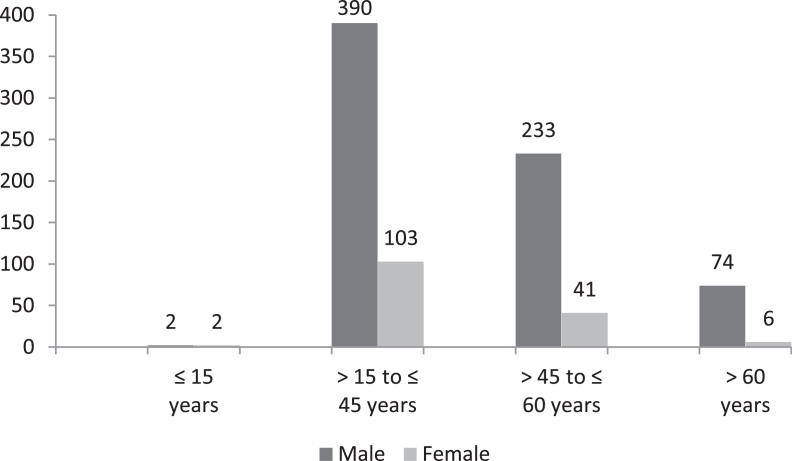Highlights
-
•
Total 558 multidrug resistant, 293 RIF mono resistant and 923 INH mono resistant tuberculosis were detected from the 12,786 MDR suspects samples.
-
•
Most frequent mutations were found in the region of MUT: S531L as 50.5% and 55.6% specimens out of 558 MDR-TB and 293 rifampicin monoresistant cases.
-
•
The rate of occurrences of mutations was found widely in the Rifampicin Resistant Determination Region of rpoB gene, but the hypervariable regions of 530–533 were alarming in the specification.
-
•
Six strains had novel MUT3A (T8C) mutation for isoniazid drug.
Keywords: Mycobacterium tuberculosis, Multidrug resistant, Rifampicin monoresistant, Hypervariable region, Isoniazid monoresistant
Abstract
Purpose: To analyze prevalence of mutations in genes associated with rifampicin and isoniazid resistance in Mycobacterium tuberculosis clinical isolates from patients with possible MDR TB of Puducherry, South India and to explore the association of specific mutations conferring rifampicin (RIF) resistance.
Methods: We performed a commercial Genotype MDBDRplus V.2.0 assay for the rapid detection of rifampicin and isoniazid resistance directly on sputum specimens of patients with possible MDR TB.
Results: Totally 558 multidrug resistant, 293 RIF mono resistant and 923 INH mono resistant tuberculosis were detected from the 12,786 patients with possible MDR TB samples. The 50.5% mutations were observed in the region of S531L in MDR TB patients and 55.6% in rifampicin monoresistant cases. In total isoniazid monoresistant, 68.0% mutations were detected in katG gene, which is more prevalent in comparison to inhA gene 32.0%. There were about 57.9% and 32.2% MDR TB cases diagnosed in the age group of > 15 to ≤ 45 years and > 45 to ≤ 60 years respectively.
Conclusions: The rate of occurrences of mutations were found widely in the Rifampicin Resistant Determination Region (81 bp) of rpoB gene and the hypervariable region 530–533 codons of rpoB gene is alarming in the specification. The higher frequency of mutation in codons of rpoB (S531L) and katG (S315T) gene help to design simple, new and less expensive molecular techniques to use in peripheral laboratories.
Introduction
The emergence and spread of multi-drug resistant tuberculosis (MDR-TB) is menacing to global tuberculosis control. According to WHO, nearly 50% of the world's burden of MDR-TB cases is in India and China [33]. The prevalence of MDR-TB is increasing throughout the world both among new tuberculosis cases as well as among previously treated cases [34]. The World Health Organization has estimated that India accounted for 26% of the total number of TB cases worldwide in 2015, with 3.9% and 21% of the new and retreatment cases respectively being caused by multi drug resistant strains [16].
In the majority of drug-resistant M. tuberculosis clinical isolates, drug resistance is due to mutations in genes or promoters region of genes activating the drug or encoding the drug targets. Studies have pointed out that the M. tuberculosis becomes resistant to RIF due to the mutations in rpoB, INH due to katG and inhA [30]. Resistance to rifampicin in mycobacterium results from point mutations mainly located in the 507–533 region of the rpoB polypeptide [12], [13], [26]. The most common mutations observed in rifampicin resistant M. tuberculosis isolates are Ser531Leu, His526Asp or Tyr, and Asp516Val [37]. Rifampicin resistance in M. tuberculosis strains is conferred by a diverse group of mutations within a hypervariable region of the rpoB gene, which codes for a β-subunit of RNA polymerase [3], [7]. More than 95% of rifampicin resistant isolates possess mutations within this hyper variable regions of the rpoB gene [10].Resistance to isoniazid is mostly associated with the amino acid substitution Ser315Thr in katG (in roughly 70% of INH-resistant strains) and the −15 C-to-T mutation in the inhA promoter (in 15–35% of INH-resistant strains) [8], [18], [25].
