Summary
Iron (Fe) and zinc (Zn) deficiencies are a global human health problem that may worsen by the growth of crops at elevated atmospheric CO 2 concentration (eCO 2). However, climate change will also involve higher temperature, but it is unclear how the combined effect of eCO 2 and higher temperature will affect the nutritional quality of food crops. To begin to address this question, we grew soybean (Glycine max) in a Temperature by Free‐Air CO 2 Enrichment (T‐FACE) experiment in 2014 and 2015 under ambient (400 μmol mol−1) and elevated (600 μmol mol−1) CO 2 concentrations, and under ambient and elevated temperatures (+2.7°C day and +3.4°C at night). In our study, eCO 2 significantly decreased Fe concentration in soybean seeds in both seasons (−8.7 and −7.7%) and Zn concentration in one season (−8.9%), while higher temperature (at ambient CO 2 concentration) had the opposite effect. The combination of eCO 2 with elevated temperature generally restored seed Fe and Zn concentrations to levels obtained under ambient CO 2 and temperature conditions, suggesting that the potential threat to human nutrition by increasing CO 2 concentration may not be realized. In general, seed Fe concentration was negatively correlated with yield, suggesting inherent limitations to increasing seed Fe. In addition, we confirm our previous report that the concentration of seed storage products and several minerals varies with node position at which the seeds developed. Overall, these results demonstrate the complexity of predicting climate change effects on food and nutritional security when various environmental parameters change in an interactive manner.
Keywords: soybean, seed mineral concentrations, iron, zinc, boron, canopy position, climate change, elevated atmospheric CO2, elevated air temperature
Significance statement
Mineral deficiencies are a global health problem that may be exacerbated by crop growth at elevated atmospheric CO2 concentration (eCO2) that can reduce micronutrient concentrations in grains and seeds. However, our soybean studies suggest that the potential threat to human nutrition by increasing CO2 concentration may not be realized when plants grow at eCO2 and higher temperatures that more realistically mimic future climate conditions.
Introduction
Atmospheric CO2 is increasing, and is projected to reach between 730 and 1020 μmol mol−1 by the end of the century (Collins et al., 2013). The increase in CO2 would be expected to increase leaf photosynthesis, and this has been documented experimentally for many C3 species (Ainsworth and Long, 2005; Bernacchi et al., 2005, 2006; Ainsworth and Rogers, 2007) using free‐air CO2 enrichment (FACE) technology (Long et al., 2004; Leakey et al., 2009). As a result, biomass production and seed yield are increased, but often to a lesser extent than the increase in light‐saturated photosynthesis (Long et al., 2004; Leakey et al., 2009). In addition to increasing yield, eCO2 has the potential to reduce the concentration of minerals in seeds and thereby threaten human nutrition, as highlighted in two recent meta‐analysis studies. Across a range of C3 plants, Loladze (2014) reported that eCO2 reduced the concentrations of several minerals in foliar and edible tissues by approximately 8%, with the exception of Mn that was not reduced. Overall, the patterns of mineral changes were similar between foliar and edible tissue, with the exception that K was reduced only in edible tissues (Loladze, 2014). The second study (Myers et al., 2014) focused on C3 grains and legumes, and reported that concentrations of Zn and Fe were decreased from ~5 to 10% at elevated atmospheric CO2 concentration (eCO2). Both studies concluded that eCO2 may result in crops that are less nutritious as a result of reduced mineral concentrations, which would have enormous implications for the use of crops for human food as well as animal feed (Weigel, 2014).
The mechanisms responsible for the effects of eCO2 on seed mineral concentrations are not clear, but many biological and physical processes could contribute. For example, reduced transpiration as a result of partial stomatal closure at eCO2 (Bernacchi et al., 2007; Hussian et al., 2013) would be expected to reduce the uptake of minerals that are acquired by mass flow, but have less effect on those where movement to the root surface occurs by diffusion (McGrath and Lobell, 2013). Following uptake into the plant, mineral transport continues in the xylem with distribution among stems and leaves (Marschner, 1995). In grasses, nutrient distribution among vegetative organs is controlled independently of transpiration rate (Yamaji and Ma, 2017), and it can be expected to occur in dicots as well. The mineral content of seeds is thought to reflect import from the phloem as a result of continued uptake by roots (followed by xylem‐to‐phloem transfer of minerals), as well as redistribution from vegetative tissues such as leaves. Conceivably, many of these steps could be impacted by eCO2. Furthermore, dilution by enhanced carbohydrate production at eCO2 or dilution by increased seed production could contribute to the reduced mineral concentrations observed. Dilution by enhanced carbohydrate production cannot explain all of the effects of eCO2 (McGrath and Lobell, 2013), but the impact of changes in seed yield on the content of minerals on a plant basis (Singh et al., 2016) has not been broadly considered. However, it is reasonable to assume that if a micronutrient is obtained in controlled or limited amounts it would be diluted when distributed amongst a greater number of seeds produced at eCO2, such that the concentration would decrease while the mineral seed content per plant would not change.
Elevated temperature is another environmental factor that will impact future crop production (Ruiz‐Vera et al., 2013, 2015; Siebers et al., 2015, 2017; Köhler et al., 2016). Increased atmospheric accumulation of CO2 along with other greenhouse gases will be accompanied by an increase in terrestrial surface air temperatures between 1 and 6°C by 2050, relative to 1961–1990, depending on geographic location (Rowlands et al., 2012). The impact of elevated temperature is expected to have a greater effect on reproductive development, as soybean has an optimum temperature of ~30°C for vegetative growth (Hesketh et al., 1973), whereas reproductive development has an optimal temperature of 22–24°C (Hatfield et al., 2011). Furthermore, an increase in temperature will generally lead to an increase in vapor pressure deficit (VPD) that would increase transpiration (assuming water supply is sufficient), and as a result the uptake of minerals would be driven by mass flow. Thus, elevated temperature could impact the concentration of minerals in plant tissues including seeds and in an opposite manner to eCO2.
