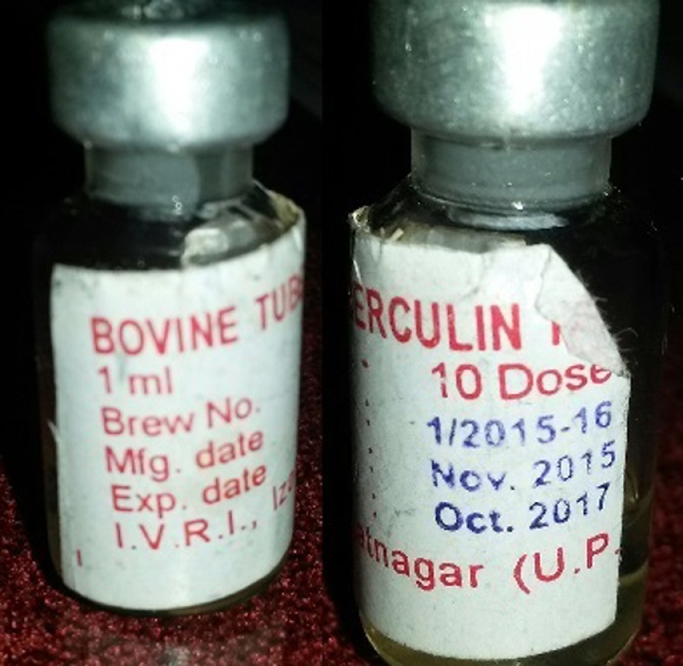Abstract
The close association of cattle/buffaloes to farmers and their family members is a well-known phenomenon in rural India. Cattle are major contributors to the income for the sustenance of families, and for many landless farmers, often the only source of livelihood. The animals are sheltered under the same roof where the family members sleep, cook and eat. This close proximity of humans to cattle/buffaloes exposes farmers and family members to tuberculosis (TB), especially if they are vulnerable (e.g. malnourished children along with adults, diabetics, people with HIV/AIDS, people with addiction to alcohol, or smokers) . Several studies have examined the risk factors that are associated with TB, such as crowding (slum dwellers), poor living conditions, alcohol, and tobacco . Other studies have found a significant proportion of diabetic patients with coexistent TB. This study examines the spread of TB from animals to humans and its public health significance. To achieve this, selected numbers of cattle/buffaloes were tested for TB and the association with humans and animals was examined.
Keywords: Tuberculosis, Zoonotic transmission, Tuberculin antigen, Animal positive
Introduction
Tuberculosis in humans is a public health problem; India has the highest burden of TB in the world. The World Health Organization (WHO) TB statistics for India for 2015 mentions an estimated incidence of 2.2 million cases out of a global incidence of 9.6 million. The estimated TB prevalence in 2015 was 2.5 million. It is estimated that about 40% of the Indian population is infected with TB; the vast majority of whom have latent TB rather than active TB disease [1]. Antimicrobial drug resistance along with HIV/AIDS have added further challenges to this public health problem. In 1997, the Government of India initiated the Revised National Tuberculosis Control Programme (RNTPC), which focuses mainly on early detection and initiation of treatment [2] with an objective of controlling the spread rather than eradication.
In 2015, there were an estimated 149,000 new human cases of zoonotic (bovine) TB globally, with 13,400 deaths due to zoonotic TB. Africa carries the heaviest burden of disease and death due to zoonotic TB, followed by South-East Asia. The true burden of zoonotic TB is likely to be underestimated due to a lack of routine surveillance data from most countries [3]. Many countries lack effective data collection and reporting mechanisms; this is an urgent need to address the problem of zoonotic TB and needs to be put in place with information technology methods.
Bovine TB is not as major a public health problem in the United States and western European countries, where historically (around the year 1950) a different strategy has been adopted that includes mass tuberculin testing on animals and isolation or culling of the herd if an animal is found positive. This approach has been largely neglected in Asian and African countries, which may explain why bovine TB still continues to be a major cause of morbidity and mortality.
This fieldbased cross-sectional study examines the spread of TB to humans from cattle/buffaloes (herein after referred to as ‘cattle’). The study was planned and implemented by a team of veterinarians, medical doctors and a statistician.
