Abstract
Revascularization in the setting of anastomotic “blow-out” in the groin is a technically demanding and morbid undertaking, often mandating transabdominal or retroperitoneal exposure of the iliac artery for proximal control or anastomosis, or both. The Gore Hybrid Vascular Graft (W. L. Gore and Associates Inc, Flagstaff, Ariz) is an expanded polytetrafluoroethylene graft with an external nitinol stent on one end designed for remote venous implantation for proximal axillary vein dialysis access outflow. We recently used this device to treat femoral anastomotic disruptions in two postoperative patients.
The Gore Hybrid Vascular Graft (GHVG; W. L. Gore and Associates Inc, Flagstaff, Ariz) is an expanded polytetrafluoroethylene (ePTFE) vascular prosthesis that provides a streamlined solution for hemodialysis access procedures.1 With the patients' express permission to publish, we present the first cases in which the GHVG was used in the management of a femoral artery anastomotic disruption.
Case reports
Patient 1
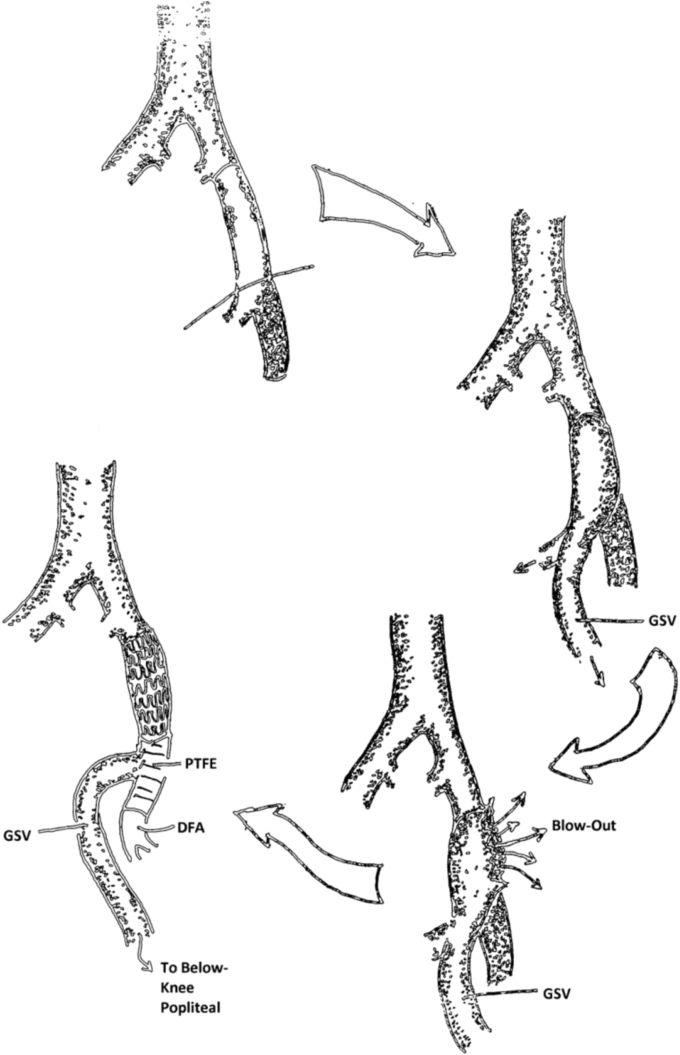
A 51-year-old woman with disabling claudication presented after failed endovascular interventions for multilevel infrainguinal disease. As depicted in the Fig, she underwent an extensive left common femoral artery (CFA) and deep femoral artery (DFA) endarterectomy with vein patch angioplasty and CFA-to-below-knee popliteal artery bypass with in situ great saphenous vein (GSV).
Fig.
Schematic shows the sequence of events in patient 1. Occluded superficial femoral artery → in situ great saphenous vein (GSV) bypass → anastomotic blowout → management using the Gore Hybrid Vascular Graft (GHVG; W. L. Gore and Associates Inc, Flagstaff, Ariz) bypass from the common femoral artery (CFA) to the deep femoral artery (DFA; profunda) and reimplantation of the previous GSV bypass graft onto the polytetrafluoroethylene (PTFE) segment of the GHVG.
She was discharged on postoperative day 3. However, while getting out of her car on day 7, she developed abrupt severe groin pain. She presented to the emergency department with a pulsatile hemorrhage from her left groin. She denied fevers, chills, erythema, or other signs of infection.
In the hybrid vascular operating suite, we encountered hemorrhage emanating from a 2-cm anastomotic disruption in the femoral anastomosis. There were no gross signs of infection, and am intraoperative gram stain was negative for any organisms. Proximal control was obtained with a Fogarty catheter. The patient had a large pannus, and severe calcification of the iliac arteries had been noted at the prior surgery.
We proceeded with in-line prosthetic reconstruction using the GHVS. Retrograde access was obtained passing a 12F Peel-Away sheath (Cook Medical, Bloomington, Ind) over a guidewire into the external iliac artery under fluoroscopic guidance. Iliofemoral arteriography revealed diffuse calcific disease. A Fogarty balloon was inserted alongside the 12F Peel-Away sheath and deflated as we deployed the 7-mm stent segment of the GHVG. The vein patch was excised, and the DFA was débrided to its primary bifurcation. The free distal end of the GHVG was spatulated and anastomosed to the DFA while the vein graft was transposed end-to-side onto the ePTFE graft. The sartorius muscle was mobilized from the anterior superior iliac spine and used to cover the reconstruction. The skin was loosely approximated and a negative pressure dressing applied.
