Abstract
Granulomatous inflammation is a histologic pattern of tissue reaction which appears following cell injury. Granulomatous inflammation is caused by a variety of conditions including infection, autoimmune, toxic, allergic, drug, and neoplastic conditions. The tissue reaction pattern narrows the pathologic and clinical differential diagnosis and subsequent clinical management. Common reaction patterns include necrotizing granulomas, non necrotizing granulomas, suppurative granulomas, diffuse granulomatous inflammation, and foreign body giant cell reaction. Prototypical examples of necrotizing granulomas are seen with mycobacterial infections and non-necrotizing granulomas with sarcoidosis. However, broad differential diagnoses exist within each category. Using a pattern based algorithmic approach, identification of the etiology becomes apparent when taken with clinical context.
The pulmonary system is one of the most commonly affected sites to encounter granulomatous inflammation. Infectious causes of granuloma are most prevalent with mycobacteria and dimorphic fungi leading the differential diagnoses. Unlike the lung, skin can be affected by several routes, including direct inoculation, endogenous sources, and hematogenous spread. This broad basis of involvement introduces a variety of infectious agents, which can present as necrotizing or non-necrotizing granulomatous inflammation. Non-infectious etiologies require a thorough clinicopathologic review to narrow the scope of the pathogenesis which include: foreign body reaction, autoimmune, neoplastic, and drug related etiologies. Granulomatous inflammation of the kidney, often referred to as granulomatous interstitial nephritis (GIN) is unlike organ systems such as the skin or lungs. The differential diagnosis of GIN is more frequently due to drugs and sarcoidosis as compared to infections (fungal and mycobacterial).
Herein we discuss the pathogenesis and histologic patterns seen in a variety of organ systems and clinical conditions.
Keywords: Foreign-body, Granulomatous inflammation, Granuloma, Mycobacterial, Sarcoidal, Tuberculous
1. Introduction
Granulomatous inflammation is a distinctive form of chronic inflammation produced in response to various infectious, autoimmune, toxic, allergic, and neoplastic conditions (Table 1). It is defined by the presence of mononuclear leukocytes, specifically histiocytes (macrophages), which respond to various chemical mediators of cell injury. This pattern of injury response occurs in all age groups and within all tissue sites. Through light microscopy, the activated histiocytes appear as epithelioid cells with round to oval nuclei, often with irregular contours and abundant granular eosinophilic cytoplasm with indistinct cell borders (Fig. 1). They may also coalesce to form multinucleated giant cells. Identification and classification of the granulomatous inflammation pattern can be helpful in narrowing a clinical differential diagnosis. In a study of pulmonary granulomas, 23% of diagnoses could not identify the specific etiology via hematoxylin and eosin (H&E) at the time of biopsy. In this series, etiology identification improved to 90.8% with clinical features, radiographic findings, and improved laboratory methodologies, including molecular techniques, culture, immunohistochemical profiles, and serologic values [1].
Table 1.
Patterns of granulomatous inflammation and commonly associated etiologies.
| Pattern of Inflammation | Associated Etiology |
|---|---|
| Foreign Body | Talc, starch, suture, hyaluronic acid (and other injectable fillers) |
| Necrotizing Granulomas |
Infectious:Coccidioides immitis/C. posadasii, Cryptococcus neoformans/C. gattii, Histoplasma capsulatum, Blastomyces dermatitidis, Aspergillus spp., Mucorales, Mycobacterium tuberculosis, Non-tuberculous mycobacteria, Brucella spp., Nocardia spp., Yersinia spp., Bartonella henselae, Pneumocystis jiroveci, Echinococcus granulosus, xanthogranulomatous pyelonephritis+ |
| Autoimmune: Rheumatoid nodule, granuloma annulare, necrobiosis lipoidica, granulomatosis with polyangiitis | |
| Non-Necrotizing Granulomas |
Infectious*:Candida albicans (hepatosplenic candidiasis), C. immitis/C. posadasii, Coxiella burnetii, cytomegalovirus, M. tuberculosis, non-tuberculous mycobacteria including M. leprae (tuberculoid forms), Schistosoma spp., Toxoplasma gondii, Rickettsia spp., Salmonella typhi, hepatitis A & C virii, Autoimmune: Sarcoidosis, Churg Strauss, giant cell arteritis, systemic lupus erythematous, Crohn disease, primary biliary cirrhosis, orofacial granulomatosis, rosacea, granuloma annulare Toxic: actinic granuloma, berylliosis, zirconium, hot tub lung Drug: Bacillus Calmette-Guérin, Non-steroidal anti-inflammatory drugs, antibiotics, methotrexate Other: Lymphoid interstitial pneumonia, hypersensitivity pneumonitis, chronic lymphocytic leukemia |
| Suppurative Granulomas | Infectious: Actinomyces spp., Dirofilaria spp., Acanthamoeba spp., Balamuthia mandrillaris, B. henselae, B. dermatitidis, Brucella spp., Chlamydia trachomatis (serotypes L1, L2, L3 causing lymphogranuloma venereum), dematiaceous fungi causing chromoblastomycoses and phaeohyphomycosis, non-tuberculous mycobacteria, Francisella tularensis, Prototheca spp., Sporothrix schenckii, Paracoccidioides brasiliensis, Yersinia spp., Enterobius vermicularis |
| Histiocytic response, no granulomas |
Infectious: Tropheryma whipplei, Listeria monocytogenes, non-tuberculous mycobacteria including M. leprae (lepromatous forms), H. capsulatum, Leishmania spp., Rhodococcus spp. (with malakoplakia) Other: Langerhans cell histiocytosis, granulomatous mycosis fungoides, juvenile xanthogranuloma, reticulohistiocytoma, Rosai Dorfman, pineal germinoma, seminoma/dysgerminoma, dendritic cell sarcoma, Erdheim-Chester disease, hemophagocytic lymphohistiocytosis, histiocytic sarcoma, interdigitating cell sarcoma, Langerhans cell sarcoma |
*Entities may appear as well formed granulomas or histiocytic response.+Can present as necrotizing or non-necrotizing.
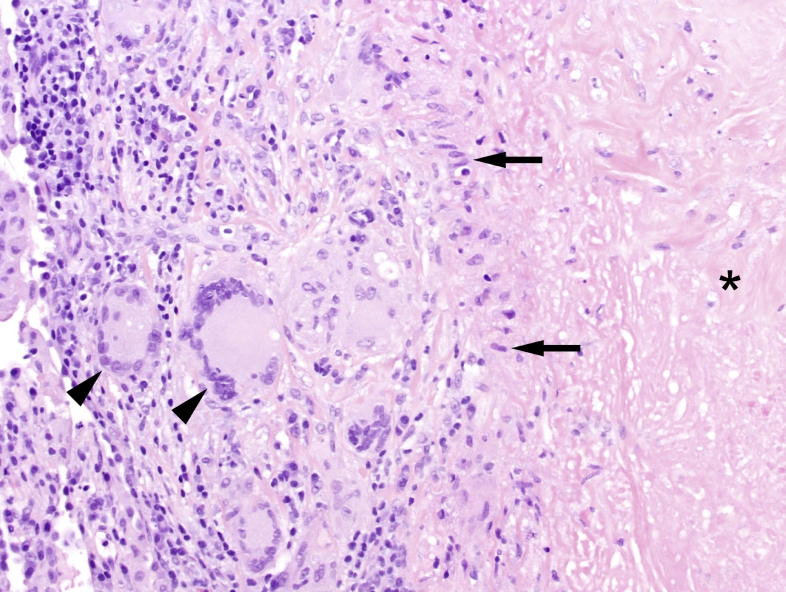
Fig. 1.