Because future climate change is projected to involve both rising CO2 and temperature, it is important to evaluate both environmental factors together. Recently, the Temperature by Free‐Air CO2 Enrichment (T‐FACE) facility was used to explore the independent and interactive effects of eCO2 and elevated temperature on soybean photosynthesis and productivity (Ruiz‐Vera et al., 2013; Köhler et al., 2016). In general, photosynthesis, aboveground biomass and seed yields were increased by eCO2 and decreased by elevated temperature; the combination of eCO2 plus elevated temperature had a variable effect (likely dependent on ambient temperature during the growing season), but the generalized conclusion was that eCO2 attenuated the negative impact of elevated temperature (Ruiz‐Vera et al., 2013). However, there is little information about the impact of eCO2 in combination with elevated temperature on seed mineral concentrations. Consequently, the overall objective of the present study was to determine the individual and combined effect of eCO2 and elevated temperature on soybean seed composition in terms of storage products (protein and oil) and important minerals. Furthermore, we examined seeds produced at different positions along the main stem because seed composition varies with nodal position (Huber et al., 2016).
Results
Plant growth and seed yield
Soybean plants were grown in the field under conditions of eCO2 and elevated temperature projected for midway through this century for terrestrial areas (Rowlands et al., 2012). In both seasons of our study (2014 and 2015), eCO2 increased plant height (Figure 1a) and the number of main stem nodes (Figure 1b) compared with plants at ambient [CO2], whereas elevated temperature had the opposite effect but only in 2014 (Table 1).
Figure 1.
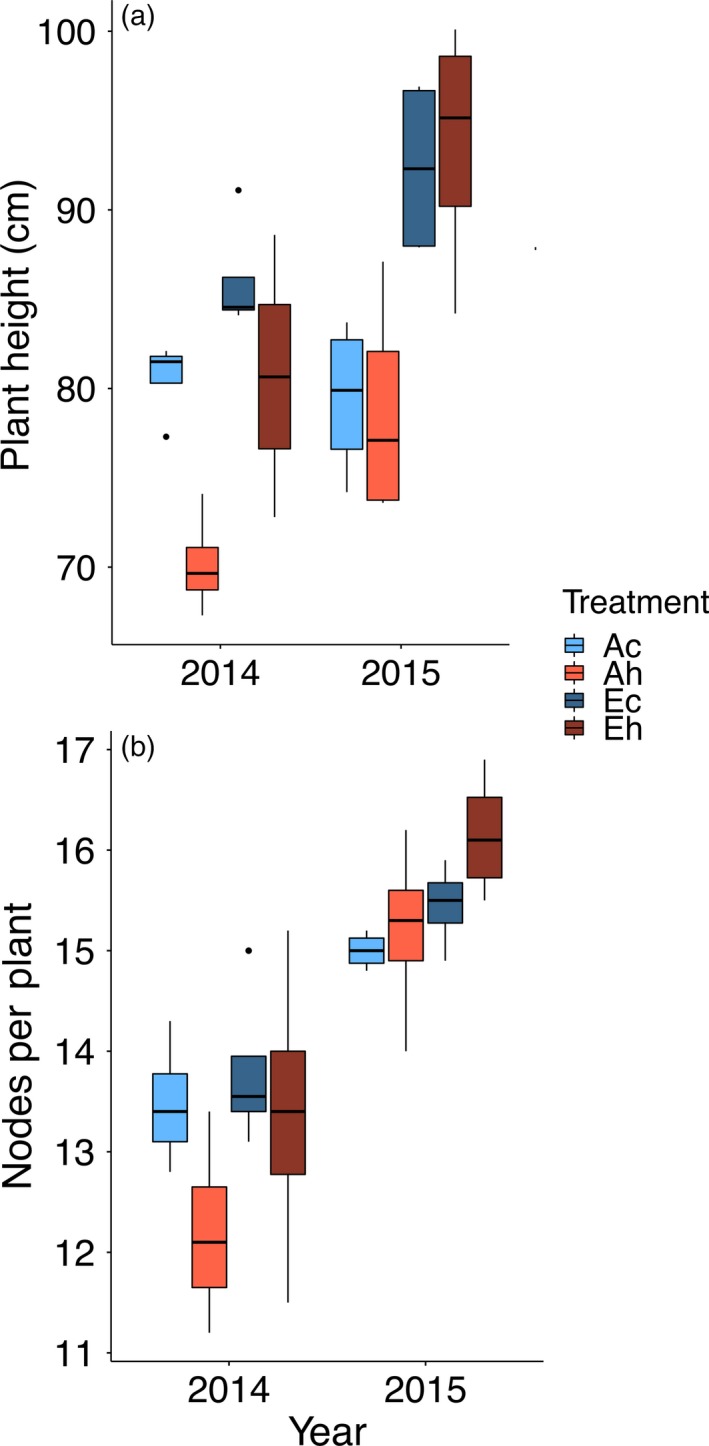
Impact of elevated atmospheric CO2 concentration (eCO 2) and elevated temperature treatments, singly and in combination, on (a) mean plant height and (b) number of main stem nodes per plant for soybean over two growing seasons. Both the CO 2 (F 1,9 = 15.95; P < 0.005) and temperature (F 1,9 = 15.66; P < 0.005) main effects were statistically significant in 2014. The CO 2 main effect (F 1,9 = 28.23) was statistically significant in 2015. There were no statistically significant interactions between CO 2 and temperature for either height or node number in either year. Ac, ambient CO 2, control temperature; Ah, ambient CO 2, heated + 3.5°C; Ec, elevated CO 2, control temperature; Eh, elevated CO 2, heated + 3.5°C. Boxplots display the median, and 25 and 75% percentiles, and whiskers extend to 1.5 × interquartile range, with outliers (if any) shown.
Table 1.
