Abstract
While the study of dispersal and connectivity in the ocean typically centres on pelagic species and planktonic larval stages of benthic species, the present work explores an overlooked locomotor means in post‐settlement benthic stages that redefines their dispersal potential.
Members of the echinoderm class Holothuroidea colonize a diversity of marine environments world‐wide, where they play key ecological and economical roles, making their conservation a priority. Holothuroids are commonly called sea cucumbers or sea slugs to reflect their slow movements and are assumed to disperse chiefly through pelagic larvae.
The present study documents and explores their unexpected ability to actively modify their buoyancy, leading them to tumble or float at speeds orders of magnitudes faster than through benthic crawling. Two focal species representing different taxonomic orders, geographic distributions and reproductive strategies were studied over several years.
Active buoyancy adjustment (ABA) was achieved through a rapid increase in water‐to‐flesh ratio by up to 740%, leading to bloating, and simultaneously detachment from the substrate. It occurred as early as 6 months post settlement in juveniles and was recorded in wild adult populations. In experimental trials, ABA was triggered by high conspecific density, decreasing salinity and increasing water turbidity. Based on field video footage, ABA‐assisted movements generated speeds of up to 90 km/day.
These findings imply that displacement during planktonic larval stages may not supersede the locomotor capacity of benthic stages, challenging the notion of sedentarity. Combining the present results and anecdotal reports, ABA emerges as a generalized means of dispersal among benthic animals, with critical implications for world‐wide management and conservation of commercially and ecologically significant species.
Keywords: benthic organisms, dispersal, echinoderm, locomotor behaviour, marine organisms, movement ecology, sea cucumber
This study documents the unexpected ability of so‐called sessile or sedentary benthic marine animals (e.g. sea cucumbers) to actively modify their buoyancy to tumble or float at speeds orders of magnitudes faster than anticipated. These findings redefine the locomotor capacity of benthic animals, challenging the notion of sedentarity, with critical implications for worldwide management and conservation of commercially and ecologically significant species.

1. INTRODUCTION
Movement is among the fundamental components of life, and a key determinant of community structure (Bie et al., 2012), population and ecosystem connectivity (Baguette, Blanchet, Legrand, Stevens, & Turlure, 2013), and ecological and evolutionary processes (Nathan et al., 2008). Understanding why and how organisms disperse is also central to wildlife management and conservation (Allen & Singh, 2016). Overall, the interplay between adaptation and dispersal determines the persistence of species in a dynamic and ever‐changing world (Berg et al., 2010). Various dispersal attributes and strategies have evolved among terrestrial and aquatic organisms to offset the associated costs of movement (Bonte et al., 2012). Some organisms are motile throughout their lives, whereas others are adapted to undergo movement at precise, limited phases of their life cycles, commonly called the dispersive phase(s) (Allen, Metaxas, & Snelgrove, 2018). The life‐history strategies of organisms are often driven by the nature and circumstances of their dispersive phases (e.g. restricted or prolonged, active or passive). In the marine realm, movement ecology focuses on large pelagic megafauna (Hays et al., 2016; Sequeira et al., 2018), with fewer data on non‐vertebrate benthic taxa (Holyoak, Casagrandi, Nathan, Revilla, & Spiegel, 2008) despite the fact that they form the bulk of marine macrofaunal biodiversity.
Most benthic organisms exhibit a complex life history, whereby the early life stages are pelagic and the adults are either sessile (permanently anchored), such as barnacles, sponges and corals, or sedentary (exhibiting limited movement) such as many molluscs and echinoderms. While benthic stages can display transient movement (Winston, 2012), dispersal is commonly presumed to occur predominantly during planktonic embryonic and larval phases lasting days to weeks (Grantham, Eckert, & Shanks, 2003). Consequently, research on marine population structures and connectivity is chiefly centred on pelagic propagules (Cowen & Sponaugle, 2009), and secondarily on rafting of benthic juveniles or adults (Macfarlane Colin, Drolet, Barbeau Myriam, Hamilton Diana, & Ollerhead, 2013; Thiel & Gutow, 2005), although a more holistic view is increasingly being advocated (Allen et al., 2018; Pilditch, Valanko, Norkko, & Norkko, 2015).
Holothuroids (Echinodermata: Holothuroidea), also commonly known as sea cucumbers or sea slugs, are ubiquitous members of benthic communities extending from the poles to the equator and from the shores to the abyssal trenches, where they may represent up to 95% of the whole biomass (Heezen & Hollister, 1971). Many species have broad geographic distributions; for instance, Holothuria scabra occurs throughout the Indo‐Pacific and along most of the tropical Asian and Eastern African coasts (Hamel, Conand, Pawson, & Mercier, 2001), and Cucumaria frondosa is common in the Arctic and on both sides of the North Atlantic Ocean (Hamel & Mercier, 2008a). Several holothuroid species, including H. scabra and C. frondosa, are also commercially exploited in several regions of the globe (Purcell, Samyn, & Conand, 2012; Purcell et al., 2013). The notorious boom‐and‐bust pattern of wild fisheries has led the most prized species to the brink of extinction, earning them a spot on the IUCN Red List of endangered species (Anderson, Flemming, Watson, & Lotze, 2011; Purcell, Polidoro, Hamel, Gamboa, & Mercier, 2014). Apart from being one of the most sought after luxury seafoods, holothuroids play critical roles in several marine environments, from bioturbation to nutrient recycling (Purcell, Conand, Uthicke, & Byrne, 2016).
Our current understanding of holothuroid biology, including life‐history strategy, population structure/connectivity and biogeography, revolves around the notion of a sedentary adult with a dispersive larval stage. Many species are classified as sessile (Grantham et al., 2003), and estimates of benthic displacement through forward crawling in juveniles and adults range from a few centimetres to a few metres a day. For instance, H. scabra was found to cover 40–80 cm/day through crawling as a juvenile (Mercier, Battaglene, & Hamel, 2000) and 1.3 m/day as an adult (Purcell & Kirby, 2006). Short‐term average movement rates were 2–8 m/day in Bohadschia argus and 5–9 m/day in Thelenota ananas, yielding a long‐term average range of 15–47 m over 2 years (Purcell, Piddocke, Dalton, & Wang, 2016). Average displacements of 3.9 m/day have been reported in Parastichopus californicus (Da Silva, Cameron, & Fankboner, 1986). In C. frondosa, 12‐month‐old juveniles were shown to move up to 5 cm/hr (Gianasi, Hamel, & Mercier, 2018), equivalent to 1.2 m/day. In addition, some coastal species (including C. frondosa) can display enhanced contractions when encountering a predator (e.g. Legault & Himmelman, 1993; Margolin, 1976; So, Hamel, & Mercier, 2010), although these escape responses are of short duration and not considered true means of locomotion.
The present study revisits the dispersal capacity of juvenile and adult holothuroids in subtidal and intertidal environments, based on evidence that they commonly and predictably rely on semi‐pelagic means of locomotion that can be orders of magnitude faster than their podia‐assisted benthic crawling movements. A combination of experimental trials and observational data from the field, gathered in two species belonging to different taxonomic orders and native environments, suggests that juveniles and adults are capable of moving as efficiently as pelagic embryos/larvae. In challenging the notion that these long‐lived macrobenthic organisms strictly disperse during a brief period in their early life history, the findings have crucial implications for management and conservation initiatives.
2. MATERIALS AND METHODS
The two holothuroid species under study, Cucumaria frondosa and Holothuria scabra, are among the well‐studied echinoderms, both from biological/ecological and commercial perspectives, including studies in wild and captive individuals. Among others, papers have been published on their reproductive cycle, embryonic and larval development, settlement, growth, population genetics, feeding, movement, prey–predator interactions, chemical composition, as well as on the effect of various environmental stressors on their health (e.g. Hamel & Mercier, 2008a,b; Hamel et al., 2001; Mercier & Hamel, 2013).
