Abstract
Chronic kidney disease (CKD) affects more than 20 million people in the United States and the global burden of this disorder is increasing. Many affected individuals will progress to end stage kidney disease necessitating dialysis or transplantation. CKD is also a major independent contributor to the risk of cardiovascular morbidity and mortality. Tubulointerstitial fibrosis is a final common pathway for most causes of progressive CKD. Currently, there are no clinically available therapies targeting fibrosis that can slow the decline in kidney function. Although it has long been known that TGF-β signaling is a critical mediator of kidney fibrosis, translating this knowledge to the clinic has been challenging. In this review, we highlight some recent insights into the mechanisms of TGF-β signaling that target activation of this cytokine at the site of injury or selectively inhibit pro-fibrotic gene expression. Molecules directed at these targets hold the promise of attaining therapeutic efficacy while limiting toxicity seen with global inhibition of TGF-β. Kidney injury has profound epigenetic effects leading to altered expression of more than a thousand genes. We discuss how drugs targeting epigenetic modifications, some of which are in use for cancer therapy, have the potential to reprogram gene regulatory networks to favor adaptive repair and prevent fibrosis. The lack of reliable biomarkers of kidney fibrosis is a major limitation in designing clinical trials for testing CKD treatments. We conclude by reviewing recent advances in fibrosis biomarker development.
INTRODUCTION
Acute and chronic kidney disease epidemiology: scope of the problem.
Chronic kidney disease (CKD) affects ~15% of the US population. In many affected individuals, there is progression to end stage renal failure leading to a need for dialysis and transplantation.1 CKD is also a potent, independent risk factor for cardiovascular morbidity and mortality.2 Globally, the burden of kidney disease is also substantial and affects countries that span the range from low to high sociodemographic indices. In the United States, the crude incidence of end stage kidney disease (ESKD) is predicted to increase by 11%–18% through 2030.3 The contribution of CKD to years of life lost was forecasted to shift from the 16th to the 5th leading cause in the next 2 decades4; this is in sharp contrast to other top causes of years of life lost, which have stayed the same or declined. These epidemiological data highlight the importance of understanding mechanisms of CKD and translating this knowledge to effective therapies. In this review, we will highlight pathogenic mechanisms of kidney fibrosis that may be targeted with several drugs which are currently being evaluated in clinical development for various fibrotic disease indications (Table 1), or which might be targeted with various clinical-stage epigenetic modifier agents.
Table 1.
Selected antifibrotic agents in clinical development that modulate TGF-β activation, expression, or signaling
| Drug (company) | Type | Target(s) or mechanism | Last known status | Clinicaltrials.gov identifier or reference |
|---|---|---|---|---|
| GLPG1690 (Galapagos NV) | Small molecule | autotaxin | Ph III (recruiting) | |
| FG-3019/pamrevlumab (Fibrogen) | Antibody | CTGF | Ph II (completed) | |
| BG00011 (Biogen) | Antibody | αvβ6 | Ph II (recruiting) | |
| VPI-2690B (Vascular Pharmaceuticals) | Antibody | αvβ3 | Ph II (completed) | |
| IDL-2965 (Indalo Therapeutics) | Small molecule | αvβ1/3/6 | Ph I (active) | Company website |
| PLN-74809 (Pliant Therapeutics) | Small molecule | αvβ1/6 | Ph I (active) | Company website |
| (Morphic Therapeutics/Abbvie) | Small molecule | αvβ6 | IND enabling | Company website |
| TRK-250 (Toray) | siRNA | TGF-β1 | Ph I (recruiting) | |
| BBT-877 (Bridge Biotherapeutics) | Small molecule | autotaxin | Ph I (recruiting) | |
| RG-012 (Genzyme/Sanofi) | Oligonucleotide | miR-21 | Ph II (paused) |
Abbreviations: IND, investigational new drug; miR-21, microRNA 21; Ph, phase; siRNA, small interfering ribonucleic acid.
Nephrologists typically consider acute and chronic kidney failure to be distinct entities in clinical practice. However, we now recognize that these entities are interconnected syndromes.5 While the renal tubular epithelium has the capacity to regenerate after acute injury, multiple studies demonstrate that maladaptive repair leading to fibrosis is common. Moreover, individuals with CKD are more prone to acute kidney injury (AKI), which in turn accelerates the progression of CKD. AKI affects ~8%–16% of patients admitted to a hospital and its incidence is on the rise.6 AKI is associated with an 8.8-fold increase risk for CKD and a 3.3-fold increase in ESKD.7 It may account for up to 20% of the incidence of new chronic dialysis patients.7,8 However, there are no known effective therapies to prevent development of fibrosis after AKI. The 13th acute dialysis quality initiative concluded that research on the best treatment strategies for targeting progression after AKI is a major priority.9 Because AKI typically occurs in the hospital setting, there is a potential to intervene during the acute or early recovery phase to prevent the development of fibrosis and CKD.
Fibrosis is a final common pathway of progressive kidney disease.
Although a broad range of insults can initiate kidney injury, interstitial fibrosis is a final common pathologic mechanism of most causes of progressive CKD. Studies in animal models and humans support the conclusion that the degree of interstitial fibrosis and tubular atrophy are strongly correlated with the severity of CKD in diverse forms of kidney disease,10,11 even after adjusting for estimated glomerular filtration rate (eGFR), proteinuria, and clinicopathologic diagnosis.12 Thus, targeting pro-fibrogenic pathways is an important strategy to slow the progression of CKD. Diabetic kidney disease and hypertensive nephrosclerosis are leading causes of CKD and account for the majority of ESKD. However, with the exception of renin-angiotensin (RAAS) blockers, introduced more than 2 decades ago for treatment of glomerular diseases, there are currently no specific therapies to ameliorate fibrosis and slow the decline in renal function. Moreover, while RAAS blockers slow the progression of glomerular diseases associated with proteinuria, they do not arrest the disease or prevent the progression to ESKD.
MECHANISMS OF RENAL FIBROSIS
Overview of renal fibrosis mechanisms.
Genome-wide unbiased approaches have identified multiple pathways that are dysregulated in experimental models of kidney injury and human CKD tissue samples (reviewed in ref. 13). The application of single cell RNA sequencing to human kidney biopsies is likely to refine our understanding of signaling pathways and cell types involved in tubulointerstitial fibrosis.14 However, to date, much of our knowledge about mechanisms of fibrosis comes from studies in rodent models investigating specific signaling pathways. Persistent reactivation of WNT, Notch, and Hedgehog pathways, which are required for formation of multiple organs including the kidney, promote kidney fibrosis in various injury models. However, delineating the functional contribution of these pathways to fibrosis in experimental models is not straightforward. For example, the canonical Wnt pathway has pleiotropic effects that can promote regeneration, repair or fibrosis. The outcome of Wnt activation after injury appears to vary, depending on the cell type, stage, and type of injury (reviewed in ref. 15). Notch activation is associated with dedifferentiation of renal tubular epithelia, leading to expression of collagens, fibronectin, and other matrix proteins.16 The Snail and Twist transcription factors are also implicated in promoting fibrosis by inducing renal epithelial cell dedifferentiation or “partial” epithelial-mesenchymal transition (EMT). Recent studies demonstrate that dedifferentiated epithelial cells undergo G2-M cell cycle arrest and adopt a phenotype of cell senescence, which is associated with secretion of pro-fibrotic and inflammatory mediators.17 Among the secreted paracrine factors are hedgehog ligands, which promote differentiation of perivascular cells into activated myofibroblasts, key mediators of interstitial fibrosis.18,19 The functional importance of G2-M renal epithelial cell cycle arrest in kidney fibrosis was recently demonstrated by showing that deletion of cyclin G1 prevented G2-M arrest and ameliorated fibrosis.20 Recent studies also highlight the complex role of macrophages in renal injury and chronic fibrosis (reviewed in ref. 21). Macrophages exhibit significant plasticity and can adopt a variety of phenotypes. While M1 macrophages are pro-inflammatory and promote injury, the division into M1 and M2 subtypes likely represents an oversimplification. Different M2 subtypes may have opposing effects in determining the balance between renal repair and fibrosis. As has been the case with interstitial cells, as we refine our understanding of the heterogeneity of these cells and identify rare cell types, we will gain new insights into the role of macrophages in kidney fibrosis.
The above discussion illustrates that multiple factors and cell types participate in fibrogenesis. Moreover, the crosstalk between signaling pathways and cellular compartments adds to the complexity of these processes. However, since the seminal studies of Border,22 a large body of evidence has consistently demonstrated, with few exceptions, that TGF-β signaling plays a central role in promoting fibrosis in multiple organs in response to diverse types of injury. The challenge has been how to target this pathway to ameliorate fibrosis. In this review, we focus on 2 promising strategies to specifically disrupt TGF-β pro-fibrotic signaling. The first is the inhibition of integrins that mediate TGF-β activation and the recruitment and maintenance of myofibroblasts at sites of injury. Second, we examine the potential to dampen TGF-β-dependent gene expression through epigenetic modifications. These approaches have the potential to reduce pathologic fibrogenesis while limiting effects on the action of this cytokine in other physiological processes that may lead to undesirable effects.
TGF-β as a therapeutic target in CKD.
