Abstract
Latent tuberculosis infection (LTBI) screening and preventive treatment is one of the components of the World Health Organization (WHO) End TB strategy, and particularly relevant for low tuberculosis (TB) incidence countries, i.e. less than 100 TB cases per million population. The Netherlands is such a low-incidence country with traditionally a strong emphasis on programmatic management of LTBI, e.g. examining contacts of infectious TB patients by the public health services. Increasingly, curative services are involved in LTBI management of clinical risk groups. The country recently adopted a five-year strategic national plan recommending LTBI screening of high-risk migrants populations. A monitoring and evaluation system is already in place to measure programme performance and guide policy. Research on LTBI screening of migrants is on-going and results should inform future decisions in scaling-up this intervention. Several challenges remain for programmatic LTBI management, such as securing financial resources and the right professional cadre for implementation; availability of screening tests and drugs; collecting additional data for monitoring and evaluation, in line with the WHO indicators for LTBI programmatic management; developing cultural-sensitive and client-centred education for migrants; reducing patient costs for LTBI screening and preventive treatment; and assessing cost-effectiveness and impact on TB epidemiology.
Keywords: Elimination, Latent tuberculosis infection, Prevention, Screening, Tuberculosis
Keywords: MPHS Municipal Public Health Service
Abbreviations
- CPT
Committee for Practical Tuberculosis Control
- EU
European Union
- HIV
Human Immunodeficiency Virus
- IGRA
Interferon Gamma Release Assay
- LTBI
Latent tuberculosis infection
- NTCP
National Tuberculosis Control Plan
- NTR
Netherlands TB Register
- PTB
Pulmonary Tuberculosis
- TB
Tuberculosis
- TNF
Tumour Necrosis Factor
- TST
Tuberculin Skin Test
- WHO
World Health Organization
Introduction
In 2014, the World Health Assembly adopted the World Health Organization (WHO) post-2015 tuberculosis (TB) strategy [1]. The End TB Strategy, as it is now called, is the third long-term strategy of the WHO after TB was declared a public health emergency in 1993 [2], [3]. The End TB Strategy (2016–2035) has three pillars: integrated, patient-centred care and prevention; bold policies and supportive systems and intensified research and innovations. The three pillars altogether are composed of ten components. It is for the first time that preventive treatment of persons with a latent TB infection (LTBI) at high risk for active TB is a component of a WHO TB strategy (under the first pillar).
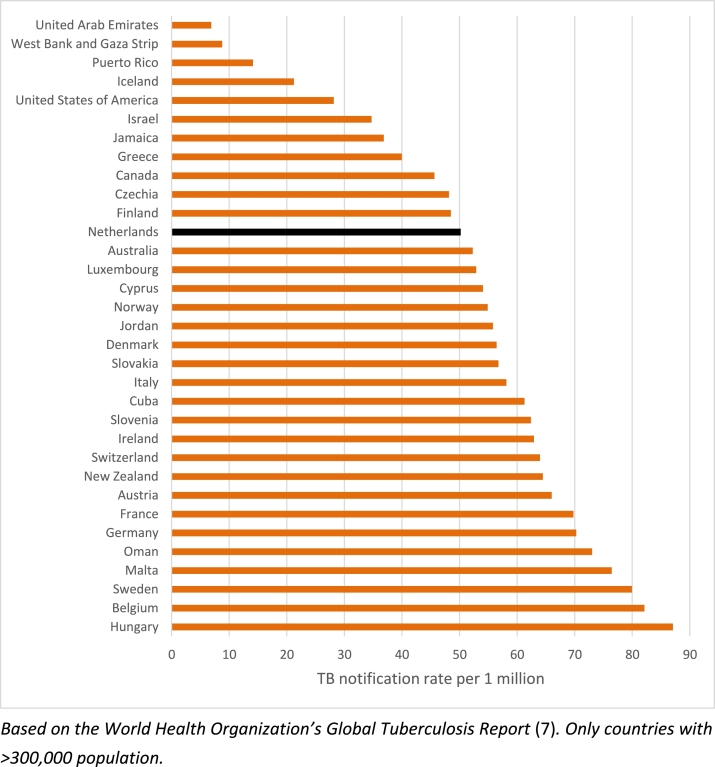
In 2014, WHO and the European Respiratory Society (ERS) also developed a framework for TB elimination in low-incidence countries [4], [5]. One of the eight priority areas is screening for active TB and LTBI in TB contacts and selected high-risk groups, and provide appropriate treatment. The Framework also includes (new) definitions of low incidence, pre-elimination and elimination, i.e. less than 100, 10 and 1 notified TB cases per 1 million population. The Netherlands is one of the 33 low-incidence countries and territories (with > 300,000 population) worldwide, with a TB notification rate of 50 per million population in 2015 (Fig. 1) [6], [7]. Programmatic management of LTBI is supported by the WHO Guideline on this topic released in 2014 [8], [9], and the WHO Global Task Force on LTBI initiated in 2015 [10].
Fig. 1.
Tuberculosis (TB) notification rate in countries with a WHO estimated TB incidence <10/100,000, (only countries with population >300,000), 2015.
The Global TB Programme of WHO, in collaboration with the Republic of Korea's Centers for Disease Control and Prevention and International Tuberculosis Research Center, organized on 27–28 April 2016, in Seoul, Republic of Korea, a global consultation on the management of LTBI to present and discuss challenges to, opportunities for, and best practices on the programmatic management of LTBI, and to consider recommendations to facilitate its implementation in both low- and high-burden countries [11]. The Netherlands was one of the countries sharing its experiences with programmatic management of LTBI, and these are elaborated on in this manuscript.
The Netherlands and its health infrastructure
The Netherlands is a member state of the European Union (EU). In 2016, the population increased to more than 17 million inhabitants, of whom 19.6% were not born in the country. The country is densely populated with 409 persons per km2 (surface area is 41,543 km2) [12]. The Netherlands is a high-income country and among the world's top 20 in terms of gross domestic product per capita [13], [14].