2.1. Field studies
2.1.1. Adults of Cucumaria frondosa in Newfoundland and Nova Scotia (Eastern Canada)
At‐sea monitoring in Newfoundland was conducted off the south coast on St. Pierre Bank in the Northwest Atlantic Fisheries Organization (NAFO) Subdivision 3Ps (around 46°13′N 56°30′W). A first survey was conducted in August 2004 aboard the CCGS Shamook at depths of 45–52 m (3.5–4.5°C), and a second survey was conducted in August 2005 aboard the CCGS Templeman at depths of 41–57 m (2.9–5.0°C). Video footage of the seafloor was collected using a benthic sled (Lauth, Wakefield, & Smith, 2004) deployed over the stern of the vessel. An underwater camera (Simrad OE 1367) was mounted on the front of the sled and was angled slightly downward. Digital video was stored on an autonomous recording unit (Underwood, Winger, & Legge, 2012). Descent of the sled was monitored using a SCANMAR depth sensor, and bottom temperature was recorded using a VEMCO Minilog‐TR thermograph. Successful video transects were conducted at a total of 3 stations in 2004 and 6 in 2005, at a towing speed of 2.0 knots with on‐bottom durations ranging from 0.09 to 0.62 hr. All transects were made during daylight hours.
The video survey in Nova Scotia was conducted in the Shortland Canyon on the Scotian Shelf in NAFO Subdivision 4Vs (around 44°14′N: 58°28′W) using a Campod unit deployed from the CCGS Hudson between 220 and 300 m depth in July 2008. Campod, which is a static observation platform equipped with a high‐resolution video camera for viewing the seabed directly below and an oblique video camera for viewing the seabed ahead (Gordon et al., 2000), was deployed while the ship was slowly drifting (<1 knot; ~2 m from the seafloor) over the investigation site for 20–30 min on each set. The ship's position was used as a proxy for on‐bottom location.
For both locations, video footage was used to conduct an analysis of displacement and behaviour in C. frondosa. The number of individuals tumbling or floating, as well as their proportion relative to other individuals present on the seafloor, was assessed. Tumbling individuals are defined as those that drift with the current while remaining in constant or partial contact with the substrate (i.e. rolling and bouncing movements). Floating individuals are those that drift with the current without touching the substrate. Displacement speeds of individuals were calculated as the number of body lengths travelled per interval of 15 s relative to a reference point on the bottom (successive measures were taken to obtain a mean for each focal individual). The speed value over 15 s was extrapolated to m/min and daily displacement (km/day). Because only individuals travelling parallel to the viewpoint were considered, speed sample sizes (number of individuals analysed) varied as provided in the results. Based on their estimated length of 21–26 cm mouth‐anus (CSAR, 2006), individuals in the videos from Newfoundland and Nova Scotia were all adults.
2.1.2. Holothuria scabra in Madagascar
This study was conducted in offshore enclosures (Indian Ocean Trepang, IOT) located off Belaza, 25 km south of Toliara, Madagascar (23°29′S; 43°45′E). They were spread over 100 Ha, in the upper intertidal zone, 0.5–1.5 km from the high water mark, consistent with the typical habitat of H. scabra. Each of the seven focal enclosures covered an area of ~15,000 m2 and was seeded with a mean of 24,500 (± 5,500 SD) individuals obtained from spawning local wild broodstock. They were submerged with 1.5–2.5 m of water at high tide and 0.10 m at low tide. Freshwater channels a few metres wide and a few centimetres deep fed the enclosures at low tide (decreasing salinity to 18–30 psu). The other enclosures not under the influence of freshwater runoff were at 35–36 psu. Juveniles and adults of H. scabra were maintained in these enclosures at a density not exceeding 2 ind/m2, which is at the low end of densities reported for individuals in the wild (Mercier et al., 2000).
Every 15 days between April 2014 and November 2016, the number of tumbling/floating individuals was counted in 9–10 subsamples per enclosure (using standardized effort), and mean proportions derived from counts based on the total number of individuals held in each enclosure (established at night on a weekly basis). In addition, video footage was taken at night when most individuals were surfacing and tumbling/floating events were detected, towards the end of ebb tide when the water level was 0.3 m. The speed of displacement of tumbling or floating individuals was calculated using video clips, whereby the time to travel across 0.7‐m markers was measured.
2.2. Laboratory studies
2.2.1. Early juveniles of Cucumaria frondosa
Adults of C. frondosa (n = 200) measuring 11.0 ± 1.7 cm (± SD; n = 30) contracted body length were kept in two 500‐L tanks supplied with running ambient sea water (20 L/hr). Males and females spawned freely during the natural breeding season. Embryos and larvae (n = 150 per vessel) were incubated in three rearing vessels (~0.4 embryo/ml) consisting of 4‐L round containers with meshed openings (1 mm in diameter), placed inside a 40‐L tank supplied with running ambient sea water (20 L/hr). Natural light was provided through large windows following ambient photoperiod (from 15‐hr light/9‐hr dark in the summer to 8‐hr light/16‐hr dark during winter). Natural instances of active buoyancy adjustment or ABA (e.g. bloating, floating) were visually recorded on a daily basis in several cohorts of juveniles between post‐settlement until 2 years of age (~5–6 mm long). Photographs and measurements were taken under an automated stereomicroscope (Leica M205FA) using the associated software (Leica LAS‐X).
2.2.2. Adults of Cucumaria frondosa
To identify the drivers of ABA, trials were conducted in 20‐L tanks. The response of adult individuals (as described above) to ecologically relevant factors (from general knowledge of the species and preliminary experiments) was measured, using 4 population densities (1, 5, 10 and 15 individual/m2), 3 salinities [32, 26 and 22 psu; based on threshold tolerance for the species in So et al. (2010)] and 2 turbidity levels (pristine vs. 1‐L solution of detritic organic matter; mimicking turbidity flows generated by storms, tidal currents or trawling activities close to the seafloor). Except when otherwise mentioned, trials were conducted in triplicate with 4 individuals per tank following 2 hr of acclimation. The salinity was adjusted by slowly adding freshwater (~50 ml/min) to the tank; salinity was measured with a multiparameter probe (YSI 556 MPS). The load of turbidity water (suspended sediment concentration) was adjusted by adding the whole volume once; the number of particles and bacteria was established to be 1.4–2.2 × 109/ml (measured with a hemocytometer). Response metrics were recorded for 2 hr after the targeted parameter of each treatment was established, including orientation of the tentacles and their level of extension (relative to the substrate and the water surface), the shape of the body (degree of bloating) as well as the orientation of the anus (relative to the substrate and the water surface).
In distinct experiments, individuals of a similar size (as described above) that were either in the normal state or undergoing ABA (showing signs of morphological and behavioural changes) were compared for the strength of their attachment to the substrate, the amount of water they contained, and the rhythm of their cloacal respiration. The strength of attachment to the substrate was assessed in 24 normal individuals and in individuals undergoing ABA (22 stimulated by high conspecific density, 17 by turbidity and 20 by low salinity). This was done by gently placing a zip‐tie around their middle section to hook them to a digital precision spring balance (WeiHeng®) as per a method used with sea urchins (Santos & Flammang, 2007). The force (in Newton) necessary to detach the individual from the substrate was determined, and the number of podia used for anchorage was counted through the transparent glass wall of the tank. Another set of individuals exhibiting either weak ABA (induced by conspecific density) or severe ABA (from turbidity) were used to assess behaviour and importance of water intake during ABA. The shapes of the two groups of individuals (n = 19 of each) were monitored, including the precise orientation of the tentacles and anus relative to the rest of the body, the level of tentacle extension, and the ratio between total length (distance mouth‐anus) and mid‐body diameter. After they had reached the maximum size established during preliminary observations, their wet weight (bloated) was measured before making a longitudinal slit across the body wall to expose the organs (Polian vesicle, intestine, respiratory tree), which were punctured and drained. The water content was collected and weighed. The same procedure was repeated on a group of normal individuals (n = 18). A final experiment was undertaken to measure cloacal respiration rate (opening/min) in weakly and severely bloated individuals (n = 9 of each, induced by density and turbidity, respectively). The dilatation of the anus was also measured with a ruler at different times during ABA. Triplicates of all measurements were obtained from each individual. The same procedures were repeated on 7 individuals under normal holding conditions, which were firmly attached to the substrate and not showing signs of ABA.