Forced expression of TGF-β in transgenic mice is sufficient to cause renal interstitial fibrosis and glomerulosclerosis,23,24 whereas inhibition of TGF-β by neutralizing antibodies or antisense oligonucleotides ameliorates kidney fibrosis in vivo.25 TGF-β expression is also correlated with progression of kidney disease in humans.26,27 This cytokine affects multiple pathways and cell types that influence the development of fibrosis and progressive decline in kidney function. TGF-β is a highly potent inducer of extracellular matrix (ECM) production and an inhibitor of enzymes that degrade matrix components. It promotes the differentiation and proliferation of quiescent myofibroblast precursors into activated collagen-secreting cells that lay down scar tissue.19,28 TGF-β promotes expression of the transcription factors Snail1 and Twist1 that drive a partial EMT in response to injury.29,30 Damaged epithelial cells in a dedifferentiated state of partial-EMT arrest in G2-M and secrete pro-fibrotic and pro-inflammatory cytokines, including TGF-β itself.17 A recent study demonstrated that TGF-β is a potent chemoattractant for macrophages in response to AKI. Disruption of this signaling pathway by genetic deletion of the TGF-β receptor type II reduced renal macrophage infiltration in 2 AKI models and ameliorated kidney fibrosis.31 In addition to activation of pro-fibrotic signaling in myofibroblasts and damaged epithelia, and promoting macrophage infiltration, TGF-β1 also mediates metabolic reprogramming in injured kidneys, thereby contributing to maladaptive repair. Human kidney tissue-derived tubules and mouse models of kidney fibrosis showed reduced expression of key enzymes and regulators of fatty acid oxidation.32 TGF-β1 administration reduced fatty acid oxidation and led to lipid accumulation in proximal tubule cells by reducing expression of PPARA and PPARGC1A, implicating TGF-β1 in metabolic reprogramming in response to kidney injury.
Thus, a large body of evidence in animal models and humans supports the conclusion that TGF-β plays a critical role in promoting kidney fibrosis by affecting multiple pathways and cell types, making it a prime therapeutic target. However, because TGF-β has such a broad range of functions in normal human physiological and pathologic processes, developing effective therapies that target this cytokine has proved very challenging. Global inhibition of TGF-β is associated with deleterious effects, especially with chronic fibrotic diseases such as CKD which require long-term treatment (reviewed in refs. 33 and 34). Moreover, global blockade of TGF-β may inhibit other actions, such as its anti-inflammatory properties that may promote proper repair.34 While multiple preclinical studies indicate therapeutic efficacy of TGF-β inhibition, including an additive effect to RAAS blockade,35,36 the few human studies undertaken have not shown beneficial clinical effects. A phase 2 clinical trial sponsored by Lilly using a neutralizing TGF-β antibody in diabetic kidney disease did not show clinical benefit.37 However, this trial had a number of significant limitations. A large percentage of the randomized patients (159/417) discontinued therapy before receiving the intended 12 months of treatment. Mean serum creatinine changes during the short follow-up period of 9 – 12 months reported in this study are not likely to have had adequate sensitivity to detect a beneficial effect in patients with diabetic kidney disease. The average increase in serum creatinine in the placebo group was only 0.33 ± 0.67 mg/dL during the study period. In addition, a substantial fraction of placebo-treated patients did not have any worsening of renal function during this short study period. As acknowledged by the study authors, this trial does not exclude a role for novel TGF-β inhibitors in diabetic kidney disease. The study highlights some of the challenges in testing novel treatments for chronic fibrotic diseases like CKD. The absence of sensitive biomarkers of progressive tissue fibrosis and the need for long-term follow-up to account for nonlinear and variable rates of disease progression are practical limitations. Another challenge is the lack of detailed phenotypic information resulting in a heterogeneous study population. Patients with diabetes, the most common cause of CKD, are rarely biopsied and a significant number of patients in clinical trials for diabetic kidney disease may have a different etiology for their CKD.38 We must also acknowledge that TGF-β may not be the main driver of fibrosis for all forms of kidney disease, and its role may vary between individual patients. The efficacy of TGF-β inhibition may depend on the type of injury, the main cell type it targets, and the stage of evolution of the fibrotic process.39 There may be a specific window of time in which inhibition of TGF-β activity can impact the disease process and this may only apply to a subset of patients. Therefore, to select patients likely to benefit from TGF-β inhibition, we need to evolve toward molecularly guided therapy for individual patients.40 Analogous to approaches in the cancer and rheumatology fields, we likely will also need multidrug regimens targeting nonoverlapping pathways to achieve therapeutic efficacy. Moreover, emergence of drug resistance, a major barrier in cancer therapy, could be a significant and underappreciated factor in treatment of fibrotic diseases. A recent comprehensive review summarized candidate therapies targeting inflammation, oxidative stress, and other processes implicated in the pathogenesis of progressive kidney disease.13 Targeting these processes in combination with TGF-β and RAAS blocking agents or selectively at different stages of disease when informed by molecular analysis may ultimately be necessary to achieve therapeutic efficacy.
NEW THERAPEUTIC APPROACHES FOR RENAL FIBROSIS
Targeting TGF-β activation and myofibroblasts in fibrotic diseases via inhibition of RGD integrins.
An emerging consensus in the field is that optimal targeting of TGF-β will need to be directed at selective signaling pathways, guided by more precise knowledge about mechanisms of TGF-β actions in specific cell types.33,34 A promising approach is to selectively target TGF-β in its extracellular microenvironment and prevent the transition from an inactive (latent) to an active state.41 Increased expression of TGF-β itself is not sufficient to increase its function, because TGF-β is synthesized and secreted by cells in an inactive state sustained by noncovalent association with Latent Associated Peptide (LAP). Together with LAP, TGF-β binds to latent TGF-β binding protein-1 (LTBP-1) which is a component of the ECM. The latent complex is abundantly present in most tissues,42 and therefore activation may be a more important mechanism of regulating the biological effects of TGF-β than its expression. Measuring total TGF-β in serum or even in kidney tissue is not likely to be nearly as informative for fibrosis compared with measurements of activated TGF-β or changes in its receptor-mediated signaling pathways.
In many organ injury states that lead to fibrosis, locally upregulated members of the integrin family are the primary mediators of latent TGF-β activation.33,43,44 Integrins are transmembrane cell adhesion and signaling receptors consisting of α and β subunits that connect the cytoskeleton with the ECM.45 These interactions do not simply serve an adhesive function. Rather they also convey to the integrin-expressing cell critically important information about its local environment. This occurs via finely tuned outside-in signal transduction pathways which when integrated with other signals such as those from growth factor or cytokine receptors can modify the balance between normal and pathologic responses. In mammals, 18 α and 8 β subunits noncovalently associate to form 24 different integrin heterodimers. Of these, all 5 incorporating the αv (alpha-vee) subunit (αvβ1, αvβ3, αvβ5, αvβ6, αvβ8) have been shown in vitro to bind and activate latent TGF-β through the amino acid sequence Arg-Gly-Asp (RGD) in the LAP. Once activated, TGF-β itself induces the expression of many of the same integrin subunits,46,47 thereby establishing a local positive amplification loop which can perpetuate fibrogenesis until the loop is disrupted by drugs or processes that interfere with TGF-β activation (Fig 1). TGF-β also participates in a positive feedback loop with Snail1, maintaining damaged renal epithelia in a dedifferentiated state.29 The existence of these autoamplification loops at the site of injury suggests that moderate reduction in TGF-β activity could have significant therapeutic benefits while limiting side effects seen with global shut down of TGF-β.
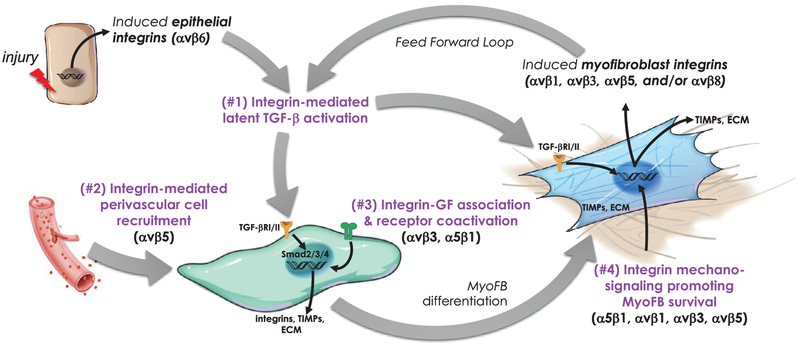
Fig 1.
Model illustrating 4 potential roles of RGD-binding integrins in the initiation, development, and perpetuation of organ fibrosis.1 Acute or chronic tissue injury locally upregulates the expression of integrins such as αvβ6 on epithelial cells which is a potent activator of the latent TGF-β stored in the surrounding ECM. The activated cytokine binds to its receptor on unactivated fibroblasts and initiates a SMAD-dependent signaling pathway culminating in the induction of dozens of target genes including the subunits of other RGD integrins, collagen, and other fibrotic matrix components, inhibitors of matrix degradation, and a constellation of other factors that promote transdifferentiation to a myofibroblastic phenotype.2 Evidence suggests myofibroblasts have a predominantly perivascular origin and that αvβ5 may have an important role in their recruitment to sites of developing fibrosis in kidney.3 Growth factors such as CTGF are capable of interacting with integrins on the cell surface and may provide cooperative signaling with TGF-β in the developing fibrotic response. Myofibroblasts exhibit expression of additional αv-containing integrins capable of activating more latent TGF-β setting up a feed forward loop.1,4 The myofibroblast integrins also act as sensors of ECM composition and stiffness which is important for maintaining their phenotype and survival, thus preserving the fibrotic niche and inhibiting resolution. ECM, extracellular matrix; myoFB, myofibroblast; TIMPs, tissue inhibitor of metalloproteases; TGF-βR1/RII, transforming growth factor receptor-I/II.
