Abstract
Miliary tuberculosis (TB), is a fatal form of disseminated TB characterized by tiny tubercles evident on gross pathology similar to innumerable millet seeds in size and appearance. Global HIV/AIDS pandemic and increasing use of immunosuppressive drugs have altered the epidemiology of miliary TB. Keeping in mind its protean manifestations, clinicians should have a low threshold for suspecting miliary TB. Careful physical examination should focus on identifying organ system involvement early, particularly TB meningitis, as this has therapeutic significance. Fundus examination for detecting choroid tubercles can help in early diagnosis as their presence is pathognomonic of miliary TB. Imaging modalities help in recognizing the miliary pattern, define the extent of organ system involvement and facilitate image guided fine-needle aspiration cytology or biopsy from various organ sites. Sputum or BAL fluid examination, pleural, pericardial, peritoneal fluid and cerebrospinal fluid studies, fine needle aspiration cytology or biopsy of the lymph nodes, needle biopsy of the liver, bone marrow aspiration and biopsy, testing of body fluids must be carried out. GeneXpert MTB/RIF, line probe assay, mycobacterial culture and drug-susceptibility testing must be carried out as appropriate and feasible. Treatment of miliary TB should be started at the earliest as this can be life saving. Response to first-line anti-TB drugs is good. Screening and monitoring for complications like acute respiratory distress syndrome (ARDS), adverse drug reactions like drug-induced liver injury, drug-drug interactions, especially in patients co-infected with HIV/AIDS, are warranted. Sparse data are available from randomized controlled trials regarding optimum regimen and duration of anti-TB treatment.
Keywords: Miliary tuberculosis, Human immunodeficiency virus, Diagnosis, Treatment, Complications
1. Introduction
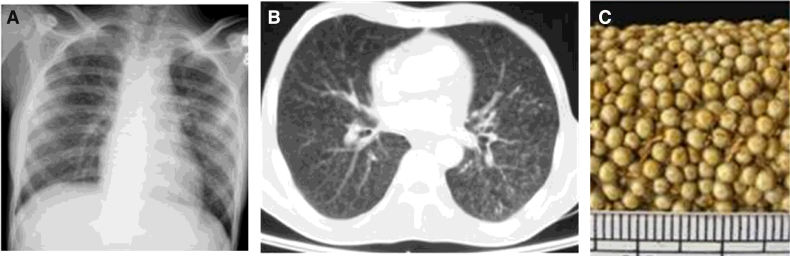
Tuberculosis (TB) is still a global public health problem in spite of worldwide control efforts (Table 1). As per the year 2014 estimates published in the Global Tuberculosis Report in 2015 [1], an estimated 9.6 million people developed TB and 1.5 million died from the disease globally. Miliary TB is a fatal form of disseminated TB that results from a massive lymphohematogeneous dissemination from a Mycobacterium tuberculosis-laden focus [2], [3], [4], [5], [6]. Radiologically, the miliary pattern has been defined as “a collection of tiny discrete pulmonary opacities that are generally uniform in size and widespread in distribution, each of which measures 2 mm or less in diameter” (Fig. 1) [7].
Table 1.
Epidemiology of tuberculosis 2014.
| Variable | Estimate |
|---|---|
| Incidence* | 133 (126–141) |
| Prevalence* | 174 (158–190) |
| Prevalence of HIV in incident TB cases* | 12 (11–13) |
| Mortality* | 16 (13–18) |
| HIV-positive TB mortality* | 5.3 (4.8–5.9) |
| % of new cases with MDR-TB† | 3.3 (2.2–4.4) |
| % of retreatment cases with MDR-TB† | 20 (14–27) |
| Prevalence of XDR-TB among MDR-TB cases† | 9.7 (7.4–12) |
HIV = human immunodeficiency virus; TB = tuberculosis; MDR-TB = multidrug-resistant tuberculosis; XDR-TB = extensively drug-resistant tuberculosis.
Expressed as estimate (lower and upper bounds of 95% uncertainty levels)/100,000 population.
Numbers in parentheses indicate 95% confidence intervals.
Source: Ref. [1].
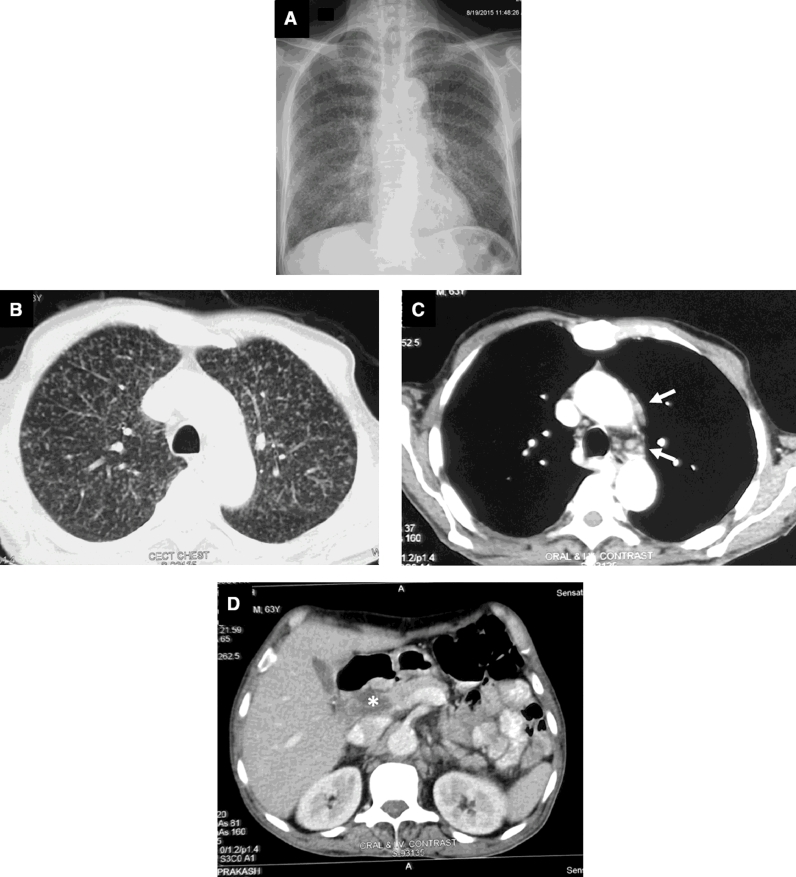
Fig. 1.
Chest radiograph (postero-anterior view) (A) and chest CT (lung window) (B) showing classical miliary pattern. The nodules (<2 mm) evident in miliary tuberculosis resemble the grains of pearl millet (Pennisetum typhoides, bajra) (C).
Manget, is credited to have coined the term “miliary TB” in 1700 [8]. He likened the tiny tubercles evident on gross pathological examination to that of innumerable millet seeds in size and appearance [miliarius (Latin), translation: related to millet seed]. Miliary TB is uniformly fatal if untreated. Due to varied clinical manifestations, atypical radiographic findings and difficulties in establishing TB as the aetiological diagnosis, even today, miliary TB remains a formidable diagnostic and therapeutic challenge [2], [9]. In this review, we have attempted to provide an overview regarding the changing clinical picture of miliary TB and issues related to diagnosis and management.
2. Epidemiology
2.1. Methodological issues
Reliable community-based epidemiological data are not available on the prevalence of miliary TB. Some of the methodological issues include different denominators used, lack of a “gold standard” for diagnosis, variations in the choice and nature of invasive diagnostic methods used for securing tissue to confirm the diagnosis among others. These issues should be kept in mind while comparing and interpreting published epidemiological data on miliary TB.
2.2. Clinical and autopsy studies
In various clinical studies among immunocompetent adults, miliary TB accounts for less than 2% of all cases of TB and up to 20% of all extra-pulmonary TB (EPTB) cases [10], [11], [12], [13], [14], [15]. In late HIV infection, EPTB accounts for more than 50% of all cases of TB and miliary TB is more frequently encountered [2], [3], [4], [5]. In autopsy studies in adults, miliary TB was documented in a higher proportion of patients, accounting for 0.3%–13.3% of all autopsies and 11.9%–40.5% of all cases of TB [16], [17], [18], [19], [20], [21], [22].
2.3. Demographic trends
Before the advent of anti-TB treatment, miliary TB was predominantly seen as a disease of infants and children [23], [24]. However, since the 1980s a changing epidemiological trend has been observed and miliary TB is increasingly being recognized in adults also. Two peaks are evident, one involving adolescents, young adults and another later in life among elderly individuals [2], [3], [4], [5], [14], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50]. This epidemiological change has been thought to be due to global pandemic of human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), increasing occurrence of organ transplantation, use of immunosuppressive drugs including anti-TNF-α, chronic hemodialysis program, among others.
In pediatric as well as adult series [2], [3], [4], [5], [14], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], male gender seems to be more frequently affected by miliary TB. In some adult series on miliary TB [14], [22], [34], [39] a female preponderance has been documented. A higher occurrence of miliary TB among African Americans has been observed in some of the earlier publications from the USA, though such a trend is not evident in later studies [2], [3], [4], [5], [29], [40].
3. Predisposing, associated conditions
In patients with miliary TB, several predisposing or associated conditions have been documented. Some of these conditions include childhood infections, malnutrition, HIV/AIDS, alcoholism, chronic kidney disease, dialysis, post-gastrectomy status, organ transplantation, immunosuppressive drug use, connective tissue disorders, pregnancy, postpartum, presence of an underlying malignancy, and silicosis [2], [3], [4], [5]. Recent evidence has brought into focus diabetes mellitus and tobacco smoking as newer emerging risk factors in the causation of TB [9].
In addition to glucocorticoids, immunosuppressive, cytotoxic drugs that are known to predispose to the development of miliary TB, use of immunomodulatory biologicals has been reported to cause fatal TB including miliary TB [51], [52], [53], [54]. In a prospective study among patients who received anti-tumor necrosis factor (anti-TNF) therapy [51], disseminated and miliary TB accounted for 27.5% of all TB cases and 44% of EPTB. In this study [51], it was also observed that the rate of development of TB was higher for adalimumab (136 events/100,000 person-years) and infliximab (144 events/100,000 person-years) than for etanercept (39 events/100,000 person-years). In a prospective population-based national cohort study (2002–2011) from Sweden [53] the rate of incident TB in the general population and in a cohort of biological-naïve and biological-exposed patients diagnosed with RA was estimated. In comparison to the general population, patients with RA who were not exposed to biologicals had a four-fold increased risk of TB (hazard ratio [HR] 4.2; 95% confidence intervals [CI] 2.7–6.7), which did not decline over calendar time. Further, compared to etanercept, the HRs for most recent exposure to adalimumab and infliximab were 3.1 (95% CI 0.8–12.5) and 2.7 (95% CI 0.7–10.9), respectively; and the HR for etanercept compared with biological-naïve RA was 1.7 (95% CI 0.6–4.6). In another recent study [54] that investigated incidence of TB following anti-TNF therapy from Korea, a significantly lower incidence of TB was observed in patients treated with etanercept (reference), compared with those treated with infliximab (incidence rate ratio [IRR] 6.8, 95% CI 3.74–12.37) and adalimumab (IRR 3.45, 95% CI 1.82–6.55). A systematic review and meta-analysis [55] of randomized controlled trials also reported a higher risk of developing TB with adalimumab and infliximab than with etanercept.
Several procedures and interventions, such as, ureteral catheterization, extracorporeal shockwave lithotripsy, laser lithotripsy, cardiac valve homograft replacement, have been implicated in the causation of miliary TB [56], [57], [58], [59], [60]. Further, the uncommon occurrence of miliary disease caused by intravesical bacille Calmette–Guerin (BCG) therapy for urinary bladder carcinoma has also been documented [58].
4. Immunopathogenesis
Miliary TB is thought to result as a consequence of the inadequate effector T-cell (Teff) response in containing the tubercle bacillus [61], [62], [63], [64]. In the context of immunopathogenesis of miliary TB, Th1 cell activation, is central to protective immunity and the Th2 cell activation represents impaired immunity. Evidence is available suggesting that chemokine directed selective homing of Th2 cells may play a critical role in the development of miliary TB. In a susceptible host, immune responses skewed towards Th2 cross-inhibit protective responses, such as granuloma formation and this failure in limiting the disease activity at the local site favors dissemination. Miliary TB probably results from the Th2-biased response occurring as a default pathway [63], [64].
Recent data indicate that interleukin-4 (IL-4), with its ability to downregulate inducible nitric oxide synthase (iNOS), toll-like receptor 2 (TLR2) and macrophage activation, determine whether infection with Mycobacterium tuberculosis becomes latent or progressive [2], [3], [4], [5], [61], [62]. It is postulated that Mycobacterium tuberculosis either fails to evoke the protective response or drives the protective mechanisms and then deliberately ‘sabotage’ these leading to progressive disease [61], [62], [63], [64]. When the balance of homing of regulator T (Treg) cells and Teff cells shifts towards the former, there is a state of local immunosuppression leading to disease dissemination [2], [3], [4], [5], [63], [64].
Molecular mechanisms thought to be responsible for disease dissemination and development of miliary TB have been described [2], [3], [4], [5], [65], [66], [67], [68], [69], [70]. These include impaired expansion of γ/δ T-cells [65], failure to generate adequate cell-mediated immunity (CMI) [66], presence of human leucocyte antigen (HLA)-Bw15 [67], HLA-DRB1*15/16, DRB1*13, and DQB1*0602 [68], absence of HLA-Cw6, HLA-DRB1*10, and DQB1*0501 [68], impaired MHC class II restricted target cell lysis, and over-exuberant lysis of target cell macrophages [69] and LTA+368 G/A polymorphisms [70], among others.