The rapid diagnosis of multidrug resistant tuberculosis patients, place them on treatment regimens is indispensable in controlling the MDR-TB in a community and limit the nosocomial spread of MDR-TB through proper infection control methods. The World Health Organization (WHO) recommended Line-probe assays (LPAs), which can simultaneously identify the M. tuberculosis complex and detect genetic mutations in the rpoB gene region related to rifampicin resistance, katG and inhA gene regions related isoniazid resistance [24]. The main objective of this study the prevalence of mutations in genes associated with rifampicin and isoniazid resistance in Mycobacterium tuberculosis clinical isolates from patients with possible MDR TB of Puducherry, South India and to explore the association of specific mutations conferring rifampicin (RIF) resistance.
Materials and methods
Specimen collection and processing
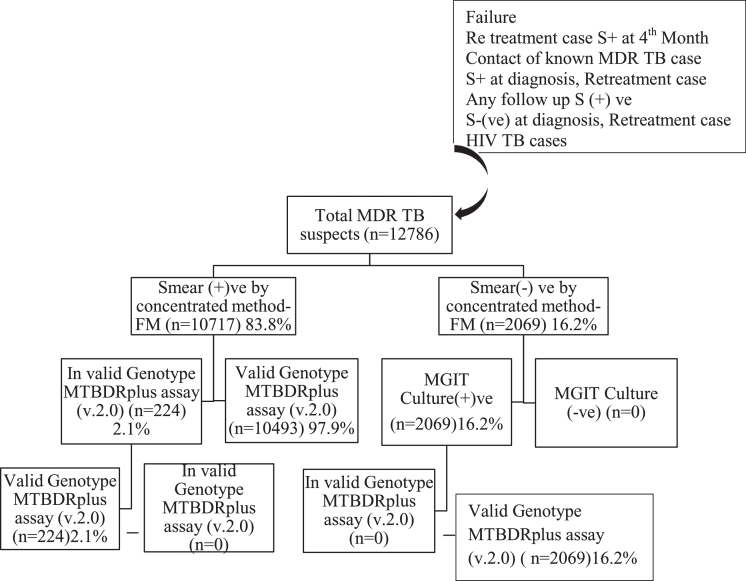
The study was conducted retrospectively in the Intermediate Reference TB Laboratory at Government Hospital for Chest Diseases, Puducherry for a span of 42 months between July 2012 and December 2015. The two sputum samples were collected in 50 ml sterile falcon tubes for each patient and transported through cold chain mechanism from the nine districts (Villupuram, Tanjore, Nagapattinam, Thiruvarur, Cuddalore, Dindigul, Perambalore, and Trichy) of Tamil Nadu state in addition to Puducherry state as per the criterias of Revised National Tuberculosis Control Programme, India. Twelve thousands seven hundred and eight six (12,786) sputum samples were collected from various age groups for this study, which included ≤15 years (n-117), >15 to ≤45 years (n-7060), >45 to ≤60 years (n-4357) and >60 years (n-1252) as described in Table 1. The sputum samples received at Intermediate Reference TB Laboratory were assigned lab number and consecutively screened for acid fast bacilli (AFBs) using Fluorescence (FM) microscopy [32].The smear positive sputum samples in Fluorescence microscopy were directly processed by GenoType MTBDRplus V.2.0 assay (Hain Life Sciences). The smear negative samples were subjected to liquid culture using the BACTEC MGIT 960 system (Becton Dickinson Microbiology Systems, Cockeysville, MD, USA) under stringent conditions. The culture positive samples from the MGIT system were in turn subjected to the GenoType MTBDRplus V.2.0 assay (Fig. 1). All the laboratory bench works related with potentially infectious specimens were performed in a Class II biosafety cabinet placed at Bio Safety Level III facility. All processed specimens were stored at −20 °C for the duration of the study to allow for re-testing of specimens giving discrepant results.
Table 1.
MDR TB suspects on gender and age wise.
| RNTCP criteria | Sex | Total no of MDR TB suspects received |
Total MDR TB suspects received | Grand total | |||
|---|---|---|---|---|---|---|---|
| ≤15 years | >15 ≤ to 45 years | >45 to ≤60 years | > 60 years | ||||
| Failure | Male | 2 | 251 | 164 | 51 | 468 | 557 |
| Female | 1 | 71 | 13 | 4 | 89 | ||
| Re treatment case S+ at 4th month | Male | 1 | 258 | 190 | 53 | 502 | 590 |
| Female | 3 | 62 | 18 | 5 | 88 | ||
| Contact of known MDR TB case | Male | 5 | 41 | 14 | 5 | 65 | 104 |
| Female | 6 | 27 | 6 | 0 | 39 | ||
| S+ at diagnosis, re treatment case | Male | 10 | 2600 | 1961 | 576 | 5147 | 6035 |
| Female | 13 | 592 | 223 | 60 | 888 | ||
| Any follow up smear positive | Male | 3 | 1125 | 836 | 247 | 2211 | 2702 |
| Female | 7 | 326 | 120 | 38 | 491 | ||
| S - at diagnosis, re treatment case | Male | 2 | 430 | 374 | 149 | 955 | 1266 |
| Female | 5 | 186 | 92 | 28 | 311 | ||
| HIV TB case | Male | 32 | 716 | 256 | 28 | 1032 | 1532 |
| Female | 27 | 375 | 90 | 8 | 500 | ||
| Total | Male | 55 | 5421 | 3795 | 1109 | 10380 | 12786 |
| Female | 62 | 1639 | 562 | 143 | 2406 | ||
| Grand total | 117 | 7060 | 4357 | 1252 | 12786 | ||
Fig. 1.