Independent of this direct biochemical role in TGF-β activation, RGD-binding integrins are important mechanosensors and transducers that provide cells such as myofibroblasts and their precursors information regarding the components and stiffness of their local ECM48,49 (Fig 1). There is intriguing evidence that fibrotic ECM adapts cells to a pro-fibrotic phenotype rather than vice versa.50 Furthermore, the RGD-containing protein fibronectin, which is bound and assembled by integrin α5β1, is robustly deposited in remodeling tissue and is essential to collagen matrix deposition and maintenance.51 Other studies have shown that upon limited proteolysis, collagen itself can become a ligand for αvβ3 and another unknown β1 integrin by exposure of a cryptic RGD motif, and that the resulting interaction with myofibroblasts confers dependence for their growth during the transition from quiescent to activated states.52,53 Similar matricryptic sites for RGD integrin binding have been described in tenascin, which may be significant as this extracellular matrix protein is expressed at high levels in the fibrotic kidney.54,55 Thus, interference with cellular signaling from the fibrotic matrix represents a novel second mechanism by which integrin antagonists may mitigate myofibroblast differentiation, migration, function, and survival.
Role of integrins in kidney disease.
Alpha v (αv) integrins are ubiquitously expressed in the adult kidney56 so there is potential for functional redundancy when these receptors are activated during repair processes. Complementary integrin-dependent roles also likely exist for driving fibrosis and other renal pathology. αvβ6 integrin is expressed on renal tubular epithelia and is upregulated in response to injury.57,58 Genetic deletion of the integrin β6 subunit confers significant protection from development of fibrosis and activation of TGF-β signaling in unilateral ureteral obstruction.57 In the Col4a3−/− mouse model of Alport syndrome, genetic deficiency of integrin β6 or administration of αvβ6 integrin blocking antibodies inhibited TGF-β-dependent gene expression and reduced renal fibrosis.59 In addition to Alport syndrome, recent studies showed that RGD integrin inhibition reduced proteinuria due to glomerular sclerosis in models of diabetes and focal segmental glomerulosclerosis.60,61 Kidney myofibroblasts isolated from mice have been shown to express 3 αv integrins (αvβ1, αvβ3, and αvβ5).62 Cell-selective αv deletion from the myofibroblast lineage protected in several organ injury models including kidney.44 In a model of chronic nephrotoxic injury due to adenine, a small molecule inhibitor of αvβ1 and some other β1-containing integrins ameliorated kidney dysfunction and fibrosis.62,63 As discussed above, all αv integrins are formally capable of activating latent TGF-β by direct binding, but may also have TGF-β-independent actions mediated by their ECM interactions, binding of growth factors, and vascular effects that support fibrosis (Fig 1).50,64–66 For example, in a rat ischemia-reperfusion injury model, specific antibody neutralization of αvβ5 diminished renal damage that correlated with decreased kidney pericyte adhesion, migration, and vascular permeability.66 The diverse interactions of integrins with pro-mitotic, proangiogenic, and pro-fibrotic growth factors and their roles in modulating their signaling and endocytosis have been reviewed previously.67,68 Connective tissue growth factor (CTGF), a strongly pro-fibrotic cytokine that is currently being clinically assessed as a target for treatment of lung fibrosis and other indications (Table 1), is a ligand for both α5β1 and αvβ3.64,69 Some studies suggest selective inhibition or knockout of certain integrins may have opposing effects in kidney.70,71 However, treatment with a small molecule that provided potent simultaneous antagonism of all RGD integrins shown in Fig 1 provided powerful efficacy in blocking or reversing fibrosis in many diverse murine organ injury models,44,72,73 including a model of fibrosis induced by acute nephrotoxic kidney injury from aristolochic acid (MR & DG, unpublished).
RGD integrin antagonists as antifibrotic agents.
Several integrin antagonists, both small molecules and biologics, have been successfully approved by the FDA for treatment of patients with cardiovascular and inflammatory conditions (eg, Reopro, Integrilin, Entyvio) and more are in active clinical trials.74 Other drugs, such as the αvβ3/αvβ5 inhibitor cilengitide and the α5β1 inhibitor volociximab were explored previously in cancer clinical trials and while generally shown to be safe, efficacy in this notoriously difficult population was disappointing.75–78 However, these agents were designed primarily to target tumor angiogenesis, and subsequent studies using knockout mice have shown the roles of these integrins in angiogenesis to be more complex than originally surmised.79,80 Indeed, in contrast to the inhibition of angiogenesis and tumor growth by cilengitide and some other αvβ3 inhibitors observed when cell or tissue exposures were maintained at high concentrations, low concentrations of these agents were paradoxically observed to promote angiogenesis in vitro and in vivo by stimulating the αvβ3-dependent recycling of VEGFR2 to the surface of endothelial cells.81 These data make it difficult to predict how angiogenesis in the context of the diseased kidney may be impacted by RGD integrin treatments. This is further complicated by the nature of angiogenesis and inflammation as interactive and interrelated processes. While angiogenesis that counters the microvasculature attrition found in many renal diseases would clearly be beneficial, persistent angiogenesis may become pathologic in supporting continued recruitment of inflammatory cells. Several studies have demonstrated efficacy of treatment with antiangiogenic agents in models of diabetic nephropathy.82–84 Finally, some of the same integrins expressed on the angiogenic endothelial cells are also expressed in vascular pericytes, which are strongly implicated as the predominant source of myofibroblasts that are mobilized into the interstitium during renal fibrosis. As was noted earlier in this review, αvβ5 antagonism improved vascular function in a renal model of ischemia-reperfusion,66 supporting it as an interesting target with the potential to confer protection from capillary dropout.
The rising excitement around the emerging data for the integrin roles in fibrosis has stimulated the recent advancement of several new candidates to clinical trials (Table 1). An antibody targeting αvβ6 (BG0001, Biogen) is in a phase II trial to treat pulmonary fibrosis, while a blocking antibody against αvβ3 (VPI-2690B, Vascular Pharmaceuticals) is currently being tested in a phase 2 trial of diabetic nephropathy. Because multi-integrin antagonists have been effective and well tolerated in animal models, at least 2 companies are actively pursuing clinical development of such agents, although it appears not initially in renal disease indications. Pliant Therapeutics has opened a Phase I trial for PLN-74809, an orally administered small molecule described as a dual αvβ1/αvβ6 inhibitor. Indalo Therapeutics is developing an orally administered small molecule that inhibits epithelial αvβ6 and myofibroblast-expressed RGD integrins. In contrast, a third company is performing IND-enabling studies with a selective αvβ6 small molecule inhibitor (Morphic Therapeutic).
Targeting TGF-β-SMAD signaling.
Pirfenidone has been approved for treatment of idiopathic pulmonary fibrosis. While its mechanism of action has not been fully elucidated, the drug reduces TGF-β expression and TGF-β-stimulated scar collagen production by myofibroblasts, in addition to anti-inflammatory properties.85 There are other drugs in various stages of investigation that modulate targets that act on or intersect with the TGF-β pathway. Autotaxin is a secreted enzyme that generates bioactive lipid mediators with profibrotic effects that include stimulating integrin-mediated TGF-β activation.86,87 Bone morphogenetic protein 7 (BMP) expression was suppressed after kidney injury and administration of recombinant BMP7 ameliorated kidney fibrosis in various preclinical models.88 The renoprotective action of BMP7 was attributed to its ability to antagonize TGF-β-mediated SMAD2/3 activity via the type I BMP receptor activin-like kinase 3 (Alk3).88,89 Kielin-chordin like protein (KCP) is a secreted factor that sequesters TGF-β from its receptor thereby reducing signaling, while also promoting ligand–receptor interaction and activity for BMP. Transgenic mice overexpressing KCP in the kidney reduced fibrosis in UUO and ischemia reperfusion injury models.90 Development of recombinant BMP7 or KCP proteins for clinical use is complicated by challenges with synthesis and potentially harmful effects of administering sufficient amount of protein to achieve therapeutic efficacy. Synthetic small molecule BMP agonists represent a viable alternative approach. A small peptide agonist of Alk3-mediated BMP signaling reversed fibrosis in multiple acute and chronic kidney injury models.89 A high throughput screen found that a benzoxazole compound potently stimulated canonical SMAD1/5/9-dependent BMP signaling91 and could be tested as an antifibrotic agent. The orphan nuclear receptor NR4A1 was shown to be an endogenous inhibitor of TGF-β/SMAD-dependent pro-fibrotic gene expression by recruiting a Sin3A-HDAC containing corepressor complex.92 Small molecule NR4A1 agonists effectively diminished fibrosis in several organs after injury, including the kidney.