5. Clinical presentation
5.1. Adults
The clinical manifestations of miliary TB in adults are non-specific and can be obscure till late in the disease. Classically, fever with evening rise of temperature of several weeks duration, anorexia, weight loss, weakness and cough are evident [2], [3], [4], [5]. Occurrence of daily morning fever spikes [71] has also been reported. Chills and rigors, similar to that described in patients with malaria and bacteremia have been frequently described in adult patients with miliary TB [2], [3], [4], [5]. Night sweats are common; sweat engraved the patient's silhouette on the bed, closely resembling a body's shadow (damp shadow sign) has been described in miliary TB [72].
5.1.2. Cryptic miliary TB
In some patients, fever may be absent and the patients may present with a progressive wasting strongly mimicking a metastatic carcinoma. This presentation, termed “cryptic miliary TB” [73] is increasingly being reported in older individuals [23], [24].
5.1.3. Organ system involvement
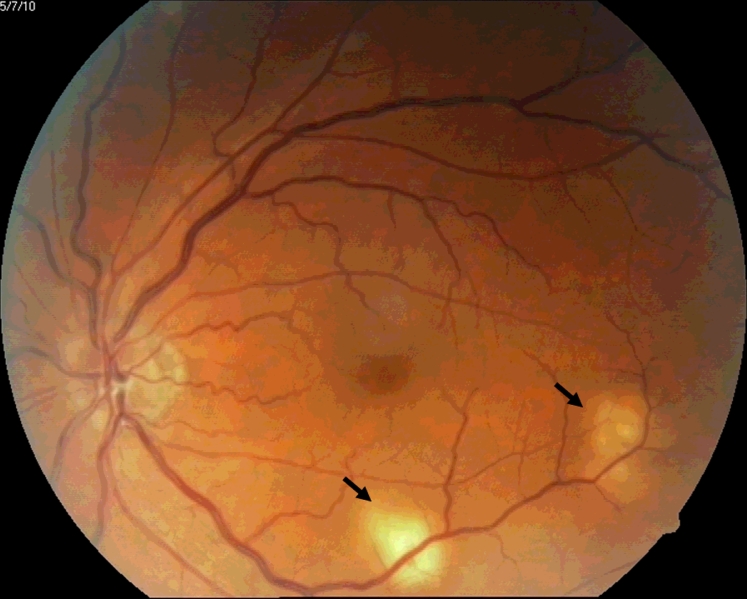
Consistent with the systemic nature of the disease, patients with miliary TB present with symptoms and signs referred to various organ systems [2], [3], [4], [5]. In the present era, imaging modalities have made it possible to detect organ system involvement in patients with miliary TB. Dry cough, scanty sputum and dyspnea are frequently evident. Rarely, hemoptysis can occur. Skin lesions, namely, eythematous macules and papules (tuberculosis miliaria cutis), may offer a valuable clue to the diagnosis [2], [3], [4], [5]. Choroidal tubercles are bilateral, pale, gray–white or yellowish lesions usually less than one quarter of the size of the optic disc and are located within 2 cm of the optic nerve. When present, choroidal tubercles are considered to be pathognomonic of miliary TB [2], [3], [4], [5], [45]. A systematic ophthalmoscopic examination is after mydiatric administration thus warranted in all patients with suspected miliary TB (Fig. 2).
Fig. 2.
Ophthalmoscopic picture showing choroid tubercles (arrows). (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.)
TB meningitis (TBM) has been described in 10%–30% adult patients with miliary TB [14], [21], [26], [27], [28], [29], [30], [31], [32], [34], [36], [38], [45], [47], [48]; about one-third of patients presenting with TBM have underlying miliary TB [74]. Neurological involvement in adult patients with miliary TB includes TB meningitis with and without tuberculoma; and thoracic transverse myelopathy, among others [75]. Miliary TB presenting with overt adrenal insufficiency manifesting as Addison's disease at the time of initial presentation, or during anti-TB treatment has also been documented [76], [77].
5.2. Children
Certain important differences have been observed in the clinical presentation of miliary TB in children compared to adults. Miliary TB develops less frequently in children who have received the BCG vaccination [2], [3], [4], [5]. In children with miliary TB, chills and night sweats, hemoptysis, productive cough are less common; peripheral lymphadenopathy and hepatosplenomegaly are more frequent compared with adults. TBM is more frequently seen in children with miliary TB (20%–40%) [2], [3], [4], [5], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44] compared to adults (15%–30%) [14], [21], [26], [27], [29], [30], [31], [32], [34], [36], [42], [43], [44], [45], [46], [47], [48], [50].
5.3. Immunosuppressed individuals
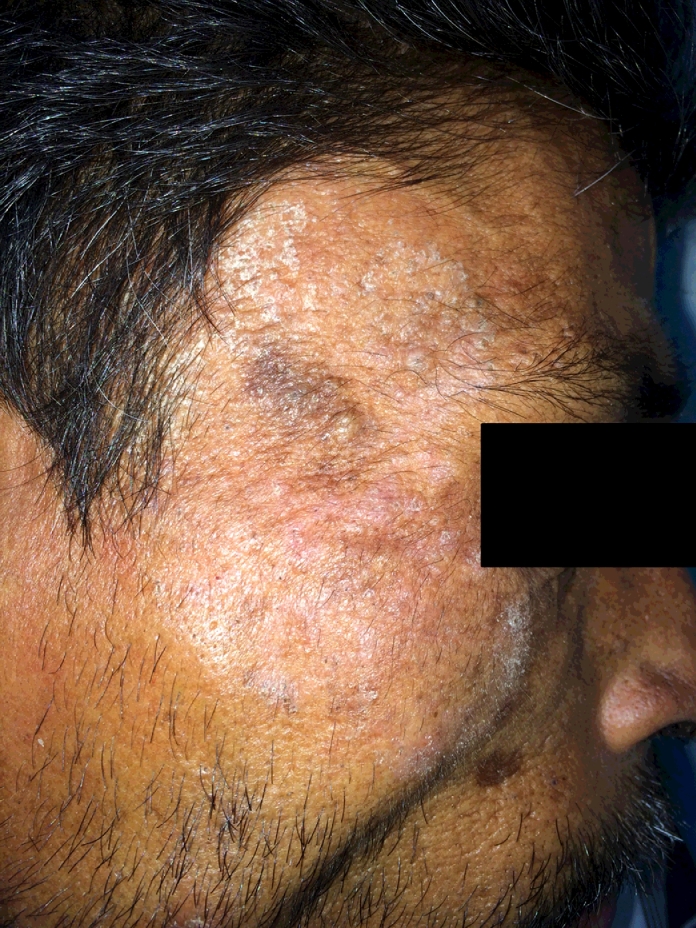
In patients with profound immunosuppression, such as those co-infected with HIV, certain important differences are evident in the presentation of miliary TB compared to immunocompetent individuals. Clinical presentation of miliary TB in persons with early HIV infection (CD4+ cell counts >200 cells/mm3), is similar to that observed in immunocompetent individuals. In late, advanced stage of HIV infection miliary TB is seen more often [2], [3], [4], [5], [47], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87]. Cutaneous involvement, rarely seen in HIV-seronegative patients with miliary TB are more frequently seen in HIV-seropositive and other immunosuppressive patients [2], [3], [4], [5], [78], [79], [80], [81], [82], [83], [84], [85]. These include tiny papules or vesiculopapules, (tuberculosis cutis miliaris disseminata, tuberculosis cutis acuta generalisita), and disseminated tuberculosis of the skin. Macular, pustular, or purpuric lesions, indurated ulcerating plaques (Fig. 3), and subcutaneous abscesses have also been reported [86]. In late HIV-infection, intrathoracic lymphadenopathy and tuberculin anergy are more common; sputum smears are seldom positive and blood culture may grow Mycobacterium tuberculosis [2], [3], [4], [5], [78], [79], [80], [81], [82], [83], [84], [85], [87].
Fig. 3.
Clinical photograph of a HIV-seropositive patient who also had poorly controlled type 2 diabetes mellitus with miliary TB showing papulonodular cutaneous lesions over the face
HIV = human immunodeficiency virus; TB = tuberculosis.
Kind courtesy: Professor D.R. Reddy, Department of Dermatology, Venereology and Leprosy, Teerthanker Mahaveer Medical College & Research Centre, Moradabad, Uttar Pradesh, India.
5.4. Rare manifestations and complications
Several rare clinical manifestations and complications have been reported in miliary TB. Sometimes, complications like acute respiratory distress syndrome (ARDS) or myocarditis may in fact be the initial presentation. Miliary TB presenting with ARDS, air-leak syndromes, acute kidney injury (AKI), hepatic and gastrointestinal manifestations is increasingly being reported from the emergency department and intensive care unit (ICU). These rare manifestation may be a component of the multiorgan dysfunction syndrome (MODS) due to TB or as a manifestation of immune reconstitution inflammatory syndrome (IRIS) [88], [89], [90], [91], [92], [93], [94]. Atypical clinical presentation often delays the diagnosis and treatment. While ARDS may develop anytime during the disease course, it is usually seen at the time of initial presentation. Occurrence of ARDS has been observed to be a poor prognostic marker [88], [89], [90], [91], [92], [93], [94].
Pneumothorax, which may sometimes be bilateral has been described in miliary TB either at the time of initial presentation, during the course of the disease or as a complication in TB-ARDS patients receiving mechanical ventilation [2], [3], [4], [5], [95], [96]. Pneumomediastinum with subcutaneous emphysema may develop due to intrapulmonary rupture of alveoli and consequent air-leak that traverses into the mediastinum after spreading along the vascular sheath and this may be fatal [97].
AKI may occur due to direct renal parenchymal involvement in patients with miliary TB [2], [3], [4], [5], [98] or as a manifestation of IRIS [99] in HIV co-infected patients. Rarely, renal failure due obstructive uropathy has been described [78]. Other rare initial presenting manifestations can be secondary hemophagocytic lymphohistiocytosis (HLH), macrophage-activating syndrome (MAS), clinical syndromes characterized by pancytopenia, hypertriglyceridemia and/or hypofibrinogenemia, evidence of hemophagocytosis in bone marrow, spleen or lymph nodes; other associated laboratory abnormalities include hyperferritinemia and elevated blood levels of lactate dehydrogenase [100], [101].
Miliary TB presenting with fulminant hepatic failure has been reported [102]. An extrapulmonary focus discharging Mycobacterium tuberculosis into the portal circulation, resulting in hepatic miliary TB is thought to be the underlying mechanism for this presentation. Peritoneal involvement (ascites), intestinal involvement (diarrhea or altered bowel habit) small intestinal perforations [33], [103] have also been described. In patients with miliary TB, especially those co-infected with HIV, associated intrathoracic and intraabdominal lymphadenopathy involving portahepatis, pre- and paraaortic and mesenteric lymph nodes; retroperitoneal lymphadenopathy may be seen.
Other important clinical lesions include Pott's spine with or without myelopathy, paraspinal cold abscess. Pelvic ascites, tubo-ovarian masses, pyosalpinx can be seen in women with miliary TB [2], [3], [4], [5].
Life-threatening cardiovascular complications have been documented in patients with miliary TB. These include myocarditis [104], congestive heart failure [104], native [105], [106], [107] and prosthetic valve [108] endocarditis, pericarditis, intracardiac mass [104], mycotic aneurysm [109], infection of a pacemaker pulse-generator pocket [110], infection of ventriculoatrial shunt causing miliary TB [111] and sudden cardiac death [112], [113], [114] among others.
IRIS, reported in about one-third of patients co-infected with HIV and TB usually manifests within days to weeks of the initiation of highly active antiretroviral therapy (HAART) [115], [116]. Unmasking and paradoxical IRIS are two subtypes. The clinical course of IRIS can be brief or a prolonged course with multiple recurrent episodes has also been described. Miliary TB, sometimes presenting with complications such as AKI, ARDS can occur as a manifestation of IRIS [88], [100], [117]. In HIV-seronegative TB patients, paradoxical worsening (deterioration) has been described, especially in lymph node TB and TB pleural effusion [78], [79]. Fatal paradoxical worsening of TB in a HIV-seronegative patient with generalized TB lymphadenopathy to cryptic miliary TB has been reported [118].