Specimen flow schematic.
GenoType MTBDRplus V.2.0 assay
The GenoType MTBDRplus V.2.0 assay was performed according to the manufacturer's protocol ([15]). The test is based on DNA strip technology and has three steps: DNA extraction, multiplex PCR amplification, and reverse hybridization. All three steps were performed as per the WHO recommendations [29], [39].
BACTEC MGIT 960 culture
The smear negative and discrepant samples were processed in MGIT 960 culture tube. A 500 µl sample was taken out from decontaminated sample and inoculated in BACTEC MGIT 960 tube. After the culture flashed positive, MGIT tubes were confirmed for acid fast bacilli by ZN staining and further subjected to confirm as M. tuberculosis complex using Capilia TB Neo (TAUNS Corporation, Japan) and checked for contamination by growth on blood agar medium for 48 h at 37 °C ([6], [11], [21]). The confirmed positive MGIT tube was processed with the Genotype MTBDRplus V.2.0 assay (Hain Life Science, Nehren, Germany) as per the manufacturer's protocol.
Results
The sputum samples were collected in 50 ml sterile falcon tubes from each person with possible MDR TB mainly based on the criteria of Revised National Tuberculosis Control Programme and totally 12,786 person's with possible MDR TB sputum samples from different age groups (Table 1) were processed for the Auromine O phenol staining. Among them, (83.8%) sputum samples were smear positive,(16.2%) samples were smear negative. The Conventional BACTEC MGIT 960 procedure was performed for all smear negative TB person's with possible MDR TB samples and no results/invalid obtained from Genotype MTBDRplus V.2.0 assay. The 2% contamination samples are received on request for the further processing by the Genotype MTBDRplus V.2.0 assay. In total, 1774 (13.87%) drug resistant strains were identified by Genotype MTBDRplus assay V.2.0; among them 558 were multidrug resistant, 293 were RIF mono resistant and 923 were INH mono resistant from high-risk patients. The number of MDR TB cases diagnosed from each criteria were tabulated as shown in Table 2.
Table 2.
MDR TB diagnosed on gender and age wise.