Edge of a necrotizing granuloma seen in mycobacterial tuberculosis showing a peripheral rim of epithelioid histiocytes (arrows) surrounding the central necrotic region (asterisk; H&E, 200x). Some histiocytes are also forming multinucleated giant cells (arrow heads). External to the rim of histiocytes is an outer rim of lymphocytes and plasma cells.
1.1. Pathogenesis
The origin of the epithelioid histiocytes begins within the bone marrow as myeloid precursors mature into monocytes, which enlarge and enter peripheral circulation. When recruited into tissues, mature monocytes are renamed as histiocytes. The activation of histiocytes, via the innate immune response, gives the cells their characteristic epithelioid appearance (Fig. 1) as compared to the inactivated, reniform histiocytes with distinct cell borders. As histiocytes are unable to efficiently phagocytize the foreign agent; dendritic cells (antigen presenting cells) and major histocompatibility complex II orchestrate a combined innate and Th1-dominant adaptive immune response. Physiologic activation of histiocytes occurs within 24–48 hours of an injury via activation of complement (C3b, C5a), helper T cells (Th1) which release chemokines, and cytokines (TNF, IL-1, IL-6, IL-17, and IFN gamma) to promote, recruit, and direct macrophages to the site of injury [2], [3], [4]. Activated histiocytes persist beyond the acute phase and dominate the chronic inflammatory response and subsequent tissue repair. This persistence into the chronic phase supports the hypothesis that two types of histiocytes exist, (1) to balance the inflammatory effect and promote phagocytosis, and (2) aid in wound repair, fibrosis, and angiogenesis [5].
1.2. Histologic subtypes
Granulomatous inflammation is commonly characterized by the formation of distinct granulomas composed of aggregates of epithelioid histiocytes, with a peripheral cuff of lymphocytes and plasma cells, and occasionally a necrotic center (Fig. 1). However, the term “granulomatous inflammation” encompasses a spectrum of findings, ranging from well-formed granulomas to loose collections of histiocytes admixed with other inflammatory cells. The latter is employed by pathologists to describe the chronic injury and healing response, histologically described as an admixed lymphohistiocytic inflammation with edema, neovascularization, and early stage fibrosis. This loose form of inflammation is a non-specific finding, whereas defined granulomas offer potential diagnostic etiologies (Table 1). In addition, granulomatous entities can be subdivided by commonly affected organ system such as the lung, skin, kidney, liver, and lymph node (Table 2).
Table 2.
Granulomatous inflammation organized by commonly affected organ system.
| Lung | Skin | Kidney | Liver | Lymph Node |
|---|---|---|---|---|
| Infectious | Infectious | Infectious | Infectious | Infectious |
| Bacterial | Bacterial | Bacterial | Bacterial | Bacterial |
| Actinomyces spp. | Actinomyces spp. | Bartonella henselae | Actinomyces spp. | Actinomyces spp. |
| Brucella spp. | Bartonella henselae | Brucella spp. | Bartonella henselae | Bartonella henselae |
| Francisella tularensis | Chlamydia trachomatis (L1, L2, L3 serovars) | Malakoplakia (various bacteria) | Brucella spp. | Brucella spp. |
| Mycobacterium tuberculosis | Malakoplakia (various bacteria) | Mycobacterium tuberculosis | Chlamydia trachomatis (L1, L2, L3 serovars) | Chlamydia trachomatis (L1, L2, L3 serovars) |
| Mycoplasma pneumonia | Mycobacterium tuberculosis | Non-tuberculous mycobacteria | Coxiella burnetii | Coxiella burnetii |
| Nocardia spp. | Non-tuberculous mycobacteria | Xanthogranulomatous pyelonephritis | Francisella tularensis | Francisella tularensis |
| Non-tuberculous mycobacteria | Fungal | Fungal | Listeria monocytogenes | Listeria monocytogenes |
| Rhodococcus equi (malakoplakia) | Aspergillus spp. | Aspergillus spp. | Malakoplakia (various bacteria) | Malakoplakia (various bacteria) |
| Fungal | Blastomyces dermatitidis | Blastomyces dermatitidis | Mycobacterium tuberculosis | Mycobacterium tuberculosis |
| Aspergillus spp. | Coccidioides spp. | Coccidioides spp. | Non-tuberculous mycobacteria | Non-tuberculous mycobacteria |
| Blastomyces dermatitidis | Cryptococcus spp. | Histoplasma capsulatum | Rickettsia spp. | Yersinia granulomatosis |
| Coccidioides spp. | Dematiaceous fungi causing chromoblastomycosis | Mucorales | Salmonella typhi | Fungal |
| Cryptococcus spp. | Histoplasma capsulatum | Non-Infectious | Yersinia spp. | Aspergillus spp. |
| Histoplasma capsulatum | Mucorales | Autoimmune | Fungal | Blastomyces dermatitidis |
| Mucorales | Sporothrix shenckii | Crohn Disease | Aspergillus spp. | Coccidioides spp. |
| Viral and Parasitic | Viral and Parasitic | Granulomatosis with polyangiitis | Blastomyces dermatitidis | Cryptococcus spp. |
| Acanthamoeba spp. | Acanthamoeba spp. | Sarcoid | Candida spp. | Histoplasma capsulatum |
| Balamuthia mandrillaris | Balamuthia mandrillaris | Tubulointerstitial nephritis and uveitis (TINU) | Coccidioides spp. | Mucorales |
| Cytomegalovirus | Cytomegalovirus | Neoplastic | Histoplasma capsulatum | Sporothrix schenckii |
| Dirofilaria spp. | Dirofilaria spp. | Chronic lymphocytic leukemia | Mucorales | Viral and Parasitic |
| Toxoplasma gondii | Leishmania spp. | Other | Viral and Parasitic | Cytomegalovirus |
| Non-Infectious | Prototheca spp. | Chronic pyelonephritis | Cytomegalovirus | Epstein-Barr virus |
| Autoimmune | Non-Infectious | Drugs | Echinococcus spp. | Leishmania spp. |
| Churg Strauss | Autoimmune | Enterobius vermicularis | Toxoplasma gondii | |
| Granulomatosis with polyangiitis | Churg Strauss | Epstein-Barr virus | Non-Infectious | |
| Lymphoid interstitial pneumonia | Crohn disease | Hepatitis A | Autoimmune | |
| Sarcoid | Granuloma annulare | Hepatitis C | Churg Strauss | |
| Neoplastic | Granulomatosis with polyangiitis | Leishmania spp. | Granulomatosis with polyangiitis | |
| Hodgkin Lymphoma | Orofacial granulomatosis | Schistosoma spp. | Sarcoid | |
| Langerhans cell histiocytosis | Rheumatoid nodule | Toxoplasma gondii | Neoplastic | |
| Metastasis | Sarcoid | Non-Infectious | Dendritic cell sarcoma | |
| Rosai-Dorfman disease | Systemic lupus erythematosus | Autoimmune | Erdheim-Chester Disease | |
| Other | Neoplastic | Chronic granulomatous disease | Hemophagocytic lymphohistiocytosis | |
| Chronic granulomatous disease | Granulomatous mycosis fungoides | Crohn's disease | Histiocytic sarcoma | |
| Chronic pneumonia | Hodgkin lymphoma | Primary biliary cirrhosis | Hodgkin lymphoma | |
| Drugs | Juvenile xanthogranuloma | Sarcoid | Interdigitating cell sarcoma | |
| Foreign body reaction | Langerhans cell histiocytosis | Neoplastic | Langerhans cell histiocytosis | |
| Pneumoconioses | Metastasis | Hemophagocytic lymphohistiocytosis | Langerhans cell sarcoma | |
| Reticulohistiocytoma | Hodgkin lymphoma | Metastasis | ||
| Rosai-Dorfman disease | Metastasis | Rosai-Dorfman disease | ||
| Other | Rosai-Dorfman disease | Other | ||
| Actinic granuloma | Other | Foreign body reaction | ||
| Chronic granulomatous disease | Berylliosis | |||
| Drugs | Drugs | |||
| Foreign body reaction | Foreign body reaction | |||
| Necrobiosis lipoidica | ||||
| Rosacea |
Two broad forms of well-defined granuloma exist, defined by their etiology: foreign-body giant cell granulomas and immune granulomas. Foreign-body giant cells are histiocytic reactions to otherwise inert material without an adaptive immune response, for example, suture, talc, and food material. A collection of histiocytes surround the foreign material and as single histiocytes are unable to phagocytize the foreign material alone (Fig. 2). The foreign material can be visualized by light microscopy and often exhibits birefringence using polarized light. Immune granulomas are the result of a variety of etiologies (Table 1). Each etiology has an identifiable histologic appearance allowing pathologists to narrow the differential diagnosis. Immune granulomas are further histologically characterized as necrotizing or non-necrotizing “naked.” The term “caseous” refers to a type of necrotizing granulomas in which the central necrotic material has a “cheese-like” consistency (Fig. 3). Microscopically, necrotizing granulomas distinctly have central necrosis with a palisaded lymphohistiocytic reaction and a cuff of chronic inflammation (Fig. 1, Fig. 3, Fig. 4).