The Dutch health care system includes about 8000 general practitioners, 88 hospitals (including 8 university hospitals) and 25 Municipal Public Health Services (MPHS). Hospitals provide curative services to patients, mostly after referral by general practitioners, and MPHSs provide public health services, including infectious disease prevention and control activities. The role and responsibility of municipalities and MPHSs is outlined in a Public Health Act.
Citizens in the Netherlands are obliged to take a health insurance. The health insurance covers costs of medical treatment, but patients have to pay the first amount of medical costs – excluding costs for General Practitioner visits – incurred during a year (385 Euro's in 2016). TB and LTBI treatment costs are not exempted. Children and adolescents < 18 years however are exempted from this contribution to medical costs, including TB/LTBI diagnosis and treatment.
Tuberculosis control in the Netherlands
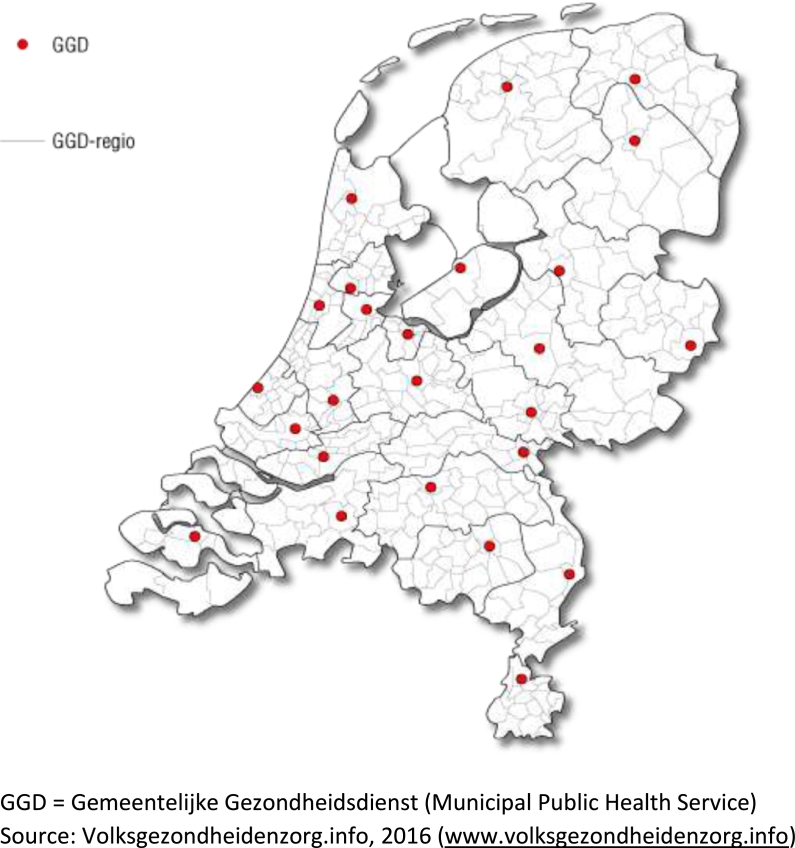
TB control started as a private initiative of benevolent societies in early 1900 s and these organizations were incorporated in the MPHSs in the 1980 s. Currently, 23 MPHSs implement TB control activities, while two MPHSs have outsourced these activities to a larger MPHS (Fig. 2). TB departments of MPHSs are staffed by public health TB control physicians, specially trained for a combination of public health and clinical TB responsibilities, who read chest radiographs and diagnose and treat TB and LTBI patients, among other tasks; specialized TB nurses who support patients, supervise their medication and conduct contact investigation; and medical technical assistants who perform tuberculin skin testing (TST), take blood for interferon gamma release assays (IGRAs), make chest radiographs and perform administrative tasks. The MPHSs conduct radiographic screening of risk groups, such as immigrants, asylum seekers and prisoners, and perform LTBI screening, e.g. in contact investigation.
Fig. 2.
Coverage of Municipal Public Health Services in The Netherlands.
Clinical specialists, mainly pulmonologists and infectious disease specialists, diagnose and treat TB (about 75–80% of all notified patients) and LTBI patients in the curative sector. LTBI screening and preventive treatment has become an increasing part of these hospitals specialists’ responsibilities due to an increase of immunosuppressed patients, e.g. patients with HIV, those starting medication such as Tumour Necrosis Factor (TNF)-alpha blocking therapy, and transplantation patients receiving immunosuppressive drugs. In the context of a reducing TB caseload, with the potential of diminishing expertise, and the increased relevance of LTBI management in the clinical setting, a training program was set up in 2011 for pulmonologists and infectious disease specialists to train them in the function of hospital TB coordinator. 82% of all hospitals in the Netherlands now have such a dedicated hospital TB coordinator. MPHS public health TB control physicians, TB nurses and hospital TB coordinators work closely together.
Two hospitals have facilities for long-term admission and specialized treatment of TB patients, so-called modern sanatoria. All MDR-TB patients and, on indication, clinically complex or socially problematic TB cases are admitted in these centres, as well as patients with a court order for mandatory isolation.