| Criteria | Sex | Total MDR TB diagnosed on age and criteria basis |
Total MDR TB | Grand total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤15 years |
>15 ≤ to 45 years |
>45 to ≤60 years |
>60 years |
|||||||||
| RH | R | RH | R | RH | R | RH | R | RH | R | |||
| Failure | Male | 0 | 0 | 25 | 17 | 17 | 5 | 3 | 1 | 45 | 23 | 68 |
| Female | 0 | 0 | 2 | 3 | 0 | 2 | 0 | 0 | 2 | 5 | 7 | |
| Re treatment case S+ at 4th month | Male | 0 | 0 | 10 | 10 | 9 | 6 | 2 | 1 | 21 | 17 | 38 |
| Female | 1 | 0 | 7 | 2 | 0 | 2 | 1 | 1 | 9 | 5 | 14 | |
| Contact of known MDR TB case | Male | 0 | 0 | 7 | 1 | 1 | 1 | 0 | 0 | 8 | 2 | 10 |
| Female | 0 | 0 | 4 | 0 | 1 | 0 | 0 | 0 | 5 | 0 | 5 | |
| S+ at diagnosis, re treatment case | Male | 0 | 2 | 105 | 83 | 78 | 45 | 29 | 14 | 212 | 144 | 360 |
| Female | 0 | 0 | 39 | 12 | 14 | 8 | 3 | 1 | 56 | 21 | 77 | |
| Any follow up smear positive | Male | 0 | 0 | 73 | 19 | 34 | 18 | 14 | 6 | 121 | 43 | 164 |
| Female | 0 | 0 | 20 | 1 | 9 | 3 | 0 | 0 | 29 | 4 | 33 | |
| S - at diagnosis, re treatment case | Male | 0 | 0 | 15 | 4 | 9 | 3 | 2 | 1 | 26 | 8 | 34 |
| Female | 0 | 0 | 4 | 3 | 1 | 1 | 0 | 0 | 5 | 4 | 9 | |
| HIV TB case | Male | 0 | 0 | 11 | 10 | 3 | 4 | 1 | 0 | 15 | 14 | 29 |
| Female | 0 | 1 | 4 | 2 | 0 | 0 | 0 | 0 | 4 | 3 | 7 | |
| Total | Male | 0 | 2 | 246 | 144 | 151 | 82 | 51 | 23 | 448 | 251 | 699 |
| Female | 1 | 1 | 80 | 23 | 25 | 16 | 4 | 2 | 110 | 42 | 152 | |
| Grand total | 1 | 3 | 326 | 167 | 176 | 98 | 55 | 25 | 558 | 293 | 851 | |
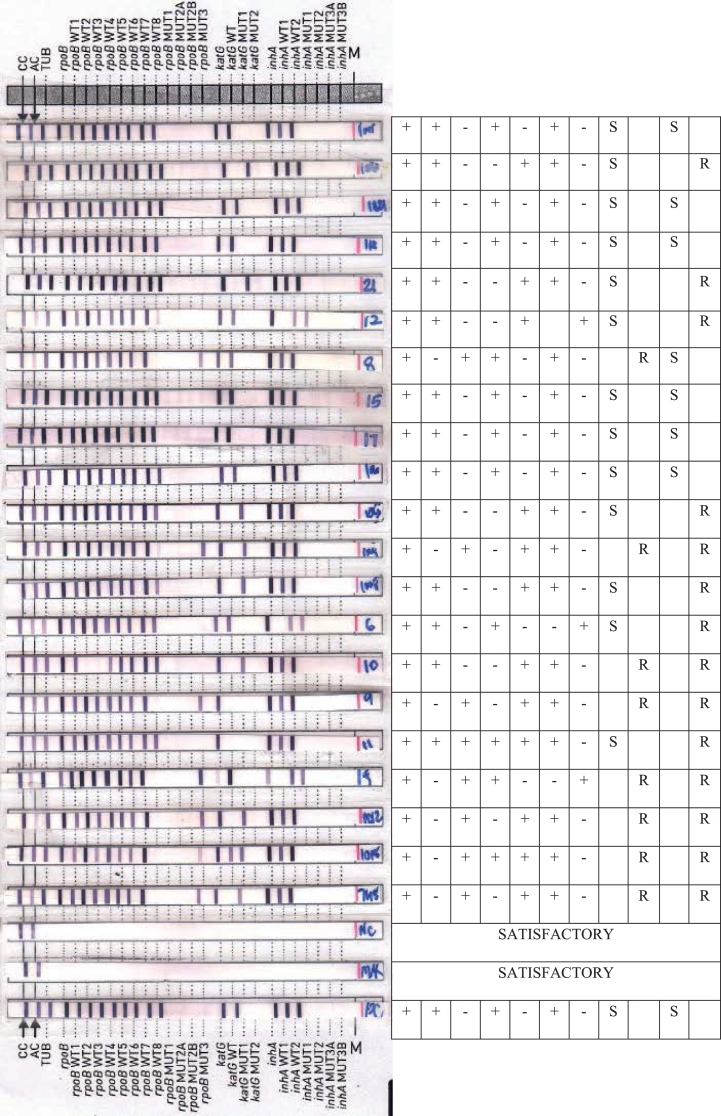
Overall, 83.8% smear positive specimens gave interpretable results within 2–3 days by Genotype MTBDRplus V.2.0 assay and only 16.2% of the samples gave smear negative results by Fluorescence Microscopy and those samples are further established with conventional BACTEC MGIT 960 culture procedures. Overall, 83.8% smear positive specimen's results effectively including the results of repeated testing in 224 (2.1% invalid) cases as shown in Fig. 1. The results of Genotype MTBDRplus V.2.0 assay is interpreted as shown in the Fig. 2.
Fig. 2.
The interpretation of LPA results with various resistant/sensitive pattern.