To fine‐tune our understanding of the relationship between flow regime and the occurrence of ABA, trials were conducted in a large mesocosm. A population of ~1,000 adults of C. frondosa was held in a 34,500‐L flow‐through tank (11.5 m long × 2.5 m wide × 1.2 m deep) in ambient running sea water (30–60 L/min). An experimental flow‐through raceway (8.25 m long × 2.5 m wide × 0.85 m deep) was covered with gravel (1–3 cm diameter) to mimic the natural habitat where C. frondosa can be found. Grid markings were made on the dividing plate and the raceway, at 50‐cm intervals to allow measurement of movements and speed of displacement.
For the trials, 100 individuals of similar size (described earlier) were evenly spread, at a density considered equivalent to the high‐density level in experiments described above, and left to acclimate for 5 hr in static conditions before effecting a nominal flow of 200 cm/s (at the inflow). Precise flows were measured around focal individuals in the experimental arena using a hand‐held flow probe (Global Water, FP211). A time‐lapse video camera (Brinno, TLC 200 Pro) placed above the arena took one picture every minute for 3 hr. Pictures were automatically stitched together into a video output. To minimize the possibility of tank effects, 4 successive replicates were conducted, alternatingly placing the inflow in different locations. No individual was ever used for two successive trials. Based on analysis of the video footage, the proportions of crawling, tumbling and floating individuals were determined for the various flow regimes, and their respective speeds of displacement were measured.
2.3. Data analysis
Data on strength of attachment in adults of C. frondosa did not meet the assumptions of normal distribution and equal variance; the effect of ABA type on this variable was therefore tested using one‐way analysis of variance (ANOVA) on ranks followed by pairwise comparisons using Dunn's method. Data on water‐to‐flesh ratio (weight based) among independent groups of C. frondosa adults exhibiting different ABA levels were not normally distributed but exhibited equal variance and were thus tested using one‐way ANOVA followed by Holm–Sidak pairwise comparisons (nonparametric counterparts yielded the same results). The same approach was applied to proportions of tumbling individuals among different water flows. In H. scabra, data on proportions of individuals displaying tumbling/floating across locations did not meet the normality and equal variance assumptions; analysis was conducted using one‐way ANOVA on ranks followed by pairwise comparisons (Tukey's method). Data on the intensity of ABA reaction across seasons and lunar cycles in H. scabra were normally distributed and displayed equal variance, and were thus compared using two‐way ANOVA and Holm–Sidak post hoc tests. All data in the text are reported as mean and standard deviation (SD).
3. RESULTS
3.1. Adults of Cucumaria frondosa in the field
Occurrences of tumbling individuals (Supporting Information Video S1) were recorded in two geographic locations (located >400 km apart) in eastern Canada. In Newfoundland, the proportion of tumbling individuals ranged from 1.22% to 45% with individuals moving at speeds of 30 ± 6 m/min (n = 13). In Nova Scotia, the proportion of tumbling individuals reached 100% and they were determined to move faster, around 55 ± 9 m/min (n = 21). The holothuroids in Nova Scotia were tumbling across an area of muddy sand where no attached individuals could be detected. Conversely, a mix of attached and tumbling individuals was observed in Newfoundland, where the substrate was mostly composed of pebbles, small boulders or compacted sand. Based on the calculated speeds, it was estimated that individuals could travel 41.1 ± 7.7 km/day (Newfoundland) and 79.3 ± 7.6 km/day (Nova Scotia). In both locations, tumbling was recorded in the daytime (no data obtained at night). No special weather events (e.g. storms) or unusual environmental conditions were noted in the study areas at the time. Tumbling individuals were characteristically bloated with ambulacral podia and tentacles retracted (Supporting Information Video S1).
3.2. Juveniles of Cucumaria frondosa in the laboratory
A capacity to expand the entire body into a balloon shape was first detected when juveniles were 6 months old, in ~35% of the population (all individuals exhibiting this behaviour were doing it synchronously in three independent culture vessels). Bloated individuals were up to 3.8 times larger in volume than the normal juveniles (Figure 1a,b). The length of normal juveniles (mouth‐anus) was 1.8 ± 0.2 mm and their height (maximum distance from the dorsal to the ventral side) was 0.4 ± 0.1 mm compared to 2.1 ± 0.2 mm in length and 1.3 ± 0.1 mm in height for bloated individuals, representing an average increase of ~17% in length and ~225% in height. The ossicles covering the body wall of normal individuals were tightly packed and often overlapped. However, ossicles of bloated juveniles were spaced out over the body wall (Figure 1a,b). In the absence of current, the majority of bloated juveniles remained attached to the wall of the rearing tanks; however, 9% of individuals (n = 14) were seen floating at the surface of the water. The ABA reaction persisted for ~2 days before individuals resumed their normal body shape and position on the bottom of the tanks. No mortality was observed as a result of this reaction. Sporadic ABA was also noticed in older juveniles (>6 months of age); however, the frequency of occurrence was lower (~1% of the population). This behaviour was recorded both during the day and at night. No perceptible change in environmental factors (temperature, salinity) was noted while juveniles were exhibiting ABA.
Figure 1.

Illustration of posturing and behaviour involved during active buoyancy adjustment (ABA) in Cucumaria frondosa. (a) Juvenile in normal state exhibiting typical elongated shape, with tentacles deployed (right). (b) Juvenile exhibiting bloating (balloon shape) typical of ABA, with podia (p) and tentacles (t) deployed, and space visible between ossicles (o) on the surface of the body wall. (c) Adult in normal state with ventral podia attached, oral end elevated and tentacles deployed (t). (d) Adult detaching from the substrate and assuming a bloated shape during initiation of ABA. (e) Adult in middle stage of ABA with anus (a) becoming elevated. (f,g) Posture at culmination of ABA in adult, that is fully bloated, completely detached, neutrally buoyant, with anal end pointing upwards. Scale bars in A and B represent 0.5 mm; individuals in C to G are ~24 cm long (relaxed)
3.3. Adults of Cucumaria frondosa in the laboratory
Undisturbed (normal) individuals of C. frondosa remained firmly attached to the substrate with their tentacles either retracted or extended; the oral end occupied the highest position, that is farthest from the substrate, or closest to the water surface (Figure 1c).
After the treatments (density, salinity, turbidity) were applied, the following changes in general behaviour were recorded: decrease in the strength of attachment to the substrate, retraction of the tentacles (when initially extended), bloating of the whole body, and in some cases, change in orientation whereby the anus was in an upright position (above the oral end), followed by tumbling or floating when water current was present. These behaviours were categorized in two distinct intensity levels that were determined to be related to the intensity of the stimulus. The responses recorded during these experiments highlighted the fact that, depending on the severity of the stressor (from mild to life‐threatening), the sequence and/or intensity of responses varied.
3.3.1. Normal state (baseline metrics)
When kept in pristine natural sea water at low population densities (≤5 ind/m2) and normal salinity (~32–34 psu), individuals remained firmly attached, requiring a force of 803.4 ± 57.9 g (7.87 ± 0.57 Newtons) to detach them from the substrate (Figure 2). The number of podia used for anchorage fluctuated around 390 ± 55; several of these podia were torn during forcible detachment. The average wet weight of normal individuals was 352 ± 32 g, corresponding to an underwater weight of 6.7 g, ~12.5 cm length and 6.5 cm diameter (contracted condition). Water on average represented 31 ± 2% of the wet weight of normal individuals corresponding to a water:flesh ratio of 0.45 (Figure 3), and their tentacle crown was consistently upward (Figure 1c). The opening diameter of the anus was 1.7 ± 0.2 mm in the normal state, with a cloacal respiration rate of 0.99 ± 0.13 opening/min.
Figure 2.

Box plot of strength of attachment to the substrate in Cucumaria frondosa measured as force (N) necessary to forcibly pull away individuals in different states (at the peak of the reaction), comparing normal adults to adults exhibiting active buoyancy adjustment (ABA) in response to high conspecific density (weak ABA), water turbidity and low salinity (severe ABA). The box shows the mean in white (n = 17–24) with upper and lower quartiles, the whiskers show minimum and maximum values, and the circles show the outliers (5th and 95th percentile). Different letters denote statistically significant differences (one‐way ANOVA on ranks, H = 73.51, df = 3, p < 0.001; post hoc Dunn's method, p < 0.05)
Figure 3.