Epidemiology of tuberculosis in the Netherlands
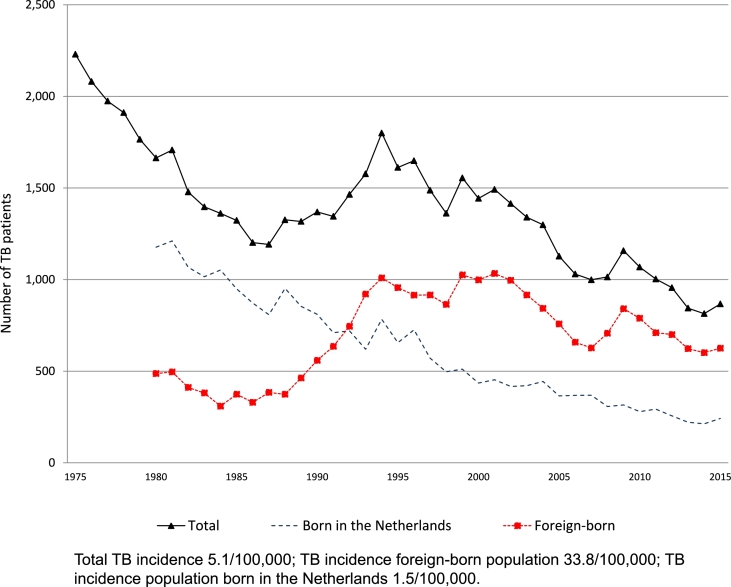
At the end of the 19th century, TB was the major killer disease in Western countries [15], with 0.25% of the population dying annually of TB in the period 1866–1875 [16]. In the 1950 s, apart from mortality registration, a morbidity notification system was set up for active TB. After the availability of an effective combination therapy, notification rates declined until the mid-1980 s with about 8% per year. Fig. 3 shows the upsurge of TB in the period 1985–1995, predominantly as an effect of increased migration. The last twenty years, the TB notification rate declined relatively steady, although in 2015, it increased with 6%, mainly as a result of a high influx of asylum seekers in the Netherlands (867 patients, TB notification rate 51 per million in 2015) [6].
Fig. 3.
Number and rate of tuberculosis notification, The Netherlands, 1975–2015.
The number of TB patients born in the Netherlands declined over the last forty years and their stratified notification rate almost reached the pre-elimination level of 10 cases per 1 million population. In 2015, 234 patients born in the Netherlands were reported to the Netherlands TB Register (NTR), translating in an overall notification rate of 15 cases per million population, of whom 24.8% had one or both parents born in a foreign country. However, the TB notification rate for the population born in the Netherlands for those younger than 65 years was 10 per million and for those at least 65 years old 22 per million. It is expected that the decline in the older population will continue due to the cohort effect [17], [18].
Among the foreign-born population the notification rate is much higher (338 per million). Although the TB notification rate is highest in the first years upon arrival in the Netherlands, the majority (58%) of foreign-born TB cases resided more than two years in the Netherlands at the time of diagnosis [6]. In a previous study, 52% of the foreign-born patients with culture-confirmed TB, who resided more than two years in the Netherlands, had a unique DNA fingerprint suggesting that even among TB patients having lived in the Netherlands for a longer time, at least half of them had a reactivation of a TB infection acquired in the country of origin [19].
National strategic tuberculosis control plan
The Ministry of Health commissioned the National Institute for Public Health and the Environment (RIVM) for the second time to develop a five-year National TB Control Plan (NTCP), together with KNCV Tuberculosis Foundation and other stakeholders. The previous NTCP 2011–2015 focused on concentration and specialization of TB control, laboratory and curative services due to the decline of the number of TB cases, while maintaining quality services [20]. The current NTCP 2016–2020 describes the efforts needed to reduce TB incidence in the Netherlands [21], in line with the WHO End TB Strategy and the WHO European Region Tuberculosis Action Plan 2016–2020 [1], [22]. The target in the WHO European Region TB action plan to reduce TB incidence with 25% by 2020 was adopted. A national target was set to also reduce in-country TB transmission with 25% over the next five years. The main new intervention to reach these targets is to screen new immigrants and asylum seekers for LTBI and provide preventive treatment to those infected. The following objectives/activities related to LTBI are listed in the NTCP 2016–2020:
-
1.
The radiographic screening of newly arrived immigrants will be gradually replaced or supplemented by LTBI screening, with priority for migrant children and immigrants from high-incidence countries (TB incidence > 200/100,000). The implementation, cost-effectiveness and impact of LTBI screening is to be evaluated in 2018, and thereafter a decision is made whether LTBI screening can be extended to other immigrants.
-
2.
By 2018, all hospitals should have an approved protocol for the diagnosis and treatment of TB and LTBI in clinical risk groups. Data of hospital LTBI screening policy needs to be collected and analysed at national level.
-
3.
Criteria should be defined for supervision of TB and LTBI patients, including the new target group for LTBI screening, i.e. recently arrived immigrants. Patients are explicitly involved in defining these criteria. The criteria need to be evaluated two years after implementation, again with input of (former) patients.
Policy and practices of latent tuberculosis infection management
TB guidelines are developed in the Netherlands by a multi-disciplinary Committee for Practical TB Control (CPT). The guidelines are regularly evaluated and revised, and compiled in a yearly updated TB Manual [23]. LTBI diagnosis is mainly done by a two-step approach, i.e. an initial TST followed by an IGRA if TST ≥ 5 mm. Both tests are recommended simultaneously for LTBI diagnosis in clinical risk groups (HIV patients, patients starting TNF-alpha blocking medication and candidate organ transplant patients) to increase sensitivity. LTBI is treated with 3 months’ isoniazid/rifampicin (3HR), or 6 months’ isoniazid (6H) or 4 months’ rifampicin (4R) [8], [9], [24], [25], [26], [27], [28]. Persons initiating LTBI treatment should be screened for potential risk factors for possible adverse effects, e.g. transaminases in persons with a history of liver disease, alcohol abuse, HIV infection, older than 35 years, pregnancy and during the first three months of post-partum. Patients on preventive treatment should be evaluated every month for adverse effects and treatment adherence. Preventive treatment is extended to 4HR and 9H in patients with immunosuppressive disorders or starting immunosuppressive medication and those with pulmonary fibrotic lesions. For the latter condition, it is crucial that active TB is ruled out [29], which is defined in the Netherlands by unaltered radiological findings over a period of three months, absence of symptoms during this period and negative results of sputum culture for Mycobacterium tuberculosis complex, or, based on the clinician's decision, on material obtained by sputum induction or bronchoscopy [30].