Epigenetic regulation of kidney fibrosis.
Epigenetics can be defined as a heritable change in phenotype that does not involve a change to DNA. Epigenetic regulation of gene expression encompasses DNA methylation, nucleosome remodeling, post-translational modifications of histones, histone variants, and noncoding RNAs. DNA methylation at promoters can silence gene expression in a heritable pattern. Instances of epigenetic transmission due to DNA methylation are also clearly seen in imprinted genes, such as the Igf2/H19 locus, where differential methylation affects the activity of genomic enhancers. It has long been known that the proteins that bind DNA can modify gene expression. Post-translational modifications of amino-terminal histone tails play important roles in promoting regions of active and silent chromatin. For example, histone lysine 27 tri-methylation (H3K27me3) mediated by the Polycomb complex is typically associated with silent chromatin, while acetylation at this same residue (H3K27ac) is considered a marker of an active enhancer. However, increasingly, exceptions to these general rules are being discovered and therefore caution should be taken in inferring gene expression based on the pattern of histone modifications alone.93,94 Another important epigenetic mechanism involves chromatin-remodeling complexes, which can use the energy of ATP to alter the structure or change the position of nucleosomes, the basic repeating unit of chromatin. Nucleosomes can create a barrier to the transcriptional machinery. Multiprotein complexes, such as the Nucleosome Remodeling and Deactylase (NuRD) complex can mobilize nucleosomes and act together with enzymes that modify histones to alter the epigenetic landscape, thereby regulating genes expression. The discovery of the enzymes that mediate all these epigenetic modifications has created the potential for novel therapies. Drugs that target epigenetic regulation have entered the clinic in cancer treatment and they are attractive candidates for treatment of kidney fibrosis. Kidney injury leads to significant changes in gene regulatory networks resulting in pro-fibrotic gene expression and inhibition of pathways that promote proper repair of injured tissue. In theory, drugs that target the epigenome have the potential to reprogram deleterious gene expression and thereby prevent ongoing fibrosis.
DNA methylation.
Methylation of cytosine residues in CpG islands located in promoters is typically, though not uniformly associated with repression of gene expression. This biochemical modification is mediated by DNA methyltransferases (DNMTs 1–3) which transfer a methyl group from S-adenyl methionine to DNA. While previously considered a stable modification, studies show that DNA methylation is a dynamic process. The ten-eleven-translocation (Tet) family of enzymes mediate active oxidative demethylation ultimately resulting in replacement of 5-hydroxymethylcytosine with cytosine (Fig 2). Several studies in mice and humans point to an important role of DNA methylation in kidney fibrosis. Oba et al found promoter hypomethylation of TGF-β in mesangial cells from ob/ob diabetic mice leading to its increased expression95; reversal of demethylation decreased TGF-β mRNA expression and ameliorated glomerulosclerosis. Post-AKI fibrosis induced by folic acid increased Dnmt1 expression and heterozygous deficiency of Dnmt1 reduced fibrosis.96 These authors identified the ras inhibitor, Rasl1 as a target gene whose promoter was hypermethylated in kidney fibroblasts persistently activated after injury and thereby promoting fibrosis. TGF-β1 induced Dnmt1 expression, resulting in hypermethylation of Rasl1 in activated primary renal fibroblasts from injured kidneys, thereby linking TGF-β1 to epigenetic changes during fibrogenesis. In contrast, in an AKI-to-CKD ischemia model of kidney fibrosis Tet-3 expression was significantly reduced, enabling Rasl1 promoter methylation to persist.97 Hydralazine at low doses that does not affect blood pressure restored expression of Rasl1 and Bmp7 and reduced kidney fibrosis.97,98 This effect was mediated by induction of Tet-3-dependent demethylation of these genes.99 While hydralazine has long history for treatment of hypertension and heart failure based on its vasodilator properties, no studies have tested its efficacy for kidney fibrosis and CKD progression. While reversal of DNA methylation by Tet enzyme activation is a potential strategy to treat fibrosis, the large number of genes that are targets of these enzymes is a limitation if the goal is to selectively affect genes that are involved in fibrosis. To circumvent this problem, an alternative approach is to use CRISPR-Cas9 to target the Tet catalytic activity to specific gene loci. Using this approach to demethylate Rasal1 in interstitial cells and Klotho in tubular epithelial cells, Xu et al were able to restore gene expression and ameliorate fibrosis in an UUO model.100
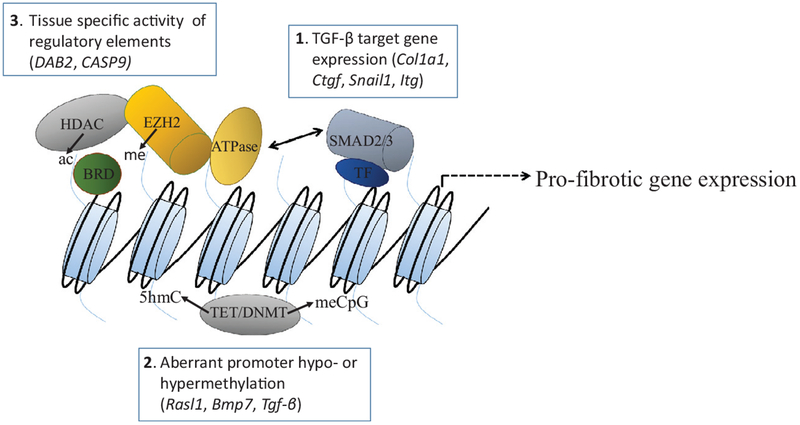
Fig 2.
Regulation of gene expression in response to kidney injury.1 SMAD2/3 in association with other transcription factors (TF) binds DNA and mediates TGF-β-dependent gene expression. Target genes include scar forming collagens (Col1a1), the profibrotic cytokine CTGF, and the gene encoding the Snail1 transcription factor, which mediates “partial” EMT/dedifferentiation of renal tubular epithelia. Increased expression of RGD-binding integrins (Itg genes) amplifies the activation of TGF-β. BMP7, another TGF-β family member, and other transcriptional regulators such as the orphan nuclear receptor NR4A1 (see text) can antagonize SMAD2/3 pro-fibrotic gene expression stimulated by TGF-β. Small molecule agonists of NR4A1 ameliorated fibrosis in an animal model.2 Methylation of promoters (meCpG) by DNA methyltransferases (DNMT1–3) can result in reduced expression of genes such as Rasl1 and Bmp7 that suppress deleterious pro-fibrotic and pro-inflammatory signaling or stimulate adaptive repair. In contrast, aberrant promoter hypomethylation leads to increased Tgfb expression, thereby promoting fibrosis. Ten-eleven-translocation (Tet) enzymes catalyze reactions that ultimately lead to hypomethylated DNA via 5-hydroxymethyl cytosine (5hmC) and other intermediate products. Therapeutic strategies that target aberrant DNA hypo- or hypermethylation at specific genomic loci have the potential to reduce fibrosis and enhance repair.3 TFs interact with multiprotein complexes that modify chromatin. These modifications alter the activity of regulatory elements, leading to tissue-specific changes in expression of genes such as DAB2 and CAS9 that impact repair/fibrosis. Histone deacetylases (HDACs) remove acetyl groups from lysine tails. Enhancer of Zeste Homolog (EZH2), an enzymatic component of Polycomb repressive complex 2 (PRC2) catalyzes histone lysine 27 trimethlyation, an epigenetic mark of repressed chromatin. Bromodo-main-containing proteins (BRD) bind acetylated lysine acetylated histones and recruit other proteins to chromatin to regulate gene transcription. Chromatin remodeling complexes contain helicases that use the energy of ATP to slide nucleosomes, thereby opening the chromatin to transcription factor binding to regulate gene expression. HDAC, BRD/BET, and EZH2 inhibitors ameliorated fibrosis in preclinical models of kidney injury (see text).
Interestingly, high inorganic phosphate leads to DNMT1 phosphorylation and consequent fibroblast activation, suggesting that the association of elevated serum phosphate and faster rates of CKD progression may be due in part to this mechanism.101 While not a direct epigenetic effect, the increase in DNMT1 activity by this post-translational modification is associated with aberrant CpG island methylation. Studies of kidney allograft tissue at the time of harvest for transplantation revealed that changes in DNA methylation attributed to cold ischemic time predicted fibrosis and allograft dysfunction 1 year after transplant.102 CpG hypermethylation due to reduced Tet-1 activity in allografts caused reduced expression of genes that suppress fibrosis. The fact that these epigenetic changes and associated alterations in gene expression preceded fibrosis indicates that these epigenetic marks could serve as biomarkers and highlights the potential for therapy targeting methylation to prevent fibrosis and progressive CKD.