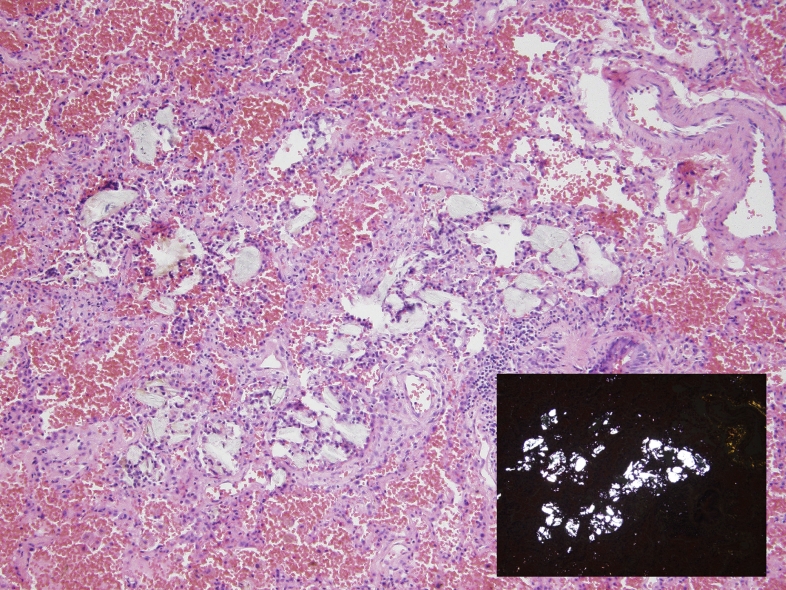
Fig. 2.
Foreign body giant cell reaction within the lung alveoli, with macrophages engulfing inhaled talc (H&E, 200x). The inset highlights refractile foreign material with plane polarizable light (H&E with polarized light, 200x).
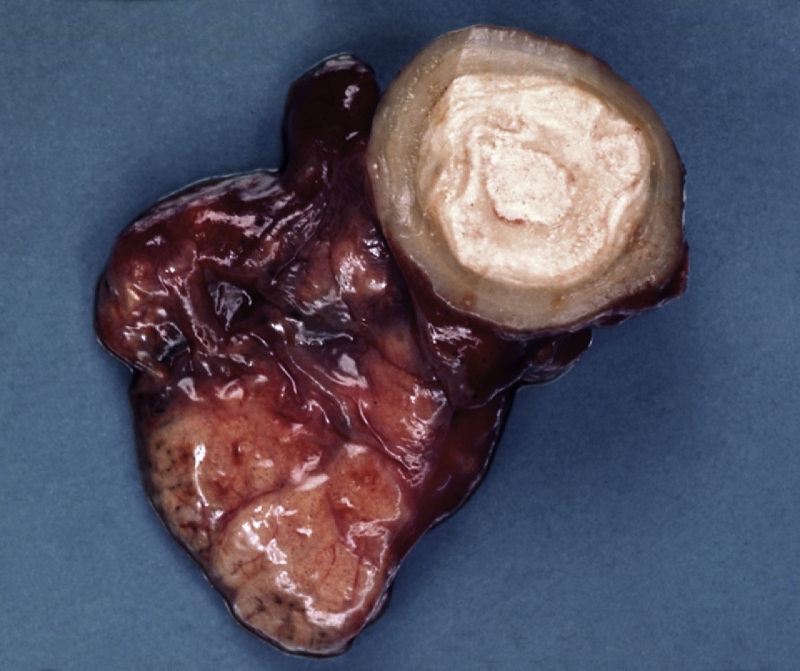
Fig. 3.
Laminated Coccidioides spp. granuloma (coccidioidoma) of the lung, showing a central necrotic focus.
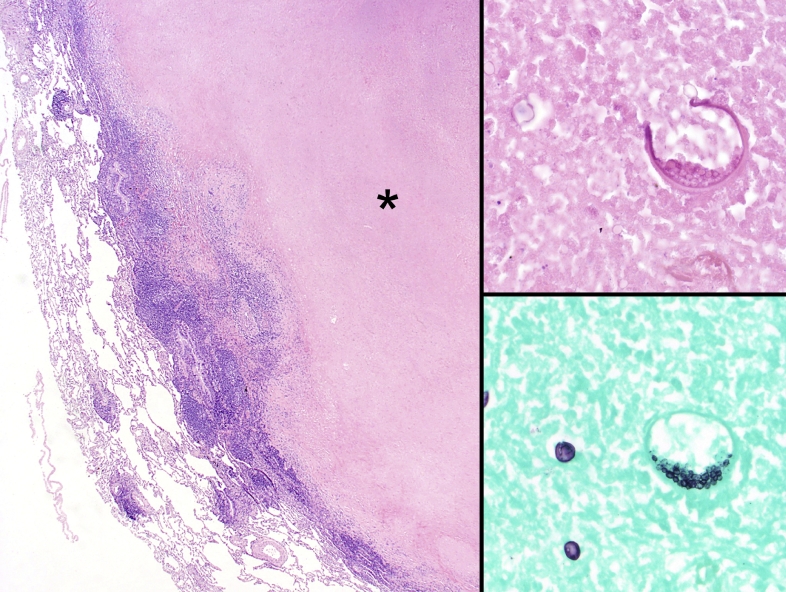
Fig. 4.
Edge of a necrotizing granuloma formed by Coccidioides immitis showing a large caseating necrotic center (asterisk) and outer rim of epithelioid histiocytes and lymphoplasmacytic inflammation (Left panel, H&E, 20x). On higher magnification (400x), a spherule containing endospores can be seen on H&E (upper right panel) and GMS (lower right panel). Note that the GMS also highlight maturing spherules to the left of the mature spherule.
Careful hematoxylin and eosin (H&E) or histochemical (IHC) evaluation can yield identifiable organisms within the necrosis or within histiocytes depending on the etiology. A prototypical example is Coccidioides species with organisms classically found within the necrotic center (Fig. 4). Non-necrotizing granulomas are characterized by the collection of epithelioid histiocytes and giant cells with minimal peripheral chronic inflammation; the prototypical example is sarcoidosis (Fig. 5).