LTBI screening is recommended for contacts exposed to bacteriological (smear-positive and/or culture-confirmed) pulmonary TB, clinical risk groups, occupational risk groups (pre-employment screening of health care workers with previous high-risk for TB exposure and periodically, those with increased risk during work) and travellers returning from high-incidence countries. In December 2015, the CPT recommended to screen migrants < 18 years (also) for LTBI, which is now gradually implemented countrywide. Illicit drug users and homeless persons are not (yet) included as target groups for LTBI screening in national guidelines, but this is being piloted by the MPHS Amsterdam, and hence radiographic screening for those uninfected or treated for LTBI is discontinued. Since this pilot started on 1 April 2015, until 30 June 2016, 160 persons were screened for LTBI, 22 (14%) diagnosed with LTBI, 16 (73%) started preventive treatment and 14 (88%) completed the treatment.
Box 1 provides a practice of LTBI management in one of the hospitals in the Netherlands, including agreement on screening pathways, responsibilities for treatment and reporting and recording of the results. Similar initiatives with dedicated clinics for LTBI examination and preventive treatment of clinical risk groups are developed in other hospitals.
Box 1. Practice of latent tuberculosis infection management in a hospital in The Netherlands.
Pulmonologists, rheumatologists, dermatologists and gastroenterologists of the Gelderse Vallei Hospital, a 600-bed hospital in the central-eastern part of the Netherlands, agreed in 2013 that all patients initiating TNF-alpha blocking medication should be tested for LTBI and TB. Patients were first screened with an IGRA and a chest radiograph, and then referred to the pulmonology department for a TST and to complete a questionnaire with clinical risk factors for TB and factors influencing test results. Based on all test results, the pulmonologist decides whether the patient can start TNF-alpha blocking medication, or whether the patient first should start treatment for LTBI (or TB). When preventive treatment is started, both specialists agree on the start of TNF-alpha blocking medication, which generally is delayed until the patient has been on treatment for a minimum of two months.
Since November 2015, also pre-transplantation patients, mainly for kidney transplantation, follow the same work-up. Newly diagnosed HIV patients are referred to another hospital for case management, including TB/LTBI screening.
Pulmonologists treat all LTBI patients and report the cases to the MPHS for registering in the NTR. In the years 2013–2015, annually 80, 81 and 83 patients were screened for LTBI, and 88 patients in 2016 (until 30 September 2016). In 2015 and 2016, 10 patients (5.8%) were notified with LTBI to the MPHS in the area, and started preventive treatment.
Monitoring and evaluation of latent tuberculosis infection
LTBI is reported on a voluntary basis to the NTR. This register started in 1993 as a paper case-based registry for TB and LTBI cases and was integrated in 2005 into a central web-based register for infectious disease surveillance [31]. At that time, also a new case definition for LTBI cases to be reported, was agreed upon, based on the eligibility for preventive treatment according to national guidelines [32]. This includes individuals with a positive LTBI test with 1) a high likelihood of recent infection (< 2 years ago), 2) pulmonary fibrotic lesions consistent with active TB in the past and without adequate treatment, 3) severe immunosuppressive disorders (e.g. HIV infection), and 4) planned immunosuppressive therapy (pre-TNF-alpha blocking therapy/pre-organ transplantation).
Characteristics of notified TB and LTBI cases are annually described in the TB Surveillance Report [6]. In 2015, 1433 LTBI cases were reported to the NTR (8.5 per 100,000 persons), 61% were identified through contact investigation, 20% by pre-employment or pre-travel screening and 14% by screening of individuals with immunosuppressive disorders or starting immunosuppressive treatment (Table 1). 1128 (79%) started preventive treatment, 14% of cases were followed-up for two years with chest radiography and in 7% of cases the LTBI management policy was not yet known. As with TB cohort analysis, LTBI treatment results of the previous year are also reported in the TB Surveillance Report. Of the 1013 LTBI cases that started preventive treatment in 2014, 89% completed treatment, 5% defaulted, and 4% discontinued treatment due to side effects. Information of TB and LTBI cases and case management is electronically available at www.tbc-online.nl.
Table 1.
Latent tuberculosis infection indicators in The Netherlands, 2015.
| Target group for LTBI screening in the Netherlands | Number eligible for LTBI screening | Number screened for LTBI | Screening coverage | Number LTBI cases diagnosed and reported to NTR | Number cases that initiated LTBI treatment | LTBI treatment coverage | Number completed LTBI treatment (2014) | LTBI completion rate (2014) | Number with active TB reported to NTR | TB incidence among target group (per 100,000) |
|---|---|---|---|---|---|---|---|---|---|---|
| All contacts of TB patients | * | * | * | 867 | 679 | 78% | 505/575 | 88% | 65 | |
| People living with HIV | 865 ‡ | Unknown | – | 3 | 3 | 100% | 4/4 | 100% | 36 | |
| Patients initiating / on TNF-alpha blocking treatment | Unknown | Unknown | – | 192** | 177** | 92% | 151/169** | 89% | 14 | |
| Patients preparing for (or with) organ or hematologic transplantation | Unknown | Unknown | – | 5 | ||||||
| Health care workers | Unknown | Unknown | – | 21 | 17 | 81% | 27/29 | 93% | 6 | |
| Travelers | Unknown | Unknown | – | 22 | 18 | 82% | 20/20 | 100% | ||
| Others*** | Unknown | Unknown | – | 328 | 234 | 79% | 194/216 | 90% | ||
| Total | Unknown | Unknown | – | 1433 | 1128 | 79% | 901/1013 | 89% |
Note: There is no policy in the Netherlands to screen patients with renal insufficiency/ dialysis or with silicosis for LTBI.
LTBI: latent tuberculosis infection; NTR: Netherlands Tuberculosis Register; TB: tuberculosis.
Data is available of the number of contacts eligible for TB examination in source tracing and contact investigation (n= 7162) related to a TB patient diagnosed in 2014, the number of contacts actually screened for LTBI (n= 5446) and the screening coverage (76%). The dataset allows a breakdown in intensity of contacts, i.e. household and close contacts, but not (yet) for age group, such as children.