Pie charts showing per cent water content and box plot depicting water‐to‐flesh ratio (weight based) in normal adults and in bloated adults exhibiting weak or severe active buoyancy adjustment (ABA). The pies show mean proportions. Each box shows the mean in white (n = 18–19) with upper and lower quartiles, whiskers show minimum and maximum values, and the circles show the outliers (5th and 95th percentile). Different letters denote statistically significant differences between conditions (one‐way ANOVA, F 2,53 = 671.03, p < 0.001; post hoc Holm–Sidak method, p < 0.05)
3.3.2. Weak reactions
When exposed to high densities of 10 or 15 ind/m2, individuals generally displayed a posture and behaviour similar to those of the normal holothuroids except that the number of podia involved was reduced and the corresponding strength of attachment was significantly weakened (Figure 2). Inside a 20‐min period, the number of anchored podia varied from 53.7 ± 14.1 overall, and the force required to detach them varied between 38.0 ± 24.6 g (0.37 ± 0.24 Newtons; Figure 2) with a cloacal respiration rate of 0.9 ± 0.1 opening/min and an opening diameter of the anus of 1.7 ± 0.3 mm. Water content increased by ~75% compared to the normal state, representing on average 39 ± 5% of the body weight of individuals (Figure 3), which displayed a more rounded shape. The water: flesh ratio increased to 0.65 (Figure 3). A light water current was enough to detach such individuals.
3.3.3. Severe reactions
Compared to weak reactions induced by high conspecific density, ABA reactions to turbidity and low salinities (22–26 psu) developed more quickly and were more severe (Figure 2). Firstly, the number of attached podia decreased to 0.8 ± 2.2 within 5 min. The body shape started to become rounded, and the cloacal respiration increased to 3.9 ± 0.2 openings/min until maximum bloating of the whole body, after which it decreased to 0.7 ± 0.4 opening/min. The opening diameter of the anus increased to a maximum of 3.7 ± 0.4 mm at the peak of the bloated state. The reaction culminated with retraction of all podia and complete detachment of the bloated individuals from the substrate (Figure 1d,e) inside 10‐15 min, corresponding to an almost null or null force of attachment (1.4 ± 2.7 g, 0.01 ± 0.03 Newtons; Figure 2). Finally, there was a consistent change in the body orientation, switching from mouth‐up (Figure 1c) to anus‐up (Figure 1f,g). The total wet weight increased to an average of 1044 ± 134 g in fully bloated individuals; their underwater weight was close to 0 g; the average body length was 20.0 cm and the diameter 12.5 cm. Severely bloated individuals displayed a ~742% increase on average in their water:flesh ratio relative to normal individuals; water filled the respiratory tree, intestine, Polian vesicle and coelomic cavity, representing 78 ± 5% of the whole weight on average or a water:flesh ratio of 3.79 (Figure 3). These individuals were extremely buoyant, and the slightest water movement was enough to carry them away.
3.3.4. Flow experiments
There was a clear increment in the proportion of tumbling individuals from ABA responses induced by increased current (Figure 4). At flow regimes ≤40 cm/s, most individuals exhibited forward crawling and a few were rolling on their side while remaining in contact with the substrate (but not using their ambulacral podia). When exposed to flows between 41 and 120 cm/s, a majority of individuals started tumbling (Figure 4), and many were bouncing (periodically losing contact with the substrate). The ranges of displacement speeds measured in the tumbling individuals were 9.3 ± 3.0, 22.0 ± 5.2, 39.3 ± 13.2 and 54.7 ± 26.3 cm/s when the flow speeds were 0–20, 21–40, 41–80 and 81–120 cm/s, respectively. In normal individuals, the maximum forward crawling speeds averaged 0.013 ± 0.006 cm/s.
Figure 4.

Proportion of tumbling individuals of Cucumaria frondosa under various flow regimes in a large mesocosm. Data shown as mean (± SD) across 4 replicate trials. Different letters denote statistically significant differences between conditions (one‐way ANOVA, F 3,12 = 19.19, p <0.001; post hoc Holm–Sidak method, p <0.05)
3.4. Juveniles and adults of Holothuria scabra
In contrast to adults of C. frondosa where tumbling close to the bottom was the main ABA behaviour detected in the field, adults of H. scabra (>270 g wet weight) displayed both tumbling and floating (Supporting Information Video S2). Thousands of individuals were seen displaying ABA in 2014–2016, whereby they either floated near the sea surface at low tide (in 0.2–0.7 m depth) or tumbled on the sediment, and got carried away by the current. The speed of floating H. scabra was estimated to be 0.1 m/s, equivalent to 6 m/min, whereas tumbling speed was around 0.02 m/s. Normal individuals of this species exhibit a length:width ratio of 2:3, depending on contraction state, whereas bloated individuals had a 1:3 ratio. The wet weight of tumbling/floating individuals was 329.3 ± 91.0 g, compared to normal values of 298.7 ± 53.4 g. When tumbling or floating, both males and females became flabby, crescent‐shaped and slightly transparent under direct light (Supporting Information Video S2). ABA was related to the uptake of sea water in the respiratory tree and coelomic cavity. Tumbling and floating behaviours were only recorded during ebb tides (never during flood tides).
Between 33 and 73 tumbling/floating individuals of H. scabra were recorded by each observer during rounds conducted at the full or new moons every month for over 2 years, which was extrapolated to constitute between 0.5 and 4% of the entire surveyed population on any occasion. Instances of tumbling/floating occurred at unequal frequencies in the different enclosures monitored (mean conspecific density of 1.5 ind/m). The individuals housed in the offshore enclosures (35–36 psu) did not exhibit the behaviour (data not illustrated), whereas those in the six focal enclosures closest to land (18–27 psu) displayed the behaviour at frequencies that varied between 0.8% ± 0.1% and 2.9% ± 0.6% (Figure 5) with an average of 2.0% ± 0.8% (n = 294) over the course of the study. Enclosure B11 was the least affected by drops of salinities (27–30 psu), being located 600 m from the shore; it also showed the lowest proportion of individuals with ABA, that is 0.8% ± 0.1% (Figure 5). Overall, occurrences of ABA were higher during full moons than during new moons and more frequent during the cool than during the warm season (Figure 6). Although most frequent at night, ABA also occurred during the day. The location of tumbling/floating individuals at the full moon and at the new moons carried them to the west side of the enclosures where they accumulated, especially in the northwest corner, corresponding to the open sea. Several floating individuals found their way over the fences (Supporting Information Material S3) and were later seen hundreds of metres out at sea.
Figure 5.
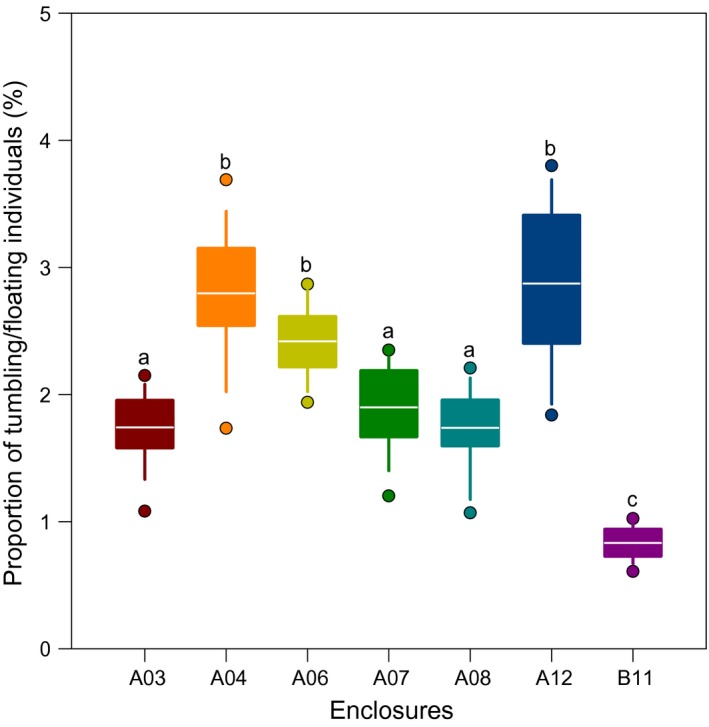
Proportion of individuals of Holothuria scabra displaying tumbling/floating from active buoyancy adjustment (ABA) in 7 focal enclosures around the full and new moons over the study. The box shows the mean in white (n = 42) with upper and lower quartiles, the whiskers show minimum and maximum values, and the circles show the outliers (5th and 95th percentile). Different letters denote statistically significant differences between pens (one‐way ANOVA on ranks, H = 219.16, df = 6, p < 0.001; post hoc Tukey's method, p < 0.05)
Figure 6.