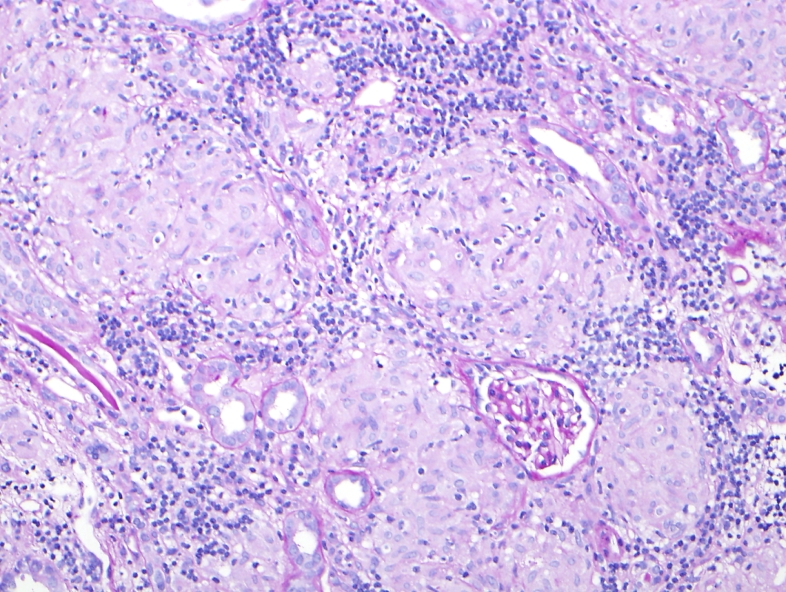
Fig. 5.
The renal parenchyma is replaced by numerous non-necrotizing granulomas in a patient with sarcoidosis (Periodic-acid Schiff, 200X).
1.3. Ancillary tests
1.3.1. Special stains
Once H&E sections have been carefully evaluated, special stains can be employed to improve diagnostic sensitivity. As mycobacterium and fungal organisms are the most common culprits for granulomas, stains directed at either organism are prioritized. Grocott methenamine silver (GMS) stain (Fig. 4) and Ziehl–Neelsen (AFB) are most commonly employed for the identification of fungi and acid fast bacilli. Periodic acid-Schiff (PAS) is sometimes preferred for fungal identification due to the reduced background staining artifact. Additionally, a modified acid fast stain (Fite or Fite-Faraco method) is employed for detection of partially-acid fast mycobacteria (M. leprae), Nocardia, and Rhodococcus species (Fig. 6). Another technique for acid fast organisms are the auramine or auramine-rhodamine fluorescence stains which have superior sensitivity to the aforementioned acid-fast bacillus stains, but require the use of fluorescence microscope [6]. It is important to note that all special stains (and H&E) require careful and thorough evaluation of the granulomas at various depths of the tissue block as organisms can be missed when reviewing single sections of tissue or using only low magnification (<400x). Definitive mycobacterial species identification requires culture or molecular methods since the mycobacterial species cannot be definitively differentiated by morphology alone.
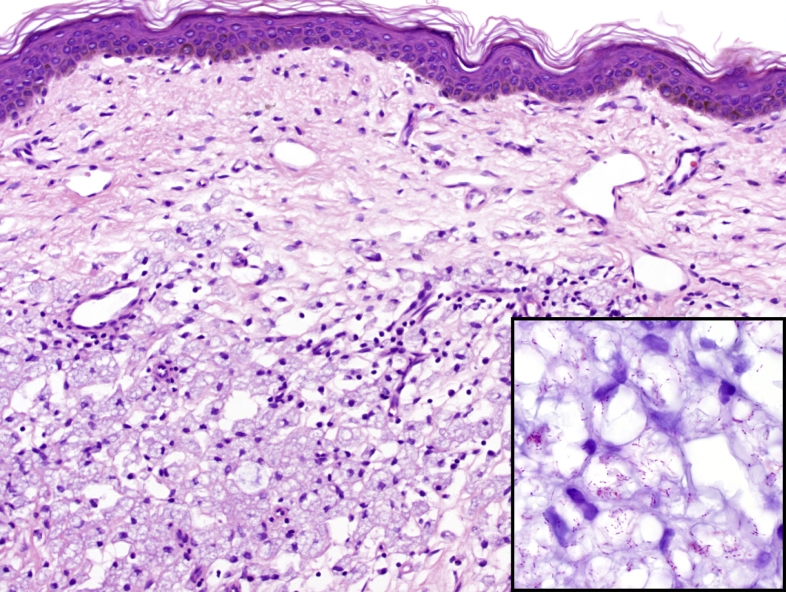
Fig. 6.
Skin biopsy in a patient with lepromatous leprosy showing a dermal infiltrate of histiocytes containing numerous bacilli of Mycobacterium leprae (H&E, 200x). The organisms are visible using a Fite stain which highlights partially acid fast organisms (inset, 1000x).
1.3.2. Molecular testing
Though culture has traditionally been the gold-standard for the diagnosis of infectious etiologies, molecular methods from culture growth or directly from the fresh or formalin-fixed specimen have vastly improved the specificity and identification of organisms, particularly mycobacteria [7]. Broad-range PCR and sequencing for bacteria and fungi are now commercially available and can be performed on both fresh and formalin-fixed, paraffin-embedded tissues, although fresh tissue provides greater diagnostic sensitivity. In addition to rapid identification through nucleic acid amplification methods, drug resistance screening may also possible through PCR identification of specific resistance mutations [7]. Despite these advances in diagnostics, culture is still required for the complete antimicrobial susceptibility testing of most organisms.
2. Etiology of granulomatous inflammation
The etiology of granulomatous inflammation is broad and expands many etiologies including infectious, autoimmune, toxic, allergic, and neoplastic entities (Table 1). Herein is a discussion of patterns of granulomatous inflammation within commonly affected organ systems: lung, skin and kidneys [8].
3. Necrotizing granuloma
3.1. Infectious etiologies of necrotizing granuloma
3.1.1. Mycobacteria spp.
Mycobacteria species are the most common etiologies of necrotizing granulomas worldwide. Tuberculosis and non-tuberculous mycobacterial infections have distinct clinical and histologic presentations, although their clinical and radiographic appearance may be confused with other entities, including malignancy [9]. As a result of overlapping clinical presentations or coincident co-infections, biopsy or resection can occur without concurrent culture, thus making the recognition of histologic findings critical.
Mycobacterium tuberculosis is an acid fast bacillus and strict obligate aerobe which can proliferate within histiocytes. Within the lung, inhalation of this bacterium initiates a histologic granulomatous response mediated by similar cytokine mediated mechanisms as described above. Chronic granulomatous response limits the extent of inflammation, activates histiocytes, and forms a rim of histiocytes and peripheral lymphocytes around a necrotic center (Ghon focus). The primary lesion will spread to regional lymph nodes and undergo latency with subsequent fibrosis and calcification (Ghon complex). If the immune state changes within the patient, the rare residual organisms can reactivate and give rise to secondary disease. Cavitation or erosion into adjacent structures can occur to create a more favorable environment for the bacteria to grow and disseminate. Mycobacterium tuberculosis can affect any tissue through direct inoculation or hematogenous spread such as miliary tuberculosis or as the localized cavitary form. For example, tuberculosis remains the leading cause of granulomatous interstitial nephritis (GIN) in developing countries such as India, as compared to other infectious etiologies [10], [11], [12]. The granulomata in tuberculous GIN are typically caseating. Necrosis, demonstration of acid fast bacilli, blood interferon gamma release assay, and urine culture are not sensitive for the diagnosis of tuberculosis in GIN. Tissue PCR for tuberculosis performed in an appropriate clinical setting is useful in the diagnostic evaluation [12].
Non-tuberculous mycobacteria (NTM) commonly present as chronic infections which include a host of pathologic organisms including: M. kansasii, M. marinum, M. gordonae, M. scrofulaceum, Mycobacterium avium-intracellulare complex (MAC), M. ulcerans, M. fortuitum, M. chelonae, and M. abscessus [13]. The organisms are ubiquitous in soil and water which serve as primary sources of infection. Thus, NTM can present in immune competent or compromised individuals making the clinical presentation variable, as in the radiographic findings [13]. Two common clinical scenarios seen with NTM infection of the lung include cavitary lesions seen in patients with existing structural lung disease and thin females with thoracic anomalies and evolving nodular radiographic opacities [13], [14]. Within the kidney, Mycobacterium avium intracellular infection can occur in immunocompromised patients, such as renal allograft recipients. Histologically, these organisms have an indistinct appearance with AFB positive mycobacteria laden histiocytes and well-formed granulomas with or without caseous necrosis.