LTBI screening data in the NTR has been combined for patients initiating TNF-alpha blocking therapy and preparing for transplantation.
Others: mainly pre-employment, pre-travel and other low-risk LTBI screening.
Results of 21 years’ programmatic management of LTBI in the Netherlands (1993–2013) were recently published [32]. In that period, 37,729 persons were registered with LTBI; 77% started preventive treatment and 82% of these cases completed treatment. Two-thirds of the notified LTBI cases were detected through contact investigation. Preventive treatment initiation was highest in groups more likely to progress to active TB, such as TB contacts, children and immunosuppressed persons. Children and immunosuppressed persons were also more likely to complete preventive treatment, as were individuals treated with the rifampicin or isoniazid/rifampicin regimens. Male gender, age younger than 15 years and rifampicin containing short regimens were associated with a lower risk to discontinue the treatment due to serious adverse events. A study, following-up 14,231 persons notified with LTBI (2005–2013) in the Netherlands, found that 134 (0.9%) were notified with active TB [33]. Effect of preventive treatment reduced TB incidence among TB contacts in the first two years after diagnosis at least 2-fold.
Recently, WHO published a list of core global, core national and optional indicators to monitor and evaluate LTBI management [7]. We applied this framework to the Dutch LTBI surveillance system (Table 1). The number of people eligible for screening and the number actually screened is unknown for most of the target groups for LTBI screening. Persons with clinical risk factors are mainly screened in hospitals and information on diagnosed LTBI cases and management is incomplete, although there is an increase of reporting, mainly those with immunosuppressive disorders or candidates for immunosuppressive therapy [6].
Contact investigation is included in the list of WHO LTBI indicators. Source tracing and contact investigation has been evaluated systematically in the Netherlands for the years 2006–2010 [34]. In this period, contact investigations were done around 1062 smear-positive pulmonary TB (PTB) patients and in 613 smear-negative culture-positive PTB patients, according to the stone-in-the-pond principle [35]. This yielded 2587 and 331 LTBI cases respectively (Table 2), in addition to 208 and 47 secondary active TB cases respectively (data not shown). The yield of contact investigations for 2011–2015 will be evaluated in 2017.
Table 2.
Contacts screened for and diagnosed with latent tuberculosis infection in contact investigations in The Netherlands, 2006–2010.
| Smear-positive pulmonary PTB |
Smear-negative culture-positive pulmonary PTB |
|||||
|---|---|---|---|---|---|---|
| Number screened for LTBI (n) | Number with LTBI (n) | Proportion infected (%) | Number screened for LTBI (n) | Number with LTBI (n) | Proportion infected (%) | |
| 1st Ring | 10,108 | 1359 | 13.4 | 2512 | 184 | 7.3 |
| 2nd Ring | 14,651 | 878 | 6.0 | 2523 | 114 | 4.5 |
| 3rd Ring | 11,233 | 350 | 3.1 | 918 | 33 | 3.6 |
| Total | 35,992 | 2587 | 7.2 | 5954 | 331 | 5.6 |
LTBI = latent tuberculosis infection; PTB = pulmonary tuberculosis.
Research on latent tuberculosis infection
Over the last decade, the Netherlands Organization for Research and Development (ZonMw) financed three large research (PhD) projects addressing the prevention of LTBI among the immigrant population. In the PREDICT studies (2005–2008), the predictive value of TST and IGRA in immigrant contacts was studied [36], [37], [38]. In the TB PERSPECTIVE studies (2009–2012), the role of TST and IGRA in screening immigrants in contact investigation or at arrival in the Netherlands was subject of research [39], [40], [41], [42], as well as the cost-effectiveness of TB and LTBI screening and modelling its impact. In 2013, using remaining budget, we investigated the feasibility of LTBI screening of immigrants arriving in the country. In total, 726 immigrants younger than 35 years were tested, either with TST followed by IGRA if TST ≥ 5 mm, or with IGRA only. Of those, 88 (12%) persons were diagnosed with LTBI, and one person was diagnosed with active TB. 73 persons were offered preventive treatment; 34/73 (47%) initiated preventive treatment of whom 26 (76%) completed the treatment.
Recently, ZonMw financed the TB ENDPoint project (2016–2019) which addresses the perceived barriers for implementation of LTBI screening and preventive treatment among immigrants from countries with a TB incidence > 50/100.000. So far, 583 immigrants have been enrolled for LTBI screening, enrolment of 800 asylum seekers for LTBI screening is currently taking place, followed by 300 immigrants with a high-risk for TB will be enrolled in a community screening project. After completion of the three pilots, the project will also assess the cost-effectiveness of the intervention under optimized conditions. The data from this research will be the basis for a business case document, which will guide policy makers in the decision whether or not to extend LTBI screening to other immigrants.
Challenges to scale up latent tuberculosis infection management
The Netherlands, with already a long history of LTBI screening and treatment, faces several challenges to scale up LTBI programmatic management, such as:
-
1)
Political commitment, with maintaining and adapting the public health infrastructure for TB control. The decline of the number of TB cases in this century also caused a decrease in financial and human resources. The current emphasis on programmatic management of LTBI requires an adaptation of the system, with securing adequate financial resources and the right human resource cadre to implement it. TB nurses and nurse physician assistants can play a stronger role in LTBI management.
-
2)
Availability of LTBI screening tests and drugs. The main supplier of tuberculin in the EU had irregular supplies over the last years and recently stopped to supply this diagnostic, creating shortages in EU countries [43]. IGRA tests are costly for scaling up LTBI screening. New screening tests are at the horizon [44], but better tests/biomarkers to predict disease are urgently needed. The pharmaceutical company manufacturing isoniazid in the Netherlands stopped producing the drug after the Fukoshima nuclear disaster, from which area the substance for drug development was procured, and only recently restarted the isoniazid drug production. Rifapentine, already registered with the Drug and Food Administration in the United States since 2014, is not yet available on the European market. The drug is promising since it will only require patients to take 12 (weekly) doses of LTBI treatment [45]. KNCV Tuberculosis Foundation, European organisations, including patient organisations, and individuals from 20 different European countries signed a letter requesting Sanofi, the company producing rifapentine, to take all steps to register the drug in Europe (www.kncvtbc.org/rifapentine).