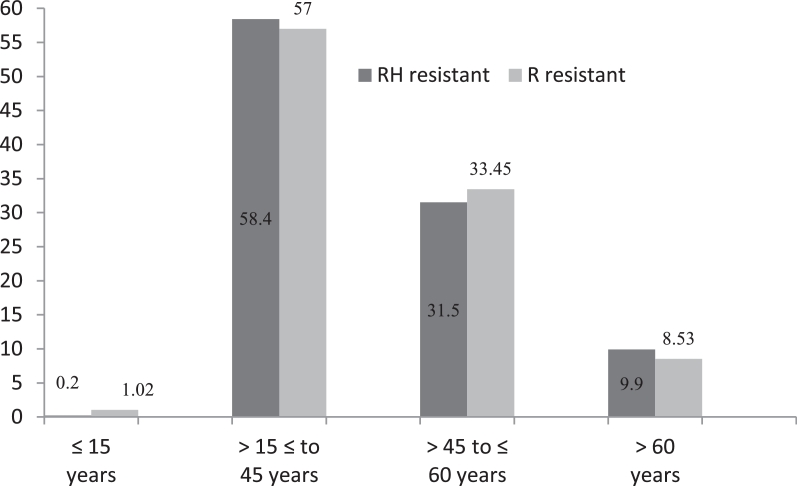
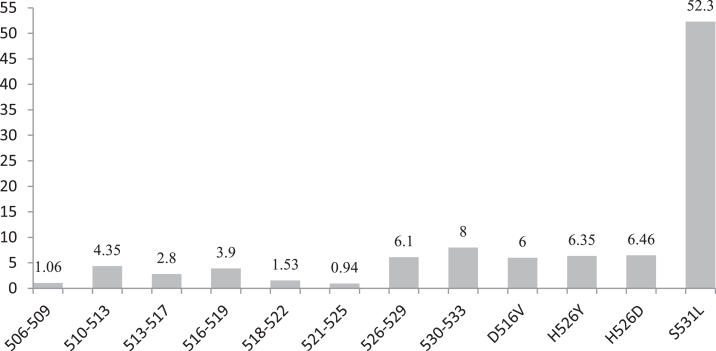
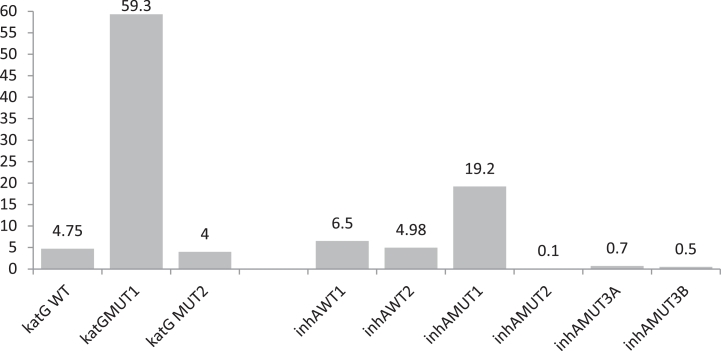
Among 851 diagnosed MDR tuberculosis, 90% cases were diagnosed from age group of >15 to ≤45 years and >45 to ≤60 years respectively (Fig. 3) and rifampicin mono resistant were diagnosed equal proportion to multi drug resistant from these age group only (Fig. 4). The diagnosed MDR TB cases in pediatric age group were 0.47% and 9.4% MDR TB cases were diagnosed in the age group of >60 years. The 50.5% mutations were observed in the region of S531L in MDR TB patients and 55.6% in rifampicin monoresistant cases (Fig. 5). In total isoniazid monoresistant, 68.0% mutations were detected in katG gene, which is more prevalent in comparison to inhA gene 32.0% (Fig. 6). In isoniazid monoresistant strains, the particular type of mutations were observed uniformly, which highly specified the efficacy of the detection of mutations in katG and inhA genes. This difference in prevalence of mutations in MDR strains compared with INH monoresistant strains was significant for katG but not in inhA. Surprisingly, only two strains had mutations in both the katG and inhA genes.
Fig. 3.
Total no of multi drug resistant on age and sex wise.
Fig. 4.
Percentage of multi drug and Rif mono resistant on age wise.
Fig. 5.
Frequency of mutations on codons of hyper variable region of rpoB gene.
Fig. 6.
Frequency of mutations on codons of katG and InhA genes.
Discussion
In this study, the performance of the GenoType MTBDRplus V.2.0 assay was assessed that offers the simultaneous identification of M. tuberculosis and its resistance to rifampicin (RIF) and isoniazid (INH) by detecting the most common mutations in the rpoB and katG genes. As reported widely elsewhere, rifampicin resistance was highly associated with mutations in the 81 base pair region of the rpoB gene [35], [36]. DNA sequencing studies have shown that greater than 95% of the RIF-resistant strains have mutations within an 81 base pair hot-spot region (codons 507–533) of the rpoB gene [9]. Though more than 50 mutations within this region have been characterized by automated DNA sequencing, the majority involve point mutations at codons 516, 526, or 531 [1]. Priyanka et al. [27] found a higher (89.65%) proportion of RMP resistance due to S531L mutations. Barnard et al. [5] also found higher proportion (70.5%) RMP resistance due to S531L in their study. Similar to both above mentioned studies, Ravindran et al. (20,012) also found codon 531 of the rpoB was the most frequently encountered (84.6%). Raj et al. [28] found 72% RMP resistance due to S531L in their study. But we found a 52.3% proportion of RMP resistance due to S531L mutations. The reasons for lesser proportion of RMP resistant due to S531L mutations are sample volume and selection of sample criteria. A merely equal proportion of rifampicin resistance (52.3%) due to S531L mutations as what has been reported in other geographical locations (between 36.1 and 56.7%) [17], [22]. Viveiros et al. [38], Marinus et al. [20], Florence et al. [14] reported the “false” resistant strains in their study as absence of RIF WT2 probe, interestingly; the same sort of mutation was found in this study made justification for the possibility of true resistance as of 4.3% in MDR cases and two (4.4%) in RIF-mono resistant strains. These results indicated that the GenoType MTBDRplus V.2.0 assay is also capable of revealing the presence of rare mutations.