Intensity of active buoyancy adjustment (ABA) reaction across seasons and lunar cycles in Holothuria scabra, shown as mean proportion (± SD) of individuals scored as “floating” or “tumbling” while monitoring the seven enclosures every 15 days in 2015–2016. Sample size is shown on each bar. Different lowercase letters denote statistically significant differences between moon phases in each season (two‐way ANOVA, F 1,38 = 20.43, p < 0.001; post hoc Holm–Sidak method, p < 0.005), and different capital letters denote statistically significant differences between seasons (two‐way ANOVA, F 1,38 = 22.73, p < 0.001)
4. DISCUSSION
Movement ecology represents a strategic link between animal behaviour and population dynamics that can be defined as the interplay between an individual's internal state, its motion capacity, its navigation capacity and external factors (Nathan et al., 2008). These topics are understudied in the marine realm compared to the terrestrial environment, in part because movement patterns in the ocean are uniquely shaped by the pelago‐benthic life history of most animal species, whereby adults are benthic and early life stages are pelagic (Walther, Munguia, & Fuiman, 2015). While focus has been placed on pelagic larvae that drift with the currents, it has been postulated that benthic adults may be the missing link in population connectivity relevant to fisheries biology and marine conservation studies (Frisk, Jordaan, & Miller, 2014). A recent review of movement ecology in marine animals with complex life cycles (i.e. the bulk of marine faunal biodiversity) further emphasized the importance of holistically considering all life stages (Allen et al., 2018). Interestingly, the movement of sessile or sedentary benthic adults through physical transport by currents was presented only as being either passive, that is following dislodgment, or indirect through dispersal of colonized substrates, known as rafting (Allen et al., 2018).
The present study highlights a different strategy, in the form of active alternation between self‐directed motion and physical transport, which evokes potentially more frequent and more predictable patterns of benthic locomotion under environmental control. Two species from drastically different climes and environments (C. frondosa and H. scabra) were shown to consistently react in a matter of minutes to undesirable environmental conditions and/or abnormally high conspecific densities. Instead of using their podia to crawl away, they actively underwent a change in body shape, water content (buoyancy) and strength of attachment to the substrate that allowed them to be carried away passively at speeds >1,000 times greater than crawling. Thereby, population metrics of sedentary organisms may be responding more quickly than expected to environmental, social and reproductive imperatives. Overall, a change in our perception of sedentarity in marine benthic animals appears to be warranted, towards a deeper integration of how behavioural adaptations modulate mobility and dispersal across all life stages.
Until now, accounts of “swimming” in adult holothuroids were thought to be unique to certain deep‐sea species (Rogacheva, Gebruk, & Alt, 2012), except for burst responses to predator encounters (Legault & Himmelman, 1993; Margolin, 1976). The unprecedented evidence provided here for subtidal/intertidal species, from combined experimental and observational work, underscores that dispersal in holothuroids (and other macrobenthic animals) may not be limited to the pelagic larval phase, but also occur during the benthic juvenile/adult stages through ABA. Such findings shed new light on a growing body of anecdotal reports (Supporting Information Material S3), including “ballooning” in cultured holothuroid juveniles (C. Hair pers. comm., University of the Sunshine Coast, Australia), “balling” in asteroid echinoderms (Sheehan & Cousens, 2017) and “inflating” in pennatulacean corals (Chimienti, Angeletti, & Mastrototaro, 2018), supporting that ABA‐assisted locomotion could be widespread, if not generalized. While most larval forms disperse through mid‐column or near‐surface currents, the potential contribution of bottom currents to ABA‐assisted migration suggests that dispersal of benthic species may not only occur over longer periods, but also take advantage of broader oceanographic processes than typically accounted for in plankton‐centric models (e.g. Cowen & Sponaugle, 2009). This could even lead to a shift in dispersal paradigms; over a lifetime, displacement might be equal or greater during the adult benthic stage than through larval dispersal. The present study also hints at predictable patterns of ABA‐assisted migrations (e.g. monthly, seasonally) likely to have significant impacts, from both ecological and economical perspectives.
Already, the occurrence of ABA and associated behaviours in Holothuroidea can help understand population structures, and variations thereof, which are not easily reconciled with a slow‐moving sedentary benthic lifestyle. For one, it may contribute to the broad or cosmopolitan distributions, as those seen in the focal species. In addition, large aggregations of C. frondosa have been reported around Newfoundland (CSAR, 2006). These patchy distribution patterns could be the result of mass migrations, such as those depicted here in the videos, perhaps in response to transient currents or turbidity flows (e.g. tides, storms). ABA and tumbling in C. frondosa may also explain bathymetric trends in size frequency distributions, whereby large individuals are typically found in greater number at deeper depths (Hamel & Mercier, 1996). Interestingly, sudden mass beaching (running aground) of holothuroids and other echinoderms has been reported, which could have involved ABA reactions (Supporting Information Material S3).
While the general ABA behaviours were similar in the two focal species, nuances were detected, which may or may not be related to the fact that the study on C. frondosa involved wild individuals and field surveys, whereas that on H. scabra involved captive individuals (albeit of first generation studied in close‐to‐natural settings). Only tumbling was commonly recorded in C. frondosa, whereas tumbling and floating were both consistently documented in H. scabra. In general, C. frondosa is found at subtidal depths on rocky or gravely bottoms (Hamel & Mercier, 1996), while H. scabra typically occurs on soft substrate at shallower depths, sometimes <1 m (Hamel et al., 2001). The latter therefore have a greater likelihood of coming in contact with air, which is suspected to assist in the floating behaviour by combining ABA with accumulation of air in the body cavity. Another difference between the focal species was the more predictable ABA behaviour evidenced in H. scabra juveniles and adults during certain lunar/tidal phases, which may be a dispersal strategy to counter drops in the quality or quantity of benthic food supply (organic matter) in sea‐ranching settings. In contrast, C. frondosa is a suspension feeder that captures plankton carried by the currents, virtually eliminating any need for periodic movement related to localized depletion of food.
The experimental segment on C. frondosa highlighted different strengths of ABA, which might afford a certain level of control over its use and outcome. When triggered by an increase in conspecific density (weak response), only minimum bloating (~40% increase in water‐to‐flesh ratio) and detachment were noted in C. frondosa. Under laboratory conditions, this behaviour persisted for days or weeks, as long as conspecific density was not decreased, and it disrupted normal feeding (which requires firm attachment to the substrate and deployment of the oral tentacles). This weak ABA response may be akin to a standby state, allowing the individual to be carried away opportunistically. This mechanism may prevent accumulations of holothuroids at bottlenecks or boundaries by helping to redistribute individuals more evenly after mass physical‐migration events. The latter appear to occur in response to greater stress, such as a sudden decrease in salinity or surge in turbidity, which triggered a more severe ABA response in the trials (>700% increase in water:flesh ratio). Like other echinoderms, holothuroids have a limited capacity for osmoregulation that makes them susceptible to low salinities (Meng, Dong, Dong, Yu, & Zhou, 2011). Under such adverse conditions, ABA was also linked to body reorientation in C. frondosa, from a normal upward to an unusual downward (anus‐up) posture, persisting as long as the stressor was present. Moreover, cloacal respiration, which is a known indicator of stress level in C. frondosa (Gianasi, Verkaik, Hamel, & Mercier, 2015), increased immediately after exposure to low salinity or high turbidity, possibly to help fill the body cavity and aid ABA. Once the response was established, slower cloacal respiration rates were recorded, presumably to limit exchanges with the adverse external environment.