Studies in CKD patients also support a pathogenic role for DNA methylation at selected genomic loci. Analysis of DNA methylation patterns in peripheral mononuclear cells in the chronic renal insufficiency cohort study revealed significant differences between patients with stable compared with rapid progression of CKD, including at TGF-β1 and other promoters.103 Ko et al compared DNA methylation patterns in healthy transplant kidneys with nephrectomy kidneys that exhibited moderate degrees of glomerulosclerosis and interstitial fibrosis.104 Differentially methylated regions occurred mostly at putative kidney enhancer regions and correlated with downstream gene expression. SMAD3 and SMAD6, mediators of TGF-β profibrotic gene expression, were among the hypomethylated genes that showed increased expression. In a follow-up study, the Susztak group analyzed expressed quantitative trait loci (eQTLs) in the glomerular and tubular compartments in relation to fibrosis in human kidney tissue.105 Interestingly, most of the candidate fibrosis-associated eQTLs were compartment specific, with the most significant enrichment (39%) for expression in proximal tubules, including PGAP3, CASP9, and MANBA. eQTL variants identified by GWAS were often localized to putative distal regulatory regions. Among the most promising candidate genes identified in this study was DAB2, which encodes an adaptor protein for the TGF-β pathway. Increased DAB2 expression correlated with fibrosis and a disease-causing variant of this gene localized to a kidney-specific distal enhancer defined by H3K27 acetylation. In mouse models of kidney injury, genetic reduction of Dab2 expression ameliorated fibrosis. Moreover, knockdown of Dab2 in primary proximal tubules cells reduced TGF-β-dependent SMAD2/3 phosphorylation, and collagen 1 and fibronectin expression.105
Histone modifications.
Several histone deacetylase (HDAC) inhibitors have been FDA approved for treatment of various cancers. Studies in animal models suggest that these drugs have therapeutic efficacy in organ fibrosis. HDACs are enzymes that regulate gene expression and protein function by removing acetyl groups from histones and from many functionally important nonhistone proteins, such as P53 (Fig 2). HDAC inhibitors have been shown to ameliorate kidney fibrosis in various models of kidney injury and fibrosis, including UUO, aristolochic acid nephrotoxicity, diabetic kidney disease, lupus nephritis, and transplant nephropathy.106–109 Many of these studies have been performed using compounds, such as trichostatin, vorinostat, and CG200745 that inhibit both class I and class II HDACs, although selective class I HDAC inhibitors, such as MS-275 (Entinostat) also reduced kidney fibrosis.110 deCaesteker and Hukriede used a zebrafish screen for compounds that increased embryonic kidney progenitor proliferation after gentamicin-induced AKI. This phenotypic screen identified the HDAC inhibitor phenylthiobutanoic acid (PTBA), which was subsequently shown to enhance survival of zebrafish after AKI.111 UPHD25, an analog of PTBA, was able to reduce fibrosis and enhance renal tubular epithelial repair when administered 24 hours after ischemic-reperfusion injury, and 4 days after aristolochic acid induced injury in mice.112 The fact that fibrosis could be ameliorated even if treatment was begun after injury, is a promising indicator for potential clinical utility. Because HDAC inhibitors have broad effects on gene expression, both activation and repression, and regulate the function of nonhistone proteins, multiple mechanisms have been proposed for its antifibrotic effects. Transcriptional profiling of human kidney tissue and mouse models of kidney fibrosis reveal altered expression in a large number of genes. Therefore, it is possible that the ability of HDACs to reprogram gene expression via deacetylation of lysine tails of histones plays a major role in its therapeutic efficacy. Among signaling pathways known to promote fibrosis, TGF-β and EGFR activity were suppressed by HDAC inhibitors.110 However, it is important to emphasize that the acetylation status of histones and nonhistone proteins can have broad effects on gene expression and protein function, affecting both pro- and antifibrotic pathways. Therefore, the efficacy of drugs that reduce or promote acetylation may be disease specific and depend of the stage of injury/repair.
Although less well studied than DNA methylation and histone deacetylation inhibitors, other drugs that target epigenetic modifiers have been investigated in kidney fibrosis. Enhancer of Zeste Homolog (EZH2), a component of the Polycomb repressive complex 2 (PRC2), catalyzes histone lysine 27 trimethylation (H3K27me3), an epigenetic mark associated with suppressed gene transcription (Fig 2). Pharmacologic inhibition of EZH2 ameliorated kidney fibrosis in the UUO model.113 Inhibition of EZH2 diminished signaling in several pathways that promote fibrosis including TGF-β/SMAD, EGFR, and PDGFR signaling. Bromodo-main and extra terminal (BET) proteins such as BRD4 recognize acetylated lysine residues on histones and recruit other proteins to chromatin to regulate gene transcription (Fig 2). Currently, BET inhibitors are in clinical trials for treatment of hematologic malignancies and some solid tumors. BET inhibition by the small molecule JQ1 reduced fibrosis and renal inflammation in several models of kidney injury.114,115
Noncoding RNAs.
Noncoding RNAs are another type of target being explored to inhibit pro-fibrotic gene expression. Wang et al discovered a kidney-enriched long noncoding RNA (lnc-TSI) that was induced in response to TGF-β.116 Lnc-TSI delivery to the kidney after UUO or ischemia reperfusion injury inhibited TGF-β/SMAD3 target gene expression and ameliorated fibrosis. Reduced expression of lnc-TSI correlated with more severe fibrosis and faster decline in renal function in a longitudinal cohort with IgA nephropathy, indicating the potential relevance of this noncoding RNA in human kidney disease. MicroRNA-21 (miR-21) is widely expressed in multiple cell types in the kidney, is upregulated by TGF-β, and has been linked to fibrosis through silencing of metabolic pathways that regulate ATP generation, reactive oxygen species production, and inflammatory signaling.117 Oligonucleotides inhibitors of this miRNA mitigated renal fibrosis and dysfunction in a mouse model of Alport disease,117 and have entered clinical testing (Table 1). Recent advances have led to improvements in the stability, specificity, and delivery platforms of oligonucleotide-based drugs, increasing their translational potential for the future.118
While the potential for epigenetic drugs to have broad effects on gene expression makes them attractive targets, this property might also cause a high likelihood of adverse effects. However, it appears that certain target genes are more sensitive to pharmacologic inhibition of the epigenome, enabling these drugs to be suitable candidates for translation to the clinic. Some have been FDA approved and others are in clinical trials mainly for cancer, but these could be tested in CKD in the future.
BIOMARKERS OF RENAL FIBROSIS
In contrast to the ready availability of noninvasive biomarkers of kidney function, biomarkers of kidney fibrosis are still in the exploratory stage. Tissue biopsy currently remains the only sure means of clinically diagnosing renal fibrosis but is not routinely performed in most CKD cases due to the discomfort and safety risks posed to patients. Moreover, samples cannot typically be obtained at multiple points over time, and the analysis of tissue from a single site may not be representative of the larger organ. Therefore, translational validation of noninvasive kidney-specific fibrosis biomarkers should be extremely useful in supporting clinical development of antifibrotic treatments by providing early mechanistic proof-of-pharmacology, improving the selection of appropriately staged patients into trials at each stage, and safely monitoring individual longitudinal responses to therapy over time.
Mansour et al recently performed a systematic review of all published clinical studies having biomarker data and identified 3 proteins detectable by ELISA in the blood or urine as having the strongest current evidence for predicting worsening renal function over time: TGF-β, MCP-1, and MMP-2.119 The authors noted several study limitations including that many of the evaluated studies were confined to specific renal disease conditions, and since biomarker performance varied across the different patient populations, caution in generalizing to the broad patient population is warranted. A study in which RNA sequencing was used to initially identify genes upregulated with fibrosis in a mouse injury model culminated in the identification of 3 candidate proteins detectable in human urine with levels that distinguished patients with CKD from healthy individuals: CDH11, MRC1, and PLTP.120 Many other exploratory biomarkers have been associated with fibrosis development and progression in animal models of AKI and CKD.
Urinary peptides.
While most data continue to support the promise of both serum and urine as useful compartments for minimally invasive sampling of a range of analytes, urinary peptides as opposed to full-length proteins appear to be emerging as particularly attractive for study.121–123 Being small, many are filtered from circulation and so are excreted under both physiological and pathologic conditions, and since they are often the products of ECM degradation, they are highly mechanistically relevant. They are expected to be more resistant than the parent proteins to further proteolytic processing at the temperature in the bladder and can be directly detected by ELISA or by liquid chromatography/mass spectrometry without need for prior tryptic digestion, thus removing a significant source of variability. Studies using clinical specimens have frequently identified collagen fragments with a negative correlation to fibrosis,123,124 and this has been hypothesized to reflect reduced ECM degradation in the face of increased deposition. In contrast, specific assays have been developed to measure specific collagen degradation fragments or neoepitopes that are elevated in the urine and/or serum in association with kidney fibrosis. For example, low molecular weight peptides C1M and C3M which are derived from the prototypical scar collagens type I and III, respectively, were increased in urine 9–100-fold in 3 different rat models of renal fibrosis and were closely correlated with collagen remodeling in tissue.125 Putative-specific biomarkers of collagen formation rather than degradation are Pro-C3, a neoepitope of the N-terminal pro-peptide of type III collagen, and endotrophin, which is released from collagen VI during its formation.126,127 Pro-C3 was shown to be significantly increased in the urine of 5/6 nephrectomy rats,125 and tracked liver fibrosis in human patients with chronic hepatitis.128 Collagen VI (COL VI) is upregulated in fibrotic kidney tissue in mouse models and human samples,129 and recent studies showed that endotrophin levels were useful to predict CKD progression.126,127 Assays have been developed for the cleavage products of collagen type IV, laminin α5, and laminin γ1 (Nordic BioScience, Denmark); these have potential to be useful to report ECM remodeling in the glomerular basement membrane and for tracking progression in glomerular diseases such as Alport syndrome.
