.
This research was supported by Grants‐In‐Aid for Scientific Research (B) (grant numbers JP20390190, JP21390212, JP24390173, JP26310111 and 17H04140) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, by Research Funding for Longevity Sciences from the National Center for Geriatrics and Gerontology (grant number 29‐42), Japan, and by a Research Grant from the Japan Foundation for Promoting Welfare of Independent Entrepreneurs.
Kitamura A, Taniguchi Y, Seino S, et al. Combined effect of diabetes and frailty on mortality and incident disability in older Japanese adults. Geriatr. Gerontol. Int. 2019;19:423–428. 10.1111/ggi.13637
References
- 1. IDF Diabetes Atlas Eighth edition 2017. Diabetes in people older than 65 years. Available from https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/134-idf-diabetes-atlas-8th-edition.html. Accessed 18 Oct 2018
- 2. Li Y, Zou Y, Wang S, et al. A Pilot Study of the FRAIL Scale on Predicting Outcomes in Chinese Elderly People With Type 2 Diabetes. J Am Med Dir Assoc 2015;16:714.e7‐714.e12. [DOI] [PubMed]
- 3. Liccini A, Malmstrom TK. Frailty and sarcopenia as predictors of adverse health outcomes in persons with diabetes mellitus. J Am Med Dir Assoc 2016; 17: 846–851. [DOI] [PubMed] [Google Scholar]
- 4. Hubbard RE, Andrew MK, Fallah N, Rockwood K. Comparison of the prognostic importance of diagnosed diabetes, co‐morbidity and frailty in older people. Diabet Med 2010; 27: 603–606. [DOI] [PubMed] [Google Scholar]
- 5. Cacciatore F, Testa G, Galizia G, et al. Clinical frailty and long‐term mortality in elderly subjects with diabetes. Acta Diabetol 2013;50:251–260. [DOI] [PubMed] [Google Scholar]
- 6. Castro‐Rodríguez M. Carnicero JA2, Garcia‐Garcia FJ3, el al. Frailty as a major factor in the increased risk of death and disability in older people with diabetes. J Am Med Dir Assoc 2016; 17: 949–955. [DOI] [PubMed] [Google Scholar]
- 7. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009;301:2129–2140. [DOI] [PubMed] [Google Scholar]
- 8. Shinkai S, Yoshida H, Taniguchi Y, et al. Public health approach to preventing frailty in the community and its effect on healthy aging in Japan. Geriatr Gerontol Int 2016;16 Suppl 1:87–97. [DOI] [PubMed]
- 9. Taniguchi Y, Shinkai S, Nishi M, et al. Nutritional biomarkers and subsequent cognitive decline among community‐dwelling older Japanese: a prospective study. J Gerontol A Biol Sci Med Sci 2014;69:1276–1283. [DOI] [PubMed] [Google Scholar]
- 10. Matsuo S, Imai E, Horio M, Yasuda Y., Tomita K., Nitta K., Yamagata K., Tomino Y., Yokoyama H., Hishida A., Collaborators developing the Japanese equation for estimated GFR .; Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53:982–992. [DOI] [PubMed] [Google Scholar]
- 11. Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig 2012;3:39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 13. Burke WJ, Roccaforte WH, Wengel SP. The short form of the Geriatric Depression Scale: a comparison with the 30‐item form. J Geriatr Psychiatry Neurol 1991; 4: 173–178. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Classification and Diagnosis of Diabetes. Diabetes Care 2016;39 Suppl 1:S13‐22. [DOI] [PubMed]
- 15. Fried LP, Tangen CM, Walston J, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146‐156. [DOI] [PubMed] [Google Scholar]
- 16. Kitamura A, Shinkai S, Taniguchi Y, et al. Impact of frailty and metabolic syndrome on the incidence of loss of independence in community‐dwelling older Japanese: the Kusatsu‐town study. Nihon Koshu Eisei Zasshi 2017;64:593–606. [ In Japanese] [DOI] [PubMed]
- 17. Tsutsui T, Muramatsu N. Care‐needs certification in the long‐term care insurance system of Japan. J Am Geriatr Soc 2005; 53: 522–527. [DOI] [PubMed] [Google Scholar]
- 18. Celis‐Morales CA, Petermann F, Hui L, et al. Associations between diabetes and both cardiovascular disease and all‐cause mortality are modified by grip strength: evidence from UK Biobank, a prospective population‐based cohort study. Diabetes Care 2017;40:1710–1718. [DOI] [PubMed] [Google Scholar]
- 19. Gregg EW, Mangione CM, Cauley JA, et al; Study of Osteoporotic Fractures Research Group. Diabetes and incidence of functional disability in older women. Diabetes Care 2002;25:61–67. [DOI] [PubMed] [Google Scholar]
- 20. Yano Y, Kario K, Ishikawa S, et al; JMS Cohort Study Group. Associations between diabetes, leanness, and the risk of death in the Japanese general population: the Jichi Medical School Cohort Study. Diabetes Care 2013;36:1186–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang SF, Lin PL. Frail phenotype and mortality prediction: a systematic review and meta‐analysis of prospective cohort studies. Int J Nurs Stud 2015; 52: 1362–1374. [DOI] [PubMed] [Google Scholar]
- 22. Avila‐Funes JA, Helmer C, Amieva H, et al. Frailty among community‐dwelling elderly people in France: the three‐city study. J Gerontol A Biol Sci Med Sci 2008;63:1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wong E, Backholer K, Gearon E, et al. Diabetes and risk of physical disability in adults: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2013;1:106–114. [DOI] [PubMed] [Google Scholar]
- 24.Laiteerapong N1, Karter AJ, Liu JY, et al. Correlates of quality of life in older adults with diabetes: the diabetes & aging study. Diabetes Care 2011;34:1749–1753, Correlates of quality of life in older adults with diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim TN, Park MS, Yang SJ, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010;33:1497–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Volpato S, Leveille SG, Blaum C, Fried LP, Guralnik JM. Risk factors for falls in older disabled women with diabetes: the women's health and aging study. J Gerontol A Biol Sci Med Sci 2005; 60: 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tesfaye S, Stevens LK, Stephenson JM, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM complications study. Diabetologia 1996;39:1377–1384. [DOI] [PubMed] [Google Scholar]
- 28. Kalyani RR, Saudek CD, Brancati FL, Selvin E. Association of diabetes, comorbidities, and A1C with functional disability in older adults: results from the National Health and Nutrition Examination Survey (NHANES), 1999‐2006. Diabetes Care 2010; 33: 1055–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Regidor E, Franch J, Seguí M, Serrano R, Rodríguez‐Artalejo F, Artola S. Traditional risk factors alone could not explain the excess mortality in patients with diabetes: a national cohort study of older Spanish adults. Diabetes Care 2012; 35: 2503–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seino S, Nishi M, Murayama H, et al. Effects of a multifactorial intervention comprising resistance exercise, nutritional and psychosocial programs on frailty and functional health in community‐dwelling older adults: a randomized, controlled, cross‐over trial. Geriatr Gerontol Int 2017;17:2034–2045. [DOI] [PubMed] [Google Scholar]