The various types of uncommon drug resistant patterns identified are; thirteen MDR strains had absence of WT8 and a H526Y mutation, five MDR strain had both H526Y and S531L mutations, four MDR strain had H526Y and H526D, two MDR strain had a H526D and S531L mutation. Other mutations in the 530–533 regions were common, as detected by the lack of binding with the WT8 probe in the presence of S531L mutation. A significantly higher proportion of RIF-monoresistant strains (10.6%) had a lack of binding with the WT8 probe and in combination with presence of MUT3 (S531L) probe (55.6%), compared with MDR strains (50.5%). There was no significant difference in the presence of other bands between MDR and RIF monoresistant strains. In previous study, Akos Somoskovi et al. [2] reported a rare mutation of WT2 (Q513L mutation), as a ‘‘false’’ RIF-resistant. The same mutation of WT2 probe was missing observed in thirty seven strains in this study also. But it was combined with absence of katG WT probes in thirteen strains and presence of MUT1 (S315T) probe in twenty four strains, thus made these strains ensemble are indicative of true resistant.
The prevalence of mutations in the katG and inhA genes seems to vary widely in different geographic locations [2]. It was observed that the prevalence of mutations in katG gene plays major role in determining of MDR-TB, when compare to inhA gene. However, in concordant with other studies, it was observed that the most common mutations at codons katG WT: 315, MUT1: S315T and inhA WT: −15/−16, MUT1: C15T. It was established that a higher proportion of INH resistance due to MUT1: S315T probe [19]. Studies from other countries have confirmed this variability in the contribution of different mutations to INH resistance [4]. In rare case six INH monoresistant had a MUT3A (T8C) mutation and it has not been published elsewhere. A high prevalence of katG mutations has been reported to account for a high proportion of MDR and INH-resistance in high-TB prevalent countries and for a much lower proportion in lower-TB prevalent settings, presumably due to on-going transmission of these strains in high-burden settings [31,23]. In INH-resistant cases region of wild types in katG and inhA genes showed repeated mutations.
Conclusion
In conclusion, the rate of occurrences of mutations were found widely in the Rifampicin Resistant Determination Region (81 bp) of rpoB gene and the hypervariable region 530–533 codons of rpoB gene is alarming in the specification. The most frequent mutations involved in resistance to Rifampicin and Isoniazid drugs was observed in this study and this higher frequency of mutation in particular codons of rpoB (S531L) and katG (S315T) gene help to design new simple less expensive molecular assay to use at peripheral laboratories in developing countries.
Conflict of interest
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Informed consent
Not applicable.
Acknowledgements
The author is grateful to Directorate of Health and Family Welfare Services, Puducherry, India.
References
- 1.Ahmad S., Mokaddas E., Fares E. Characterization of rpo B mutations in rifampin-resistant clinical Mycobacterium tuberculosis isolates from Kuwait and Dubai. Diagn Microbiol Infect Dis. 2002;44:245–252. doi: 10.1016/s0732-8893(02)00457-1. [DOI] [PubMed] [Google Scholar]
- 2.Somoskovi A., Linda M., Parsons M.S. The molecular basis of resistance to isoniazid, rifampin, and pyrazinamide in Mycobacterium tuberculosis. Respir Res. 2001;2:164–168. doi: 10.1186/rr54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alono M., Palacios J.J., Herranz M., Penedo A., Menendez A., Bouza E. Isolation of Mycobacterium tuberculosis in rpoB leading to potential mismanagement of resistance category. J Clin Microbiol. 2011;49:2688–2690. doi: 10.1128/JCM.00659-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker L.V., Brown T.J., Maxwel L.O., Gibson A.L., Fang Z., Yates M.D. Molecular analysis of isoniazid-resistant Mycobacterium tuberculosis isolates from England and Wales reveals the phylogenetic significance of the ahpC-46A polymorphism. Antimicrob Agents Chemother. 2005;49:1455–1464. doi: 10.1128/AAC.49.4.1455-1464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnard M., Albert H., Coetzee G., O'Brien R., Bosman M.E. Rapid Molecular screening for multi drug resistance Tuberculosis in a high volume public health laboratory in South Africa. Am J Respir Crit Care Med. 2008:787–792. doi: 10.1164/rccm.200709-1436OC. [DOI] [PubMed] [Google Scholar]
- 6.Becton D. & Company. BACTEC MGI-960 SIRE kit for the antimycobacterial susceptibility testing of Mycobacterium tuberculosis. http://www.bd.com/ds/technicalCenter/clsi/clsi-960sire.pdf. Becton, Dickinson & Company, Franklin Lakes, NJ.