6. Diagnostic approach
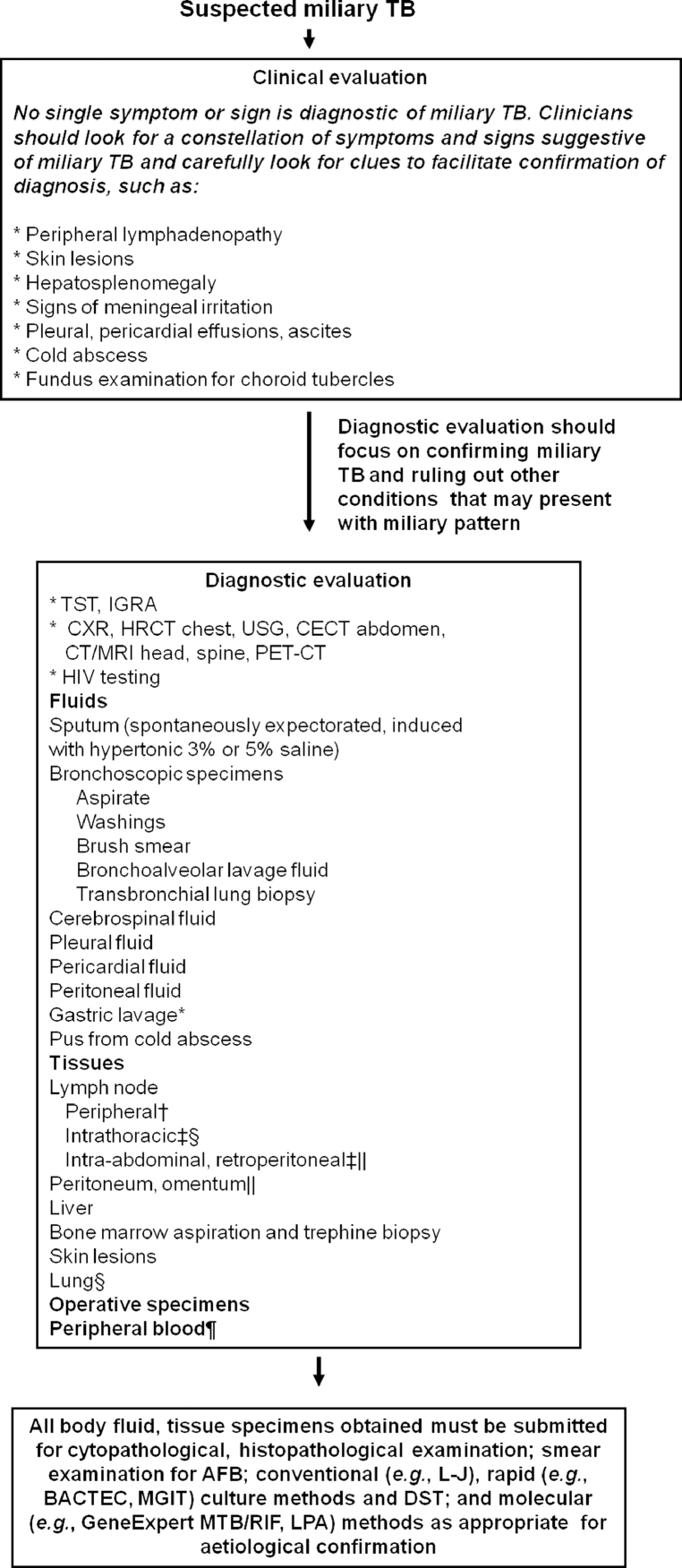
The diagnosis of miliary TB can be difficult as the clinical manifestations can be atypical and non-specific, the chest radiographs do not always reveal the classical miliary pattern at the time of initial presentation. Therefore, a high index of clinical suspicion and a systematic approach to diagnostic testing is required to establish an early diagnosis of miliary TB (Fig. 4) [2], [3], [4], [5].
Fig. 4.
Algorithm for the diagnostic work-up of a patient with suspected miliary TB. The clinical and imaging diagnostic work-up should also aim at accurately assessing the extent of extrapulmonary involvement to facilitate monitoring and ensure adequate duration of treatment. All laboratory testing, especially, antituberculosis drug-susceptibility testing must be carried out in quality assured, periodically accredited laboratories.
* Often used in children.
† FNAC/excision biopsy.
‡ Radiologically guided FNAC/biopsy.
§ Mediastinoscopic/video-assisted thoracoscopic surgery, biopsy.
|| Laparoscopic biopsy.
 Useful in advanced HIV infection.
Useful in advanced HIV infection.
TB = tuberculosis; TST = tuberculin skin test; IGRA = interferon-γ release assays; HRCT = high resolution computed tomography; CECT = contrast enhanced computed tomography; MRI = magnetic resonance imaging; FNAC = fine needle aspiration cytology; HIV = human immunodeficiency virus; AFB = acid-fast bacilli; L–J = Lowenstein–Jensen medium; DST = drug-susceptibility testing; MGIT = mycobacterial growth inhibitor tube; BACTEC = radiometric culture method; PCR = polymerase chain reaction; GeneExpert MTB/RIF = GeneXpert MTB/RIF assay (Cepheid, Sunnyvale, CA); LPA = line probe assay
Adapted and reproduced with permission from Sharma et al. [2].
Patients with miliary TB should ideally be hospitalized for a complete diagnostic work-up. Patients presenting with dyspnea should be carefully evaluated for cardiac tamponade and ARDS. Clinicians should have a low threshold for performing lumbar puncture to document TB meningitis.
Following criteria are useful for the diagnosis of miliary TB in the clinical setting: (i) clinical presentation consistent with a diagnosis of TB such as, pyrexia with evening rise of temperature, weight loss, anorexia, tachycardia and night sweats of greater than six weeks duration responding to anti-TB treatment; (ii) classical miliary pattern on chest radiograph; (iii) bilateral diffuse reticulonodular lung lesions on a background of miliary shadows demonstrable either on plain chest radiograph or high resolution computed tomography (HRCT); and (iv) microbiological, histopathological and/or molecular evidence of TB [45].
6.1. Tuberculin skin test and interferon-gamma release assays
A positive tuberculin skin test (TST) or interferon-gamma release assays (IGRA) result only indicates infection with Mycobacterium tuberculosis and does not indicate active disease. Tuberculin anergy is common in miliary TB and has ranged from 35%–74% and 20%–70% in various pediatric [2], [3], [4], [5], [26], [44] and adult series [29], [30], [31], [32], [38], [39], [40], [42], [43], respectively. In high burden TB countries, neither IGRAs nor TST have been found to be adequate in accurately identifying persons who will benefit from treatment of latent TB infection (LTBI) with high false positivity rates being reported for both [119], [120], [121], [122]. Further, even after completion of treatment, the PPD as well as IGRAs will still remain positive. A policy statement issued by the WHO [122] and the European Centre for Disease Prevention and Control guidelines [121] discourage the use of IGRAs in preference to TST, in areas where TB is highly endemic for the diagnosis of pulmonary or extra-pulmonary TB.
6.2. Laboratory testing
A number of hematological and biochemical laboratory abnormalities have been described in miliary TB [2], [3], [4], [5], [21], [26], [27], [28], [29], [30], [31], [34], [36], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47] but their significance is controversial. Disseminated intravascular coagulation (DIC) [89], [91] in patients with miliary TB in the setting of ARDS and MODS is associated with a high mortality. Immune mechanisms have been implicated to cause bone marrow suppression and this could be the cause of pancytopenia, hypoplastic anemia [2], [3], [4], [5], [123]. Hypercalcemia has occasionally been documented in miliary TB [120], [121], [122], [123], [124]. Hyponatremia may indicate the presence of TB meningitis [40] and may also be a predictor of mortality [45] in miliary TB. Several mechanisms, such as, an acquired disturbance of neurohypophyseal function resulting in unregulated antidiuretic hormone (ADH) release; an antidiuretic principle in the lung tissue affected by TB that may either produce ADH or absorb an inappropriately released hormone from the posterior pituitary [125], [126], [127] are postulated mechanisms.
6.3. Imaging studies
Imaging modalities, such as, ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), 18F labeled 2-deoxy-D-glucose (FDG) positron emission tomography-CT (FDG PET-CT) have been used to define the extent of organ involvement and evaluating response to treatment [2], [3], [4], [5].
6.3.1. Chest radiograph
Various chest radiographic abnormalities seen in miliary TB are listed in Table 2. Miliary pattern on chest radiograph [2], [3], [4], [5], [6], [7], the hall mark of miliary TB, is seen in a majority of patients. The classical miliary pattern on the chest radiograph represents summation of densities of the tubercles that are perfectly aligned and imperfectly aligned tubercles result in curvilinear densities and a reticulonodular pattern [128]. In about 10% of the cases, the nodules may be greater than 3 mm in diameter [129]. Sometimes, well-defined, linear, branching opacities (tree-in-bud appearance) may also be seen when centrilobular bronchioles are dilated, or, are filled with mucus, fluid or, pus and this pattern represents endobronchial spreading of infection [2], [3], [4], [5]. Rarely, ground glass opacities can occur due to lymphatic obstruction or infiltration or acute lung injury [130]. In some patients, predominance of lesions on one side may be evident. Sometimes, chest radiographs can be normal initially and the typical miliary pattern may evolve over the course of disease evolution. Thus, periodic repeat chest radiographic examination should be done in patients with suspected miliary TB during the course of their diagnostic evaluation [2], [3], [4], [5], [45].
Table 2.
Chest radiographic abnormalities in miliary TB.
| Classical presentation (50%) |
| Miliary pattern |
| Other pulmonary manifestations (10%–30%) |
| Asymmetrical nodular pattern |
| Coalescence of nodules |
| Mottled appearance |
| “Snow storm” appearance |
| Ground glass appearance |
| Air-space consolidation |
| Associated findings (<5%) |
| Pulmonary |
| Parenchymal lesions and cavitation |
| Segmental consolidation |
| Thickening of interlobular septae |
| Pleural |
| Pleural effusion |
| Empyema |
| Pneumothroax |
| Pneumomediastinum |
| Others |
| Intrathoracic lymphadenopathy |
| Pericardial effusion |
6.3.2. Ultrasonography
Ultrasonography facilitates detection of free or loculated ascites, focal lesions in the liver or spleen, cold abscesses, mesenteric, retroperitoneal or porta hepatis intraabdominal lymphadenopathy, involvement of other abdominal organs and pleural effusion(s). Further, diagnostic thoracic or abdominal paracentesis to procure pleural or peritoneal fluid for diagnostic testing can be done under ultrasonography guidance [2], [3], [4], [5].
HRCT, thin-section multidetector row CT (MDCT) have facilitated the antemortem diagnosis of miliary TB. Classically, HRCT reveals a mixture of both sharply and poorly defined, less than 2 mm nodules that are widely disseminated throughout the lungs associated with diffuse reticulation [131], [132]. The HRCT may reveal classical miliary pattern even when the chest radiograph looks apparently normal [2], [3], [4], [5], [45] and also facilitates identification of additional findings such as intrathoracic lymphadenopathy (Fig. 5), calcification, pleural and pericardial lesions [133].
Fig. 5.
Chest radiograph (postero-anterior view) (A) and CT chest (lung window) (B) showing classical miliary pattern. CT chest (mediastinal window) (C) of the same patient also shows pre-vascular and mediastinal lymph nodes (arrows) CT (lung window). CT abdomen (D) of the same patient showing intrabdominal lymphadenopathy (asterisk). Bronchoalvelolar fluid GeneXpert MTB/RIF detected Mycobacterium tuberculosis; there was no rifampicin resistance.
CT = computed tomography.
Miliary pattern observed on HRCT may be difficult to distinguish from that observed in miliary sarcoid and lymphangitis carcinomatosa. The lesions of miliary TB are randomly distributed and the lesions of miliary sarcoidosis are distributed along the bronchovascular bundle (lymphangitic distribution). Thus transbronchial lung biopsy gives a higher diagnostic yield in miliary sarcoidosis [2], [3], [4], [5]. On HRCT, air trapping has been described on HRCT both at presentation and during follow-up period [133] and rupture of these areas of air trapping may result in air-leak syndromes in miliary TB. The interlobular septal thickening or intralobular fine networking seen on HRCT in miliary TB reflects caseation necrosis in the alveolar walls and interlobular septa.
Abdominal CT (Fig. 5D) has been useful in identifying lesions in the liver and spleen, intestine, mesentery, peritoneum, adrenals and lymph nodes, and also detects cold abscesses [2], [3], [4], [5]. Unlike in the chest, miliary lesions in the liver and spleen may appear as discrete hypodense lesions a few of which may be confluent, sometimes with irregular peripheral rim enhancement [134]. In female patients with miliary TB, pelvic CT is useful to detect tubo-ovarian masses, hydro and pyosalpinx, fluid collection in the pouch of Douglas [2], [3], [4], [5].
6.3.3. Magnetic resonance imaging
In patients with miliary TB, TBM, spinal TB, MRI of brain and spine is very useful in documenting extent of neurological involvement. As MRI does not use ionizing radiation, it offers the advantage that it can be repeated during follow-up if required [2], [3], [4], [5].
6.3.4. Positron emission tomography
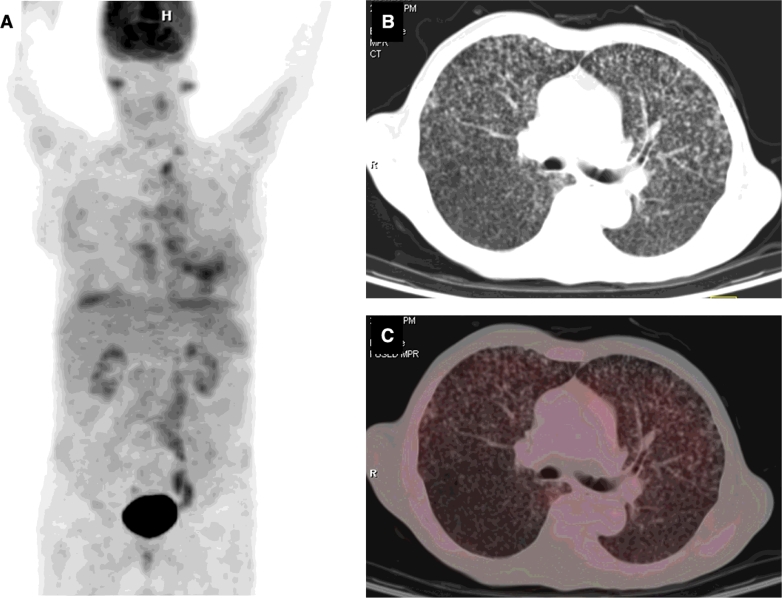
In patients with extrapulmonary TB, FDG PET-CT appears to be suited to define the extent and multiplicity of lesions at the time of initial presentation and assessing the activity of lesions during and at the end of anti-TB treatment (Fig. 6). Little published data are available on the 18FDG PET-CT findings in patients with miliary TB. Sharma et al [2] have reported that miliary as well as coalesced lesions can be evident on FDG PET-CT. The 18FDG PET-MRI is another potentially useful future imaging tool in miliary TB. As compared with 18FDG PET-CT, there is no risk of radiation exposure with 18FDG PET-MRI. The 18FDG PET-CT and PET-MRI have potential to further understanding the clinico-radiographic-functional correlation in miliary TB and merit further study.