Intravenous antibiotics were maintained for 10 days. Operative cultures grew only rare diphtheroids. The patient has done well, with sustained primary patency, a healed wound, and no infection through 12 months of follow-up.
Patient 2
A 90-year-old man presented with an expanding anastomotic aneurysm in the right groin at the junction of a prosthetic femorofemoral graft and a right in situ GSV femorotibial graft. A previous anastomotic aneurysm had been excluded with a Viabahn (W. L. Gore) stent graft 1 year prior. His comorbidities included critical aortic valvular stenosis and moderate renal insufficiency (serum creatinine, 2.9 mg/dL). He had undergone multiple previous vascular surgeries, including aortic aneurysm repair and multiple infrainguinal bypass procedures.
The patient underwent right groin exploration under local anesthesia and was found to have contained rupture of the proximal vein graft anastomosis. There was no evidence of infection, and gram stain demonstrated no organisms. Proximal dissection of the femoral artery was performed in preparation for a rifampin-soaked Dacron (DuPont, Wilmington, Del) interposition graft. However, the vessel was extremely friable and unsuitable for anastomosis, with prior stent material extending from just proximal to the inguinal ligament.
A GHVG was then deployed into the proximal CFA using a 14F Peel-Away sheath. An angiogram revealed persistent flow around the stent portion of the GHVG, which was then extended proximally with a 10-mm Viabahn stent graft. The distal end of the hybrid graft was then anastomosed to the previous GSV bypass graft. The entire wound was débrided, irrigated, and covered with a sartorius muscle flap and a negative pressure dressing.
Operative cultures were negative, wound healing was achieved, and the graft remained patent. At 5 months of follow-up, a recurrent anastomotic aneurysm was detected, and endovascular management was planned. Rupture occurred before admission. There was no gross evidence of infection, and we obtained percutaneous access and excluded the anastomotic dehiscence with a Viabahn stent graft and coil embolization of the DFA. Reconstruction was performed using cryopreserved saphenous vein allograft via bypass from the right limb of a previous aortoiliac graft to the in situ GSV bypass, followed by ligation of the DFA remnant within the wound bed. Pseudomonas aeruginosa ultimately was cultured. Unfortunately, the allograft occluded on postoperative day 2, and the patient declined amputation. Comfort care measures were applied, and he died several days later.
Discussion
The optimal management of femoral artery blow-out remains controversial, with limited controlled data published, but general principles can be applied. If an infectious etiology is established for an anastomotic blow-out, ligation and extra-anatomic revascularization, when necessary, is the accepted management. The wound bed is débrided and dressed, the patient is re-prepared for the intervention, and clean instruments are obtained. The new bypass is tunneled remote from the infected tissues. This usually requires general anesthesia for retroperitoneal or transabdominal exposure of the iliac artery and, even in the best of circumstances, may take several hours to complete. Avoidance of contamination of new graft material is difficult at best. The mortality risk is up to 20% in this setting.2 Our approach using the hybrid graft may be less invasive, less morbid, and may offer an alternative to extra-anatomic grafting. It at least provides a bridge to definitive treatment and the opportunity to optimize the patient's physiologic status and to avoid new graft contamination.
Although the first patient presented later than would have been expected for a technical etiology of the anastomotic disruption, there were no clinical symptoms or signs of sepsis, and there was no gross or microscopic evidence of infection in the operating room. Diphtheroids do not commonly cause vascular graft infection and were likely a contaminant rather than pathogenic in the anastomotic rupture. The patient did undergo an extensive endarterectomy of the left femoral bifurcation with saphenous vein patch angioplasty at the time of the initial surgery. We suspect that the anastomotic blowout may have been multifactorial and related to the extent of endarterectomy and sudden groin extension. The GHVG allowed an in-line reconstruction to uninvolved vein conduit, with patency and no evidence of reinfection to date.
Our second patient developed s recurrent pseudoaneurysm after 4.5 months, and most likely there was an infectious etiology, despite the negative cultures. Because of the patient's severe aortic valvular disease and prohibitive cardiac risk for general anesthesia, surgery was done under local anesthesia using the GHVG. Given the extensive nature of the groin hematoma and pseudoaneurysm, extra-anatomic distal revascularization was not feasible while avoiding the previous wounds and hematoma. We therefore elected to temporize the hemorrhage with a percutaneous endovascular approach and resuscitate the patient while we planned the definitive repair with an allograft. Ultimately, the extra-anatomic bypass failed, and the patient refused an amputation and died.
Conclusions
These two patients demonstrate the successful use of the GHVG in the management of a femoral anastomotic blowout. However, if a clear infectious etiology is suspected or established, then we believe that the GHVG may only be used to bridge an emergency operation to a more elective, definitive option. One case proved to be definitive management in the absence of infection, the other was a bridge to deliberate management. As with any stent crossing the inguinal ligament, this technique may also be susceptible to stent kinking at this location. We believe this is a useful tool in a complex clinical setting to avoid abdominal incision and lengthy extra-anatomic bypass.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Klonaris C., Katsargyris A., Vasileiou I., Markatis F., Liapis C., Bastounis E. Hybrid repair of ruptured infected anastomotic femoral pseudoaneurysms: emergent stent-graft implantation and secondary surgical debridement. J Vasc Surg. 2009;49:938–945. doi: 10.1016/j.jvs.2008.10.063. [DOI] [PubMed] [Google Scholar]
- 2.Demarche M., Waltregny D., van Damme H., Limet R. Femoral anastomotic aneurysm: pathogenic factors, clinical presentations and treatment. A study of 142 cases. Cardiovasc Surg. 1999;7:315–322. doi: 10.1016/s0967-2109(98)00161-6. [DOI] [PubMed] [Google Scholar]