- 7.Bicmen C., Gunduz A.T., Coskun M. Molecular identification and characterization of rifampicin-resistant Mycobacterium tuberculosis isolates by line-probe assay: an approach for rapid diagnosis of multidrug-resistant tuberculosis. Lett Appl Microbiol. 2008;47:214–220. doi: 10.1111/j.1472-765X.2008.02403.x. [DOI] [PubMed] [Google Scholar]
- 8.Bwanga F., Joloba M.L., Haile M., Hoffner S. Evaluation of seven tests for the rapid detection of multidrug-resistant tuberculosis in Uganda. Int J Tuberc Lung Dis. 2010;14:890–895. [PubMed] [Google Scholar]
- 9.Cavusoglu C., Hilmioglu S., Guneri S., Bilgic A. Characterization of rpo B mutations in rifampin-resistant clinical isolates of Mycobacterium tuberculosis from Turkey by DNA sequencing and line probe assay. J Clin Microbiol. 2002;40:4435–4438. doi: 10.1128/JCM.40.12.4435-4438.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caws M., Drobniewski F.A. Molecular techniques in the diagnosis of Mycobacterium tuberculosis and the detection of drug resistance. Ann N Y Acad Sci. 2001;953:138–145. doi: 10.1111/j.1749-6632.2001.tb11371.x. [DOI] [PubMed] [Google Scholar]
- 11.Chihota V.N., Grant A.D., Fielding K., Ndibongo B., Van Zyl A., Muirhead D. Liquid vs. solid culture for tuberculosis: performance and cost in a resource-constrained setting. Int J Tuberc Lung Dis. 2010;14:1024–1031. [PubMed] [Google Scholar]
- 12.Edwards K.J., Metherell L.A., Yates M., Saunders N.A. Detection of rpoB mutations in Mycobacterium tuberculosis by biprobe analysis. J Clin Microbiol. 2001;39:3350–3352. doi: 10.1128/JCM.39.9.3350-3352.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Hajj H.H., Marras S.A., Tyagi S., Kramer F.R., Alland D. Detection of rifampin resistance in Mycobacterium tuberculosis in a single tube with molecular beacons. J Clin Microbiol. 2001;39:4131–4137. doi: 10.1128/JCM.39.11.4131-4137.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Florence B., Nicolas V., Chantal T.P., Vincent J., Wladimir S. Performance of the genotype MTBDR line probe assay for detection of resistance to rifampin and isoniazid in strains of Mycobacterium tuberculosis with low- and high-level resistance. J Clin Microbiol. 2006;44:3659–3664. doi: 10.1128/JCM.01054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hain Lifescience GmbH. GenoTypeMTBDRplus, version 2.0 product insert. Nehren, Germany. http://www.hain-lifescience.de/en/instructions-for-use.html.
- 16.Isaakidis P., Das M., Kumar A.M.V., Peskett C., Khetarpal M. Alarming levels of drug-resistant tuberculosis in HIV-infected patients in metropolitan Mumbai, India. PLoS ONE. 2014;9(10) doi: 10.1371/journal.pone.0110461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansen I.S., Lundgren B., Sosnovskaja A., Thomsen V.O. Direct detection of multidrug-resistant Mycobacterium tuberculosis in clinical specimens in low- and high-incidence countries by line probe assay. J Clin Microbiol. 2003;41:4454–4456. doi: 10.1128/JCM.41.9.4454-4456.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung E.T., Ho P.L., Yuen H.Y., Woo W.L., Lam T.H., Kao R.Y. Molecular characterization of isoniazid resistance in Mycobacterium tuberculosis: identification of a novel mutation in inhA. Antimicrob Agents Chemother. 2006;50:1075–1078. doi: 10.1128/AAC.50.3.1075-1078.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makinen J., Marttila H.J., Marjamaki M., Viljanen M.K., Soini H. Comparison of two commercially available DNA line probe assays for detection of multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol. 2006;44:350–352. doi: 10.1128/JCM.44.2.350-352.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marinus B., Heidi A., Gerrit C., O'Brien R., Marlein E.B. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. Am J Respir Crit Care Med. 2008;177:787–792. doi: 10.1164/rccm.200709-1436OC. [DOI] [PubMed] [Google Scholar]
- 21.Master R.N. American Society for Microbiology; Washington, DC: 1992. Mycobacteriology. Clinical microbiology procedures hand book; p. 1. Section 3Isenburg HD. [Google Scholar]