World Bank and local state government facts and figures
In 2015, the incidence of human TB in India was 217 per 100,000 people [4]. The incidence of human TB in Belgaum District (Karnataka state) was 78 per 100,000 people in the year 2016 [5]. The prevalence of human TB in Belgaum District was 90 per 100,000 people in the year 2016 [6]. According to the 2011 census of the Karnataka government, the total human population in the five villages where this study was conducted was 13,085; the total cattle population per the 2016 census was 5301 [7].
Methods
This study was done in selected five villages that were selected based on the information gathered through a questionnaire. Animals were selected from households having any one or more the following: history of TB, diabetes, raw-milk consumption, and chronic respiratory diseases. The primary information about the history of TB illness and other respiratory illnesses was collected from the local government health workers. Based on this information, 27 to 48 animals in each village were screened for TB by injecting tuberculin antigen (Tables A and B).
Table A.
Animal tested and positive tuberculin according to villages in the study area.
| Sl No | Name of village | Number of animals tested | Number of positive (+ve) animals for Tuberculin testing |
|---|---|---|---|
| 1 | Budhihal SH | Total = 44, | 1 buffalo +ve. (The farmer has 2 buffalos, 1 cow tested & 1 buffalo is +ve) |
| Buffalo = 14, | |||
| Others = 30. | |||
| 2 | Avaradi | Total = 41, | 2 buffalos +ve. (The first farmer has 3 buffalos tested & 1 buffalo is +ve) (The second farmer has 1 buffalo, 1 calf tested & 1 buffalo is +ve). |
| Buffalo = 19, | |||
| Others = 22 | |||
| 3 | Kendur | Total = 48, | 3 buffalos +ve. (The first farmer has 3 buffalos tested & 1buffalo is +ve) (The second farmer has 2 buffalos tested & 1 buffalo is +ve) (The third farmer has 2 cows & 1 buffalo tested & 1 buffalo is +ve) |
| Buffalo = 19, | |||
| Others = 29. | |||
| 4 | Baragi | Total = 43 , | 3 buffalos +ve (The first farmer has 4 buffalos, 3 cows tested & 2 buffalos are +ve). (The second farmer has 2 buffalos tested & 1 buffalo is +ve) |
| Buffalo = 26 | |||
| others = 17. | |||
| 5 | Katharaki | Total = 27, | 3 buffalos +ve. (The first farmer has 3 buffalos tested and 1 buffalo is +ve). (The second farmer has 2 buffalos tested & 1 buffalo is +ve) (The third farmer has 1 buffalo & is +ve) |
| Buffalo = 21, | |||
| Others = 6. | |||
| TOTAL | 203 | 12 |
Table B.
Number of human population examined in each village from families where the animal was +ve along with detected human +ve.
| Sl No | Name of the village | Number of subjects clinically examined | Clinically detected TB |
|---|---|---|---|
| 1 | Budhihal HS | 5 | Nil |
| 2 | Avaradi | 25 | 2 |
| 3 | Kendur | 18 | Nil |
| 4 | Baragi | 15 | 2 |
| 5 | Katharki | 14 | 1 |
| TOTAL | 77 | 5 |
While doing Tuberculin testing on animals, it was found that this was not a routine procedure and so the study team faced hurdles while testing in the field. This reflects at least in the Indian context the neglect of detecting TB in animals by using Tuberculin testing and so a brief outline of the experiences is shared below.
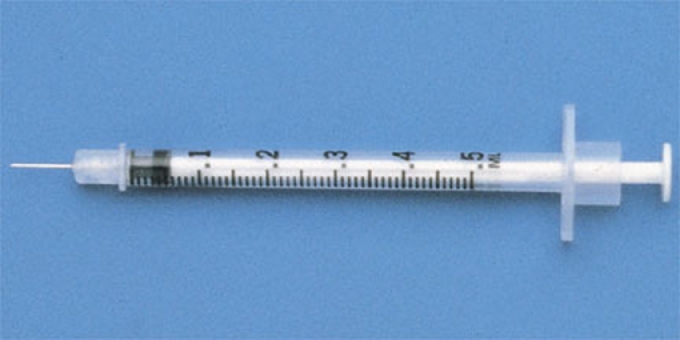
Tuberculin antigen (Picture-1) was procured from IVRI, Izzatanagar [8]; training was given to the local veterinary technicians by qualified and trained veterinarian team members before initiating testing. Tuberculin testing was performed on animals after shaving the area on the right side of the neck, throughout to keep uniformity (Picture-2). A digital Vernier Caliper (Picture-3) was used to measure the thickness of the skin before injecting the 0.1 ml of the tuberculin antigen intradermally with a Tuberculin syringe (Picture-4). During the procedure, animals were restrained by being held forcibly by the cattle owner or at times tied down (Picture-5). The reading of the skin test was done 72 hours after injecting the antigen; increase in the thickness of skin of more than 5 mm measured by using Vernier Caliper or induration or eryhthematous or tenderness changes by palpating was evaluated (Picture 6 and video). Informed consent was sought from the owners of the cattles before the procedure was undertaken.