Complete block analysis of variance of total seed yield (SY) for the main effects [CO2] concentration (400 μmol mol−1, 600 μmol mol−1), temperature (control, heat) and the interaction term
| CO2 | Temperature | CO2 × temperature | |
|---|---|---|---|
| Seed yield | |||
| 2014 | 0.0231 | 0.0003 | 0.1055 |
| 2015 | 0.0192 | < 0.0001 | 0.688 |
| Num DF | 1 | 1 | 1 |
| Den DF | 3 | 6 | 6 |
| Plant height | |||
| 2014 | 0.031 | 0.0033 | 0.2394 |
| 2015 | < 0.001 | 0.9176 | 0.7104 |
| Num DF | 1 | 1 | 1 |
| Den DF | 9 | 9 | 9 |
| Nodes per plant | |||
| 2014 | 0.0574 | 0.0311 | 0.2568 |
| 2015 | 0.0124 | 0.0746 | 0.2689 |
| Num DF | 1 | 1 | 1 |
| Den DF | 9 | 9 | 9 |
Bold values represent statistical significant (P < 0.1).
In both years, eCO2 significantly increased the total seed yield while elevated temperature significantly decreased the total seed yield in the bulk samples collected from the 3.2‐m row harvest (Figure 2; Table 1). A similar pattern was observed for the total seed yield from the replicated sub‐sampling of 10 single plants, and yield data from both sampling methods were closely correlated (R 2 = 0.82). The 10‐plant samples consisted of normal‐sized, representative plants selected for each of the treatments that were harvested at maturity and the main stems divided into thirds, with each stem fraction (bottom, middle and top) threshed separately. The distribution of seed yield among the three main stem positions is presented in Figure 3(a), and as a percentage distribution in Figure 3(b). As expected, 40–50% of the total main stem seed yield was located in the middle canopy position in both years. In 2014, a higher proportion (25–33%) of the total seed yield was located at the bottom canopy position compared with 2015 (13–23%), and consequently the opposite was observed for the proportion at the top canopy position in 2014 (24–33%) and 2015 (30–42%; Figure 3b). Elevated CO2 affected the proportional distribution of seed yield between the three canopy positions, but only in 2015, whereas the heat treatment led to a decreased seed yield at the bottom canopy position and increased seed yield at the top canopy position under both ambient and eCO2 concentrations in both seasons (Figure 3b; Table 2).
Figure 2.
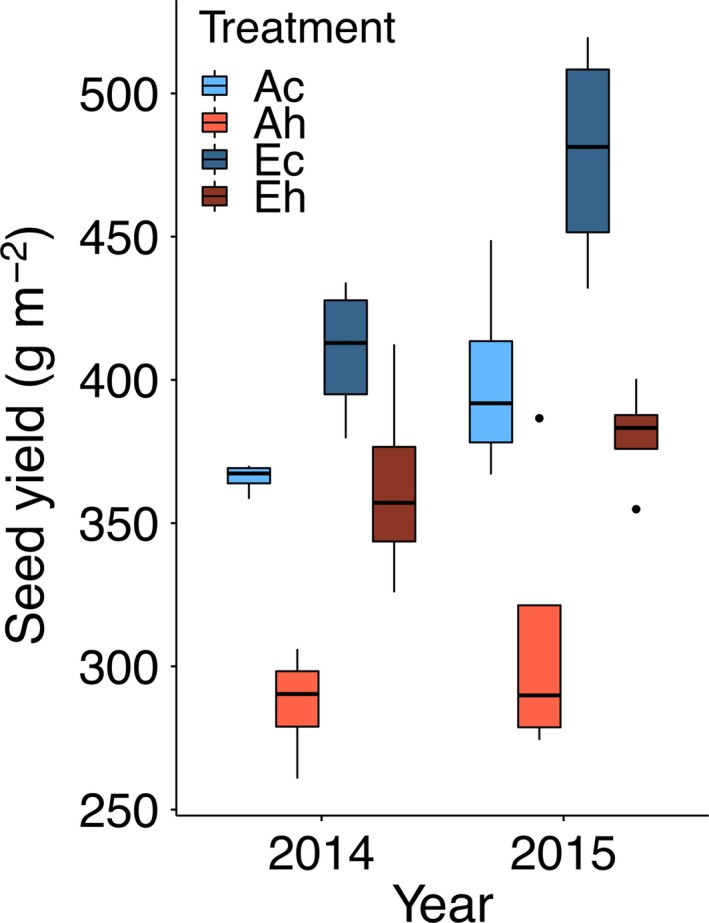
Impact of elevated atmospheric CO2 concentration (eCO 2) and elevated temperature treatments, singly and in combination, on seed yield in g m−2 as derived from sampling of 3.2‐m rows. Treatment abbreviations and boxplots as in Figure 1.
Figure 3.
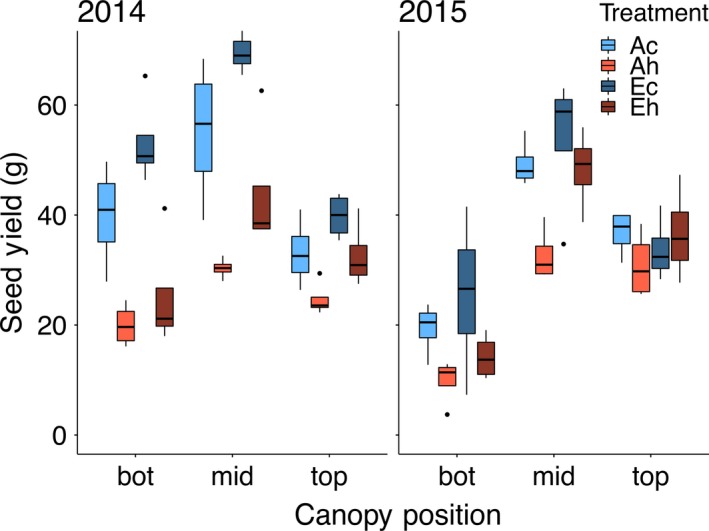
Distribution of seed yield among the bottom, middle and top canopy positions as impacted by elevated atmospheric CO2 concentration (eCO 2) and elevated temperature treatments in 2014 and 2015.