3.1.2. Coccidioides species
Coccidioides immitis and Coccidioides posadasii are the etiologic agents of coccidioidomycosis, also known as Valley Fever. These fungi are responsible for pulmonary disease in travelers or inhabitants of endemic arid regions such as the southwestern United States. The organism is a dimorphic fungus that exists as a mold stage in the environment and as a yeast-like form in humans. It is transmitted to humans from inhalation or direct inoculation of arthroconidia (hyphal segments) in the soil. They subsequently develop into thick-walled spherules formed in tissue, measuring 20–100 µM. As the spherules mature, they divide internally to produce numerous round endospores (Fig. 4). Ultimately the mature spherules rupture to release the endospores which then form additional spherules. The endospores trigger a granulomatous T-cell mediated host response, eosinophils may also be seen. As the recruited histiocytes phagocytize the endospores, they may drain into regional lymphatics, where lymphangitis can occur. Clinically, the most common manifestation is pulmonary coccidiomycosis, which presents with cough, pleuritic chest pain, fever, and rash. In affected patients, the disease course is self-limiting; however in immunocompromised individuals, systemic or disseminated disease can occur. When small yeast forms are identified using fungal stains such as GMS or PAS, the differential diagnosis may include Histoplasma capsulatum and Cryptococcus spp., as well of endospores of Coccidioides spp. All three entities can be associated with necrotizing granulomas. However, Coccidioides spp. can be differentiated by the size variation of the newly-released endospores and enlarging spherules, as well as the presence of mature spherules containing endospores. Also, Coccidioides endospores and spherules do not bud, unlike the yeasts of H. capsulatum and Cryptococcus spp. which exhibit narrow-based budding. Of note, one must be careful not to mistake the presence of two closely opposing Coccidioides spp. endospores or maturing spherules as evidence of budding. Another dimorphic fungal infection that may enter the differential in the lung is Blastomycosis. Blastomycosis dermatitidis is a larger yeast, measuring 8–15 µm in diameter, that has a thick, double-contoured cell wall. Unlike Coccidioides spp., it divides by budding and is commonly associated with a mixed granulomatous/suppurative response [15].
4. Non necrotizing granuloma
4.1. Infectious etiologies of non necrotizing granuloma
4.1.1. Mycobacterium leprae
Mycobacterium leprae, otherwise known as Hansen's disease, is primarily a tropical, mycobacterial infection which most often affects skin, nerves, and nasal mucosa. Mycobacterium leprae is an obligate intracellular gram positive, partially acid fast bacillus. Morphologically, it has a thick waxy coating which may prevent identification with traditional Gram stain. When it does stain, the organism is Gram positive. The mode of infection is suspected to be through aerosolized droplets, however direct infection from armadillos, rodents, or tattoos have been reported [16], [17], [18].
Leprosy exists as a spectrum of disease states, at each end are the paucibacillary (tuberculoid) and multibacillary (lepromatous) disease states. As the name suggests, paucibacillary is characterized by few lesions with a high resistance and paucity of organisms, whereas, multibacillary occurs with multisite involvement and a high bacterial load (Fig. 6). Periods of disease evolution have been described and include tuberculoid, borderline-tuberculoid, borderline, borderline-lepromatous, and lepromatous leprosy. Cellular response has been characterized with a similar spectrum, starting with an initial IL-2 and interferon gamma initiation (T-helper type 1) and eventually progressing to activation of T-helper type II (IL-4, 5, 10) and cyclooxygenase II [19].
Diagnosis requires skin biopsy from the active edge of a lesion. Histologically, the tuberculoid and lepromatous forms have distinct appearances [20]. Lepromatous leprosy is rich with dermal parasitized macrophages, whereas the tuberculoid form is similar to the granulomatous inflammation by which its name is ascribed; epithelioid histiocytes with multinucleated giant cells and a lymphohistiocytic cuff. In addition, mild to severe disease states show increasing involvement with chronically inflamed blood vessels, dermal appendages, and hypertrophied nerves within the superficial and deep dermis. In the mild form of tuberculoid leprosy, organisms are found in less than 50% of cases [21]. Additionally, post treatment, granulomas can persist for 18 months [22]. In the severe, lepromatous form, foamy histiocytes, eccrine structures, and endothelium are filled with bacilli. There is a relatively minor lymphocytic infiltrate seen in the lepromatous form. In rare cases, necrotizing vasculitis (Lucio's phenomenon) or a severe erythema nodosum-like histologic presentation can occur. The characteristic diagnostic triad of leprosy is hypo-pigmented skin patches with thickened nerves, definite loss of sensation, and biopsy-proven organisms [23]. Given their partial acid-fast nature, the Fite stain rather than the Ziehl–Neelsen should be used to stain M. leprae. These bacilli also stain using GMS, which non-specifically stains most fungi and bacteria.
4.2. Non necrotizing granuloma
4.2.1. Sarcoidosis
Sarcoidosis is a granulomatous disease involving multiple organ systems including the lungs, kidneys, skin, joints, muscles, and eyes [24], [25], [26]. Data from A Case Control Etiologic Study of Sarcoidosis (ACCESS), which registered 736 patients, demonstrated pulmonary and skin involvement in 95% and 15.9% of cases, respectively [24]. Of note, there is an age, sex, and race predilection to end organ involvement, with African American females most commonly affected [24], [25], [26]. Majority of patients present with pulmonary involvement, which is pathologically characterized by the presence of non-necrotizing granulomas with exclusion of other causes. The suspected etiology is hypothesized to represent an autoimmune response to infections of Mycobacteria or Propionibacteria species or an unidentified environmental agent [26]. In the lung, the suspected inhaled exposure stimulates an antigenic response where granulomas are detected clinically by endobronchial biopsy (40–71% of patients). Histologically occur around bronchovascular bundles and follow a lymphangitic distribution (nearly 70%) [27]. Lymphocytic alveolitis composed of T-helper cells is commonly identified in open lung biopsies of sarcoidosis patients and is thought to be a precursor lesion to granuloma formation [28]. The subsequent immune response is similar to that described in Section 1.1 above. A variety of non-specific inclusions have been histologically described in varying frequencies including Schaumann's bodies, asteroid bodies, birefringent crystals, and Hamazaki-Wesenberg bodies [27]. During disease progression the granulomas can heal and undergo fibrosis. In up to a third of patients progressive fibrosis can occur and result in end-stage fibrosis [27]. In nearly a third of patients, extrapulmonary sarcoidosis is the presenting symptom.
Renal sarcoidosis occurs either in association with other organ involvement, or as a renal limited form of sarcoidosis [29], [30]. The most common modes of presentation include hypercalcemia, hypercalciuria, nephrolithiasis, obstructive uropathy, and renal tubular defects [31]. GIN, is a well described manifestation of renal sarcoidosis, and the frequency with which it occurs, as estimated by post-mortem studies ranges from 7% to 27% [32]. The granulomas are usually well-formed and non-necrotizing. The presence of Schaumann and asteroid bodies are not common. There are often interstitial and tubular deposits of calcium due to hypercalcemia.
4.2.2. Cutaneous manifestations of non necrotizing granuloma
The etiology of non necrotizing granuloma is broad and can be challenging to distinguish in the skin (Table 2). Herein common non-necrotizing granulomatous entities are described.