Most ABA reactions, with the exception of positive buoyancy observed at the air–sea interface (discussed earlier), were not immediately conducive to movement, but instead dependent on the presence and strength of water flow. Therefore, ABA‐generated speeds were highly variable, but generally yielded greater displacements than the 1–2 m/day typically effected by forward crawling in the focal species (Gianasi et al., 2018; Purcell & Kirby, 2006) and the maximum crawling speeds measured here in adults of C. frondosa (12 m/day). Tumbling of C. frondosa (from camera tows) was estimated to generate displacement rates of up to ~90 km/day. In comparison, the pelagic embryos/larvae that develop over 40–45 days (Hamel & Mercier, 1996) are estimated to cover 17–20 km/day over that period (So, Uthicke, Hamel, & Mercier, 2011). Weak ABA generated by high conspecific densities was maintained as long as the stressor persisted (in the order of weeks under laboratory setting); similarly, severe ABA reactions started to subside only when conditions of turbidity or salinity returned to normal, supporting that ABA may underlie both short‐ and long‐term travel. Over the lifetime of the species, which is likely in the order of several decades (Ebert & Southon, 2003), dispersal of adults through ABA could be several orders of magnitude greater than larval dispersal. Movements through ABA are probably not rare either, since they were captured on video during random benthic surveys conducted hundreds of km and years apart. Conditions that trigger them may occur relatively frequently. Based on results obtained in the mesocosm, water flows between 20 and 120 cm/s induced tumbling behaviour. Overall, tumbling under laboratory conditions effected displacement speeds between 5 and 86 cm/s that represented ~70% of the concurrent flow regimes to which they were exposed. Tumbling events documented in the field may correspond to periods of changing tide and/or may have followed residual storm‐induced currents, which can fetch up to 110 cm/s over the Newfoundland Grand Banks (Wu, Tang, Li, & Prescott, 2011). Similarly, tumbling and floating in H. scabra were favoured by tidal currents along the coast of Madagascar, as evidenced by greater occurrence during the full/new moons and ebbing tides, although the greater intensity of ABA events at the full than at the new moon remains unresolved. Moreover, H. scabra exhibited ABA behaviour when the current was in an offshore direction, presumably to avoid being washed ashore. It was demonstrated during the mesocosm trials that individuals of C. frondosa were able to re‐anchor to the substrate after ABA events under water flows of similar strengths, emphasizing the “active” component of ABA. However, more experiments should be performed to better understand the limitation of this dispersive strategy.
While no direct comparison can be made with terrestrial taxa, due to the distinctiveness of the aquatic medium, a few interesting parallels may be drawn. Tumbling behaviour on land is best exemplified by tumbleweeds that break free of attachment to be carried by the wind (Baker, Beck, Bienkiewicz, & Bjostad, 2008). However, this adaptation involves the death of the parental unit to enable the dispersal of seeds. Indeed, much like dispersal in the ocean (Cowen & Sponaugle, 2009), dispersal in air largely centres on propagules (Cain, Milligan, & Strand, 2000; Howe & Smallwood, 1982), rather than on the adult and juvenile stages discussed here. One exception may be the transient aerial dispersal of adult arachnids using silk (dubbed “ballooning”), which has been described as a mixed evolutionary stable strategy (Bell, Bohan, Shaw, & Weyman, 2005). Overall, the drivers and implications of mixing directed small‐scale movements and undirected long‐distance dispersal (e.g. crawling and tumbling; walking and ballooning) deserve better integration in the conceptual frameworks of movement ecology (Nathan et al., 2008).
The occurrence of ABA in Holothuroidea is also of great significance for their management and conservation, since they are the target of important fisheries and aquaculture programmes (Hamel & Mercier, 2008a; Purcell et al., 2013, 2014). Rapid ABA responses could explain why well‐known fishing grounds for C. frondosa can start to yield dramatically decreased catches over short periods, and recover again (Quin Sea Ltd. meeting, 13 Dec 2017). Bottom trawling activity is known to generate increased turbidity (Palanques, Guillén, & Puig, 2001), which likely triggers ABA and momentarily reduces overall catchability (as neutrally buoyant individuals likely bounce off the gear, and could drift away). Such sudden variations in the catchability of C. frondosa due to ABA not only impact the fisheries, but also need to be factored in during stock assessments. On the other hand, certain areas may function as bottlenecks where holothuroids accumulate transiently after mass ABA events, as was suspected to occur in the highly dynamic tidal environment of the Passamaquoddy Bay (S. Rowe, pers. comm.). Should these areas become targeted by fisheries, increased catches could deplete the resource at much faster rates than estimated by monitoring programmes. The occurrence of ABA also has major implications for aquaculture and sea ranching. Until now, the loss of juveniles (seedlings) during the grow‐out phase was principally attributed to either mortality or predation. However, the main culprit may well be ABA, as shown here with H. scabra in Madagascar and suspected by other stakeholders in several regions, including Vietnam, Papua New Guinea and Malaysia (C. Hair pers. comm., World Aquaculture Conference symposium; E. Nesher pers. comm., Malaysia; Supporting Information Material S3). Moreover, climatic changes will likely drive increases in the occurrence and/or severity of storm events and rainy seasons, thereby stimulating ABA and tumbling/floating behaviours over the coming years, which could change the population dynamics and distribution range of focal holothuroids. For all these reasons, more attention should henceforth be given to ABA in painting a more holistic picture of the ecology and biogeography of so‐called sedentary benthic organisms.
AUTHORS’ CONTRIBUTIONS
J.‐F.H. and A.M. conceived and coordinated the study, analysed the data and led the writing of the manuscript; J.S., B.L.G. and E.M.M. collected and analysed laboratory data on C. frondosa; B.B. compiled the data on H. scabra; E.L.K. led the collection of field video data on C. frondosa in Nova Scotia; S.R. helped collect data on C. frondosa and provided intellectual input; P.D.W. led the collection of field video data on C. frondosa in Newfoundland; all authors contributed critically to the drafts and gave final approval for publication.
Supporting information
ACKNOWLEDGEMENTS
We would like to thank the Field Services of the Ocean Sciences Centre and the staff of the Marine Institute (Memorial University), as well as the crew and staff of Fisheries and Oceans Canada on board the CCGS Hudson in Nova Scotia, and the CCGS Templeman and CCGS Shamook in Newfoundland, for their valuable help. We are also thankful to the IOT sea pen Department for accepting to spend time wading in the water to collect these data. We would like to acknowledge the information, help and intellectual input provided by several people during the development of this study, including Cathy Hair (University of the Sunshine State), Igor Eeckhaut (University of Mons), Camille Lirette (DFO) and MacGregor Parent (Memorial University). Sincere thanks as well to two anonymous reviewers for their constructive comments. This research was partly supported by grants to AM from the Natural Sciences and Engineering Research Council of Canada and the Ocean Frontier Institute through an award from the Canada First Research Excellence Fund.
Hamel J‐F, Sun J, Gianasi BL, et al. Active buoyancy adjustment increases dispersal potential in benthic marine animals. J Anim Ecol. 2019;88:820–832. 10.1111/1365-2656.12943
DATA ACCESSIBILITY
Data associated with this study are available in the Dryad Digital Repository https://doi.org/10.5061/dryad.90103c0 (Hamel et al., 2018).