(a) Absolute seed yield expressed as g from each 10‐plant sample.
(b) Seed yield from each position expressed as a percentage of total seeds under the four treatments. Treatment abbreviations and boxplots as in Figure 1.
Table 2.
Complete block analysis of variance of the SY (g) data from the 10 plants samples and the percent distribution of seed yield (%) for the main effects [CO2] concentration (400 μmol mol−1, 600 μmol mol−1), temperature (control, heat), canopy position (bottom, middle, top) and the interaction terms
| CO2 | Temperature | CO2 × temperature | Canopy | CO2 × canopy | Temperature × canopy | CO2 × temperature × canopy | |
|---|---|---|---|---|---|---|---|
| Seed yield (g) | |||||||
| 2014 | 0.035 | 0.2657 | 0.7855 | < 0.0001 | 0.0966 | 0.0003 | 0.3225 |
| 2015 | 0.2323 | 0.0043 | 0.1293 | < 0.0001 | 0.1349 | 0.058 | 0.2827 |
| Seed yield (%) | |||||||
| 2014 | – | – | – | < 0.0001 | 0.4593 | < 0.0001 | 0.3329 |
| 2015 | – | – | – | < 0.0001 | 0.0988 | 0.0065 | 0.7987 |
| Num DF | 1 | 1 | 1 | 2 | 2 | 2 | 2 |
| Den DF | 3 | 6 | 6 | 24 | 24 | 24 | 24 |
Bold values represent statistical significant (P < 0.1).
Although there was some variation in single seed weight from the samples collected at different canopy positions in the present study (Figure S1; Köhler et al., 2016), these differences were generally small. Across all treatments, comparison of single seed weight with seed yield at the corresponding canopy position (i.e. bottom, middle or top position) produced a positive correlation but with a very low correlation coefficient (r = 0.28) that was not statistically significant. When different environmental parameters (i.e. elevated temperature or eCO2) or canopy positions were color‐coded, it was apparent that the weak correlation was valid across all of the parameters (Figure S2). The weak correlation implies that the number of seeds produced rather than changes in seed size primarily drove changes in yield.
Seed protein and oil concentrations
Canopy position significantly affected seed composition of major storage products, with higher oil concentrations (Figure 4a,b) at the bottom of the canopy and, conversely, higher protein concentrations (Figure 4c,d) at the top of the canopy. In general, the canopy gradients in protein and oil concentration were larger in 2015 compared with 2014. There was no significant effect of eCO2 on protein (Figure 4c,d) or oil (Figure 4a,b) concentrations in the three canopy layers (Table 3). In contrast, elevated temperature significantly reduced protein concentration in 2014 (Figure 4c), with the strongest effect (−1.6%, P < 0.0001) in both the bottom and middle canopy positions and a smaller effect at the top of the canopy (−0.6%, P < 0.01), whereas in 2015 a significant reduction was only observed at the middle canopy position (−0.9%, P < 0.1). Conversely, the elevated temperature treatment significantly increased oil concentration at all positions in 2014 (bottom: +1.0%, P < 0.0001; middle: +0.9%, P < 0.0001; and top: +0.8%, P < 0.0001), and at the bottom (+0.5%, P < 0.05) and middle positions (+0.5%, P < 0.05) in 2015.
Figure 4.
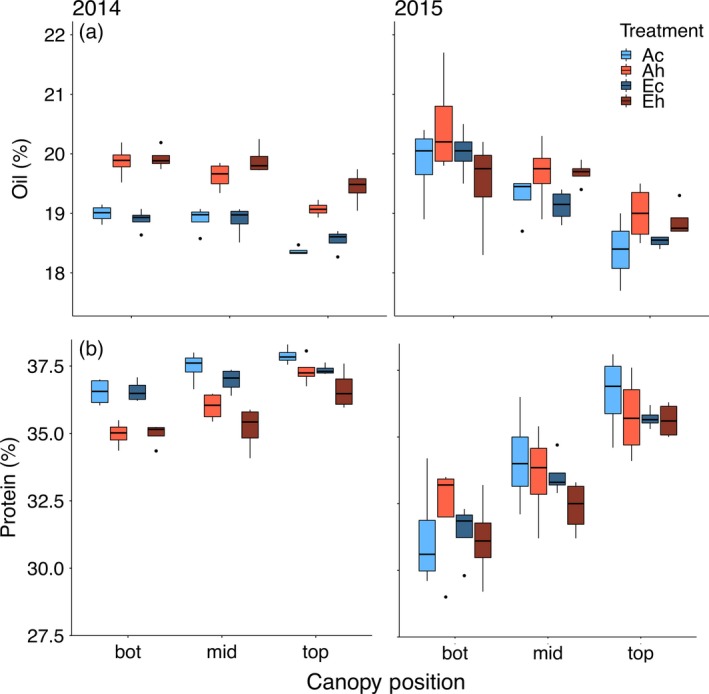
Concentration of (a) oil and (b) protein in seeds produced at the bottom, middle and top canopy positions as impacted by elevated atmospheric CO2 concentration (eCO 2) and elevated temperature treatments in 2014 and 2015.
Treatment abbreviations and boxplots as in Figure 1.
Table 3.