Overall, although biomarkers based on assays of ECM-derived peptides have many advantages, this approach is complicated in that their plasma levels may reflect remodeling in compartments throughput the whole body, and analysis of urine levels may be confounded by comorbidities and by changes in kidney filtration functions with advancing disease. MicroRNA biomarkers are being explored as an alternative approach to peptides and a recent meta-analysis provided a composite signature of seven miRNAs which appears dysregulated with renal fibrosis.130 Finally, a variety of imaging techniques continue to be actively investigated to noninvasively measure parameters such as tissue stiffness in fibrotic kidneys.131–134
SUMMARY AND CHALLENGES
The many advances in understanding the pathogenesis of kidney fibrosis has not yet resulted in new therapies being successfully translated to the clinic. CKD is typically a silent disease that progresses over years and there are no reliable biomarkers or imaging modalities to predict clinical outcomes. Consequently, clinical trial design is challenging, limiting the power of these studies to demonstrate efficacy of new treatments. In addition, inclusion of heterogeneous disease cohorts in past clinical trials reduced the likelihood of identifying a subset of patients who may respond to a particular drug. Therefore, there is a need for more precise molecular phenotyping of CKD patients, an approach that will likely require a more liberal use of kidney biopsy. A critical role for TGF-β signaling in promoting organ fibrosis has been known for many years, yet to date no therapy targeting this pathway has reached patients with CKD. Finding the right balance between antifibrotic activity and toxicity has been an issue because of the effects of this cytokine on diverse cell types and biological processes. Approaches targeting activation of TGF-β at the site of injury or pathways that selectively inhibit TGF-β/SMAD-dependent profibrotic gene expression hold some promise. Because kidney injury alters expression of many genes, attempts to reprogram gene regulatory networks to promote regeneration and repair is a viable alternative to targeting a specific signaling pathway. Drugs that modify the epigenome, some of which are FDA approved for cancer therapy, are antifibrotic agents worthy of further investigation. Finally, in designing future clinical trials, we should take into account the need to reduce cardiovascular morbidity and mortality in CKD patients.
ACKNOWLEDGMENTS
This work was supported by Merit Award to M.R. from the Department of Veterans Affairs (#BX-003674). All authors have read the journal’s authorship agreement and the manuscript has been reviewed and approved by all named authors. All authors have read the journals policy on disclosure of potential conflicts of interest. Dr Rauchman has no conflicts of interest to declare. Dr Griggs owns intellectual property rights and stock in, received grants from, and consults for Indalo Therapeutics.
Abbreviations:
- AKI
acute kidney injury
- BET
bromodomina and extra terminal protein
- BMP7
bone morphogenetic protein 7
- CKD
chronic kidney disease
- DNMT
DNA methyltransferase
- ESKD
end stage kidney disease
- EZH2
enhancer of Zeste Homolog
- HDAC
histone deacetylase
- RAAS
renin angiotensin aldosterone system
- Tet
ten-eleven-translocation
- TGF
β-transforming growth factor beta
- UUO
unilateral ureteral obstruction
REFERENCES
- 1.Hoerger TJ, Wittenborn JS, Segel JE, et al. A health policy model of CKD: 1. Model construction, assumptions, and validation of health consequences. Am J Kidney Dis 2010;55:452–62. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305. [DOI] [PubMed] [Google Scholar]
- 3.McCullough KP, Morgenstern H, Saran R, Herman WH, Robinson BM. Projecting ESRD incidence and prevalence in the United States through 2030. J Am Soc Nephrol 2019;30:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 2018;392:2052–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 2014;371:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawhney S, Fraser SD. Epidemiology of AKI: utilizing large databases to determine the burden of AKI. Adv Chronic Kidney Dis 2017;24:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 2012;81:442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coca SG. Long-term outcomes of acute kidney injury. Curr Opin Nephrol Hypertens 2010;19:266–72. [DOI] [PubMed] [Google Scholar]
- 9.Basile DP, Bonventre JV, Mehta R, et al. Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol 2016;27:687–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SY, Kim SI, Choi ME. Therapeutic targets for treating fibrotic kidney diseases. Transl Res 2015;165:512–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol 2002;283:F861–75. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava A, Palsson R, Kaze AD, et al. The prognostic value of histopathologic lesions in native kidney biopsy specimens: results from the Boston Kidney Biopsy Cohort Study. J Am Soc Nephrol 2018;29:2213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breyer MD, Susztak K. The next generation of therapeutics for chronic kidney disease. Nat Rev Drug Discov 2016;15:568–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson PC, Humphreys BD. Single-cell genomics and gene editing: implications for nephrology. Nat Rev Nephrol 2018;15:63–4. [DOI] [PubMed] [Google Scholar]
- 15.Gewin LS. Renal tubule repair: is Wnt/beta-catenin a friend or foe? Genes (Basel) 2018;9:E58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bielesz B, Sirin Y, Si H, et al. Epithelial notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest 2010;120:4040–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 2010;16:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramann R, Fleig SV, Schneider RK, et al. Pharmacological GLI2 inhibition prevents myofibroblast cell-cycle progression and reduces kidney fibrosis. J Clin Invest 2015;125:2935–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramann R, Schneider RK, DiRocco DP, et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 2015;16:51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canaud G, Brooks CR, Kishi S, et al. Cyclin G1 and TASCC regulate kidney epithelial cell G2-M arrest and fibrotic maladaptive repair. Sci Transl Med 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang PM, Nikolic-Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol 2019;15:144–58. [DOI] [PubMed] [Google Scholar]
- 22.Border WA, Okuda S, Languino LR, Sporn MB, Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature 1990;346: 371–4. [DOI] [PubMed] [Google Scholar]
- 23.Sanderson N, Factor V, Nagy P, et al. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci U S A 1995;92:2572–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopp JB, Factor VM, Mozes M, et al. Transgenic mice with increased plasma levels of TGF-beta 1 develop progressive renal disease. Lab Invest 1996;74:991–1003. [PubMed] [Google Scholar]
- 25.Border WA, Noble NA. Evidence that TGF-beta should be a therapeutic target in diabetic nephropathy. Kidney Int 1998;54: 1390–1. [DOI] [PubMed] [Google Scholar]
- 26.Bitzer M, Sterzel RB, Bottinger EP. Transforming growth factor-beta in renal disease. Kidney Blood Press Res 1998;21:1–12. [DOI] [PubMed] [Google Scholar]
- 27.Ju W, Eichinger F, Bitzer M, et al. Renal gene and protein expression signatures for prediction of kidney disease progression. Am J Pathol 2009;174:2073–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 2014;124:2299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grande MT, Sanchez-Laorden B, Lopez-Blau C, et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med 2015;21:989–97. [DOI] [PubMed] [Google Scholar]
- 30.Lovisa S, LeBleu VS, Tampe B, et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med 2015;21:998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung S, Overstreet JM, Li Y, et al. TGF-beta promotes fibrosis after severe acute kidney injury by enhancing renal macrophage infiltration. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang HM, Ahn SH, Choi P, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 2015;21:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimura SL. Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am J Pathol 2009;175:1362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sureshbabu A, Muhsin SA, Choi ME. TGF-beta signaling in the kidney: pro-fibrotic and protective effects. Am J Physiol Renal Physiol 2016:Epub Jan 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benigni A, Zoja C, Corna D, et al. Add-on anti-TGF-beta antibody to ACE inhibitor arrests progressive diabetic nephropathy in the rat. J Am Soc Nephrol 2003;14:1816–24. [DOI] [PubMed] [Google Scholar]
- 36.Gu C, Zhang J, Noble NA, Peng XR, Huang Y. An additive effect of anti-PAI-1 antibody to ACE inhibitor on slowing the progression of diabetic kidney disease. Am J Physiol Renal Physiol 2016;311:F852–f63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voelker J, Berg PH, Sheetz M, et al. Anti-TGF-beta1 antibody therapy in patients with diabetic nephropathy. J Am Soc Nephrol 2017;28:953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazzucco G, Bertani T, Fortunato M, et al. Different patterns of renal damage in type 2 diabetes mellitus: a multicentric study on 393 biopsies. Am J Kidney Dis. 2002;39:713–20. [DOI] [PubMed] [Google Scholar]
- 39.Nlandu-Khodo S, Neelisetty S, Phillips M, et al. Blocking TGF-beta and beta-catenin epithelial crosstalk exacerbates CKD. J Am Soc Nephrol 2017;28:3490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeisberg M, Zeisberg EM. Precision renal medicine: a roadmap towards targeted kidney fibrosis therapies. Fibrogenesis Tissue Repair 2015;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouchie A, DeFrancesco L. Nature biotechnology’s academic spinouts of 2014. Nat Biotechnol 2015;33:247–55. [DOI] [PubMed] [Google Scholar]