Household members of the Tuberculin positive cattle that tested tuberculin-positive and an equal number of household members with cattle that tested tuberculin-negative cattle were examined by two senior medical doctors with clinical experience of more than three decades for evidence of pulmonary and extra pulmonary tuberculosis. A proper ethical committee was set up and consent was taken from the head of the family in a form (in regional language) after duly explaining its contents and the need for clinical examination. Every effort was made to educate the family regarding the public health issues of TB. Towards this, a one page write up in the regional language was done and was distributed to each family and the public.
Chi square test was used to compare the qualitative variables in this study. The differences with p-values less than 0.05 were considered significant. A Z-test was used to evaluate the relationship between tuberculin status in the tested cattle and TB in the households associated with them [9], [10].
Results
Two-hundred-three animals were tested; 12 were positive (Tables A and B). These 12 tuberculin-positive animals were associated with households consisting of 77 individuals. Evaluation of these 77 individuals resulted in identification of 5 TB cases (Table C), 2 of which had been treated and cured, one individual was on treatment for active TB, and 2 were fresh cases.
Table C.
Clinical details of tuberculosis patients.
| Sl no. | Name of the village where the TB patient lives | Smear for AFB | HIV/AIDS status | Consumption of raw milk | Clinical impression and other related investigations | Date of the investigation |
|---|---|---|---|---|---|---|
| 1 | Katharki | Positive, 4+. | -ve | No history | ESR 92/hr. X-ray done shows scattered diffuse infiltrations. Patient is on anti TB medicines (Rifampicin+ INH +Ethambutol +Pyrazinamide+Levofloxasin since last one month). Before that RNTPC treatment for two months. | 19th July 2017 |
| 2 | Avaradi | Not done | Not done | No history | Patient was treated with anti TB medicines for four months when the patient was 9 months and had clinical respiratory symptoms. Completed the treatment and since then no health problem. X-ray done normal. Haemogram done and shows low Hb (10.3%) otherwise normal. | 26th October 2015 |
| 3 | Avaradi | Not done – scanty sputum. | -ve | No history | ESR 50 mm/hr. X-ray shows bilateral homogenous opacity in lower lobes. Needs follow up. | 18th August 2017. |
| 4 | Bargi | Positive | -ve | Consumed raw milk during childhood. | Has been treated with anti TB medicines. X-ray shows non-homogenous streaky opacity in left lung, apex and infraclavicular region suggests old pulmonary Koch on 14th July 2016. 28th January 2010 dated CT scan thorax suggestive of pulmonary Koch's with endobronchial spread. | 28th January 2010. & 14th July 2016. |
| 5 | Bargi | Negative | -ve | Same as above | X-ray shows linear opacity in right upper zone, likely sequel to previous infection/ Koch's. Prominent Broncho Vesicular Markings (BVM) suggests bronchitis. Haemogram shows Hb is low (9%) & ESR is 20 mm/1st hour. There is strong contact history with human and animals. Needs follow up. | 17th August 2017 |
Chi - Square test result did not indicate a significant association between the villages and the prevalence of TB. However, there was a significant association between tuberculin status of the tested cattle and human TB in the households associated with these cattle (calculated ‘Z – value’ was 142.076) (Table D).
Table D.
Z TEST.
| n1 = 77, P1, q1 = 0.935 |
| n2 = 77, P2 = , q2 = 0.987 |
| = 142.076 |
An equal number of individuals (77) in households with animals that were tuberculin negative were also clinically examined for evidence of TB. In this group, 1 case of treated pulmonary TB was identified.