Fig. 6.
Organ system involvement in military TB. PET maximum intensity projection image (A) showing abnormal tracer accumulation in the lung fields. Axial CT of the same patient showing randomly distributed miliary nodules in both lungs. Axial 18FDG PET-CT fused image showing the FDG uptake in the nodules.
PET = positron emission tomography; CT = computer tomography; 18FDG-PET CT = 18F labeled 2-deoxy-Dglucose positron emission tomography-computed tomography.
Kind courtesy: Dr Madhavi Tripathy, Department of Nuclear Medicine, All India Institute of Medical Sciences, New Delhi, India.
6.3.5. Image guided radiological procedures
Image guided radiological procedures such as fluid aspiration, fine needle aspiration for cytological examination (FNAC) and biopsy under CT or MRI guidance are useful for procuring tissue/body fluids for diagnostic testing.
6.4. Sputum examination
When available, sputum must be subjected to conventional methods of smear, and mycobacterial culture examination. The reader is referred to Section 6.12. for details. Use of molecular methods in sputum testing is described under Section 6.15.
6.5. Bronchoscopy
Fibreoptic bronchoscopy, bronchoalveolar lavage (BAL) fluid, bronchoscopic aspirate, brushings, and transbronchial lung biopsy (TBLB) have all been used to confirm the diagnosis of miliary TB and may provide a diagnostic clue in up to 50% of patients [27], [36], [38], [43], [45], [135]. Further, endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA) facilitates diagnostic sampling of subcarinal and mediastinal lymph nodes [2].
6.6. Mediastinoscopy
Mediastinoscopy, if available can be useful in obtaining tissue samples from intrathoracic lymph nodes for diagnosis of miliary TB.
6.7. Video-assisted thoracoscopic surgery
Video-assisted thoracoscopic surgery can facilitate diagnostic sampling of pleura, intrathoracic lymph nodes and lung.
6.8. Echocardiography
In the appropriate clinical setting, two-dimensional transthoracic echocardiography is useful in detecting pericardial effusion and myocardial involvement in miliary TB.
6.9. Laparascopy
Laparascopy provides an opportunity to visualize the lesions in patients with abdominal involvement and facilitates procurement of biopsy material for diagnostic confirmation [136].
6.10. Bone marrow aspiration and biopsy
Bone marrow aspiration and biopsy specimens should be subjected to cytology, histopathology, culture, DST and molecular methods of diagnosis [2], [3], [4], [5].
6.11. Body fluids and tissue examination
Depending on the extent of organ system involvement, appropriate tissue and body fluid samples must be obtained to confirm diagnosis. When imaging studies reveal focal lesions, image-guided FNAC or tru-cut biopsy can be carried out and the tissue thus procured should be subjected to histopathological, microbiological, or molecular evaluation to confirm the aetiological diagnosis.
Bone marrow aspiration and needle biopsy obtained from the posterior superior iliac spine have been found to be useful for the diagnosis of miliary TB. Elevated serum alkaline phosphatase levels suggest diffuse liver involvement and needle biopsy of the liver can be useful in confirming the diagnosis. Where applicable, pleural fluid, pericardial fluid, ascitic fluid, CSF, urine, bronchoscopic secretions, blood and tissue biopsy specimens have all been tested to confirm the diagnosis of miliary TB [2], [3], [4], [5], [11], [14], [21], [26], [27], [29], [30], [31], [32], [34], [36], [38], [39], [40], [42], [47], [48], [50].
6.12. Mycobacterial culture and drug-susceptibility testing
Presently, liquid culture, solid culture on Lowenstein-Jensen medium and DST by phenotypic methods are the gold standard for mycobacterial culture and DST respectively. Rapid culture methods such as the BD BACTEC® 460 TB System (Becton, Dickinson; New Jersey) and the BD BACTEC® MGIT® 960 System that combines the advantages of BACTEC® with mycobacteria growth inhibitor tube (MGIT) (BD MGIT®, Becton, Dickinson; New Jersey), molecular methods (vide infra) may be useful for rapid DST [2], [3], [4], [5].
In patients with miliary TB, mycobacterial culture and drug-susceptibility testing (DST) of sputum, body fluids and tissue specimens must be carried out. DST for first-line anti-TB drugs (FLDs), second-line anti-TB drugs (SLDs) should be carried out in an accredited laboratory with external quality assurance can facilitate correct identification of multidrug-resistant TB (MDR-TB), pre-extensively drug-resistant TB (pre-XDR-TB), extensively drug-resistant TB (XDR-TB) and facilitate appropriate management [9].
6.13. Serodiagnostic methods
The World Health Organization (WHO) policy statement on the use of serodiagnostic tests strongly recommend that the currently available commercial serodiagnostic tests should not be used for the diagnosis of active pulmonary and extra-pulmonary TB disease including miliary TB [137].
6.14. Adenosine deaminase
When there is pleural effusion, ascites, elevated body fluid adenosine deaminase (ADA) and interferon-gamma levels estimation in ascitic fluid, pleural fluid point to TB as the aetiological cause [138], [139], [140], [141], [142], [143].
6.15. Molecular methods
Polymerase chain reaction (PCR) of CSF, tissue biopsy specimens and blood (especially in HIV-infected patients), has been used for diagnostic confirmation [78]. The PCR has been found to be most useful when applied to clean specimens such as CSF where its sensitivity and specificity have been reported to be 0.5–0.9 and 1.0, respectively [2], [3], [4], [5], [78].
Current evidence suggests that the GeneXpert MTB/RIF assay (Cepheid, Sunnyvale, CA) appears to be the promising rapid diagnostic test for patients with extrapulmonary TB. GeneXpert MTB/RIF utilizes a heminested real-time PCR assay to amplify an Mycobacterium tuberculosis-specific sequence of the rpoB gene which is then probed with molecular beacons for mutations within the rifampicin-resistance determining region. It can facilitate rapid diagnosis from clinical specimens in 90 minutes time [144], [145], [146], [147].
Line probe assays (LPAs), such as, the INNO-LiPA® Rif. TB kit (Innogenetics NV, Gent, Belgium) and the GenoType® MTBDRplus assay (Hain Lifescience GmbH, Nehren, Germany) have been found to be useful for rapid screening of patients at risk for multidrug-resistant TB (MDR-TB) [148], [149].
LPA that can detect the tubercle bacillus and provide information on isoniazid and rifampicin resistance (thereby identifying MDR-TB) for confirmation of diagnosis in patients with suspected miliary TB merits further study.
6.16. Pulmonary function, gas exchange abnormalities and cardiopulmonary exercise testing
As in the case of other interstitial lung diseases (ILD) miliary TB is associated with typical abnormalities of pulmonary function. The pulmonary function and gas exchange abnormalities may be of a greater magnitude than might be expected from the chest radiograph [150], [151], [152], [153]. There is impairment of diffusion which can be severe [154], [155], a mild reduction in flow rates suggestive of peripheral airways involvement [13] are evident. During the acute stage, arterial hypoxemia due to widening of the alveolar-arterial oxygen gradient and hypocapnia due to tachypnea can develop [151]. Abnormal cardiopulmonary exercise performance including lower maximum oxygen consumption, maximal work rate, anerobic threshold, peak minute ventilation, breathing reserve, and low maximal heart rate have been reported in miliary TB [2], [3], [4], [5], [151], [153]. Other abnormalities include higher respiratory frequency, peak minute ventilation at submaximal work, and high physiological dead space/tidal volume and a demonstrable fall in oxygen saturation (to 4% or more) with exercise. Following anti-TB treatment, these abnormalities reversed in a majority of the patients, though they were persisting in some of them [151], [153].
7. Differential diagnosis
Several conditions can present with a miliary pattern on the chest radiograph or CT (Table 3) and these conditions must be differentiated from miliary TB by detailed diagnostic work-up.
Table 3.
Some causes of miliary pattern on the chest radiograph.
| Common causes | Rare causes |
|---|---|
| Infections* | Infections |
| Tuberculosis | Cryptococcosis |
| Histoplasmosis | Legionellosis |
| Blastomycosis | Melioidosis |
| Coccidioidomycosis | Tularaemia |
| Mycoplasma pneumonia | Psittacosis |
| Nocardiosis | Brucellosis |
| Immunoinflammatory disorders* | Staphylococcus aureus bacteremia |
| Sarcoidosis | Toxoplasmosis |
| Malignant | Schistosomiasis |
| Bronchoalveolar carcinoma | Strongyloide stercoralis |
| Carcinoma lung with | hyperinfection |
| lymphangitis carcinomatosa | Malignant |
| Metastatic carcinoma | Bronchial carcinoid |
| Tropical pulmonary eosinophilia | Lymphoma |
| Haemosiderosis in long standing | Lymphomatoid granulomatosis |
| rheumatic heart disease, mitral | Occupational lung diseases |
| stenosis | |
| Hypersensitivity pneumonitis | |
| Drug-induced interstitial lung disease | |
| (e.g., methotrexate, chrysotherapy, | |
| cyclophosphamide, nitrofurantoin, | |
| antidepressants) |
8. Treatment
In patients with miliary TB, there are no published randomized controlled trials assessing the efficacy of the standard World Health Organization (WHO) treatment regimens used in national TB control programs worldwide [156]. In various guidelines [156], [157], [158], miliary TB is classified as “pulmonary TB” in the programmatic approach because of pulmonary involvement. This classification does not, however, truly reflect the systemic nature of miliary TB.
There is no consensus regarding the optimum duration of treatment for miliary TB. Careful evaluation of extent of organ system involvement, especially, for TBM and TB pericarditis is indicated before the initiation of anti-TB treatment as this can influence the therapeutic decision. Most guidelines, such as, the American Thoracic Society (ATS), the Centers for Disease Control and Prevention (CDC), the Infectious Disease Society of America (IDSA) [157] and National Institute for Health and Clinical Excellence (NICE) [158] guidelines from UK state that 6 months of treatment is adequate for drug-susceptible miliary TB (Table 4). However, the guidelines also suggest that when there is associated TBM, bone and joint TB the duration of treatment may have to be longer. The evidence-based Indian extrapulmonary TB guidelines (INDEX-TB guidelines) that are shortly due to be published are expected to provide clarity to these issues.
Table 4.
Daily treatment regimens for “new” adult patients presumed or known to have drug-susceptible miliary TB.
| Variable | Guideline (reference) |
||
|---|---|---|---|
| ATS-CDC-IDSA (157) | NICE (158) | WHO (156) | |
| Year of publication | 2003 | 2006 | 2010 |
| Intensive phase | RHZE for 2 months | RHZE for 2 months | RHZE for 2 months |
| Continuation phase | RH for 4 months | RH for 4 months | RH for 4 months* |
| If TBM is present, RH for 7–10 months | If TBM is present, RH for 10 months | If TBM is present, RH for 7–10 months | |
| If bone and joint TB is present, RH for 7 months | |||
| Total duration | 6 months | 6 months | 6 months |
| If TBM is present, 9–12 months | If TBM is present, 12 months | If TBM is present, 9–12 months | |
| If bone and joint TB is present, 9 months | |||
| Glucocorticoids | Not routinely recommended for miliary TB. Strongly recommended if TBM, TB pericarditis are present. | Not routinely recommended for miliary TB. | Not routinely recommended for miliary TB. |
| For TB pericarditis, glucocorticoid treatment is recommended for 11 weeks. | Recommended if TBM, TB pericarditis are present. | Recommended for TBM and TB pericarditis | |
| Daily treatment with oral prednisone (60 mg/day, or the equivalent dose of prednisolone) is given for 4 weeks, followed by 30 mg/day for 4 weeks, 15 mg/day for 2 weeks, and finally 5 mg/day for week 11 (final week). | For TB pericarditis, glucocorticoid equivalent to prednisolone 60 mg/day, gradually withdrawn after 2–3 weeks of initiation is recommended. | ||
| For TBM, glucorticoid treatment is recommended for 6 weeks. Daily treatment with oral dexamethasone, 12 mg/day, is given for 3 weeks followed by 6 mg/day for the next 3 weeks. Glucocorticoid treatment may be useful for treating respiratory failure (expert opinion) | For TBM, glucocorticoid equivalent to prednisolone 20–40 mg if on rifampicin, otherwise 10–20 mg gradually withdrawn after 2–3 weeks of initiation is recommended. | ||
ATS = American Thoracic Society; CDC = Centers for Disease Control and Prevention; IDSA = Infectious Diseases Society of America; NICE = National Institute for Health and Clinical Excellence; WHO = World Health Organization; TB = tuberculosis; R = rifampicin; H = isoniazid; Z = pyrazinamide; E = ethambutol; TBM = TB meningitis.
In populations with known or suspected high levels of isoniazid resistance, new TB patients may receive HRE as therapy in the continuation phase as an acceptable alternative to HR (weak/insufficient evidence, expert opinion).
While the guidelines provide a policy regarding treatment duration, individual patients may require longer duration of treatment. The patient depicted in Fig. 5 illustrates this point. This patient with miliary TB also has mediastinal and intraabdominal lymphadenopathy. As per the WHO guidelines [156], this patient would have been notified as pulmonary TB in which case the extrapulmonary lymph node component would have been under-notified. From a programmatic management point of view, practical difficulties exist in extending the continuation phase beyond the standard stipulated 6 months at the field level in high burden TB countries. Sometimes, in patients with drug-susceptible miliary TB, in spite of the patient meticulously adhering to therapy, an appropriate treatment response may not be evident. The utility of therapeutic drug monitoring in this scenario merits further study.