- 22.Maureen M., Shriprakash K., Laura F., Madhukar P. A commercial line probe assay for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. BMC Infect Dis. 2005;5:1471–2334. doi: 10.1186/1471-2334-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mokrousov I., Narvskaya O., Otten T., Limenschenko E., Steklova L., Vyshnevskiy B. High prevalence of katG Ser315Thr substitution among isoniazid-resistant Mycobacterium tuberculosis clinical isolates from North Western Russia. Antimicrob Agents Chemother. 2006;46:1417–1424. doi: 10.1128/AAC.46.5.1417-1424.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan M., Kalantri S., Flores L., Pai M. A commercial line probe assay for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: a systematic review and metaanalysis. BMC Infect Dis. 2005;5:62. doi: 10.1186/1471-2334-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morlock G.P., Metchock B., Sikes D., Crawford J.T., Cooksey R.C. ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother. 2003;47:3799–3805. doi: 10.1128/AAC.47.12.3799-3805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park H., Song E.J., Song E.S., Lee E.Y., Kim C.M., Jeong S.H. Comparison of a conventional antimicrobial susceptibility assay to an oligonucleotide chip system for detection of drug resistance in Mycobacterium tuberculosis isolates. J Clin Microbiol. 2006;44:1619–1624. doi: 10.1128/JCM.44.5.1619-1624.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Priyanka K., Sarala M., Nilma H., Vaishali W., Ameeta J. Rapid and economical detection of multidrug resistant (MDR) tuberculosis: can rifampicin resistance (RMPr) be a surrogate marker for MDR-TB ? RRJMB. 2013;2(3):78–84. [Google Scholar]
- 28.Raj N.Y., Binit K.S., Surendra K.S., Rohini S., Manish S., Vishnubhatla S. Comparative evaluation of GenoType MTBDRplus line probe assay with solid culture method in early diagnosis of multidrug resistant tuberculosis (MDR-TB) at a tertiary care centre in India. PLoS ONE. 2013;8(9):e72036. doi: 10.1371/journal.pone.0072036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravindran R., Wattal C., Oberoi J.K., Goel N., Datta S., Prasad K.J. Utility of GenoType MTBDRplus assay in rapid diagnosis of multidrug resistant tuberculosis at a tertiary care centre in India. Indian J Medical Microbiol. 2012;30:58–63. doi: 10.4103/0255-0857.93034. [DOI] [PubMed] [Google Scholar]
- 30.Sadiq N.K., Stefan N., Muhammad G., Mazhar Q., Saima S., Zahid S.M. Molecular characterization of multidrug-resistant isolates of Mycobacterium tuberculosis from patients in Punjab, Pakistan. J Zool. 2013;45(1):93–100. [Google Scholar]
- 31.Shah N.S., Lan N.T.N., Huyen M.N.T., Laserson K., Iademarco M.F., Binkin N. Validation of the line-probe assay for rapid detection of rifampicin-resistant Mycobacterium tuberculosis in Vietnam. Int J Tuberc Lung Dis. 2005;13:247–252. [PubMed] [Google Scholar]
- 32.Steingart K.R., Henry M., Ng V., Hopewell P.C., Ramsay A., Cunningham J. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6(9):570–581. doi: 10.1016/S1473-3099(06)70578-3. [DOI] [PubMed] [Google Scholar]
- 33.Sunil S., Abhishek M., Sunil K.D., Harpal S., Rakesh Y., Khushwinder S. Prevalence of multidrug resistance in Mycobacterium tuberculosis isolates from HIV seropositive and seronegative patients with pulmonary tuberculosis in north India. BMC Infect Dis. 2013;13:137. doi: 10.1186/1471-2334-13-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Surendra K.S., Gaurav K., Brajesh J., Ninoo G., Arora S.K., Deepak G. Prevalence of multidrug-resistant tuberculosis among newly diagnosed cases of sputum-positive pulmonary tuberculosis. Indian J Med Res. 2011;133:308–311. [PMC free article] [PubMed] [Google Scholar]
- 35.Telenti A., Imboden P., Marchesi F., Cole S., Colston M.J., Matter L. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet. 2003;341:647–650. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- 36.Van Rie A., Warren R., Mshanga I., Jordaan A., Spuy van der G.D., Richardson M. Analysis for a limited number of gene codons can predict drug resistance of Mycobacterium tuberculosis in a high-incidence community. J Clin Microbiol. 2001;39:636–641. doi: 10.1128/JCM.39.2.636-641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viader-Salvado J.M., Luna-Aguirre C.M., Reyes-Ruiz J.M., Valdez- Leal R., del Bosque-MoncayoMde L., Tijerina-Menchaca R. Frequency of mutations in rpoB and codons 315 and 463 of katG in rifampin- and/or isoniazid-resistant Mycobacterium tuberculosis isolates from northeast Mexico. Microb Drug Resist. 2003;9:33–38. doi: 10.1089/107662903764736328. [DOI] [PubMed] [Google Scholar]
- 38.Viveiros M., Leandro C., Rodrigues L. Direct application of the INNO-LiPARif.TB line-probe assay for rapid identification of Mycobacterium tuberculosis complex strains and detection of rifampin resistance in 360 smear-positive respiratory specimens from an area of high incidence of multidrug-resistant tuberculosis. J Clin Microbiol. 2005;43:4880–4884. doi: 10.1128/JCM.43.9.4880-4884.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization . World Health Organization; Geneva, Switzerland: 2008. Molecular line probe assay for rapid screening of patients at risk of multidrug-resistant tuberculosis (MDRTB)http://www.who.int/tb/features_archive/policy_statement.pdf [Google Scholar]