REFERENCES
- Allen, R. M. , Metaxas, A. , & Snelgrove, P. V. R. (2018). Applying movement ecology to marine animals with complex life cycles. Annual Review of Marine Science, 10, 3.1–3.24. [DOI] [PubMed] [Google Scholar]
- Allen, A. M. , & Singh, N. J. (2016). Linking movement ecology with wildlife management and conservation. Frontiers in Ecology and Evolution, 3, 155. [Google Scholar]
- Anderson, S. C. , Flemming, J. M. , Watson, R. , & Lotze, H. K. (2011). Serial exploitation of global sea cucumber fisheries. Fish and Fisheries, 12, 317–339. 10.1111/j.1467-2979.2010.00397.x [DOI] [Google Scholar]
- Baguette, M. , Blanchet, S. , Legrand, D. , Stevens, V. M. , & Turlure, C. (2013). Individual dispersal, landscape connectivity and ecological networks. Biological Reviews, 88, 310–326. 10.1111/brv.12000 [DOI] [PubMed] [Google Scholar]
- Baker, D. V. , Beck, K. G. , Bienkiewicz, B. J. , & Bjostad, L. B. (2008). Forces necessary to initiate dispersal for three tumbleweeds. Invasive Plant Science and Management, 1, 59–65. 10.1614/IPSM-07-009.1 [DOI] [Google Scholar]
- Bell, J. R. , Bohan, D. A. , Shaw, E. M. , & Weyman, G. S. (2005). Ballooning dispersal using silk: World fauna, phylogenies, genetics and models. Bulletin of Entomological Research, 95, 69–114. [DOI] [PubMed] [Google Scholar]
- Berg, M. P. , Kiers, E. , Driessen, G. , Van Der Heijden, M. , Kooi, B. W. , Kuenen, F. , … Ellers, J. (2010). Adapt or disperse: Understanding species persistence in a changing world. Global Change Biology, 16, 587–598. 10.1111/j.1365-2486.2009.02014.x [DOI] [Google Scholar]
- Bie, T. , Meester, L. , Brendonck, L. , Martens, K. , Goddeeris, B. , Ercken, D. , … Gucht, K. (2012). Body size and dispersal mode as key traits determining metacommunity structure of aquatic organisms. Ecology Letters, 15, 740–747. 10.1111/j.1461-0248.2012.01794.x [DOI] [PubMed] [Google Scholar]
- Bonte, D. , Van Dyck, H. , Bullock, J. M. , Coulon, A. , Delgado, M. , Gibbs, M. , … Saastamoinen, M. (2012). Costs of dispersal. Biological Reviews, 87, 290–312. 10.1111/j.1469-185X.2011.00201.x [DOI] [PubMed] [Google Scholar]
- Cain, M. L. , Milligan, B. G. , & Strand, A. E. (2000). Long‐distance seed dispersal in plant populations. American Journal of Botany, 87, 1217–1227. 10.2307/2656714 [DOI] [PubMed] [Google Scholar]
- Chimienti, G. , Angeletti, L. , & Mastrototaro, F. (2018). Withdrawal behaviour of the red sea pen Pennatula rubra (Cnidaria: Pennatulacea). The European Zoological Journal, 85, 64–70. 10.1080/24750263.2018.1438530 [DOI] [Google Scholar]
- Cowen, R. K. , & Sponaugle, S. (2009). Larval dispersal and marine population connectivity. Annual Review of Marine Science, 1, 443–466. 10.1146/annurev.marine.010908.163757 [DOI] [PubMed] [Google Scholar]
- CSAR (2006). Habitat utilization and density of sea cucumber (Cucumaria frondosa) on St. Pierre Bank, Newfoundland: Observations using a towed camera sled in 2004 and 2005, Fisheries and Marine Institute of Memorial University, St. John's, NL, Canada. [Google Scholar]
- Da Silva, J. , Cameron, J. L. , & Fankboner, P. V. (1986). Movement and orientation patterns in the commercial sea cucumber Parastichopus californicus (Stimpson) (Holothuroidea: Aspidochirotida). Marine and Freshwater Behaviour and Physiology, 12, 133–147. 10.1080/10236248609378640 [DOI] [Google Scholar]
- Ebert, T. A. , & Southon, J. R. (2003). Red sea urchins (Strongylocentrotus franciscanus) can live over 100 years: Confirmation with A‐bomb 14carbon. Fishery Bulletin, 101, 915–922. [Google Scholar]
- Frisk, M. G. , Jordaan, A. , & Miller, T. J. (2014). Moving beyond the current paradigm in marine population connectivity: Are adults the missing link? Fish and Fisheries, 15, 242–254. 10.1111/faf.12014 [DOI] [Google Scholar]
- Gianasi, B. L. , Hamel, J.‐F. , & Mercier, A. (2018). Morphometric and behavioural changes in the early life stages of the sea cucumber Cucumaria frondosa . Aquaculture, 490, 5–18. 10.1016/j.aquaculture.2018.02.017 [DOI] [Google Scholar]
- Gianasi, B. L. , Verkaik, K. , Hamel, J.‐F. , & Mercier, A. (2015). Novel use of PIT tags in sea cucumbers: Promising results with the commercial species Cucumaria frondosa . PLoS ONE, 10, e0127884 10.1371/journal.pone.0127884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, D. , Kenchington, E. , Gilkinson, K. , McKeown, D. , Steeves, G. , Chin‐Yee, M. , … Boudreau, P. (2000). Canadian imaging and sampling technology for studying marine benthic habitat and biological communities. In ICES 2000 annual science conference. ICES, Copenhagen, Bruges, Belgium.
- Grantham, B. A. , Eckert, G. L. , & Shanks, A. L. (2003). Dispersal potential of marine invertebrates in diverse habitats. Ecological Applications, 13, 108–116. 10.1890/1051-0761(2003)013[0108:DPOMII]2.0.CO;2 [DOI] [Google Scholar]
- Hamel, J.‐F. , Conand, C. , Pawson, D. L. , & Mercier, A. (2001). The sea cucumber Holothuria scabra (Holothuroidea: Echinodermata): Its biology and exploitation as beche‐de‐mer. Advances in Marine Biology, 41, 129–223. 10.1016/S0065-2881(01)41003-0 [DOI] [Google Scholar]
- Hamel, J.‐F. , & Mercier, A. (1996). Early development, settlement, growth, and spatial distribution of the sea cucumber Cucumaria frondosa (Echinodermata: Holothuroidea). Canadian Journal of Fisheries and Aquatic Sciences, 53, 253–271. 10.1139/f95-186 [DOI] [Google Scholar]
- Hamel, J.‐F. , & Mercier, A. (2008a). Population status, fisheries and trade of sea cucumbers in temperate areas of the northern hemisphere In Toral‐Granda V., Lovatelli A., & Vasconcellos M. (Eds.), Sea cucumbers. A global review of fisheries and trade. FAO Fisheries and Aquaculture Technical paper 516, Vol. 516 (pp. 257–292). Rome, Italy: FAO. [Google Scholar]
- Hamel, J.‐F. , & Mercier, A. (2008b). Precautionary management of Cucumaria frondosa in Newfoundland and Labrador, Canada In Toral‐Granda V., Lovatelli A., & Vasconcellos M. (Eds.), Sea cucumbers. A global review of fisheries and trade. FAO Fisheries and Aquaculture Technical paper 516 (pp. 293–306). Rome, Italy: FAO. [Google Scholar]
- Hamel, J.‐F. , Sun, J. , Gianasi, B. L. , Montgomery, E. M. , Kenchington, E. L. , Burel, B. , … Mercier, A. (2018). Data from: Active buoyancy adjustment increases dispersal potential in benthic marine animals. Dryad Digital Repository, 10.5061/dryad.90103c0 [DOI] [PMC free article] [PubMed]
- Hays, G. C. , Ferreira, L. C. , Sequeira, A. M. M. , Meekan, M. G. , Duarte, C. M. , Bailey, H. , … Thums, M. (2016). Key questions in marine megafauna movement ecology. Trends in Ecology & Evolution, 31, 463–475. 10.1016/j.tree.2016.02.015 [DOI] [PubMed] [Google Scholar]
- Heezen, B. C. , & Hollister, C. D. (1971). The face of the deep. London, UK: Oxford University Press. [Google Scholar]
- Holyoak, M. , Casagrandi, R. , Nathan, R. , Revilla, E. , & Spiegel, O. (2008). Trends and missing parts in the study of movement ecology. Proceedings of the National Academy of Sciences of the United States of America, 105, 19060 10.1073/pnas.0800483105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe, H. F. , & Smallwood, J. (1982). Ecology of seed dispersal. Annual Review of Ecology and Systematics, 13, 201–228. 10.1146/annurev.es.13.110182.001221 [DOI] [Google Scholar]