For previously treated patients, the guidelines advocate that specimens for culture and DST should be obtained at or before at the start of treatment. In settings where rapid molecular-based DST results are available DST should be performed for at least isoniazid and rifampicin and, these results should be used to guide the choice of regimen. It is important to accurately assess the extent of involvement clinically and radiologically failing which some patients, for e.g., those with undiagnosed TBM may receive sub-optimal treatment. While the standard duration of treatment may be sufficient for most patients, each patient needs to be assessed individually, and wherever indicated, treatment duration may have to be extended [2], [3], [4], [5].
8.1. Monitoring for adverse drug reactions
Patients with miliary TB receiving anti-TB treatment should be carefully monitored for adverse drug reactions, especially, anti-TB drug-induced liver injury (DILI). Asymptomatic rise in hepatic transaminases is common in patients with miliary TB and unless definitive evidence of DILI is present [159], [160], [161], [162], anti-TB treatment should not be withheld based on this evidence alone. In tropical countries, acute viral hepatitis must be ruled out in patients who develop anti-TB DILI [163], [164].
When patients with miliary TB develop true anti-TB DILI, the potentially hepatotoxic drugs (rifampicin, isoniazid and pyrazinamide) should be stopped [161]. These patients should be treated with non-hepatotoxic anti-TB drugs, such as ethambutol, streptomycin and a fluoroquinolone, till the liver functions normalize. In these patients, it is usually possible to reintroduce the same hepatotoxic drugs that have been implicated in the causation of DILI once the liver functions normalize. There has been a lack of consensus regarding the optimal sequence and dosage schedule for reintroduction and these issues merit future study.
8.2. Glucocorticoids
Sparse published data are available specifically evaluating the role of adjunct glucocorticoid treatment in patients with miliary TB and the results have been conflicting. While a beneficial response was documented in some studies [165], such a benefit was not evident in others [166]. Presence of adrenal insufficiency is an absolute indication for the administration adjunctive glucocorticoid treatment. Many clinicians use adjunctive glucorticoid treatment is considered to be beneficial with TBM, large pericardial effusion, IRIS, ARDS, and immune complex nephritis [2], [3], [4], [5], [167].
8.3. Antiretroviral drugs
The efficacy of standard anti-TB treatment regimens in the treatment of miliary TB in HIV co-infected individuals has not been studied in detail in real life, field setting. Treatment of miliary TB in patients co-infected with HIV requires careful consideration of drug-drug interactions between anti-TB and antiretroviral drugs [167], [168]. Rifampicin, by inducing the hepatic cytochrome P450 pathway, can result in dangerously low levels of antiretroviral agents. Rifabutin is preferred over rifampicin especially when protease inhibitors are used but is costly. Efavirenz is preferred over nevirapine [167], [168].
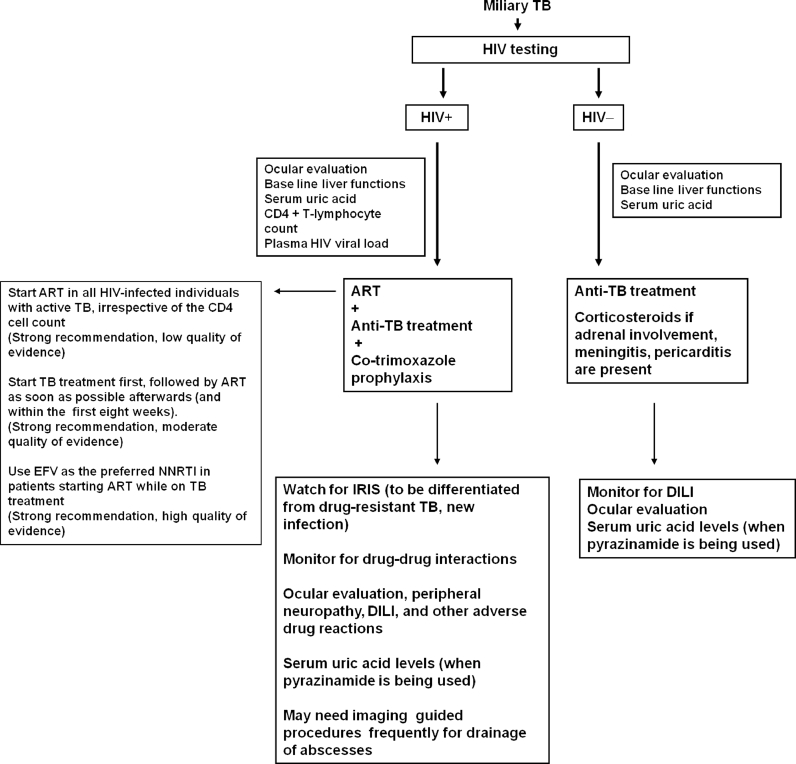
The current guidelines, such as the WHO guidelines [156] should be followed regarding the timing of starting of antiretroviral drugs, the choice of drugs and the timing of initiation in relation to institution of anti-TB treatment. These details are summarized in Fig. 7.
Fig. 7.
Algorithm for treatment of miliary TB. The anti-retroviral treatment recommendations in miliary TB patients co-infected with HIV are based on Refs. [167], [168].
TB = tuberculosis; HIV = human immunodeficiency virus; + = seropositive; − = seronegative; ART = antiretroviral treatment; IRIS = immune reconstitution inflammatory syndrome; DILI = anti-TB drug-induced liver injury; EFV = efavirenz; NNRTI = non-nucleoside reverse transcriptase inhibitors.
Adapted and reproduced with permission from Sharma et al. [2].
8.4. Intensive care
Intensive care, assisted mechanical ventilation, dialysis and other interventions may be required for the management of patients with miliary TB-ARDS and those admitted with MODS [2], [3], [4], [5], [93], [94].
8.5. Interventional radiological procedures
Interventional radiological procedures like image-guided pigtail catheter drainage for psoas abscess, coil or gel foam embolization for treating hemoptysis are useful inthe management of complications of miliary TB [2], [3], [4], [5].
8.6. Surgery
Surgery is required to procure specimens for diagnostic testing and for management of complications, such as, small bowel perforation, for relief of spinal cord compression with persistence or recurrence of neurological deficits, or instability of the spine [2], [3], [4], [5].
9. Mortality
The mortality due to miliary TB is higher in adults (25%–30%) compared to that observed in children (15%–20%) [29], [30], [31], [32], [36], [38], [39], [40], [42], [43]. Delayed initiation of specific anti-TB treatment appears to be the most important factor responsible for mortality in miliary TB.
10. Predictors of poor outcome
Several factors have been identified as predictors of poor outcome in patients with miliary TB (Table 5) [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [169]. In patients with miliary TB-ARDS [91], acute physiological and chronic health evaluation (APACHE II) score greater than 18; APACHE II score less than or equal to 18 in the presence of hyponatraemia and arterial oxygen tension (PaO2) to fraction of inspired oxygen (FIO2) ratio less than or equal to 108.5 have been identified to be predictors of death.
Table 5.
Predictors of poor outcome in patients with miliary TB.
| Demographic parameters |
|---|
| Increasing age |
| Female gender |
| Male gender |
| Co-morbid/predisposing conditions |
| Presence of any underlying co-morbid disease |
| Presence of one or more predisposing conditions |
| Clinical manifestations |
| History of cough / night sweats / dyspnea / chills |
| Altered mental status |
| Meningismus |
| Temperature >39.3 °C |
| Icterus |
| Hepatomegaly |
| Laboratory abnormalities |
| Hyponatremia |
| Hypoalbuminemia |
| Elevated serum hepatic transaminase levels |
| Elevated serum alkaline phosphatase |
| Leucopenia |
| Leucocytosis |
| Lymphopenia |
| Thrombocytopenia |
| Others |
| Presence of atypical chest radiographic patterns |
| Treatment delay |
| High nutritional risk score* |
A four-point nutritional risk score is defined according to the presence of four nutritional factors: low body mass index (<18.5 kg/m2), hypoalbuminemia (serum albumin <30 g/L), hypocholesterolemia (serum cholesterol <2.33 mmol/L) and severe lymphocytopenia (<7 × 105 cells/L). Each risk factor is assigned a value of 1 if present or 0 if absent. Patients with three or four points were classified as having a high nutritional risk score (Ref. [169])
11. Prevention
Presently, the bacille Calmette–Guerin (BCG) vaccination is the only vaccine available for TB. The BCG is effective in reducing the incidence of miliary TB, especially in children [170], [171]. However, BCG vaccine is not effective in individuals who have LTBI and should not be administered to immunosuppressed persons. Targeted tuberculin testing and treatment is practiced in countries with low prevalence of TB such as the USA [172] but anti-TB DILI is a potential risk with this intervention. The quest for a more effective vaccine than BCG is still on [173], [174].
12. Future research
Future research is required to assess the utility of newer diagnostic modalities, such as, GeneXpert MTB/RIF in the diagnosis of miliary TB and to evolve a standardized diagnostic work-up algorithm. Research should also focus on the optimal anti-TB drug treatment regimens and duration in drug-sensitive as well as drug-resistant miliary TB, including those with HIV/AIDS. Monitoring schedules for recognition and treatment of anti-TB DILI, drug, dosage and duration of glucocorticoid use in patients with miliary TB also merit future study.
References
- 1.Global tuberculosis control. WHO report 2015. WHO/HTM/ TB/2015.22. Geneva: World Health Organization; 2015.