Complete block analysis of variance of the seed protein and oil concentrations in the two seasons for the main effects [CO2] concentration (400 μmol mol−1, 600 μmol mol−1), temperature (control, heat), canopy position (bottom, middle, top) and the interaction terms
| CO2 | Temperature | CO2 × temperature | Canopy | CO2 × canopy | Temperature × canopy | CO2 × temperature × canopy | |
|---|---|---|---|---|---|---|---|
| Protein | |||||||
| 2014 | 0.2192 | < 0.0001 | 0.5433 | < 0.0001 | 0.0381 | 0.0018 | 0.8788 |
| 2015 | 0.5339 | 0.3689 | 0.6311 | < 0.0001 | 0.6459 | 0.0646 | 0.1477 |
| Oil | |||||||
| 2014 | 0.2338 | < 0.0001 | 0.2531 | < 0.0001 | 0.0524 | 0.419 | 0.8494 |
| 2015 | 0.6046 | 0.0829 | 0.2419 | < 0.0001 | 0.3068 | 0.1657 | 0.078 |
| Num DF | 1 | 1 | 1 | 2 | 2 | 2 | 2 |
| Den DF | 3 | 6 | 6 | 24 | 24 | 24 | 24 |
Bold values represent statistical significant (P < 0.1).
Seed mineral composition
Seeds of legumes such as soybeans are generally considered a good source of micronutrients (minerals) that are important for human health, but eCO2 has been reported to reduce the concentrations of important minerals in soybean seeds and other edible grains (McGrath and Lobell, 2013; Loladze, 2014; Myers et al., 2014). A significant effect of eCO2 on mineral concentrations was observed in the present study for six elements (B, Ca, Fe, K, Se, Zn), but was only consistently observed in both study years for Fe. In contrast, elevated temperature had a significant effect on 14 of the analyzed elements in at least 1 year of the study, and this effect was observed in both study years for seven of those elements (As, B, Ca, Fe, Ni, Rb, Sr). In some cases, significant interactions were also observed, in particular between temperature and canopy position. Consistent with our earlier report (Huber et al., 2016), canopy position had a significant effect on seed elemental concentrations of 17 of the 20 analyzed elements in at least 1 year of the study, and for 10 of those elements (Ca, Co, Cu, Fe, Mg, Mn, P, Rb, Sr, Zn) the effect was observed in both years of the study. For a summary of the results, see Tables 4 and 5. In the following, we present the results for nutritionally important minerals (e.g. Fe, Zn, Mg, Ca, Se and Cu; USDA, 2015) and others of particular interest in more detail below.
Table 4.
Complete block analysis of variance of the elemental mineral concentrations in soybean seedsin the two seasons for the main effects [CO2] concentration 1 (400 μmol mol−1, 600 μmol mol−1), temperature (control, heat), canopy position (bottom, middle, top) and the interaction terms
| Element | Year | CO2 | Temp | CO2 × Temp | Canopy | CO2 × Canopy | Temp × Canopy | CO2 × Temp × Canopy |
|---|---|---|---|---|---|---|---|---|
| Al | 2014 | ns | ns | ns | ns | ns | ns | ns |
| 2015 | ns | <0.05 | ns | ns | ns | ns | ns | |
| As | 2014 | ns | <0.05 | ns | ns | ns | ns | ns |
| 2015 | ns | <0.1 | ns | ns | ns | ns | ns | |
| B | 2014 | ns | <0.001 | ns | <0.1 | ns | <0.0001 | ns |
| 2015 | <0.1 | <0.0001 | <0.1 | ns | <0.01 | <0.0001 | <0.05 | |
| Ca | 2014 | <0.1 | <0.01 | ns | <0.0001 | ns | ns | ns |
| 2015 | ns | <0.01 | ns | <0.01 | ns | <0.05 | ns | |
| Cd | 2014 | ns | <0.l | ns | ns | ns | <0.01 | ns |
| 2015 | ns | ns | ns | ns | ns | <0.1 | ns | |
| Co | 2014 | ns | <0.05 | <0.1 | <0.0001 | ns | <0.001 | ns |
| 2015 | ns | ns | ns | <0.001 | ns | <0.05 | ns | |
| Cu | 2014 | ns | ns | ns | <0.0001 | <0.01 | <0.01 | <0.1 |
| 2015 | ns | ns | ns | <0.0001 | <0.05 | <0.01 | ns | |
| Fe | 2014 | <0.1 | <0.001 | ns | <0.0001 | ns | ns | ns |
| 2015 | <0.05 | <0.01 | ns | <0.0001 | ns | <0.05 | ns | |
| K | 2014 | <0.1 | ns | ns | ns | <0.05 | ns | ns |
| 2015 | ns | ns | ns | <0.0001 | ns | ns | <0.1 | |
| Mg | 2014 | ns | ns | ns | <0.01 | ns | ns | ns |
| 2015 | ns | <0.1 | ns | <0.05 | ns | <0.1 | ns | |
| Mn | 2014 | ns | ns | ns | <0.0001 | ns | ns | <0.1 |
| 2015 | ns | <0.1 | ns | <0.0001 | ns | ns | ns | |
| Mo | 2014 | ns | ns | ns | <0.0001 | <0.05 | ns | ns |
| 2015 | ns | ns | ns | ns | ns | ns | ns | |
| Na | 2014 | ns | ns | ns | <0.01 | ns | ns | ns |
| 2015 | ns | ns | ns | ns | ns | ns | ns | |
| Ni | 2014 | ns | <0.05 | ns | ns | ns | <0.01 | ns |
| 2015 | ns | <0.01 | ns | <0.05 | ns | ns | ns | |
| P | 2014 | ns | <0.001 | ns | <0.05 | <0.05 | <0.05 | ns |
| 2015 | ns | ns | ns | <0.1 | ns | <0.1 | ns | |
| Rb | 2014 | ns | <0.01 | ns | 0.05 | ns | <0.01 | ns |
| 2015 | ns | <0.05 | ns | <0.0001 | ns | ns | ns | |
| S | 2014 | ns | <0.1 | ns | ns | ns | <0.05 | ns |
| 2015 | <0.05 | ns | ns | <0.0001 | ns | ns | ns | |
| Se | 2014 | ns | ns | <0.1 | <0.1 | <0.05 | ns | ns |
| 2015 | ns | ns | <0.1 | ns | ns | <0.05 | ns | |
| Sr | 2014 | ns | <0.05 | ns | <0.0001 | <0.05 | ns | ns |
| 2015 | ns | <0.01 | ns | <0.0001 | ns | <0.1 | ns | |
| Zn | 2014 | ns | ns | ns | <0.1 | ns | <0.05 | ns |
| 2015 | <0.1 | ns | ns | 0.01 | ns | <0.1 | ns | |
| NumDF | 1 | 1 | 1 | 2 | 2 | 2 | 2 | |
| DenDF | 3 | 6 | 6 | 24 | 24 | 24 | 24 |
Table 5.