Discussion
This study, done in the five villages’ with limited resources, highlights the importance of the spread of TB from animals to humans. The study also brings forth several other dimensions that largely have remained unanswered such as whether the cattle got their infection from the people. There could also be a third dimension, such as poverty and under-nutrition. People who are poor have less food not only for themselves but also for their cattle. This may make TB more common in both people and the cattle without there being any transmission between them. Other factors like chronic smoking, alcoholism and poor housing may all be contributing factors. All these dimensions will need further investigation, which is beyond the scope of this particular study.
The spread of TB from animals to human or vice-verse has not been of much debate and research in the context of India and other developing countries. Some investigations have pointed out the risk of human infection through unpasteurised consumption of milk or using raw milk for producing cream and butter [11]. But the inhalation of airborne droplets containing mycobacteria from animals with pulmonary TB, especially in crowded and less ventilated settings, can be the most likely route of transmission from animals to man and vice-verse [12].
The disease surveillance programs in humans, especially in areas where risk factors are prevalent, need to be modified. Zoonotic TB represents a significant risk in rural communities and areas where domestic animals and humans share a common environment. The situation is critical in developing countries, as tuberculosis caused by M. bovis has been reported in patients with HIV [13].
Zoonotic TB is present in animals in most developing countries where surveillance and control activities are often inadequate or unavailable. Therefore, many epidemiologic and public health aspects of infection remain largely unknown. Ninety-four percent of the human population lives in countries where cattle and buffaloes undergo no control or only limited control for bovine TB. Within Asia, seven countries apply disease control measures as part of a test-and-slaughter policy and consider bovine TB notifiable. In the remaining 29 countries, bovine TB is partly controlled or not controlled at all [14].
On the contrary the situation in developed countries like most western European countries and the USA is rather different. For example the UK government's efforts are worth examining
England is set to apply for Officially TB-Free (OTF) status for more than half of the country next year – two years ahead of schedule – as the government's strategy to tackle bovine TB continue to deliver results. Dealing with bovine TB in England costs taxpayers over £100 million a year, required the culling of 28,000 cattle in 2015, and causes devastation and distress for rural communities. Gaining OTF status for the low risk area, covering the north and east of England, would boost trade opportunities. Herds will also require less regular testing, reducing costs for farmers. Achieving this status for the low risk area is a key step in the government's 25-year plan for the whole of the UK to be TB-free by 2038 [15].
The German government has a different strategy. From July 1st 1996, Germany was officially declared free from bovine tuberculosis, pursuant to EU Decision 97/76/EC whichreplaced EU Decision 99/467/EC. To maintain this status, at least 99.9% of cattle holdings must be officially free from tuberculosis in the respective year. Based on this disease-free status, routine nationwide tuberculin testing of cattle for the presence of tuberculosis was stopped. Pursuant to these regulations, control is mainly based on the diagnosis of clinical symptoms, official meat inspection and post mortem examination of dead animals by veterinary service laboratories and tuberculin testing in certain areas [16].
In France, the bovine tuberculosis eradication efforts commenced in 1950 when 25% of cattle herds were infected. In 2000, the operation of the program was granted the OTF status, a highly favourable situation to ensure trading. In recent years, France has become the most important live animal exporting country in the European Union and one of the most important in the world [17].
The United States Department of Agriculture, state animal health agencies, and livestock producers, have nearly eliminated bovine TB from cattle in the United States. However, bovine TB can still be found in wild animals such as bison, elk, and deer; uninfected cattle that come in-contact with these wild animals can become infected. Cattle outside the United States, particularly in developing countries, might not have the same level of inspection for M.bovis infection [18] (Figs. 1–6).
Fig. 2.
Quadrangular skin area shaved prior to injecting bovine tuberculin antigen.
Fig. 3.
Vernier calliper used for measuring the skin thickness before and after injecting bovine tuberculin antigen.
Fig. 4.
Tuberculin syringe for injecting 0.1 ml of bovine tuberculin antigen intradermally.
Fig. 5.
While injecting the bovine tuberculin antigen the animals were restrained by being held forcibly by the cattle owner or at times tied down.
Fig. 1.
Ampoule of bovine tuberculin antigen containing 10 doses.
Fig. 6.

Measuring skin thickness using the vernier calliper.