Granuloma annulare (GA) is a dermatosis classically presenting on the extremities of females as an isolated erythematous papule, or grouped in an annular or arcuate plaque (Fig. 7). GA can be seen in association with many autoimmune, neoplastic, and drug-induced states including sarcoidosis, Alagille's syndrome, thyroid disease, hepatitis B and C, Epstein Barr virus, mycosis fungoides, Hodgkin and non-Hodgkin lymphomas, gastrointestinal stromal tumors, adenocarcinomas, allopurinol, amlodipine, and curiously, TNF-alpha inhibitors [33], [34]. Th-1 type reactions have been noted with increasing levels of IL-2, 18, and interferon-gamma [35]. Two major histologic subtypes are commonly seen which include necrobiotic (palisading) and interstitial. Necrobiotic GA is a superficial and deep dermal area of central neutrophils and nuclear debris with palisading epithelioid histiocytes and lymphocytes with variable multinucleate giant cells. Additionally, a rim of collagenosis is present, likely a reparative effect. The interstitial form of GA can be subtle with a lymphohistiocytic inflammatory infiltrate involving dermal vessels and intersection collagen bundles. The differential diagnosis can be challenging when distinguishing GA from infectious etiologies or other necrobiotic granulomas including granulomatosis with polyangitis (GPA), rheumatoid nodules, necrobiosis lipodica, Well's syndrome and Churgg Strauss syndrome.
Fig. 7.
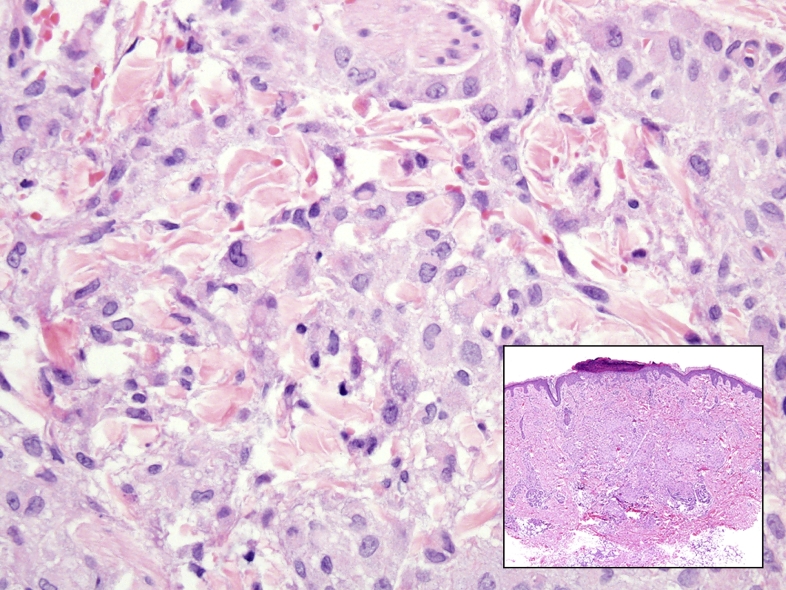
The skin punch biopsy of Langerhans cell histiocytosis (inset, H&E) shows a dermal infiltrate of non-necrotizing granulomatous inflammation with epithelioid histiocytes which have reniform nuclei and occasional nuclear grooves in a background of sparse chronic inflammation (larger image, H&E 400x).
Necrobiosis lipoidica can be distinguished by the distribution (often extending to subcutis) by the presence of plasma cells, and basophilic staining of the necrobiosis. GPA (Wegner's) classic shows necrotizing vasculitis with an unrelated granulomatosis. In addition, there is often a chronic inflammatory background seen in these cases which aid in distinction of this histologic mimic. Rheumatoid nodules typically present with stromal fibrosis and giant cells. Churgg-Strauss occurs with small vessel necrotizing vasculitis, granulomas, and extravasated eosinophils. Well's syndrome shares this histologic appearance, though typically produces characteristic aggregates of eosinophils and eosinophilic granules (“flame figures”) .
Mycosis Fungoides is one of the most common types of cutaneous T-cell lymphomas, although it makes up only 4% of non-Hodgkin lymphomas in the US [46]. A rare histologic subtype of this disease is the granulomatous form, which is characterized by epithelioid non necrotizing granulomas (> 25%) admixed with a lymphomatous epidermal and dermal infiltrate. Clinically, these lesions are indistinct and can overlap a wide spectrum of differential diagnosis [47]. In addition, granulomas may precede the histologic findings of lymphoma and can mimic the non-necrotizing granulomatous entities described in Tables 1 and 2 [47].
4.2.3. Renal manifestations of non necrotizing granuloma
Acute or chronic tubulointerstitial nephritis with uveitis, also known as Dobrin's syndrome can present as GIN. Although the etiology is uncertain, it is believed to have an autoimmune pathogenesis. Tan et al suggested that modified C- reactive protein, an autoantigen common to both the uvea and renal tubular cells, may be involved in the pathogenesis [36]. It has a female predominance and is seen in the younger population. It is associated with uveitis, and a lymphocyte and plasma cell rich interstitial nephritis in which granulomas are reported in up to 14% of patients [37], [38].
Granulomatosis with polyangiitis is one of the ANCA-associated vasculitides. This is most commonly associated with positive PR3 (proteinase 3 antibodies). Renal involvement is characterized by focal or diffuse necrotizing and crescentic glomerulonephritis with interstitial inflammation. A necrotizing, neutrophilic GIN with palisading histiocytes (geographic pattern) may be seen. A necrotizing vasculitis of interlobular arteries may also be noted.
There are several miscellaneous conditions in which GIN may be noted. These conditions include Crohns disease [39], focal granulomatous reaction to oxalate crystals [40]. Granulomatous response may occur in response to acute tubular injury and ruptured tubules. This was noted in one case of copper sulphate poisoning where severe acute tubular necrosis was the predominant picture, but a granuloma formation was seen in close proximity to a ruptured tubule [41]. Lastly, in the absence of identifying a definite etiology, the term idiopathic GIN has been employed, although the current belief is that a subset of these cases represent renal limited sarcoidosis.
4.4. Drug associated etiologies of non necrotizing granuloma
Drug associated granulomas are most commonly seen in the setting of Bacillus Calmette-Guérin (BCG) inoculation, which is most frequently encountered in the setting of urothelial carcinoma therapy. Interestingly, drug-related GIN accounts for the most common cause of GIN in three retrospective studies. Bijol reported GIN in 45% [48], Mignon in 31% [49], and Viero in 25% of cases [50]. Drug-related GIN is secondary to an immune mediated process. There are many proposed mechanisms which explain the process by which drugs (or metabolites) induce acute interstitial nephritis. The drug might bind to the tubular basement membranes and act as a hapten. Mimicry, wherein there is cross-reaction between drugs and tubular basement membrane antigens is yet another mechanism. Circulating drugs may get planted in the renal tubules or interstitium inciting an immune response. Lastly drugs and immunoglobulins might form circulating immune complexes which when deposited induce an immune reaction [51]. The drugs which can cause acute interstitial nephritis are innumerable. The drugs most commonly associated with GIN include non-steroidal anti-inflammatory drugs [48], [52], antibiotics including penicillin, ciprofloxacin [53], levofloxacin [54], and nitrofurantoin [55]. Mesalazine, which is used to treat Crohn's, has also been reported to cause GIN [56]. Drug-related GINs are characterized by non-necrotizing granulomata often associated with an eosinophil rich inflammatory infiltrate.