- 2.Sharma S.K., Mohan A., Sharma A. Challenges in the diagnosis & treatment of miliary tuberculosis. Indian J Med Res. 2012;135:703–730. [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma S.K., Mohan A., Schlossberg D. Tuberculosis and nontuberculous mycobacterial infections. 6th ed. American Society for Microbiology Press; Washington: 2011. Miliary tuberculosis; pp. 415–435. [Google Scholar]
- 4.Sharma S.K., Mohan A. Disseminated and miliary tuberculosis. In: Sharma SK, Mohan A, editors. Tuberculosis. 2nd ed. Jaypee Brothers Medical Publishers; New Delhi: 2009. pp. 493–518. [Google Scholar]
- 5.Sharma S.K., Mohan A., Sharma A., Mitra D.K. Miliary tuberculosis: new insights into an old disease. Lancet Infect Dis. 2005;5:415–430. doi: 10.1016/S1473-3099(05)70163-8. [DOI] [PubMed] [Google Scholar]
- 6.SahnS A., Neff T.A. Miliary tuberculosis. Am J Med. 1974;56:494–505. doi: 10.1016/0002-9343(74)90482-3. [DOI] [PubMed] [Google Scholar]
- 7.Tuddenham W.J. Glossary of terms for thoracic radiology: recommendations of the nomenclature committee of the Fleischner society. AJR Am J Roentgenol. 1984;143:509–517. doi: 10.2214/ajr.143.3.509. [DOI] [PubMed] [Google Scholar]
- 8.Manget J.J. Vol. 1. Cramer and Perachon; London: 1700. Sepulchretum sive anatomica practical. (Observation XLVII (3 vols)). [Google Scholar]
- 9.Sharma S.K., Mohan A. Tuberculosis: From an incurable scourge to a curable disease - journey over a millennium. Indian J Med Res. 2013;137:455–493. [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J.Y. Diagnosis and treatment of extrapulmonary tuberculosis. Tuberc Respir Dis Seoul. 2015;78:47–55. doi: 10.4046/trd.2015.78.2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurkan F., Bosnak M., Dikici B., Bosnak V., Yaramis A., Tas M.A. Miliary tuberculosis in children: a clinical review. Scand J Infect Dis. 1998;30:359–362. doi: 10.1080/00365549850160648. [DOI] [PubMed] [Google Scholar]
- 12.Hussey G., Chisholm T., Kibel M. Miliary tuberculosis in children: a review of 94 cases. Pediatr Infect Dis J. 1991;10:832–836. doi: 10.1097/00006454-199111000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Kim P.K., Lee J.S., Yun D.J. Clinical review of miliary tuberculosis in Korean children. 84 cases and review of the literature. Yonsei Med J. 1969;10:146–152. doi: 10.3349/ymj.1969.10.2.146. [DOI] [PubMed] [Google Scholar]
- 14.Long R., O'Connor R., Palayew M., Hershfield E., Manfreda J. Disseminated tuberculosis with and without a miliary pattern on chest radiograph: a clinical-pathologic-radiologic correlation. Int J Tuberc Lung Dis. 1997;1:52–58. [PubMed] [Google Scholar]
- 15.Alsoub H., Al Alousi F.S. Miliary tuberculosis in Qatar: a review of 32 adult cases. Ann Saudi Med. 2001;21:16–20. doi: 10.5144/0256-4947.2001.16. [DOI] [PubMed] [Google Scholar]
- 16.Ansari N.A., Kombe A.H., Kenyon T.A., Hone N.M., Tappero J.W., Nyirenda S.T. Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997-1998. Int J Tuberc Lung Dis. 2002;6:55–63. [PubMed] [Google Scholar]
- 17.Chapman C.B., Whorton C.M. Acute generalised miliary tuberculosis in adults. A clinicopathological study based on sixty three cases diagnosed at autopsy. N Engl J Med. 1946;235:239–248. doi: 10.1056/nejm194608222350801. [DOI] [PubMed] [Google Scholar]
- 18.Jacques J., Sloan T.M. The changing pattern of miliary tuberculosis. Thorax. 1970;25:237–240. doi: 10.1136/thx.25.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagirdar J., Zagzag D. Pathology and insights into pathogenesis of tuberculosis. In: Rom W.N., Garay S.M., editors. Tuberculosis. Lippincott Williams & Wilkins; Philadelphia: 2004. pp. 323–344. [Google Scholar]
- 20.Lewison M., Frelich E.B., Ragins O.B. Correlation of clinical diagnosis and pathological diagnosis with special reference to tuberculosis: analysis of autopsy findings in 893 cases. Am Rev Tuberc. 1931;24:152–171. [Google Scholar]
- 21.Slavin R.E., Walsh T.J., Pollack A.D. Late generalized tuberculosis: a clinical pathologic analysis and comparison of 100 cases in the preantibiotic and antibiotic eras. Medicine (Baltimore) 1980;59:352–366. [PubMed] [Google Scholar]
- 22.Vasankari T., Liippo K., Tala E. Overt and cryptic miliary tuberculosis misdiagnosed until autopsy. Scand J Infect Dis. 2003;35:794–796. doi: 10.1080/00365540310016961. [DOI] [PubMed] [Google Scholar]
- 23.Anonymous. Miliary tuberculosis: a changing pattern. Lancet. 1970;1:985–986. [PubMed] [Google Scholar]
- 24.Braun M.M., Cote T.R., Rabkin C.S. Trends in death with tuberculosis during the AIDS era. JAMA. 1993;269:2865–2868. [PubMed] [Google Scholar]
- 25.Somu N., Vijayasekaran D., Ravikumar T., Balachandran A., Subramanyam L., Chandrabhushanam A. Tuberculous disease in a pediatric referral centre: 16 years experience. Indian Pediatr. 1994;31:1245–1249. [PubMed] [Google Scholar]
- 26.Aderele W.I. Miliary tuberculosis in Nigerian children. East Afr Med J. 1978;55:166–171. [PubMed] [Google Scholar]
- 27.Al-Jahdali H., Al-Zahrani K., Amene P., Memish Z., Al-Shimemeri A., Moamary M. Clinical aspects of miliary tuberculosis in Saudi adults. Int J Tuberc Lung Dis. 2000;4:252–255. [PubMed] [Google Scholar]
- 28.Alvarez S., McCabe W.R. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Med Baltim. 1984;63:25–55. [PubMed] [Google Scholar]
- 29.Biehl J.P. Miliary tuberculosis; a review of sixty-eight adult patients admitted to a municipal general hospital. Am Rev Tuberc. 1958;77:605–622. doi: 10.1164/artpd.1958.77.4.605. [DOI] [PubMed] [Google Scholar]
- 30.Campbell I.G. Miliary tuberculosis in British Columbia. Can Med Assoc J. 1973;108:1517–1519. [PMC free article] [PubMed] [Google Scholar]
- 31.Gelb A.F., Leffler C., Brewin A., Mascatello V., Lyons H.A. Miliary tuberculosis. Am Rev Respir Dis. 1973;108:1327–1333. doi: 10.1164/arrd.1973.108.6.1327. [DOI] [PubMed] [Google Scholar]
- 32.Grieco M.H., Chmel H. Acute disseminated tuberculosis as a diagnostic problem. A clinical study based on twenty-eight cases. Am Rev Respir Dis. 1974;109:554–560. doi: 10.1164/arrd.1974.109.5.554. [DOI] [PubMed] [Google Scholar]
- 33.Jacob J.T., Mehta A.K., Leonard M.K. Acute forms of tuberculosis in adults. Am J Med. 2009;122:12–17. doi: 10.1016/j.amjmed.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Hussain S.F., Irfan M., Abbasi M., Anwer S.S., Davidson S., Haqqee R. Clinical characteristics of 110 miliary tuberculosis patients from a low HIV prevalence country. Int J Tuberc Lung Dis. 2004;8:493–499. [PubMed] [Google Scholar]
- 35.Monie R.D., Hunter A.M., Rocchiccioli K.M., White J.P., Campbell I.A., Kilpatrick G.S. Retrospective survey of the management of miliary tuberculosis in South and West Wales, 1976-8. Thorax. 1983;38:369–372. doi: 10.1136/thx.38.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J.H., Langston A.A., Gallis H.A. Miliary tuberculosis: epidemiology, clinical manifestations, diagnosis, and outcome. Rev Infect Dis. 1990;12:583–590. doi: 10.1093/clinids/12.4.583. [DOI] [PubMed] [Google Scholar]
- 37.Kim P.K., Lee J.S., Yun D.J. Clinical review of miliary tuberculosis in Korean children. 84 cases and review of the literature. Yonsei Med J. 1969;10:146–152. doi: 10.3349/ymj.1969.10.2.146. [DOI] [PubMed] [Google Scholar]
- 38.Maartens G., Willcox P.A., Benatar S.R. Miliary tuberculosis: rapid diagnosis, hematologic abnormalities, and outcome in 109 treated adults. Am J Med. 1990;89:291–296. doi: 10.1016/0002-9343(90)90340-j. [DOI] [PubMed] [Google Scholar]
- 39.Mert A., Bilir M., Tabak F., Ozaras R., Ozturk R., Senturk H. Miliary tuberculosis: clinical manifestations, diagnosis and outcome in 38 adults. Respirology. 2001;6:217–224. doi: 10.1046/j.1440-1843.2001.00328.x. [DOI] [PubMed] [Google Scholar]
- 40.Munt P.W. Miliary tuberculosis in the chemotherapy era: with a clinical review in 69 American adults. Medicine (Baltimore) 1972;51:139–155. doi: 10.1097/00005792-197203000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Noertjojo K., Tam C.M., Chan S.L., Chan-Yeung M.M. Extrapulmonary and pulmonary tuberculosis in Hong Kong. Int J Tuberc Lung Dis. 2002;6:879–886. [PubMed] [Google Scholar]
- 42.Onadeko B.O., Dickinson R., Sofowora E.O. Miliary tuberculosis of the lung in Nigerian adults. East Afr Med J. 1975;52:390–395. [PubMed] [Google Scholar]
- 43.Prout S., Benatar S.R. Disseminated tuberculosis. A study of 62 cases. S Afr Med J. 1980;58:835–842. [PubMed] [Google Scholar]
- 44.Rahajoe N.N. Miliary tuberculosis in children. A clinical review. Paediatr Indones. 1990;30:233–240. [PubMed] [Google Scholar]
- 45.Sharma S.K., Mohan A., Pande J.N., Prasad K.L., Gupta A.K., Khilnani G.C. Clinical profile, laboratory characteristics and outcome in miliary tuberculosis. QJM. 1995;88:29–37. [PubMed] [Google Scholar]
- 46.Federele M.P. Mosby-Year Book, Inc; St. Louis: 1997. editor. The year book of diagnostic radiology; pp. 91–93. [Google Scholar]
- 47.Swaminathan S., Padmapriyadarsini C., Ponnuraja C., Sumathi C.H., Rajasekaran S., Amerandran V.A. Miliary tuberculosis in human immunodeficiency virus infected patients not on antiretroviral therapy: clinical profile and response to short course chemotherapy. J Postgrad Med. 2007;53:228–231. doi: 10.4103/0022-3859.37509. [DOI] [PubMed] [Google Scholar]
- 48.Teklu B., Butler J., Ostrow J.H. Miliary tuberculosis. A review of 83 cases treated between 1950 and 1968. Ethiop Med J. 1977;15:39–48. [PubMed] [Google Scholar]
- 49.Udani P.M., Bhat U.S., Bhave S.K., Ezuthachan S.G., Shetty V.V. Problem of tuberculosis in children in India: epidemiology, morbidity, mortality and control programme. Indian Pediatr. 1976;13:881–890. [PubMed] [Google Scholar]
- 50.El Shamy A.S., Al Saidi F., Baidas G., Al Bader M., Sawy M., Hakkim R. Miliary tuberculosis in Kuwait: clinical presentation, diagnosis and treatment outcome. Kuwait Med J. 2008;40:288–292. [Google Scholar]
- 51.Dixon W.G., Hyrich K.L., Watson K.D., Lunt M., Galloway J., Ustianowski A. Control centre consortium, B.S.R.B.R., symmons, D.P.; B.S.R. biologics register. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British society for rheumatology biologics register (BSRBR) Ann Rheum Dis. 2010;69:522–528. doi: 10.1136/ard.2009.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uthman I., Kanj N., El-Sayad J., Bizri A.R. Miliary tuberculosis after infliximab therapy in Lebanon. Clin Rheumatol. 2004;23:279–280. doi: 10.1007/s10067-004-0873-z. [DOI] [PubMed] [Google Scholar]
- 53.Arkema E.V., Jonsson J., Baecklund E., Bruchfeld J., Feltelius N. ARTIS Study Group. Are patients with rheumatoid arthritis still at an increased risk of tuberculosis and what is the role of biological treatments. Ann Rheum Dis. 2015;74:1212–1217. doi: 10.1136/annrheumdis-2013-204960. [DOI] [PubMed] [Google Scholar]
- 54.Jung S.M., Ju J.H., Park M.S., Kwok S.K., Park K.S., Kim H.Y. Risk of tuberculosis in patients treated with anti-tumor necrosis factor therapy: a nationwide study in South Korea, a country with an intermediate tuberculosis burden. Int J Rheum Dis. 2015;18 doi: 10.1111/1756-185X.12530. 323–230. [DOI] [PubMed] [Google Scholar]
- 55.Xie X., Chen J., Peng Y., Gao J., Tian J., Ling G. Meta analysis of infection risks of anti-TNF-α treatment in rheumatoid arthritis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2013;38:722–736. doi: 10.3969/j.issn.1672-7347.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 56.Anyanwu C.H., Nassau E., Yacoub M. Miliary tuberculosis following homograft valve replacement. Thorax. 1976;31:101–106. doi: 10.1136/thx.31.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morano Amado L.E., Amador Barciela L., Rodriguez Fernandez A., Martinez-Sapina Llamas I., Vazquez Alvarez O., Fernandez Martin J. Extracorporeal shock wave lithotripsy complicated with miliary tuberculosis. J Urol. 1993;149:1532–1534. doi: 10.1016/s0022-5347(17)36437-6. [DOI] [PubMed] [Google Scholar]
- 58.Rabe J., Neff K.W., Lehmann K.J., Mechtersheimer U., Georgi M. Miliary tuberculosis after intravesical bacille Calmette-Guerin immunotherapy for carcinoma of the bladder. AJR Am J Roentgenol. 1999;172:748–750. doi: 10.2214/ajr.172.3.10063874. [DOI] [PubMed] [Google Scholar]
- 59.Silverblatt A., DeSimone J.A., Babinchak T.J. Acute miliary tuberculosis following laser lithotripsy. Infect Med. 2002;19:80–82. [Google Scholar]
- 60.Yekanath H., Gross P.A., Vitenson J.H. Miliary tuberculosis following ureteral catheterization. Urology. 1980;16:197–198. doi: 10.1016/0090-4295(80)90084-9. [DOI] [PubMed] [Google Scholar]
- 61.Collins H.L., Kaufmann S.H. The many faces of host responses to tuberculosis. Immunology. 2001;103:1–9. doi: 10.1046/j.1365-2567.2001.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rook G.A., Hernandez-Pando R., Dheda K., Teng Seah G. IL-4 in tuberculosis: implications for vaccine design. Trends Immunol. 2004;25:483–488. doi: 10.1016/j.it.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 63.Sharma P.K., Saha P.K., Singh A., Sharma S.K., Ghosh B., Mitra D.K. FoxP3+ regulatory T cells suppress effector T-cell function at pathologic site in miliary tuberculosis. Am J Respir Crit Care Med. 2009;179:1061–1070. doi: 10.1164/rccm.200804-529OC. [DOI] [PubMed] [Google Scholar]