Recommendations for eradication
-
1)
More Studies: There is a need for the involvement of donor agencies to focus on field-based studies and studies regarding ‘zoonotic transmission’ of TB. This is to highlight that more studies with more parameters studies need to be undertaken; civil societies need to be actively engaged.
-
2)
Involve Agriculture Ministry: The government of India under the Ministry of Agriculture and Farmers Welfare and the department of animal husbandry, dairying & fisheries (http://dahd.nic.in/) need to be sensitized on this issue of zoonotic TB.
-
3)
Involve Health Ministry: The Government of India's Ministry of Health & Family welfare's Indian Council of Medical Research (ICMR http://www.icmr.nic.in/) the apex body in India for the formulation, coordination and promotion of biomedical research needs to sensitized on this important issue of zoonotic TB.
-
4)
WHO role: The World Health Organization (WHO http://www.who.int/en/) should come out with guidelines for countries that are seeking to act on zoonotic TB.
-
5)
Meat sellers: Suspected tuberculosis tissues in butcher shops should undergo histological and serological examinations to confirm tuberculosis. For this to take place, there is a need to train butchers to identify such tissues and send them to proper laboratories.
-
6)
For vet graduates: Zoonotic TB should be part of the training of veterinary undergraduates. Moreover, veterinary research and post-graduate training institutions need to examine this in detail from an academic angle.
-
7)
For Medical Graduates: Zoonotic TB should also be taught to medical undergraduates in and researchers in infectious disease should consider involvement in zoonotic TB-related research.
-
8)
Milk Federations: Farmers milk federations and cooperatives need to advocate for tuberculin testing and proper training to all field-level staff.
-
9)
All rural and public sector banks should make it mandatory for prior tuberculin testing before giving loan for purchase of cows/buffaloes.
-
10)
The farmers’ community in India need to be made aware of not only zoonotic TB that can transmit to humans and vice-versa but also the economic loss that occurs as a result of TB in animals.
Conclusions
The study shows that there is evidence that the struggle against TB in India needs to take into account the very nature of TB as a zoonotic disease. A more holistic view is necessary in order to address TB from a public health angle. TB needs a different strategy if it has to be addressed from a public health angle in India. Mere detection and treatment as is being done now is not enough as has been the experience so far. There is an urgent need to introduce strict surveillance strategy to reduce and finally eradicate TB. This cross section study with limited resources highlights the need for further research in this neglected area. This is the most important outcome of this study as there are many areas that this study could not examine because of lack of technological inputs, resources and others.
Though isolation or culling of tuberculin positive cattle is the ideal solution, other restrictions such as on-the-spot inspection of butchers shops by trained veterinarians or consideration of tuberculin-testing as an eligibility criterion for banks loans may contribute to controlling zoonotic TB.
Acknowledgments
We would like to acknowledge with thanks the financial support by ALRA (Austrian Leprosy Relief Association-http://www.aussaetzigen-hilfswerk.at/) towards this project. Thanks to Future Greens at Bagalkot (http://www.futuregreens.co.in/) who helped us with the contacts in the villages and other field based supports. Thanks to Jagruti at Dharwad (www.jagruti.org) for helping us in maintaining our accounts and books.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jctube.2017.10.002.
Appendix. Supplementary materials
Measuring skin thickness by using vernier calliper 72 hours after injecting bovine tuberculin antigen in a positive animals.
References
- 1.TB Statistics for India . 2017. National & state statistics.http://www.tbfacts.org/tbstatistics-india/. Accessed on 29th March. [Google Scholar]
- 2.Revised National Tuberculosis Control Programme (RNTCP) 2017. Guidelines for TB control in India.http://www.searo.who.int/india/tuberculosis/topic/tb_rntcpguidelines/en/. Accessed on 29th March. [Google Scholar]
- 3.Zoonotic tuberculosis . 2017. World Health Organization (WHO)http://www.who.int/tb/zoonoticTB.pdf. Accessed on 29th March. [Google Scholar]
- 4.The World Bank . 2017. Incidence of tuberculosis.http://data.worldbank.org/indicator/SH.TBS.INCD. Accessed on 29th March. [Google Scholar]
- 5.Oral communications information from district tuberculosis officer (DTO) of Belgaum district, Karnataka state.2017.
- 6.Oral communication information from district tuberculosis officer (DTO) of Belgaum district, Karnataka state 2017.