- 42.van Laethem JL, Deviere J, Resibois A, et al. Localization of transforming growth factor beta 1 and its latent binding protein in human chronic pancreatitis. Gastroenterology 1995;108:1873–81. [DOI] [PubMed] [Google Scholar]
- 43.Worthington JJ, Klementowicz JE, Travis MA. TGFbeta: a sleeping giant awoken by integrins. Trends Biochem Sci 2011;36:47–54. [DOI] [PubMed] [Google Scholar]
- 44.Henderson NC, Arnold TD, Katamura Y, et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med 2013;19:1617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002;110:673–87. [DOI] [PubMed] [Google Scholar]
- 46.Honda E, Yoshida K, Munakata H. Transforming growth factor-beta upregulates the expression of integrin and related proteins in MRC-5 human myofibroblasts. Tohoku J Exp Med 2010;220:319–27. [DOI] [PubMed] [Google Scholar]
- 47.Zambruno G, Marchisio PC, Marconi A, et al. Transforming growth factor-beta 1 modulates beta 1 and beta 5 integrin receptors and induces the de novo expression of the alpha v beta 6 heterodimer in normal human keratinocytes: implications for wound healing. J Cell Biol 1995;129:853–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lampi MC, Reinhart-King CA. Targeting extracellular matrix stiffness to attenuate disease: from molecular mechanisms to clinical trials. Sci Transl Med 2018;10. [DOI] [PubMed] [Google Scholar]
- 49.Santos A, Lagares D. Matrix stiffness: the conductor of organ fibrosis. Curr Rheumatol Rep 2018;20:2. [DOI] [PubMed] [Google Scholar]
- 50.Parker MW, Rossi D, Peterson M, et al. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest 2014;124:1622–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zollinger AJ, Smith ML. Fibronectin, the extracellular glue. Matrix Biol 2017;60–61:27–37. [DOI] [PubMed] [Google Scholar]
- 52.Birukawa NK, Murase K, Sato Y, et al. Activated hepatic stellate cells are dependent on self-collagen, cleaved by membrane type 1 matrix metalloproteinase for their growth. J Biol Chem 2014;289:20209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou XMF, Gehdu N, Zhang J, Iredale JP, Benyon RC. Engagement of alphavbeta3 integrin regulates proliferation and apoptosis of hepatic stellate cells. J Biol Chem 2004;279:23996–4006, Epub 2004 Mar 24. [DOI] [PubMed] [Google Scholar]
- 54.Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol 2000;156: 1489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu YPM, Thill M, Yuan P, Wang NS, Csaky KG. Angiogenesis inhibition and choroidal neovascularization suppression by sustained delivery of an integrin antagonist, EMD478761. Invest Ophthalmol Vis Sci 2007;48:5184–90. [DOI] [PubMed] [Google Scholar]
- 56.Pozzi A, Zent R. Integrins in kidney disease. J Am Soc Nephrol 2013;24:1034–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma LJ, Yang H, Gaspert A, et al. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(−/−) mice. Am J Pathol 2003;163:1261–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Breuss JM, Gallo J, DeLisser HM, et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci 1995;108(Pt 6):2241–51. [DOI] [PubMed] [Google Scholar]
- 59.Hahm K, Lukashev ME, Luo Y, et al. Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol 2007;170:110–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maile LA, Busby WH, Gollahon KA, et al. Blocking ligand occupancy of the alphavbeta3 integrin inhibits the development of nephropathy in diabetic pigs. Endocrinology 2014;155: 4665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei C, Moller CC, Altintas MM, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med 2008;14: 55–63. [DOI] [PubMed] [Google Scholar]
- 62.Chang Y, Lau WL, Jo H, et al. Pharmacologic blockade of alphavbeta1 integrin ameliorates renal failure and fibrosis in vivo. J Am Soc Nephrol 2017;28:1998–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilkinson AL, Barrett JW, Slack RJ. Pharmacological characterisation of a tool alphavbeta1 integrin small molecule RGD-mimetic inhibitor. Eur J Pharmacol 2019;842:239–47. [DOI] [PubMed] [Google Scholar]
- 64.Babic AM, Chen CC, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol 1999;19:2958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao RBD. A novel integrin alpha5beta1 binding domain in module 4 of connective tissue growth factor (CCN2/CTGF) promotes adhesion and migration of activated pancreatic stellate cells. Gut 2006;55:856–62, Epub 2005 Dec 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCurley A, Alimperti S, Campos-Bilderback SB, et al. Inhibition of alphavbeta5 integrin attenuates vascular permeability and protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 2017;28:1741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ivaska J, Heino J. Cooperation between integrins and growth factor receptors in signaling and endocytosis. Annu Rev Cell Dev Biol 2011;27:291–320. [DOI] [PubMed] [Google Scholar]
- 68.Wickstrom SA, Fassler R. Regulation of membrane traffic by integrin signaling. Trends Cell Biol 2011;21:266–73. [DOI] [PubMed] [Google Scholar]
- 69.Gao R, Brigstock DR. A novel integrin alpha5beta1 binding domain in module 4 of connective tissue growth factor (CCN2/CTGF) promotes adhesion and migration of activated pancreatic stellate cells. Gut 2006;55:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khan S, Lakhe-Reddy S, McCarty JH, et al. Mesangial cell integrin alphavbeta8 provides glomerular endothelial cell cytoprotection by sequestering TGF-beta and regulating PECAM-1. Am J Pathol 2011;178:609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marek I, Lichtneger T, Cordasic N, et al. Alpha8 integrin (Itga8) signalling attenuates chronic renal interstitial fibrosis by reducing fibroblast activation, not by interfering with regulation of cell turnover. PLoS One 2016;11:e0150471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murray IR, Gonzalez ZN, Baily J, et al. Alphav integrins on mesenchymal cells regulate skeletal and cardiac muscle fibrosis. Nat Commun 2017;8:1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ulmasov B, Neuschwander-Tetri BA, Lai J, et al. Inhibitors of Arg-Gly-Asp-binding integrins reduce development of pancreatic fibrosis in mice. Cell Mol Gastroenterol Hepatol 2016;2: 499–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ley K, Rivera-Nieves J, Sandborn WJ, Shattil S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat Rev Drug Discov 2016;15:173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cirkel GA, Kerklaan BM, Vanhoutte F, et al. A dose escalating phase I study of GLPG0187, a broad spectrum integrin receptor antagonist, in adult patients with progressive high-grade glioma and other advanced solid malignancies. Invest New Drugs 2016:Epub Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hariharan S, Gustafson D, Holden S, et al. Assessment of the biological and pharmacological effects of the alpha nu beta3 and alpha nu beta5 integrin receptor antagonist, cilengitide (EMD 121974), in patients with advanced solid tumors. Ann Oncol 2007;18:1400–7. [DOI] [PubMed] [Google Scholar]
- 77.O’Day S, Pavlick A, Loquai C, et al. A randomised, phase II study of intetumumab, an anti-[alpha]v-integrin mAb, alone and with dacarbazine in stage IV melanoma. Br J Cancer 2011;105:346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ricart AD, Tolcher AW, Liu G, et al. Volociximab, a chimeric monoclonal antibody that specifically binds α5β1 integrin: a phase I, pharmacokinetic, and biological correlative study. Clin Cancer Res 2008;14:7924–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murphy PA, Begum S, Hynes RO. Tumor angiogenesis in the absence of fibronectin or its cognate integrin receptors. PLoS One 2015;10:e0120872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reynolds AR, Reynolds LE, Nagel TE, et al. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta3-integrin-deficient mice. Cancer Res 2004;64:8643–50. [DOI] [PubMed] [Google Scholar]
- 81.Reynolds AR HI, Watson AR, Welti JC, et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med 2009;15:392–400, Epub 2009 Mar 22. [DOI] [PubMed] [Google Scholar]
- 82.Ichinose K, Maeshima Y, Yamamoto Y, et al. Antiangiogenic endostatin peptide ameliorates renal alterations in the early stage of a type 1 diabetic nephropathy model. Diabetes 2005;54:2891–903. [DOI] [PubMed] [Google Scholar]
- 83.Masuda K, Tanabe K, Ujike H, et al. Deletion of pro-angiogenic factor vasohibin-2 ameliorates glomerular alterations in a mouse diabetic nephropathy model. PLoS One 2018;13: e0195779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tanabe K, Maeshima Y, Sato Y, Wada J. Antiangiogenic therapy for diabetic nephropathy. Biomed Res Int 2017;2017:5724069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun Y, Zhang Y, Chi P. Pirfenidone suppresses TGFbeta1induced human intestinal fibroblasts activities by regulating proliferation and apoptosis via the inhibition of the SMAD and PI3K/AKT signaling pathway. Mol Med Rep 2018;18:3907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu MY, Porte J, Knox AJ, et al. Lysophosphatidic acid induces alphavbeta6 integrin-mediated TGF-beta activation via the LPA2 receptor and the small G protein G alpha(q). Am J Pathol 2009;174:1264–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu T, Kooi CV, Shah P, et al. Integrin-mediated cell surface recruitment of autotaxin promotes persistent directional cell migration. Faseb J 2014;28:861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zeisberg M, Hanai J, Sugimoto H, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 2003;9:964–8. [DOI] [PubMed] [Google Scholar]