Overview of CO2, temperature and canopy layer effects (magnitude and direction) for selected mineral elements measured in this study and reported CO2 effects
| Element | Year (for our study only) | CO2 effect (%) | Temp. effect (%) | Canopy layer effect (%) | Reported CO2 effecta (%) |
|---|---|---|---|---|---|
| B | 2014 | ns | +19.8 |
+1.5 +1.6 |
−6.4 (< 0.0001) |
| 2015 | −6.0 | +17.0 |
ns ns |
||
| Ca | 2014 | −5.5 | +9.1 |
+13.9 +13.5 |
−5.8 (< 0.0001) |
| 2105 | ns | +17.9 |
+5.0 +5.2 |
||
| Cu | 2014 | ns | ns | ns | −5.7 (< 0.0001) |
| 2015 | ns | ns | ns | ||
| Fe | 2014 | −8.7 | +11.1 |
−12.5 −2.1 |
−4.1 (< 0.0001) |
| 2015 | −7.7 | +7.4 |
−12.5 −6.3 |
||
| K | 2014 | 3.1 | ns | ns | 0.1 (0.857) |
| 2015 | ns | ns |
−3.1 −0.4 |
||
| Mg | 2014 | ns | ns |
−0.2 −5.1 |
−3.5 (< 0.0001) |
| 2015 | ns | +7.0 |
−1.3 −3.4 |
||
| Mn | 2014 | ns | ns |
+8.8 +15.8 |
−1.4 (0.204) |
| 2015 | ns | +5.8 |
+10.9 +18.0 |
||
| P | 2014 | ns | −10.9 |
+1.1 +2.6 |
−0.7 (0.379) |
| 2015 | ns | ns |
−1.4 +3.9 |
||
| S | 2014 | ns | +3.6 | ns | −2.9 (< 0.0001) |
| 2015 | −9.6 | ns |
+9.4 +5.9 |
||
| Se | 2014 | ns | ns |
−0.6 −24.5 |
|
| 2015 | ns | ns |
ns ns |
||
| Zn | 2014 | ns | +3.4 |
+0.2 −5.8 |
−5.1 (< 0.0001) |
| 2015 | −8.9 | +6.4 |
−5.4 −2.4 |
Iron and magnesium
Both minerals are important for human nutrition and varied with canopy position in the same way, with higher concentrations in seeds produced at the bottom compared with the top of the canopy (Figure 5). However, only the seed concentration of iron [Fe] was affected by eCO2 and elevated temperature in both seasons. Seed [Fe] was significantly lower under eCO2 at all canopy positions (Figure 5a). On average, eCO2 decreased seed [Fe] by 8.7% in 2014 and by 7.7% in 2015. In contrast, elevated temperature significantly increased [Fe] by 11.1% in 2014 and by 7.4% in 2015 over the control temperature treatment irrespective of [CO2]. In both years and under all treatments, seed [Fe] decreased towards the top of the canopy, but in 2015 this effect was more pronounced in the control temperature treatments, with −23.2% lower [Fe] at the top compared with the bottom, while under the heat treatment this decrease was only −13%.
Figure 5.
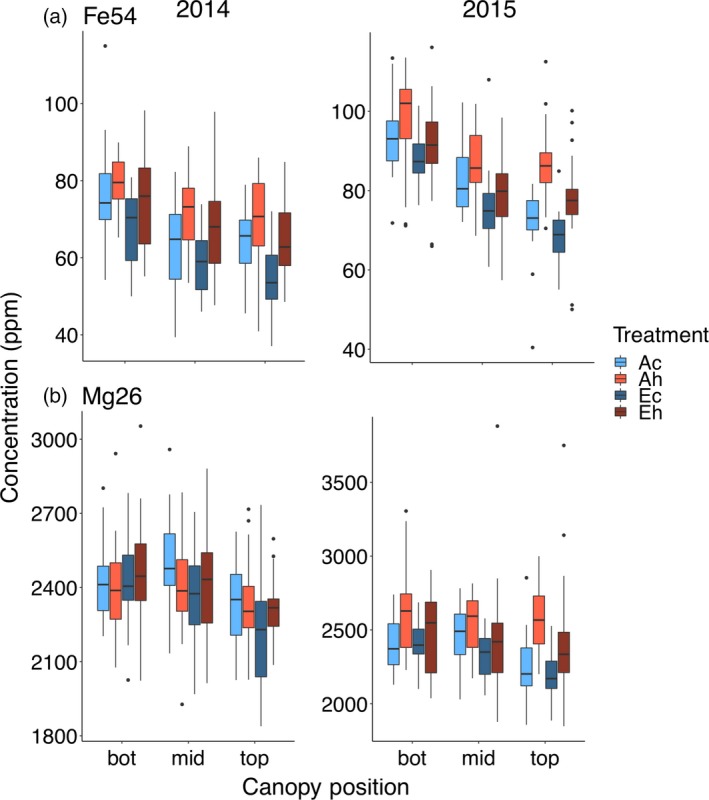
Canopy gradients of (a) seed [Fe] and (b) seed [Mg].