-
3)
Monitoring and evaluation of LTBI management. Although LTBI cases are already reported for almost 25 years in the Netherlands, there is underreporting of LTBI cases, especially those diagnosed in the clinical settings. Legislation, making LTBI a notifiable condition, could help to increase reporting coverage, as is the case in Japan [7]. Another major challenge is to measure or estimate the number of persons eligible for LTBI screening and the number actually screened. We will work towards improving this in Europe as part of a recently started European Commission co-financed project, E-DETECT TB [46], which among others aims to collect and analyse TB and LTBI migrant screening data in Italy, The Netherlands, Sweden and the United Kingdom. The project will invite other interested European countries to collaborate.
-
4)
General public and client education. LTBI is a concept that is badly understood outside the TB professional community. Awareness rising campaigns are needed to successfully introduce programmatic LTBI screening of migrants and tailored, cultural-sensitive client-centred education is necessary to ensure adherence to, and completion of, LTBI treatment [47].
-
5)
Patient cost. The Dutch health insurance system is not supportive for successful programmatic management of LTBI, because patients above 18 years have to pay the initial cost of health consumption. Thus, many patients may end-up paying the full cost of LTBI screening and treatment up to 385 Euro's. The issue has been raised to the Minister of Health, but did not lead to an exemption for TB or LTBI patients. KNCV Tuberculosis Foundation will collect case reports of impediments to access to care, analyse these and report on the barriers [21].
-
6)
Cost effectiveness and impact of programmatic management of LTBI. Several studies showed that LTBI screening and preventive treatment of migrants is cost-effective, but that it depends on the TB incidence in the country of origin, methodology and models [48], [49], [50], [51]. Obviously, when introducing LTBI screening of migrants, high-risk populations should be prioritized [50], [52]. The European Centre for Disease Prevention and Control (ECDC) embarked on a project to provide EU and European Economic Area (EEA) Member States and candidate countries with scientific advice and guidance on programmatic LTBI Control, which includes mathematical modelling and cost-effectiveness studies [47]. This work will contribute towards a guidance document that elaborates on the available options when considering programmatic LTBI control in the EU/EEA.
Conclusion
This manuscript describes elements of programmatic management of LTBI in a low-incidence country. Once countries reach this low-incidence phase, more emphasis on LTBI screening of high-risk groups is needed to move towards elimination [4], [53], [54]. In the Netherlands, with 73% of the TB patients in the foreign-born population, (newly arrived) migrants, including asylum seekers, from high-incidence countries are a relevant population to offer LTBI screening [52]. Contacts of pulmonary TB patients and clinical risk groups with a high risk of developing active TB, are also well-identifiable and need to be targeted for LTBI screening. The LTBI screening policy of risk groups needs to be regularly updated based on scientific evidence and evaluation of program performance, for which a monitoring and evaluation system is essential.
Programmatic implementation of LTBI is highly context- and country-specific [10], [47]. The Netherlands has an excellent network of public health and curative TB services with a long history of collaboration, which is now getting more directed towards LTBI screening and TB elimination. Despite these supporting conditions, several challenges and threads remain, such as the availability of diagnostic tests and drugs, the risk of losing adequate human resources, strengthening monitoring and evaluation, health insurance arrangements not supportive of scaling up programmatic LTBI screening and uncertainties about cost-effectiveness and impact. Low-incidence countries can pave the way towards elimination, but to reach the goals set by the world to reduce global TB incidence to 100 per million in 2035, investment in international TB control and the development of new tools is highly needed, which will also benefit low-incidence countries receiving migrants from high-burden countries.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared by authors.
Acknowledgment
The feasibility study on LTBI screening among immigrants was funded by the Netherlands Organization for Health Research and Development (ZonMw, Grant number: 125010011).
References
- 1.Uplekar M., Weil D., Lonnroth K., Jaramillo E., Lienhardt C., Dias H.M. WHO's new end TB strategy. Lancet. 2015;385(9979):1799–1801. doi: 10.1016/S0140-6736(15)60570-0. [DOI] [PubMed] [Google Scholar]
- 2.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72(1):1–6. doi: 10.1016/0041-3879(91)90017-m. Mar. [DOI] [PubMed] [Google Scholar]
- 3.Zaman K. Tuberculosis: A Global Health Problem. J Health Popul Nutr. Apr 2010;28(2):111–113. doi: 10.3329/jhpn.v28i2.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Framework towards tuberculosis elimination in low-incidence countries, Geneva: World Health Organization; 2014. Report No.: WHO/HTM/TB/2014.13. [PubMed]
- 5.Lönnroth K., Migliori G.B., Abubakar I., D'Ambrosio L., de Vries G., Diel R. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. Apr 2015;45(4):928–952. doi: 10.1183/09031936.00214014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuberculose in Nederland 2015 - Surveillance rapport inclusief rapportage monitoring van interventies. Bilthoven: RIVM; 2016.