- 64.Sharma S.K., Mitra D.K., Balamurugan A., Pandey R.M., Mehra N.K. Cytokine polarization in miliary and pleural tuberculosis. J Clin Immunol. 2002;22:345–352. doi: 10.1023/a:1020604331886. [DOI] [PubMed] [Google Scholar]
- 65.Barnes P.F., Grisso C.L., Abrams J.S., Band H., Rea T.H., Modlin R.L. Gamma delta T lymphocytes in human tuberculosis. J Infect Dis. 1992;165:506–512. doi: 10.1093/infdis/165.3.506. [DOI] [PubMed] [Google Scholar]
- 66.Ellner J.J. The immune response in human tuberculosis—implications for tuberculosis control. J Infect Dis. 1997;176:1351–1359. doi: 10.1086/514132. [DOI] [PubMed] [Google Scholar]
- 67.Al-Arif L.I., Goldstein R.A., Affronti L.F., Janicki B.W. HLA-Bw15 and tuberculosis in a North American black population. Am Rev Respir Dis. 1979;120:1275–1278. doi: 10.1164/arrd.1979.120.6.1275. [DOI] [PubMed] [Google Scholar]
- 68.Balamurugan A. All India Institute of Medical Sciences; New Delhi: 2002. HLA-DR restriction of Th1/Th2 cytokine profile in tuberculosis: impact of genetic diversity. [Ph.D. thesis] [Google Scholar]
- 69.Kumararatne D.S., Pithie A.S., Drysdale P., Gaston J.S., Kiessling R., Iles P.B. Specific lysis of mycobacterial antigen-bearing macrophages by class II MHC-restricted polyclonal T cell lines in healthy donors or patients with tuberculosis. Clin Exp Immunol. 1990;80:314–323. doi: 10.1111/j.1365-2249.1990.tb03287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taype C.A., Shamsuzzaman S., Accinelli R.A., Espinoza J.R., Shaw M.A. Genetic susceptibility to different clinical forms of tuberculosis in the Peruvian population. Infect Genet Evol. 2010;10:495–504. doi: 10.1016/j.meegid.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 71.Cunha B.A., Krakakis J., McDermott B.P. Fever of unknown origin (FUO) caused by miliary tuberculosis: diagnostic significance of morning temperature spikes. Heart Lung. 2009;38:77–82. doi: 10.1016/j.hrtlng.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 72.Flores-Franco R.A., Ríos-Ortiz L.A. The "damp shadow" sign: another clinical indicator of miliary tuberculosis. Heart Lung. 2010;39:87–88. doi: 10.1016/j.hrtlng.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 73.Proudfoot A.T., Akhtar A.J., Douglas A.C., Horne N.W. Miliary tuberculosis in adults. BMJ. 1969;2:273–276. doi: 10.1136/bmj.2.5652.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thwaites G.E., Nguyen D.B., Nguyen H.D., Hoang T.Q., Do T.T., Nguyen T.C. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741–1751. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 75.Garg R.K., Sharma R., Kar A.M., Kushwaha R.A., Singh M.K., Shukla R. Neurological complications of miliary tuberculosis. Clin Neurol Neurosurg. 2010;112:188–192. doi: 10.1016/j.clineuro.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 76.Braidy J., Pothel C., Amra S. Miliary tuberculosis presenting as adrenal failure. J Can Med Assoc. 1981;82:254–256. [PMC free article] [PubMed] [Google Scholar]
- 77.Yokoyama T., Toda R., Kimura Y., Mikagi M., Aizawa H. Addison's disease induced by miliary tuberculosis and the administration of rifampicin. Intern Med. 2009;48:1297–1300. doi: 10.2169/internalmedicine.48.1974. [DOI] [PubMed] [Google Scholar]
- 78.Sharma S.K., Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120:316–353. [PubMed] [Google Scholar]
- 79.Sharma S.K., Mohan A., Kadhiravan T. HIV-TB co-infection: epidemiology, diagnosis and management. Indian J Med Res. 2005;121:550–567. [PubMed] [Google Scholar]
- 80.Sharma S.K., Mohan A. Co-infection of human immunodeficiency virus (HIV) and tuberculosis: Indian perspective. Indian J Tuberc. 2004;51:5–16. [Google Scholar]
- 81.Sharma S.K., Mohan A., Gupta R., Kumar A., Gupta A.K., Singhal V.K. Clinical presentation of tuberculosis in patients with AIDS: an Indian experience. Indian J Chest Dis Allied Sci. 1997;39:213–220. [PubMed] [Google Scholar]
- 82.Lado Lado F.L., Barrio Gomez E., Carballo Arceo E., Cabarcos Ortiz de Barron A. Clinical presentation of tuberculosis and the degree of immunodeficiency in patients with HIV infection. Scand J Infect Dis. 1999;31:387–391. doi: 10.1080/00365549950163842. [DOI] [PubMed] [Google Scholar]
- 83.Kim J.Y., Jeong Y.J., Kim K.I., Lee I.S., Park H.K., Kim Y.D. Miliary tuberculosis: a comparison of CT findings in HIV-seropositive and HIV-seronegative patients. Br J Radiol. 2010;83:206–211. doi: 10.1259/bjr/95169618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zumla A., Malon P., Henderson J., Grange J.M. Impact of HIV infection on tuberculosis. Postgrad Med J. 2000;76:259–268. doi: 10.1136/pmj.76.895.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yodmalai S., Chiewchanvit S., Mahanupab P. Cutaneous miliary tuberculosis in a renal transplant patient: a case report and literature review. Southeast Asian J Trop Med Public Health. 2011;42:674–678. [PubMed] [Google Scholar]
- 86.del Giudice P., Bernard E., Perrin C., Bernardin G., R F., Boissy C. Unusual cutaneous manifestations of miliary tuberculosis. Clin Infect Dis. 2000;30:201–204. doi: 10.1086/313587. [DOI] [PubMed] [Google Scholar]
- 87.Shao C., Qu J., He L. A comparative study of clinical manifestations caused by tuberculosis in immunocompromised and non-immunocompromised patients. Chin Med J Engl. 2003;116:1717–1722. [PubMed] [Google Scholar]
- 88.Goldsack N.R., Allen S., Lipman M.C. Adult respiratory distress syndrome as a severe immune reconstitution disease following the commencement of highly active antiretroviral therapy. Sex Transm Infect. 2003;79:337–338. doi: 10.1136/sti.79.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mohan A., Sharma S.K., Pande J.N. Acute respiratory distress syndrome in miliary tuberculosis: a 12-year experience. Indian J Chest Dis Allied Sci. 1996;38:147–152. [PubMed] [Google Scholar]
- 90.Penner C., Roberts D., Kunimoto D., Manfreda J., Long R. Tuberculosis as a primary cause of respiratory failure requiring mechanical ventilation. Am J Respir Crit Care Med. 1995;151:867–872. doi: 10.1164/ajrccm/151.3_Pt_1.867. [DOI] [PubMed] [Google Scholar]
- 91.Sharma S.K., A. Mohan A., Banga S.P.K., Guntupalli K.K. Predictors of development and outcome in patients with acute respiratory distress syndrome due to tuberculosis. Int J Tuberc Lung Dis. 2006;10:429–435. [PubMed] [Google Scholar]
- 92.Lee K., Kim J.H., Lee J.H., Lee W.Y., Park M.S., Kim J.Y. Acute respiratory distress syndrome caused by military tuberculosis: a multicentre survey in South Korea. Int J Tuberc Lung Dis. 2011;15:1099–1103. doi: 10.5588/ijtld.10.0557. [DOI] [PubMed] [Google Scholar]
- 93.Valade S., Raskine L., Aout M., Malissin I., Brun P., Deye N. Tuberculosis in the intensive care unit: A retrospective descriptive cohort study with determination of a predictive fatality score. Can J Infect Dis Med Microbiol. 2012;23:173–178. doi: 10.1155/2012/361292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koegelenberg C.F., Balkema C.A., Jooste Y., Taljaard J.J., Irusen E.M. Validation of a severity-of-illness score in patients with tuberculosis requiring intensive care unit admission. S Afr Med J. 2015;105:389–392. doi: 10.7196/samj.9148. [DOI] [PubMed] [Google Scholar]
- 95.Gupta P.P., Mehta D., Agarwal D., Chand T. Recurrent pneumothorax developing during chemotherapy in a patient with miliary tuberculosis. Ann Thorac Med. 2007;2:173–175. doi: 10.4103/1817-1737.36555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lakin B.D., Riordan F.A., John C.M. Air leak in miliary tuberculosis. Am J Trop Med Hyg. 2009;80:325. [PubMed] [Google Scholar]
- 97.Krishnaswami K.V. Mediastinal emphysema in miliary tuberculosis. JAMA. 1977;69:227–229. [PubMed] [Google Scholar]
- 98.Mallinson W.J., Fuller R.W., Levison D.A., Baker L.R., Cattell W.R. Diffuse interstitial renal tuberculosis—an unusual cause of renal failure. Q JM. 1981;50:137–148. [PubMed] [Google Scholar]
- 99.Salliot C., Guichard I., Daugas E., Lagrange M., Verine J., Molina J.M. Acute kidney disease due to immune reconstitution inflammatory syndrome in an HIV-infected patient with tuberculosis. J Int Assoc Physicians AIDS Care Chic Ill. 2008;7:178–181. doi: 10.1177/1545109708320683. [DOI] [PubMed] [Google Scholar]
- 100.Le Hô H., Barbarot N., Desrues B. Pancytopenia in disseminated tuberculosis: Think of macrophage activation syndrome. Rev Mal Respir. 2010;27:257–260. doi: 10.1016/j.rmr.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 101.Henter J.I., Elinder G., Ost A. Diagnostic guidelines for hemophagocytic lymphohistiocytosis. The FHL Study Group of the Histiocyte Society. Semin Oncol. 1991;18:29–33. [PubMed] [Google Scholar]
- 102.Asada Y., Hayashi T., Sumiyoshi A., Aburaya M., Shishime E. Miliary tuberculosis presenting as fever and jaundice with hepatic failure. Hum Pathol. 1991;22:92–94. doi: 10.1016/0046-8177(91)90068-z. [DOI] [PubMed] [Google Scholar]
- 103.Seabra J., Coelho H., Barros H., Alves J.O., Goncalves V., Rocha-Marques A. Acute tuberculous perforation of the small bowel during antituberculosis therapy. J Clin Gastroenterol. 1993;16:320–322. doi: 10.1097/00004836-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 104.Rose A.G. Cardiac tuberculosis. A study of 19 patients. Arch Pathol Lab Med. 1987;111:422–426. [PubMed] [Google Scholar]
- 105.Theuer C.P., Hopewell P.C., Elias D., Schecter G.F., Rutherford G.W., Chaisson R.E. Human immunodeficiency virus infection in tuberculosis patients. J Infect Dis. 1990;162:8–12. doi: 10.1093/infdis/162.1.8. [DOI] [PubMed] [Google Scholar]
- 106.Cope A.P., Heber M., Wilkins E.G. Valvular tuberculous endocarditis: a case report and review of the literature. J Infect. 1990;21:293–296. doi: 10.1016/0163-4453(90)94029-y. [DOI] [PubMed] [Google Scholar]
- 107.Wainwright J. Tuberculous endocarditis: a report of 2 cases. S Afr Med J. 1979;56:731–733. [PubMed] [Google Scholar]
- 108.Yamane H., Fujiwara T., Doko S., Inada H., Nogami A., Masaki H. Two cases of miliary tuberculosis following prosthetic valve replacement. Kokyu To Junkan. 1989;37:803–805. [PubMed] [Google Scholar]
- 109.Felson B., Akers P.V., Hall G.S., Schreiber J.T., Greene R.E., Pedrosa C.S. Mycotic tuberculous aneurysm of the thoracic aorta. JAMA. 1977;237:1104–1108. [PubMed] [Google Scholar]
- 110.Doherty J.G., Rankin R., Kerr F. Miliary tuberculosis presenting as infection of a pacemaker pulse-generator pocket. Scott Med J. 1996;41:20–21. doi: 10.1177/003693309604100108. [DOI] [PubMed] [Google Scholar]
- 111.Shibolet S., Dan M., Jedwab M., Goldhammer Y., Baum G.L. Recurrent miliary tuberculosis secondary to infected ventriculoatrial shunt. Chest. 1979;76:328–330. doi: 10.1378/chest.76.3.328. [DOI] [PubMed] [Google Scholar]
- 112.Tseng H.L., Roulet F. Cardiac involvement in miliary tuberculosis. Am Rev Tuberc. 1953;68:771–774. doi: 10.1164/art.1953.68.5.771. [DOI] [PubMed] [Google Scholar]
- 113.Biedrzycki O.J., Baithun S.I. TB-related sudden death (TBRSD) due to myocarditis complicating miliary TB: a case report and review of the literature. Am J Forensic Med Pathol. 2006;27:335–336. doi: 10.1097/01.paf.0000233633.16185.32. [DOI] [PubMed] [Google Scholar]
- 114.Wallis P.J., Branfoot A.C., Emerson P.A. Sudden death due to myocardial tuberculosis. Thorax. 1984;39:155–156. doi: 10.1136/thx.39.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meintjes G., Lawn S.D., Scano F., Maartens G., French M.A., Worodria W. International Network for the Study of HIV-associated IRIS. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sharma S.K., Dhooria S., Barwad P., Kadhiravan T., Ranjan S., Miglani S. A study of TB-associated immune reconstitution inflammatory syndrome using the consensus case-definition. Indian J Med Res. 2010;131:804–808. [PubMed] [Google Scholar]
- 117.Jehle A.W., Khanna N., Sigle J.P., Glatz-Krieger K., Battegay M., Steiger J. Acute renal failure on immune reconstitution in an HIV-positive patient with miliary tuberculosis. Clin Infect Dis. 2004;38:e32–e35. doi: 10.1086/381441. [DOI] [PubMed] [Google Scholar]
- 118.Man M.A., Arghir O.C., Man S., Streba C.T., Olteanu M., Nitu M. Fatal paradoxical cryptic miliary tuberculosis and immune reconstitution disease in a young non-HIV immunocompromised male patient: case report with autopsy findings. Rom J Morphol Embryol. 2014;55:453–457. [PubMed] [Google Scholar]