Seeds produced at the bottom, middle and top third of the main stem were analyzed from plants grown at elevated atmospheric CO2 concentration (eCO 2) and elevated temperature. Boxplots display the median, and 25 and 75% percentiles, and whiskers extend to 1.5 × interquartile range, with outliers (if any) shown. Treatment abbreviations as in Figure 1.
Magnesium concentration was not significantly affected by eCO2 (Figure 5b). Seed [Mg] decreased towards the top of the canopy in 2014 (top versus bottom: −5.2%) and 2015 (top versus bottom: −4.7%). In 2015, higher seed [Mg] was observed under the heat treatment (+7.0%) in comparison to the control temperature treatment, and this effect was larger in seeds at the top of the canopy (+11.9%) compared with the middle (+4.2%) or bottom canopy (+5.1%) positions.
Zinc
Elevated [CO2] decreased seed [Zn], but the decrease was only significant in 2015 (−8.9%), while elevated temperature significantly increased [Zn] in 2014 (+3.4%) and 2015 (+6.4%; Figure 6). Seed [Zn] decreased towards the top of the canopy in 2014 (top minus bottom: −5.6%) and 2015 (top minus bottom: −7.7%), and this effect was influenced by temperature, with a more pronounced decrease under the control temperature treatment (top minus bottom: 2014, −11.6%; 2015, −12.8%) than under the heat treatment (top minus bottom: 2014, 0.3%; 2015, −2.6%; Table 4).
Figure 6.
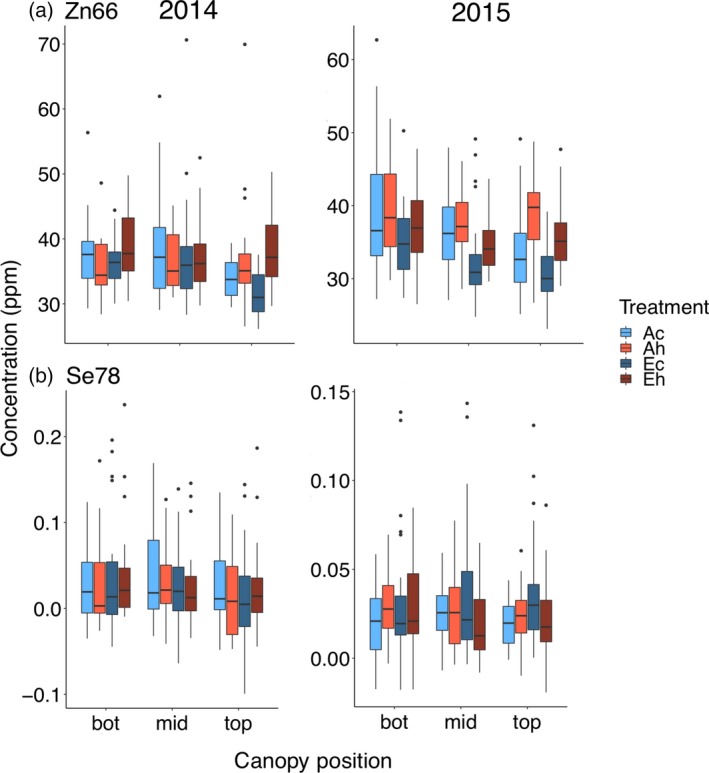
Canopy gradients of (a) seed [Zn] and (b) [Se].
Seeds produced at the bottom, middle and top third of the main stem were analyzed from plants grown at elevated atmospheric CO2 concentration (eCO 2) and elevated temperature. Treatment abbreviations and boxplots as in Figure 1.
Selenium
In contrast to Zn, Se is present at substantially lower concentrations, and no significant main effects of eCO2 or elevated temperature were observed (Figure 6). Variation in the range of seed [Se] was much higher in 2014, and there was a significant effect of canopy position on seed [Se] only in 2014 (top minus bottom: −25.0%).
Calcium, manganese and strontium
Calcium, Mn and Sr, which is included in the group because it is a chemical analog of Ca and therefore frequently correlated (Baxter, 2009), are present at very different concentrations in seeds (Ca >> Mn > Sr). However, they responded similarly in terms of variation with canopy position, and response to CO2 concentration and temperature (Figure 7). For example, the seed concentrations of Ca, Mn and Sr were not affected by eCO2, with the exception that seed [Ca] was reduced by 5.5% in just 1 year (2014). Conversely, elevated temperature increased seed [Ca] and [Sr] in both years, but [Mn] only in 2015. As noted above, seed [Ca] increased significantly towards the top of the canopy in 2014 (top minus bottom position: +29.2%), but in 2015 this effect was mainly observed in the heat treatment (top minus bottom: +16.9%) and not for the control temperature treatment (top minus bottom: +3.2%). A significant increase of Mn towards the top of the canopy was observed in both seasons (top minus bottom: +26.0% in 2014, +30.9% in 2015). Sr also increased significantly towards the top of the canopy in 2014 (top minus bottom position: +74.0%) and to a lesser extent in 2015 (top minus bottom position: +38.4%).
Figure 7.