- 7.World Health Organization . World Health Organization; Geneva: 2016. Global tuberculosis control: WHO report 2016 (WHO/HTM/TB/2016.13) [Google Scholar]
- 8.Guidelines on the management of latent tuberculosis infection. Geneva: World Health Organization; 2014. Report No.: WHO/HTM/TB/2015.01. [PubMed]
- 9.Getahun H., Matteelli A., Abubakar I., Aziz M.A., Baddeley A., Barreira D. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46(6):1563–1576. doi: 10.1183/13993003.01245-2015. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Getahun H., Matteelli A., Abubakar I., Hauer B., Pontali E., Migliori G.B. Advancing global programmatic management of latent tuberculosis infection for at risk populations. Eur Respir J. May 2016;47(5):1327–1330. doi: 10.1183/13993003.00449-2016. [DOI] [PubMed] [Google Scholar]
- 11.Report of the Global Consultation on the Programmatic Management of Latent Tuberculosis Infection, 27-28 April 2016, Seoul, Republic of Korea [Internet]. Geneva: World Health Organization; 2016. Report No.: WHO/HTM/TB/2016.08. Available from: http://apps.who.int/iris/bitstream/10665/246106/1/WHO-HTM-TB-2016.08-eng.pdf
- 12.Schäfer W., Boerma W., van den Berg M., Westert G., Devillé W., van Ginneken E. The Netherlands: health system review. Health Syst Transit. 2010;12(1):1–229. [PubMed] [Google Scholar]
- 13.World Economic Outlook Database-April 2016, International Monetary Fund. http://www.imf.org/external/data.htm. 2016.
- 14.GDP per capita (current US$), World Bank. http://databank.worldbank.org/data/reports.aspx?source=2&series=NY.GDP.PCAP.CD&country=. 2016.
- 15.Dubos R.J., Dubos J. Littel, Brown; Boston MA: 1952. The White Plague: Tuberculosis, Man, and Society. [Google Scholar]
- 16.Droeze H. Jr., J.J. De sterfte aan phthisis in Nederland: academisch proefschrift ter verkrijging van den graad van Doctor in de Geneeskunde aan de Rijksuniversiteit te Leiden. 1879.
- 17.Borgdorff M.W., van der Werf M.J., de Haas P.E., Kremer K., van Soolingen D. Tuberculosis elimination in the Netherlands. Emerg Infect Dis. 2005;11:597–602. doi: 10.3201/eid1104.041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borgdorff M.W., van den Hof S., Kremer K., Verhagen L., Kalisvaart N., Erkens C. Progress towards tuberculosis elimination: secular trend, immigration and transmission. Eur Respir J. 2010;36:339–347. doi: 10.1183/09031936.00155409. [DOI] [PubMed] [Google Scholar]
- 19.de Vries G., Slump E., Schimmel H., Erkens C. “Know your epidemic”: de tbc-situatie in Nederland. Tegen Tuberc. 2014;110(2):3–7. [Google Scholar]
- 20.National Tuberculosis Control Plan 2011-2015. Bilthoven: RIVM; 2010.
- 21.National Tuberculosis Control Plan 2016-2020. Towards Elimination [Internet]. Bilthoven: RIVM; 2016. Available from: http://www.rivm.nl/en/Documents_and_publications/Scientific/Reports/2016/maart/National_Tuberculosis_Control_Plan_2016_2020_Towards_elimination.
- 22.Tuberculosis action plan for the WHO European Region 2016–2020. Denmark: World Health Organization Regional Office for Europe; 2015.
- 23.Handboek Tuberculose 2016. Den Haag: KNCV Tuberculosefonds; 2016.
- 24.Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. International Union Against Tuberculosis Committee on Prophylaxis. Bull World Health Organ. 1982;60(4):555–64. [PMC free article] [PubMed]
- 25.A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. Am Rev Respir Dis. 1992 Jan;145(1):36–41. [DOI] [PubMed]
- 26.Smieja M.J., Marchetti C.A., Cook D.J., Smaill F.M. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst Rev. 2000;(2) doi: 10.1002/14651858.CD001363. CD001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma S.K., Sharma A., Kadhiravan T., Tharyan P. Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB. Cochrane Database Syst Rev Online. 2013 doi: 10.1002/14651858.CD007545.pub2. Jul 5;7:CD007545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stagg H.R., Zenner D., Harris R.J., Muñoz L., Lipman M.C., Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med. 2014 doi: 10.7326/M14-1019. Aug 12. [DOI] [PubMed] [Google Scholar]
- 29.Solsona Peiró J., de Souza Galvão M.L., Altet Gómez M.N. Inactive fibrotic lesions versus pulmonary tuberculosis with negative bacteriology. Arch Bronconeumol. 2014;50(11):484–489. doi: 10.1016/j.arbres.2013.07.009. Nov. [DOI] [PubMed] [Google Scholar]
- 30.CPT-leidraad voor beleid bij fibrotische afwijkingen die bij radiologische screening worden vastgesteld. Den Haag Commissie voor Praktische Tuberculosebestrijding, KNCV Tuberculosefonds; 2014.
- 31.Ward M., Brandsema P., van Straten E., Bosman A. Electronic reporting improves timeliness and completeness of infectious disease notification. The Netherlands. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2005;10(1):27–30. 2003. Jan. [PubMed] [Google Scholar]
- 32.Erkens C.G.M., Slump E., Verhagen M., Schimmel H., de Vries G., Cobelens F. Monitoring latent tuberculosis infection diagnosis and management in the Netherlands. Eur Respir J. 2016;47(5):1492–1501. doi: 10.1183/13993003.01397-2015. May. [DOI] [PubMed] [Google Scholar]
- 33.Erkens C.G.M., Slump E., Verhagen M., Schimmel H., Cobelens F., van den Hof S. Risk of developing tuberculosis disease among persons diagnosed with latent tuberculosis infection in the Netherlands. Eur Respir J. Nov 2016;48(5):1420–1428. doi: 10.1183/13993003.01157-2016. [DOI] [PubMed] [Google Scholar]
- 34.Evaluatie bron- en contactonderzoek bij tuberculosepatiënten in Nederland, 2006-2010. Den Haag: KNCV Tuberculosefonds; 2014.