5. Histiocytic Response, lacking granulomas
Granulomatous response can be seen in neoplastic as well as reactive entities. Within multiple organ systems, a neoplastic granulomatous differential diagnosis can include Rosai–Dorfman disease, and Langerhans cell histiocytosis (LCH) (Fig. 7). These entities have a distinctly different pathogenesis, presentation, and histology. Extra-nodal Rosai–Dofman disease (sinus histiocytosis with massive lymphadenopathy) can be seen in nearly 40% of cases [42]. The skin is a common site of involvement that clinically evolves from papules to plaques and coalesces into nodules which resolve with fibrosis and spontaneous regression [43]. Histologically, a dense admixture of large pale histiocytes, lymphocytes, plasma cells, and granulocytes (predominantly eosinophils) fill the dermis. The pathognomonic finding is emperipolesis, whereby inflammatory cells are identified within the cytoplasm of histiocytes [42].
LCH is a misnomer, as it is not derived from Langerhans cells but shares the histologic appearance characterized by ovoid cells with pale cytoplasm and reniform nuclei (Fig. 7) [44]. Differentially, the neoplastic cells are myeloid dendritic cells which express similar antigens as detected by immunohistochemisty, but are genetically distinct from the Langerhan cells found within the skin and mucosal sites [44]. Classically, LCH presents are lytic bone lesions, however in nearly 40% of cases cutaneous sites are involved with a variety of clinical presentations [45].
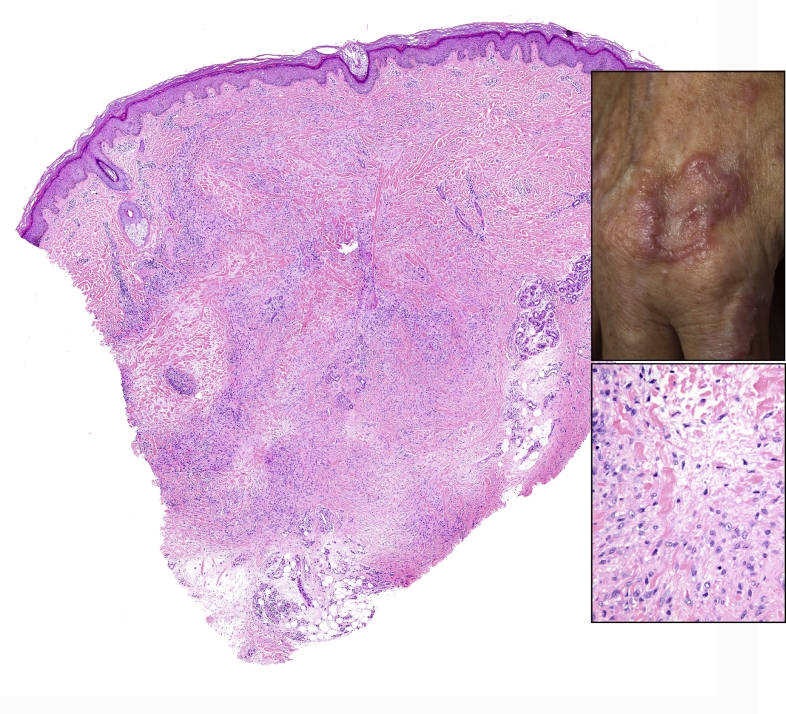
Fig. 8.
Skin punch biopsy (left) showing a palisaded granulomatous inflammation dispersed throughout the dermis diagnostic of granuloma annulare (H&E, 10x). The inset (top) shows the corresponding clinical image with an erythematous annular plaque. The bottom inset (400x) highlights the palisading histiocytes cuffing degenerative collagen.
6. Conclusion
The histologic identification of granulomatous inflammation is a helpful predictor of diagnostic etiology. Histologic patterns (foreign-body, necrotizing, non-necrotizing, suppurative, and a diffuse histiocytic reaction) can narrow the clinical differential diagnosis. A definitive diagnosis can often be made with the aid of ancillary testing (special stains and or molecular diagnostics). An open clinical dialogue between the clinician and the pathologist allows for appropriate use of ancillary laboratory techniques and ultimately accurate and effective patient care.
References
- 1.Mukhopadhyay S. Pulmonary necrotizing granulomas of unknown cause: clinical and pathologic analysis of 131 patients with completely resected nodules. Chest. 2013;144(3):813–824. doi: 10.1378/chest.12-2113. [DOI] [PubMed] [Google Scholar]
- 2.Gao J.L. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med. 1997;185(11):1959–1968. doi: 10.1084/jem.185.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imhof B.A., Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4(6):432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- 4.Kunkel S.L. Cellular and molecular aspects of granulomatous inflammation. Am J Respir Cell Mol Biol. 1989;1(6):439–447. doi: 10.1165/ajrcmb/1.6.439. [DOI] [PubMed] [Google Scholar]
- 5.Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 6.Kommareddi S. Nontuberculous mycobacterial infections: comparison of the fluorescent auramine-O and Ziehl–Neelsen techniques in tissue diagnosis. Hum Pathol. 1984;15(11):1085–1089. doi: 10.1016/s0046-8177(84)80253-1. [DOI] [PubMed] [Google Scholar]
- 7.Caulfield A.J., W.N. Diagnosis of active tuberculosis disease: from microscopy to molecular techniques. J Clin Tuberculosis Other Mycobacterial Dis. 2016;4:33–43. doi: 10.1016/j.jctube.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodard B.H. Incidence and nature of primary granulomatous inflammation in surgically removed material. Am J Surg Pathol. 1982;6(2):119–129. doi: 10.1097/00000478-198203000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Miller W.T., Jr. Spectrum of pulmonary nontuberculous mycobacterial infection. Radiology. 1994;191(2):343–350. doi: 10.1148/radiology.191.2.8153304. [DOI] [PubMed] [Google Scholar]
- 10.Naidu G.D. Granulomatous interstitial nephritis: our experience of 14 patients. Indian J Nephrol. 2013;23(6):415–418. doi: 10.4103/0971-4065.120336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta P. Renal failure due to granulomatous interstitial nephritis in native and allograft renal biopsies: experience from a tertiary care hospital. Ren Fail. 2014;36(9):1468–1470. doi: 10.3109/0886022X.2014.950975. [DOI] [PubMed] [Google Scholar]
- 12.Agrawal V. Etiological diagnosis of granulomatous tubulointerstitial nephritis in the tropics. Clin Kidney J. 2015;8(5):524–530. doi: 10.1093/ckj/sfv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffith D.E. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 14.Prince D.S. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321(13):863–868. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- 15.Mukhopadhyay S., Gal A.A. Granulomatous lung disease: an approach to the differential diagnosis. Arch Pathol Lab Med. 2010;134(5):667–690. doi: 10.5858/134.5.667. [DOI] [PubMed] [Google Scholar]
- 16.McDougall A.C., Cologlu A.S. Lepromatous leprosy in man; depth of the cellular infiltrate and bacillary mass in relation to the possibility of transmission of leprosy by biting arthropods. Ann Trop Med Parasitol. 1983;77(2):187–193. doi: 10.1080/00034983.1983.11811696. [DOI] [PubMed] [Google Scholar]
- 17.Porritt R.J., Olsen R.E. Two simultaneous cases of leprosy developing in tattoos. Am J Pathol. 1947;23(5):805–817. [PMC free article] [PubMed] [Google Scholar]
- 18.Rees R.J., McDougall A.C. Airborne infection with Mycobacterium leprae in mice. J Med Microbiol. 1977;10(1):63–68. doi: 10.1099/00222615-10-1-63. [DOI] [PubMed] [Google Scholar]
- 19.Yamamura M. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 20.Ridley D.S. Histological classification and the immunological spectrum of leprosy. Bull World Health Organ. 1974;51(5):451–465. [PMC free article] [PubMed] [Google Scholar]
- 21.Lockwood D.N. Comparing the clinical and histological diagnosis of leprosy and leprosy reactions in the INFIR cohort of Indian patients with multibacillary leprosy. PLoS Negl Trop Dis. 2012;6(6):e1702. doi: 10.1371/journal.pntd.0001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lockwood D.N. The histological diagnosis of leprosy type 1 reactions: identification of key variables and an analysis of the process of histological diagnosis. J Clin Pathol. 2008;61(5):595–600. doi: 10.1136/jcp.2007.053389. [DOI] [PubMed] [Google Scholar]