- 89.Sugimoto H, LeBleu VS, Bosukonda D, et al. Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nat Med 2012;18:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soofi A, Zhang P, Dressler GR. Kielin/chordin-like protein attenuates both acute and chronic renal injury. J Am Soc Nephrol 2013;24:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bradford STJ, Ranghini EJ, Grimley E, Lee PH, Dressler GR. High-throughput screens for agonists of bone morphogenetic protein (BMP) signaling identify potent benzoxazole compounds. J Biol Chem 2019;294:3125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palumbo-Zerr K, Zerr P, Distler A, et al. Orphan nuclear receptor NR4A1 regulates transforming growth factor-beta signaling and fibrosis. Nat Med 2015;21:150–8. [DOI] [PubMed] [Google Scholar]
- 93.Pherson M, Misulovin Z, Gause M, et al. Polycomb repressive complex 1 modifies transcription of active genes. Sci Adv 2017;3:e1700944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rickels R, Herz HM, Sze CC, et al. Histone H3K4 monomethylation catalyzed by Trr and mammalian COMPASS-like proteins at enhancers is dispensable for development and viability. Nat Genet 2017;49:1647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oba S, Ayuzawa N, Nishimoto M, et al. Aberrant DNA methylation of Tgfb1 in diabetic kidney mesangial cells. Sci Rep 2018;8:16338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bechtel W, McGoohan S, Zeisberg EM, et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med 2010;16:544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tampe B, Steinle U, Tampe D, et al. Low-dose hydralazine prevents fibrosis in a murine model of acute kidney injury-to-chronic kidney disease progression. Kidney Int 2017;91: 157–76. [DOI] [PubMed] [Google Scholar]
- 98.Tampe B, Tampe D, Muller CA, et al. Tet3-mediated hydroxymethylation of epigenetically silenced genes contributes to bone morphogenic protein 7-induced reversal of kidney fibrosis. J Am Soc Nephrol 2014;25:905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tampe B, Tampe D, Zeisberg EM, et al. Induction of Tet3-dependent epigenetic remodeling by low-dose hydralazine attenuates progression of chronic kidney disease. EBioMedicine 2015;2:19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu X, Tan X, Tampe B, et al. High-fidelity CRISPR/Cas9-based gene-specific hydroxymethylation rescues gene expression and attenuates renal fibrosis. Nat Commun 2018;9:3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tan X, Xu X, Zeisberg EM, Zeisberg M. High inorganic phosphate causes DNMT1 phosphorylation and subsequent fibrotic fibroblast activation. Biochem Biophys Res Commun 2016;472:459–64. [DOI] [PubMed] [Google Scholar]
- 102.Heylen L, Thienpont B, Naesens M, et al. Ischemia-induced DNA hypermethylation during kidney transplant predicts chronic allograft injury. J Am Soc Nephrol 2018;29:1566–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wing MR, Devaney JM, Joffe MM, et al. DNA methylation profile associated with rapid decline in kidney function: findings from the CRIC study. Nephrol Dial Transplant 2014;29: 864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ko YA, Mohtat D, Suzuki M, et al. Cytosine methylation changes in enhancer regions of core pro-fibrotic genes characterize kidney fibrosis development. Genome Biol 2013;14:R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qiu C, Huang S, Park J, et al. Renal compartment-specific genetic variation analyses identify new pathways in chronic kidney disease. Nat Med 2018;24:1721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Advani A, Huang Q, Thai K, et al. Long-term administration of the histone deacetylase inhibitor vorinostat attenuates renal injury in experimental diabetes through an endothelial nitric oxide synthase-dependent mechanism. Am J Pathol 2011;178: 2205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Choi HS, Song JH, Kim IJ, et al. Histone deacetylase inhibitor, CG200745 attenuates renal fibrosis in obstructive kidney disease. Sci Rep 2018;8:11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mishra N, Reilly CM, Brown DR, Ruiz P, Gilkeson GS. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J Clin Invest 2003;111:539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Novitskaya T, McDermott L, Zhang KX, et al. A PTBA small molecule enhances recovery and reduces postinjury fibrosis after aristolochic acid-induced kidney injury. Am J Physiol Renal Physiol 2014;306:F496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu N, He S, Ma L, et al. Blocking the class I histone deacetylase ameliorates renal fibrosis and inhibits renal fibroblast activation via modulating TGF-beta and EGFR signaling. PLoS One 2013;8:e54001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hukriede N, Vogt A, de Caestecker M. Drug discovery to halt the progression of acute kidney injury to chronic kidney disease: a case for phenotypic drug discovery in acute kidney injury. Nephron 2017;137:268–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Skrypnyk NI, Sanker S, Brilli-Skvarca L, et al. Delayed treatment with PTBA analogs reduces post injury renal fibrosis after kidney injury. Am J Physiol Renal Physiol 2015, Epub Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou X, Zang X, Ponnusamy M, et al. Enhancer of Zeste Homolog 2 inhibition attenuates renal fibrosis by maintaining SMAD7 and phosphatase and tensin homolog expression. J Am Soc Nephrol 2016;27:2092–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suarez-Alvarez B, Morgado-Pascual JL, Rayego-Mateos S, et al. Inhibition of bromodomain and extraterminal domain family proteins ameliorates experimental renal damage. J Am Soc Nephrol 2017;28:504–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou B, Mu J, Gong Y, et al. Brd4 inhibition attenuates unilateral ureteral obstruction-induced fibrosis by blocking TGF-beta-mediated Nox4 expression. Redox Biol 2017;11:390–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang P, Luo ML, Song E, et al. Long noncoding RNA lnc-TSI inhibits renal fibrogenesis by negatively regulating the TGF-beta/Smad3 pathway. Sci Transl Med 2018;10. [DOI] [PubMed] [Google Scholar]
- 117.Gomez IG, MacKenna DA, Johnson BG, et al. Anti-micro-RNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J Clin Invest 2015;125:141–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blokhin I, Khorkova O, Hsiao J, Wahlestedt C. Developments in lncRNA drug discovery: where are we heading? Expert Opin Drug Discov 2018;13:837–49. [DOI] [PubMed] [Google Scholar]
- 119.Mansour SG, Puthumana J, Coca SG, Gentry M, Parikh CR. Biomarkers for the detection of renal fibrosis and prediction of renal outcomes: a systematic review. BMC Nephrol 2017;18:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Craciun FL, Bijol V, Ajay AK, et al. RNA sequencing identifies novel translational biomarkers of kidney fibrosis. J Am Soc Nephrol 2016;27:1702–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barratt J, Topham P. Urine proteomics: the present and future of measuring urinary protein components in disease. Can Med Assoc J 2007;177:361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Genovese F, Manresa AA, Leeming DJ, Karsdal MA, Boor P. The extracellular matrix in the kidney: a source of novel noninvasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair 2014;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Klein J, Bascands JL, Mischak H, Schanstra JP. The role of urinary peptidomics in kidney disease research. Kidney Int 2016;89:539–45. [DOI] [PubMed] [Google Scholar]
- 124.Magalhaes P, Pejchinovski M, Markoska K, et al. Association of kidney fibrosis with urinary peptides: a path towards noninvasive liquid biopsies? Sci Rep 2017;7:16915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Papasotiriou M, Genovese F, Klinkhammer BM, et al. Serum and urine markers of collagen degradation reflect renal fibrosis in experimental kidney diseases. Nephrol Dial Transplant 2015;30:1112–21. [DOI] [PubMed] [Google Scholar]
- 126.Fenton A, Jesky MD, Ferro CJ, et al. Serum endotrophin, a type VI collagen cleavage product, is associated with increased mortality in chronic kidney disease. PLoS One 2017;12:e0175200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rasmussen DGK, Fenton A, Jesky M, et al. Urinary endotrophin predicts disease progression in patients with chronic kidney disease. Sci Rep 2017;7:17328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nielsen MJ, Veidal SS, Karsdal MA, et al. Plasma Pro-C3 (N-terminal type III collagen propeptide) predicts fibrosis progression in patients with chronic hepatitis C. Liver Int 2015;35:429–37. [DOI] [PubMed] [Google Scholar]
- 129.Rasmussen DGK, Hansen TW, von Scholten BJ, et al. Higher collagen VI formation is associated with all-cause mortality in patients with type 2 diabetes and microalbuminuria. Diabetes Care 2018;41:1493–500. [DOI] [PubMed] [Google Scholar]
- 130.Gholaminejad A, Abdul Tehrani H, Gholami Fesharaki M. Identification of candidate microRNA biomarkers in renal fibrosis: a meta-analysis of profiling studies. Biomarkers 2018;23:713–24. [DOI] [PubMed] [Google Scholar]
- 131.Kirpalani A, Hashim E, Leung G, et al. Magnetic resonance elastography to assess fibrosis in kidney allografts. Clin J Am Soc Nephrol 2017;12:1671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leung G, Kirpalani A, Szeto SG, et al. Could MRI be used to image kidney fibrosis? A review of recent advances and remaining barriers. Clin J Am Soc Nephrol 2017;12:1019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lin HY, Lee YL, Lin KD, et al. Association of renal elasticity and renal function progression in patients with chronic kidney disease evaluated by real-time ultrasound elastography. Sci Rep 2017;7:43303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Morrell GR, Zhang JL, Lee VS. Magnetic resonance imaging of the fibrotic kidney. J Am Soc Nephrol 2017;28:2564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]