- 35.Veen J. Microepidemics of tuberculosis: the stone-in-the-pond principle. Tuber Lung Dis. 1992;73:73–76. doi: 10.1016/0962-8479(92)90058-R. [DOI] [PubMed] [Google Scholar]
- 36.Kik S.V., Franken W.P.J., Mensen M., Cobelens F.G.J., Kamphorst M. Predictive value for progression to tuberculosis by IGRA and TST in immigrant contacts. Eur Respir J. 2010;35(6):1346–1353. doi: 10.1183/09031936.00098509. Jun 1. [DOI] [PubMed] [Google Scholar]
- 37.Kik S.V., Franken W.P.J., Arend S.M., Mensen M., Cobelens F.G.J., Kamphorst M. Interferon-gamma release assays in immigrant contacts and effect of remote exposure to Mycobacterium tuberculosis. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2009;13(7):820–828. Jul. [PubMed] [Google Scholar]
- 38.Kik S.V. University of Amsterdam; Amsterdam: 2009. Tuberculosis Transmission in the Netherlands: The Role of Immigration and Travel. [Google Scholar]
- 39.Mulder C., Erkens C.G.M., Kouw P.M., Huisman E.M., Meijer-Veldman W., Borgdorff M.W. Missed opportunities in tuberculosis control in The Netherlands due to prioritization of contact investigations. Eur J Public Health. 2012;22(2):177–182. doi: 10.1093/eurpub/ckr017. Apr. [DOI] [PubMed] [Google Scholar]
- 40.Mulder C., van Deutekom H., Huisman E.M., Toumanian S., Koster BFP J., Meijer-Veldman W. Role of the QuantiFERON(R)-TB Gold In-Tube assay in screening new immigrants for tuberculosis infection. Eur Respir J. 2012;40(6):1443–1449. doi: 10.1183/09031936.00010612. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulder C., Mulleners B., Borgdorff M.W., van Leth F. Predictive value of the tuberculin skin test among newly arriving immigrants. PloS One. 2013;8(3):e60130. doi: 10.1371/journal.pone.0060130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulder C. University of Amsterdam; Amsterdam: 2013. Tuberculosis control among immigrants. [Google Scholar]
- 43.Tebruegge M., Buonsenso D., Brinkmann F., Noguera-Julian A., Pavić I., Arbore A.S. European shortage of purified protein derivative and its impact on tuberculosis screening practices. Int J Tuberc Lung Dis. Oct 1 2016;20(10):1293–1299. doi: 10.5588/ijtld.15.0975. [DOI] [PubMed] [Google Scholar]
- 44.Hoff S.T., Peter J.G., Theron G., Pascoe M., Tingskov P.N., Aggerbeck H. Sensitivity of C-Tb: a novel RD-1-specific skin test for the diagnosis of tuberculosis infection. Eur Respir J. 2016;47(3):919–928. doi: 10.1183/13993003.01464-2015. [DOI] [PubMed] [Google Scholar]
- 45.Sterling T.R., Villarino M.E., Borisov A.S., Shang N., Gordin F., Bliven-Sizemore E. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155–2166. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 46.https://e-detecttb.eu/ [Internet]. Available from: https://e-detecttb.eu/
- 47.Sandgren A., Vonk Noordegraaf-Schouten J.M., Oordt-Speets A.M., van Kessel G.B., de Vlas S.J., van der Werf M.J. Identifying components for programmatic latent tuberculosis infection control in the European Union. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2016;21(34) doi: 10.2807/1560-7917.ES.2016.21.34.30325. Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oxlade O., Schwartzman K., Menzies D. Interferon-gamma release assays and TB screening in high-income countries: a cost-effectiveness analysis. Int J Tuberc Lung Dis. 2007;11(1):16–26. Jan 1. [PubMed] [Google Scholar]
- 49.Pareek M., Watson J.P., Ormerod L.P., Kon O.M., Woltmann G., White P.J. Screening of immigrants in the UK for imported latent tuberculosis: a multicentre cohort study and cost-effectiveness analysis. Lancet Infect Dis. 2011;11(6):435–444. doi: 10.1016/S1473-3099(11)70069-X. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zammarchi L., Casadei G., Strohmeyer M., Bartalesi F., Liendo C., Matteelli A. A scoping review of cost-effectiveness of screening and treatment for latent tubercolosis infection in migrants from high-incidence countries. BMC Health Serv Res. 2015 doi: 10.1186/s12913-015-1045-3. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4581517/ [Internet]Sep 24 [cited 2016 Oct 15];15. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oxlade O., Pinto M., Trajman A., Menzies D. How methodologic differences affect results of economic analyses: a systematic review of interferon gamma release assays for the diagnosis of LTBI. PLoS ONE. 2013 doi: 10.1371/journal.pone.0056044. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3591384/ [Internet]Mar 7 [cited 2016 Oct 15];8(3). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Vries G., van Rest J., Meijer W., Wolters B., van Hest R., Kimerling M. Preventing and controlling tuberculosis among refugees in Europe: more needed for high-risk populations. Eur Respir J. 2016;48(1):274–276. doi: 10.1183/13993003.00575-2016. Jul 1. [DOI] [PubMed] [Google Scholar]
- 53.Dara M., Solovic I., Goletti D., Sotgiu G., Centis R., D'Ambrosio L. Preventing and controlling tuberculosis among refugees in Europe: more is needed. Eur Respir J. 2016;48(1):272–274. doi: 10.1183/13993003.00329-2016. Jul 1. [DOI] [PubMed] [Google Scholar]
- 54.Dara M., Solovic I., Sotgiu G., D'Ambrosio L., Centis R., Tran R. Tuberculosis care among refugees arriving in Europe: a ERS/WHO Europe Region survey of current practices. Eur Respir J. 2016;48(3):808–817. doi: 10.1183/13993003.00840-2016. Sep. [DOI] [PubMed] [Google Scholar]
- 55.HIV Monitoring Report . 2016. Stichting HIV Monitoring. [Google Scholar]
- 56.CPT-richtlijn Tuberculose-HIV. Den Haag: Commissie voor Praktische Tuberculosebestrijding, KNCV Tuberculosefonds; 2016.