- 23.Britton W.J., Lockwood D.N. Leprosy. Lancet. 2004;363(9416):1209–1219. doi: 10.1016/S0140-6736(04)15952-7. [DOI] [PubMed] [Google Scholar]
- 24.Baughman R.P. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 25.Mahevas M., Lescure F.X., Jean-Jacques B. Renal sarcoidosis: clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine. 2009;88(2):98–106. doi: 10.1097/MD.0b013e31819de50f. [DOI] [PubMed] [Google Scholar]
- 26.Baughman R.P., Lower E.E., du Bois R.M. Sarcoidosis. Lancet. 2003;361(9363):1111–1118. doi: 10.1016/S0140-6736(03)12888-7. [DOI] [PubMed] [Google Scholar]
- 27.Rosen Y. Pathology of sarcoidosis. Semin Respir Crit Care Med. 2007;28(1):36–52. doi: 10.1055/s-2007-970332. [DOI] [PubMed] [Google Scholar]
- 28.Ginns L.C. T-lymphocyte subsets in peripheral blood and lung lavage in idiopathic pulmonary fibrosis and sarcoidosis: analysis by monoclonal antibodies and flow cytometry. Clin Immunol Immunopathol. 1982;25(1):11–20. doi: 10.1016/0090-1229(82)90160-x. [DOI] [PubMed] [Google Scholar]
- 29.Robson M.G. Seven cases of granulomatous interstitial nephritis in the absence of extrarenal sarcoid. Nephrol Dialysis, Transplantation: Off Publ Eur Dialysis Transplant Assoc - Eur Renal Assoc. 2003;18(2):280–284. doi: 10.1093/ndt/18.2.280. [DOI] [PubMed] [Google Scholar]
- 30.O'Riordan E. Isolated sarcoid granulomatous interstitial nephritis: review of five cases at one center. Clin Nephrol. 2001;55(4):297–302. [PubMed] [Google Scholar]
- 31.Berliner A.R., Haas M., Choi M.J. Sarcoidosis: the nephrologist's perspective. Am J Kidney Dis. 2006;48(5):856–870. doi: 10.1053/j.ajkd.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 32.Longcope W.T., Freiman D.G. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital. Medicine. 1952;31(1):1–132. [PubMed] [Google Scholar]
- 33.Ratnarathorn M., Raychaudhuri S.P., Naguwa S. Disseminated granuloma annulare: a cutaneous adverse effect of anti-tnf agents. Indian J Dermatol. 2011;56(6):752–754. doi: 10.4103/0019-5154.91847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avitan-Hersh E. Does infection play a role in the pathogenesis of granuloma annulare? J Am Acad Dermatol. 2013;68(2):342–343. doi: 10.1016/j.jaad.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 35.Mempel M. T-cell receptor repertoire and cytokine pattern in granuloma annulare: defining a particular type of cutaneous granulomatous inflammation. J Invest Dermatol. 2002;118(6):957–966. doi: 10.1046/j.1523-1747.2002.01783.x. [DOI] [PubMed] [Google Scholar]
- 36.Tan Y. Modified C-reactive protein might be a target autoantigen of TINU syndrome. Clin J Am Soc Nephrol. 2011;6(1):93–100. doi: 10.2215/CJN.09051209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandeville J.T., Levinson R.D., Holland G.N. The tubulointerstitial nephritis and uveitis syndrome. Surv ophthalmol. 2001;46(3):195–208. doi: 10.1016/s0039-6257(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 38.Herlitz L.C. Uveitis and acute renal failure. Kidney Int. 2007;72(12):1554–1557. doi: 10.1038/sj.ki.5002410. [DOI] [PubMed] [Google Scholar]
- 39.Colvin R.B. Granulomatous interstitial nephritis as a manifestation of Crohn disease. Arch Pathol Lab Med. 2014;138(1):125–127. doi: 10.5858/arpa.2012-0224-CR. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki M. Oxalate nephropathy with a granulomatous lesion due to excessive intake of peanuts. Clin Exp Nephrol. 2008;12(4):305–308. doi: 10.1007/s10157-008-0046-5. [DOI] [PubMed] [Google Scholar]
- 41.Chugh K.S. Acute renal failure following copper sulphate intoxication. Postgrad Med J. 1977;53(615):18–23. doi: 10.1136/pgmj.53.615.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foucar E., Rosai J., Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai–Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7(1):19–73. [PubMed] [Google Scholar]
- 43.Wang K.H. Cutaneous Rosai–Dorfman disease: clinicopathological profiles, spectrum and evolution of 21 lesions in six patients. Br J Dermatol. 2006;154(2):277–286. doi: 10.1111/j.1365-2133.2005.06917.x. [DOI] [PubMed] [Google Scholar]
- 44.Allen C.E. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184(8):4557–4567. doi: 10.4049/jimmunol.0902336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grois N. Risk factors for diabetes insipidus in Langerhans cell histiocytosis. Pediatr Blood Cancer. 2006;46(2):228–233. doi: 10.1002/pbc.20425. [DOI] [PubMed] [Google Scholar]
- 46.Criscione V.D., Weinstock M.A. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143(7):854–859. doi: 10.1001/archderm.143.7.854. [DOI] [PubMed] [Google Scholar]
- 47.Gallardo F. Cutaneous lymphomas showing prominent granulomatous component: clinicopathological features in a series of 16 cases. J Eur Acad Dermatol Venereol. 2009;23(6):639–647. doi: 10.1111/j.1468-3083.2008.03020.x. [DOI] [PubMed] [Google Scholar]
- 48.Bijol V. Granulomatous interstitial nephritis: a clinicopathologic study of 46 cases from a single institution. Int J Surg Pathol. 2006;14(1):57–63. doi: 10.1177/106689690601400110. [DOI] [PubMed] [Google Scholar]
- 49.Mignon F. Granulomatous interstitial nephritis. Adv Nephrol Necker Hosp. 1984;13:219–245. [PubMed] [Google Scholar]
- 50.Viero R.M., Cavallo T. Granulomatous interstitial nephritis. Hum Pathol. 1995;26(12):1347–1353. doi: 10.1016/0046-8177(95)90300-3. [DOI] [PubMed] [Google Scholar]
- 51.Rossert J. Drug-induced acute interstitial nephritis. Kidney Int. 2001;60(2):804–817. doi: 10.1046/j.1523-1755.2001.060002804.x. [DOI] [PubMed] [Google Scholar]
- 52.Jung J.H. Nonsteroidal antiinflammatory drug induced acute granulomatous interstitial nephritis. BMC Res Notes. 2015;8:793. doi: 10.1186/s13104-015-1788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lien Y.H. Ciprofloxacin-induced granulomatous interstitial nephritis and localized elastolysis. Am J Kidney Dis. 1993;22(4):598–602. doi: 10.1016/s0272-6386(12)80936-x. [DOI] [PubMed] [Google Scholar]
- 54.Ramalakshmi S., Bastacky S., Johnson J.P. Levofloxacin-induced granulomatous interstitial nephritis. Am J Kidney Dis. 2003;41(2):E7. doi: 10.1053/ajkd.2003.50064. [DOI] [PubMed] [Google Scholar]
- 55.Namagondlu G. Acute renal failure from nitrofurantoin-induced acute granulomatous interstitial nephritis. QJM. 2010;103(1):49–52. doi: 10.1093/qjmed/hcp146. [DOI] [PubMed] [Google Scholar]
- 56.Itano S. [A case of drug-induced granulomatous interstitial nephritis during the long course of Crohn's disease] Nihon Jinzo Gakkai Shi. 2013;55(2):167–171. [PubMed] [Google Scholar]