- 7.References of the villages:-(a) Budhihal HS:- http://villageinfo.in/karnataka/bagalkot/bilgi/budihal.html: (b) Avaradi - http://www.census2011.co.in/data/village/598398-awaradi-karnataka.html (c) Baragi - http://www.census2011.co.in/data/village/598585-baragi-karnataka.html (d) Kendur;- http://www.census2011.co.in/data/village/598738-kendur-karnataka.html(e) Katharaki;- http://www.census2011.co.in/data/village/598512-katarki-karnataka.html. 2017.
- 8.Indian Veterinary Research Institute (IVRI), Izatnagar, Bareilly 243122, Uttar Pradesh, India. http://ivri.nic.in/. Accessed on 29th March 2017.
- 9.Vishweshwara Rao K. 1st ed. 2010. Biostatistics in brief made easy. (reference book)Page numbers 113 to 115 and 118 to 121. [Google Scholar]
- 10.Prabhakara GN, ‘Biostatistics’(reference book), 3rd ed., Chapter 6, page 53-73, published 2008.
- 11.Srivastava K., Chauhan DS, Gupta P. Isolation of Mycobacterium bovis&M.tuberculosis from cattle of some farms in north India – Possible relevance in human health. Ind J Med Res. 2017;128:28–31. http://icmr.nic.in/ijmr/2008/july/0706.pdf. July 2008. Accessed on 29th March. [PubMed] [Google Scholar]
- 12.Prasad HK, Singhal A., Mishra A., Singh N.P. Bovine tuberculosis in India: potential basis for zoonosis. Tuberculosis. 2017 doi: 10.1016/j.tube.2005.08.005. September 2005. http://www.researchgate.net/publication/7512493_Bovine_tuberculosis_in_India_Pot ential_basis_for_zoonosisAccessed on 29th March. [DOI] [PubMed] [Google Scholar]
- 13.Challu V.K. Zoonotic importance of tuberculosis. NTI Bull. 2007;43(3&4):37–40. https://ntiindia.kar.nic.in/ntibulletin/NTI%20BULLETIN%2020062011/NTI%20BULLETIN%20Vol%2043_3_4_2007/pages/pdf/Zoonotic%20Importa nce%20of%20tuberculosis.pdf. Accessed on 29th March 2017. [Google Scholar]
- 14.Cosivi O., Grange JM, Daborn CJ. Zoonotic tuberculosis due to mycobacterium bovis in developing countries. emerging infectious diseases. Centres Disease Control Prevent. 2017;4(1) doi: 10.3201/eid0401.980108. https://wwwnc.cdc.gov/eid/article/4/1/98-0108_article. March 1998. Accessed on 29th March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GOV.UK . 2017. TB strategy ahead of schedule as England set to apply for officially TBfree status for half the country.https://www.gov.uk/government/news/tb-strategyahead-of-schedule-as-england-set-to-apply-for-officially-tb-free-status-for-half-thecountry. Accessed on 29th March. [Google Scholar]
- 16.National Veterinary Reference Laboratory for Bovine Tuberculosis (Mycobacterium bovis and Mycobacterium caprae) Federal Research Institute for Animal Health; 2017. Institute of Molecular Pathogenesis (IPH), Friedrich-Loeffler-Institute (FLI)https://www.fli.de/en/institutes/institute-of-molecular-pathogenesis-imp/referencelaboratories/nrl-for-bovine-tuberculosis/. Accessed on 29th March. [Google Scholar]
- 17.Boschiroli ML, Benet JJ. Bovine tuberculosis eradication in France. In: Thoen C.O., Steele JH, Kaneene JB, editors. Zoonotic tuberculosis: mycobacterium bovis and other pathogenic mycobacteria. 3rd ed. John Wiley & Sons, Inc; Chichester, UK: 2014. http://onlinelibrary.wiley.com/doi/10.1002/9781118474310.ch29/summary. Accessed on 29thMarch 2017. [Google Scholar]
- 18.Mycobacterium bovis (Bovine Tuberculosis) in Humans . 2017. CDC.https://www.cdc.gov/tb/publications/factsheets/general/mbovis.pdf. Accessed on 29th March. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Measuring skin thickness by using vernier calliper 72 hours after injecting bovine tuberculin antigen in a positive animals.