- 119.Rangaka M.X., Wilkinson K.A., Glynn J.R., Ling D., Menzies D., Mwansa-Kambafwile J. Predictive value of interferon-g release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45–55. doi: 10.1016/S1473-3099(11)70210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.National Collaborating Centre for Chronic Conditions and the Centre for Clinical Practice at NICE . NICE clinical guideline London. Royal College of Physicians of London; England: 2011. Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for Its Prevention and Control. [Google Scholar]
- 121.European Centre for Disease Prevention and Control . European Centre for Disease Prevention and Control; Stockholm: 2011. Use of interferon-gamma release assays in support of TB diagnosis. [Google Scholar]
- 122.World Health Organization . World Health Organization; Geneva: 2011. Use of tuberculosis interferon-gamma release assays (IGRAs) in low- and middle-income countries: policy Statement. WHO/HTM/TB/ 2011.18. [PubMed] [Google Scholar]
- 123.Singh K.J., Ahluwalia G., Sharma S.K., Saxena R., Chaudhary V.P., Anant M. Significance of haematological manifestations in patients with tuberculosis. J Assoc Physicians India. 2001;49:790–794. [PubMed] [Google Scholar]
- 124.Chan C.H., Chan T.Y., Shek A.C., Mak T.W., Lui S.F., Lai K.N. Severe hypercalcaemia associated with miliary tuberculosis. J Trop Med Hyg. 1994;97:180–182. [PubMed] [Google Scholar]
- 125.Shalhoub R.J., Antoniou L.D. The mechanism of hyponatremia in pulmonary tuberculosis. Ann Intern Med. 1969;70:943–962. doi: 10.7326/0003-4819-70-5-943. [DOI] [PubMed] [Google Scholar]
- 126.Vorherr H., Massry S.G., Fallet R., Kaplan L., Kleeman C.R. Antidiuretic principle in tuberculous lung tissue of a patient with pulmonary tuberculosis and hyponatremia. Ann Intern Med. 1970;72:383–387. doi: 10.7326/0003-4819-72-3-383. [DOI] [PubMed] [Google Scholar]
- 127.Winkler A.W., Crankshaw D.F. Chloride depletion in conditions other than Addison's disease. J Clin Invest. 1938;17:1–6. doi: 10.1172/JCI100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jamieson D.H., Cremin B.J. High resolution CT of the lungs in acute disseminated tuberculosis and a pediatric radiology perspective of the term “miliary”. Pediatr Radiol. 1993;23:380–383. doi: 10.1007/BF02011965. [DOI] [PubMed] [Google Scholar]
- 129.Lee K.S., Kim T.S., Han J., Hwang J.H., Yoon J.H., Kim Y., Yoo S.Y. Diffuse micronodular lung disease: HRCT and pathologic findings. J Comput Assist Tomogr. 1999;23:99–106. doi: 10.1097/00004728-199901000-00022. [DOI] [PubMed] [Google Scholar]
- 130.Price M. Lymphangitis reticularis tuberculosa. Tubercle. 1968;49:377–384. doi: 10.1016/s0041-3879(68)80018-2. [DOI] [PubMed] [Google Scholar]
- 131.McGuinness G., Naidich D.P., Jagirdar J., Leitman B., McCauley D.I. High resolution CT findings in miliary lung disease. J Comput Assist Tomogr. 1992;16:384–390. doi: 10.1097/00004728-199205000-00009. [DOI] [PubMed] [Google Scholar]
- 132.Sharma S.K., Mukhopadhyay S., Arora R., Verma K., Pande J.N., Khilnani G.C. Computed tomography in miliary tuberculosis: comparison with plain films, bronchoalveolar lavage, pulmonary functions and gas exchange. Australas Radiol. 1996;40:113–118. doi: 10.1111/j.1440-1673.1996.tb00363.x. [DOI] [PubMed] [Google Scholar]
- 133.Pipavath S.N., Sharma S.K., Sinha S., Mukhopadhyay S., Gulati M.S. High resolution CT (HRCT) in miliary tuberculosis (MTB) of the lung: correlation with pulmonary function tests & gas exchange parameters in north Indian patients. Indian J Med Res. 2007;126:193–198. [PubMed] [Google Scholar]
- 134.Yu R.S., Zhang S.Z., Wu J.J., Li R.F. Imaging diagnosis of 12 patients with hepatic tuberculosis. World J Gastroenterol. 2004;10:1639–1642. doi: 10.3748/wjg.v10.i11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Willcox P.A., Potgieter P.D., Bateman E.D., Benatar S.R. Rapid diagnosis of sputum negative miliary tuberculosis using the flexible fibreoptic bronchoscope. Thorax. 1986;41:681–684. doi: 10.1136/thx.41.9.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ibrarullah M., Mohan A., Sarkari A., Srinivas M., Mishra A., Sundar T.S. Abdominal tuberculosis: diagnosis by laparoscopy and colonoscopy. Trop Gastroenterol. 2002;23:150–153. [PubMed] [Google Scholar]
- 137.World Health Organization . World Health Organization; Geneva: 2011. Commercial serodiagnostic tests for diagnosis of tuberculosis: policy statement. WHO/HTM/TB/2011.5. [PubMed] [Google Scholar]
- 138.Riquelme A., Calvo M., Salech F., Valderrama S., Pattillo A., Arellano M. Value of adenosine deaminase (ADA) in ascitic fluid for the diagnosis of tuberculous peritonitis: a meta-analysis. J Clin Gastroenterol. 2006;40:705–710. doi: 10.1097/00004836-200609000-00009. [DOI] [PubMed] [Google Scholar]
- 139.Sharma S.K., Suresh V., Mohan A., Kaur P., Saha P., Kumar A. A prospective study of sensitivity and specificity of adenosine deaminase estimation in the diagnosis of tuberculosis pleural effusion. Indian J Chest Dis Allied Sci. 2001;43:149–155. [PubMed] [Google Scholar]
- 140.Sharma S.K., Banga A. Diagnostic utility of pleural fluid IFN gamma in tuberculosis pleural effusion. J Interferon Cytokine Res. 2004;24:213–217. doi: 10.1089/107999004323034088. [DOI] [PubMed] [Google Scholar]
- 141.Sharma S.K., Banga A. Pleural fluid interferon-gamma and adenosine deaminase levels in tuberculosis pleural effusion: a cost-effectiveness analysis. J Clin Lab Anal. 2005;19:40–46. doi: 10.1002/jcla.20054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sharma S.K., Tahir M., Mohan A., Smith-Rohrberg D., Mishra H.K., Pandey R.M. Diagnostic accuracy of ascitic fluid IFN-gamma and adenosine deaminase assays in the diagnosis of tuberculous ascites. J Interferon Cytokine Res. 2006;26:484–488. doi: 10.1089/jir.2006.26.484. [DOI] [PubMed] [Google Scholar]
- 143.Rana S.V., Chacko F., Lal V., Arora S.K., Parbhakar S., Sharma S.K., Singh K. To compare CSF adenosine deaminase levels and CSF-PCR for tuberculous meningitis. Clin Neurol Neurosurg. 2010;112:424–430. doi: 10.1016/j.clineuro.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 144.Maynard-Smith L., Larke N., Peters J.A., Lawn S.D. Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary and pulmonary tuberculosis when testing non-respiratory samples: a systematic review. BMC Infect Dis. 2014;14:709. doi: 10.1186/s12879-014-0709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Denkinger C.M., Schumacher S.G., Boehme C.C., Dendukuri N., Pai M., Steingart K.R. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2014;44:435–446. doi: 10.1183/09031936.00007814. [DOI] [PubMed] [Google Scholar]
- 146.Sharma S.K., Kohli M., Chaubey J., Yadav R.N., Sharma R., Singh B.K. Evaluation of Xpert MTB/RIF assay performance in diagnosing extrapulmonary tuberculosis among adults in a tertiary care centre in India. Eur Respir J. 2014;44:1090–1093. doi: 10.1183/09031936.00059014. [DOI] [PubMed] [Google Scholar]
- 147.World Health Organization . World Health Organization; Geneva: 2011. Rapid implementation of the Xpert MTB/RIF diagnostic test: technical and operational ‘How-to’; practical considerations. WHO/HTM/TB/2011.2. [Google Scholar]
- 148.World Health Organization. Policy statement. Molecular line probe assays for rapid screening of patients at risk of multidrug-resistant tuberculosis (MDR-TB). Available at URL:< http://www.who.int/tb/laboratory/lpa_policy.pdf>. [accessed 31.08.16] 2016.
- 149.Ling D.I., Zwerling A.A., Pai M. Rapid diagnosis of drug-resistant TB using line probe assays: from evidence to policy. Expert Rev Respir Med. 2008;2:583–588. doi: 10.1586/17476348.2.5.583. [DOI] [PubMed] [Google Scholar]
- 150.Sharma S.K., Pande J.N., Singh Y.N., Verma K., Kathait S.S., Khare S.D. Pulmonary function and immunologic abnormalities in miliary tuberculosis. Am Rev Respir Dis. 1992;145:1167–1171. doi: 10.1164/ajrccm/145.5.1167. [DOI] [PubMed] [Google Scholar]
- 151.Sharma S.K., Ahluwalia G. Effect of antituberculosis treatment on cardiopulmonary responses to exercise in miliary tuberculosis. Indian J Med Res. 2006;124:411–418. [PubMed] [Google Scholar]
- 152.McClement J.H., Renzetti Jr A.D., Carroll D., Himmelstein A., Cournand A. Cardiopulmonary function in hematogenous pulmonary tuberculosis in patients with streptomycin therapy. Am Rev Tuberc. 1951;64:588–601. doi: 10.1164/art.1951.64.6.583. [DOI] [PubMed] [Google Scholar]
- 153.Sharma S.K., Ahluwalia G. Exercise testing in miliary tuberculosis–some facts. Indian J Med Res. 2007;125:182–183. [PubMed] [Google Scholar]
- 154.Ainslie G.M., Solomon J.A., Bateman E.D. Lymphocyte and lymphocyte subset numbers in blood and in bronchoalveolar lavage and pleural fluid in various forms of pulmonary tuberculosis at presentation and during recovery. Thorax. 1992;47:513–518. doi: 10.1136/thx.47.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Williams N.H., Jr, Kane C., Yoo O.H. Pulmonary function in miliary tuberculosis. Am Rev Respir Dis. 1973;107:858–860. doi: 10.1164/arrd.1973.107.5.858. [DOI] [PubMed] [Google Scholar]
- 156.World Health Organization . 4th ed. World Health Organization; Geneva: 2010. Treatment of tuberculosis. Guidelines. WHO/HTM/ TB/2009.420. [Google Scholar]
- 157.Blumberg H.M., Burman W.J., Chaisson R.E., Daley C.L., Etkind S.C., Friedman L.N. American thoracic society, centers for disease control and prevention and the infectious diseases society. American thoracic society/centers for disease control and prevention/infectious diseases society of America. Treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 158.National Institute for Health and Clinical Excellence . In: Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. Royal College of Physicians; London: 2006. National Collaborating Centre for Chronic Conditions. Management of non-respiratory tuberculosis; pp. 63–76. [Google Scholar]
- 159.Sharma S.K., Mohan A. Antituberculosis treatment induced hepatotoxicity: from bench to bed-side. In: Gupta S.B., editor. Medicine update. Association of Physicians of India; Mumbai: 2005. pp. 479–484. [Google Scholar]
- 160.Sharma S.K. Antituberculosis drugs and hepatotoxicity. Infect Genet Evol. 2004;4:167–170. doi: 10.1016/j.meegid.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 161.Saukkonen J.J., Cohn D.L., Jasmer R.M., Schenker S., Jereb J.A., Nolan C.M. An official ATS statement: Hepatotoxicity of anti tuberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 162.Singla R., Sharma S.K., Mohan A., Makharia G., Sreenivas V., Jha B. Evaluation of risk factors for antituberculosis treatment induced hepatotoxicity. Indian J Med Res. 2010;132:81–86. [PubMed] [Google Scholar]
- 163.Sarda P., Sharma S.K., Mohan A., Makharia G., Jayaswal A., Pandey R.M. Role of acute viral hepatitis as a confounding factor in antituberculosis treatment induced hepatotoxicity. Indian J Med Res. 2009;129:64–67. [PubMed] [Google Scholar]
- 164.Sharma S.K., Singla R., Sreenivas V., Kumar S., Jha B., Rathored J. Acute viral hepatitis as a confounding factor in patients with antituberculosis treatment induced hepatotoxicity. Indian J Med Res. 2009;130:200–201. [PubMed] [Google Scholar]
- 165.Sun T.N., Yang J.Y., Zheng L.Y., Deng W.W., Sui Z.Y. Chemotherapy and its combination with corticosteroids in acute miliary tuberculosis in adolescents and adults: analysis of 55 cases. Chin Med J Engl. 1981;94:309–314. [PubMed] [Google Scholar]
- 166.Massaro D., Katz S., Sachs M. Choroidal tubercles. A clue to hematogenous tuberculosis. Ann Intern Med. 1964;60:231–241. doi: 10.7326/0003-4819-60-2-231. [DOI] [PubMed] [Google Scholar]
- 167.Pozniak A.L., Coyne K.M., Miller R.L., Lipman M.C.I., Freedman A.R., Ormerod L.P. BHIVA guidelines subcommittee. British HIV association guidelines for the treatment of TB/HIV coinfection 2011. HIV Med. 2011;12:517–524. doi: 10.1111/j.1468-1293.2011.00954.x. [DOI] [PubMed] [Google Scholar]
- 168.World Health Organization . World Health Organization; Geneva: 2010. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach – 2010 revision. [PubMed] [Google Scholar]
- 169.Kim D.K., Kim H.J., Kwon S.Y., Yoon H.I., Lee C.T., Kim Y.W. Nutritional deficit as a negative prognostic factor in patients with miliary tuberculosis. Eur Respir J. 2008;32:1031–1036. doi: 10.1183/09031936.00174907. [DOI] [PubMed] [Google Scholar]
- 170.Mangtani P., Abubakar I., Ariti C., Beynon R., Pimpin L., Fine P.E. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58:470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 171.Trunz B.B., Fine P., Dye C. Effect of BCG vaccination on childhood tuberculousmeningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 172.American Thoracic Society Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm Rep. 2000;49(RR-6):1–51. [PubMed] [Google Scholar]
- 173.Kaufmann S.H. Tuberculosis vaccine development at a divide. Curr Opin Pulm Med. 2014;20:294–300. doi: 10.1097/MCP.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 174.Ahsan M.J. Recent advances in the development of vaccines for tuberculosis. Ther Adv Vaccines. 2015;3:66–75. doi: 10.1177/2051013615593891. [DOI] [PMC free article] [PubMed] [Google Scholar]