- Lauth, R. R. , Wakefield, W. W. , & Smith, K. (2004). Estimating the density of thornyheads, Sebastolobus spp., using a towed video camera sled. Fisheries Research, 70, 39–48. 10.1016/j.fishres.2004.06.009 [DOI] [Google Scholar]
- Legault, C. , & Himmelman, J. H. (1993). Relation between escape behaviour of benthic marine invertebrates and the risk of predation. Journal of Experimental Marine Biology and Ecology, 170, 55–74. 10.1016/0022-0981(93)90129-C [DOI] [Google Scholar]
- Macfarlane Colin, B. A. , Drolet, D. , Barbeau Myriam, A. , Hamilton Diana, J. , & Ollerhead, J. (2013). Dispersal of marine benthic invertebrates through ice rafting. Ecology, 94, 250–256. 10.1890/12-1049.1 [DOI] [PubMed] [Google Scholar]
- Margolin, A. S. (1976). Swimming of the sea cucumber Parastichopus californicus (Stimpson) in response to sea stars. Ophelia, 15, 105–114. 10.1080/00785326.1976.10425452 [DOI] [Google Scholar]
- Meng, X.‐L. , Dong, Y.‐W. , Dong, S.‐L. , Yu, S.‐S. , & Zhou, X. (2011). Mortality of the sea cucumber, Apostichopus japonicus Selenka, exposed to acute salinity decrease and related physiological responses: Osmoregulation and heat shock protein expression. Aquaculture, 316, 88–92. 10.1016/j.aquaculture.2011.03.003 [DOI] [Google Scholar]
- Mercier, A. , Battaglene, S. C. , & Hamel, J.‐F. (2000). Periodic movement, recruitment and size‐related distribution of the sea cucumber Holothuria scabra in Solomon Islands. Hydrobiologia, 440, 81–100. 10.1023/A:1004121818691 [DOI] [Google Scholar]
- Mercier, A. , & Hamel, J.‐F. (2013). Sea cucumber aquaculture: Hatchery production, juvenile growth and industry challenges In Allan G., & Burnell G. (Eds.), Advances in aquaculture hatchery technology (pp. 431–454). Cambridge, UK: Woodhead Publishing Ltd; 10.1533/9780857097460.2.431 [DOI] [Google Scholar]
- Nathan, R. , Getz, W. M. , Revilla, E. , Holyoak, M. , Kadmon, R. , Saltz, D. , & Smouse, P. E. (2008). A movement ecology paradigm for unifying organismal movement research. Proceedings of the National Academy of Sciences of the United States of America, 105, 19052–19059. 10.1073/pnas.0800375105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanques, A. , Guillén, J. , & Puig, P. (2001). Impact of bottom trawling on water turbidity and muddy sediment of an unfished continental shelf. Limnology and Oceanography, 46, 1100–1110. 10.4319/lo.2001.46.5.1100 [DOI] [Google Scholar]
- Pilditch, C. A. , Valanko, S. , Norkko, J. , & Norkko, A. (2015). Post‐settlement dispersal: The neglected link in maintenance of soft‐sediment biodiversity. Biology Letters, 11, 20140795 10.1098/rsbl.2014.0795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell, S. W. , Conand, C. , Uthicke, S. , & Byrne, M. (2016). Ecological roles of exploited sea cucumbers. Oceanography and Marine Biology: An Annual Review, 54, 367–386. [Google Scholar]
- Purcell, S. W. , & Kirby, D. S. (2006). Restocking the sea cucumber Holothuria scabra: Sizing no‐take zones through individual‐based movement modelling. Fisheries Research, 80, 53–61. 10.1016/j.fishres.2006.03.020 [DOI] [Google Scholar]
- Purcell, S. W. , Mercier, A. , Conand, C. , Hamel, J.‐F. , Toral‐Granda, V. , Lovatelli, A. , & Uthicke, S. (2013). Sea cucumber fisheries: Global review of stock status, management measures and drivers of overfishing. Fish and Fisheries, 14, 34–59. 10.1111/j.1467-2979.2011.00443.x [DOI] [Google Scholar]
- Purcell, S. W. , Piddocke, T. P. , Dalton, S. J. , & Wang, Y.‐G. (2016). Movement and growth of the coral reef holothuroids Bohadschia argus and Thelenota ananas . Marine Ecology Progress Series, 551, 201–214. 10.3354/meps11720 [DOI] [Google Scholar]
- Purcell, S. W. , Polidoro, B. A. , Hamel, J.‐F. , Gamboa, R. U. , & Mercier, A. (2014). The cost of being valuable: Predictors of extinction risk in marine invertebrates exploited as luxury seafood. Proceedings of the Royal Society B: Biological Sciences, 281, 20133296 10.1098/rspb.2013.3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell, S. W. , Samyn, Y. , & Conand, C. (2012). Commercially important sea cucumbers of the world FAO Species Catalogue for Fishery Purposes No 6, Rome.
- Rogacheva, A. , Gebruk, A. , & Alt, C. H. S. (2012). Swimming deep‐sea holothurians (Echinodermata: Holothuroidea) on the northern Mid‐Atlantic Ridge. Zoosymposia, 7, 213–224. [Google Scholar]
- Santos, R. , & Flammang, P. (2007). Intra‐and interspecific variation of attachment strength in sea urchins. Marine Ecology Progress Series, 332, 129–142. 10.3354/meps332129 [DOI] [Google Scholar]
- Sequeira, A. M. M. , Rodríguez, J. P. , Eguíluz, V. M. , Harcourt, R. , Hindell, M. , Sims, D. W. , … Thums, M. (2018). Convergence of marine megafauna movement patterns in coastal and open oceans. Proceedings of the National Academy of Sciences of the United States of America, 115, 3072 10.1073/pnas.1716137115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan, E. V. , & Cousens, S. L. (2017). “Starballing”: A potential explanation for mass stranding. Marine Biodiversity, 47, 617–618. 10.1007/s12526-016-0504-3 [DOI] [Google Scholar]
- So, J. J. , Hamel, J.‐F. , & Mercier, A. (2010). Habitat utilisation, growth and predation of Cucumaria frondosa: Implications for an emerging sea cucumber fishery. Fisheries Management and Ecology, 17, 473–484. 10.1111/j.1365-2400.2010.00747.x [DOI] [Google Scholar]
- So, J. J. , Uthicke, S. , Hamel, J.‐F. , & Mercier, A. (2011). Genetic population structure in a commercial marine invertebrate with long‐lived lecithotrophic larvae: Cucumaria frondosa (Echinodermata: Holothuroidea). Marine Biology, 158, 859–870. 10.1007/s00227-010-1613-3 [DOI] [Google Scholar]
- Thiel, M. , & Gutow, L. (2005). The ecology of rafting in the marine environment. II. The rafting organisms and community. Oceanography and Marine Biology: An Annual Review, 43, 279–418. 10.1201/CRCOCEMARBIO [DOI] [Google Scholar]
- Underwood, M. J. , Winger, P. D. , & Legge, G. (2012). Development and evaluation of a new high definition self‐contained underwater camera system to observe fish and fishing gears in situ. Journal of Ocean Technology, 71, 59–70. [Google Scholar]
- Walther, B. D. , Munguia, P. , & Fuiman, L. A. (2015). Frontiers in marine movement ecology: Mechanisms and consequences of migration and dispersal in marine habitats. Biology Letters, 11, 20150146 10.1098/rsbl.2015.0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston, J. E. (2012). Dispersal in marine organisms without a pelagic larval phase. Integrative and Comparative Biology, 52, 447–457. 10.1093/icb/ics040 [DOI] [PubMed] [Google Scholar]
- Wu, Y. , Tang, C. C. L. , Li, M. Z. , & Prescott, R. H. (2011). Modelling extreme storm‐induced currents over the grand banks. Atmosphere‐Ocean, 49, 259–268. 10.1080/07055900.2011.605271 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study are available in the Dryad Digital Repository https://doi.org/10.5061/dryad.90103c0 (Hamel et al., 2018).