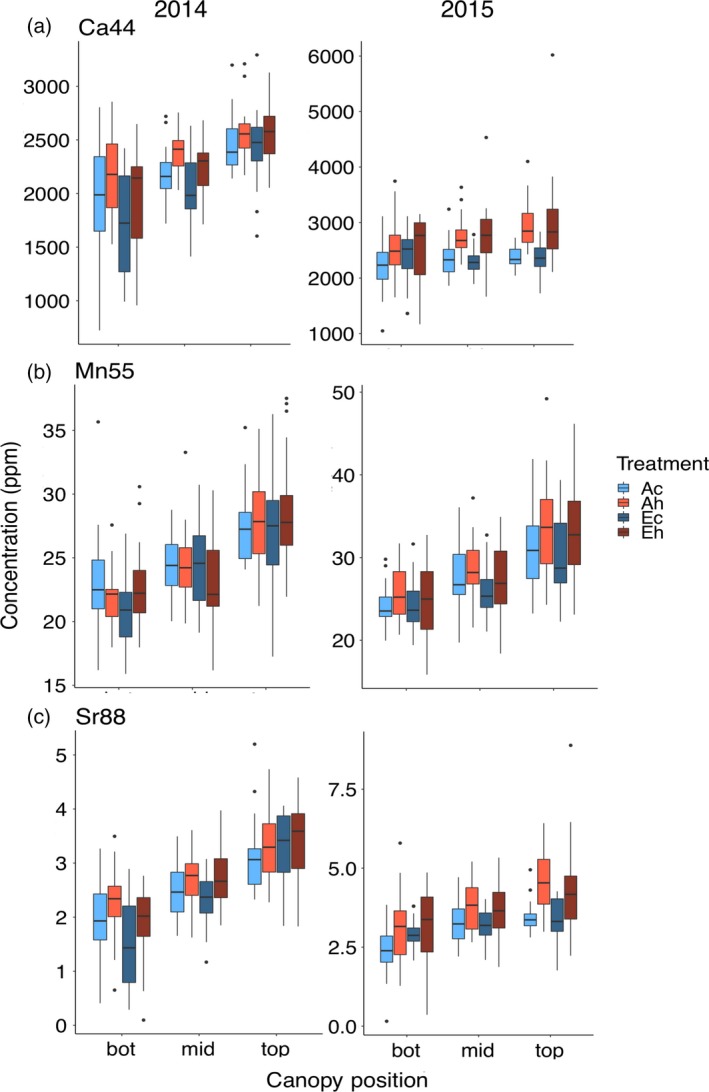
Canopy gradients of (a) seed [Ca], (b) [Mn] and (c) [Sr].
Seeds produced at the bottom, middle and top third of the main stem were analyzed from plants grown at elevated atmospheric CO2 concentration (eCO 2) and elevated temperature. Treatment abbreviations and boxplots as in Figure 1.
Boron
At ambient temperature, seed [B] tended to decrease from the bottom to the top of the canopy (top minus bottom: 2014, −8.5%; 2015, −11.4%). Elevated CO2 significantly decreased [B] by 6.0% in 2015, but this effect was not observed in 2014. In contrast, elevated temperature significantly increased seed [B] in both years (2014, 19.8%; 2015, 17.0%), and there was a significant temperature by canopy interaction. As noted above, seed [B] tended to decrease towards the top of the canopy under the control temperature treatment, but increased towards the top of the canopy under the high temperature treatment (top minus bottom: 2014, +14.0%; 2015, +9.9%). While seed [B] was increased by elevated temperature at all canopy positions, the greatest impact was observed at the top of the canopy (Figure 8).
Figure 8.
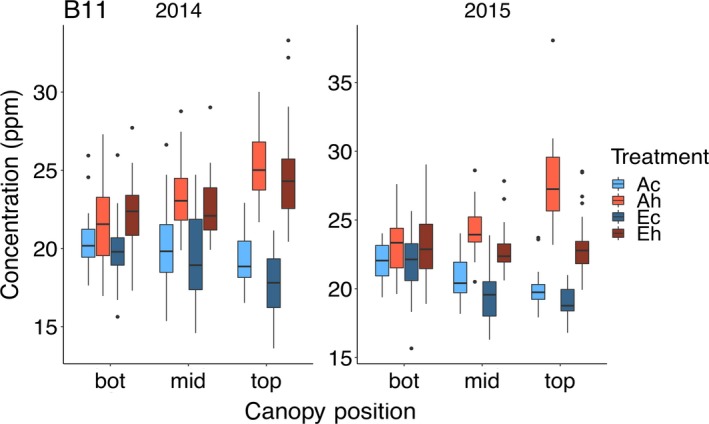
Canopy gradients of seed [B].
Seeds produced at the bottom, middle and top third of the main stem were analyzed from plants grown at elevated atmospheric CO2 concentration (eCO 2) and elevated temperature. Treatment abbreviations and boxplots as in Figure 1.
Correlations between seed mineral concentrations and seed yield
In order to determine whether changes in seed yield influenced seed mineral concentrations, we compared seed mineral concentrations with seed yields from the corresponding canopy position, and the results are summarized in Table 6. With a few exceptions where there was a positive correlation (i.e. 1 year of study each for P and S), in the majority of cases the correlations were not strong and were not statistically significant (P < 0.10). However, two exceptions emerge with seed [B] and [Fe], where significant negative correlations were observed in both years of the study (Figure 9; Table 6). These results suggest that B, Fe and, perhaps to a lesser extent, Mg and Ca are in limited or fixed supply to the developing seeds, and that changes in concentrations will reflect, in part at least, changes in the number of seeds produced in a given environment.
Table 6.
Overview of correlation analysis between seed mineral concentration and total seed yield at the corresponding canopy position. Where statistically significant (P < 0.10) the P‐values are shown parenthetically
| Element | 2014 | 2015 |
|---|---|---|
| r value | ||
| B | −0.52 (P < 0.001) | −0.36 (P = 0.012) |
| Na | ns | ns |
| Mg | ns | −0.25 (P = 0.089) |
| Al | ns | ns |
| P | 0.51 (P < 0.001) | ns |
| S | ns | 0.33 (P = 0.024) |
| K | ns | −0.27 (P = 0.059) |
| Ca | −0.40 (P < 0.005) | ns |
| Fe | −0.37 (P < 0.001) | −0.60 (P < 0.001) |
| Mn | ns | ns |
| Co | ns | ns |
| Ni | −0.26 (P = 0.075) | ns |
| Cu | ns | ns |
| Zn | ns | ns |
| As | ns | −0.36 (P = 0.011) |
| Se | ns | ns |
| Rb | ns | ns |
| Sr | −0.30 (P = 0.04) | ns |
| Mo | ns | ns |
| Cd | −0.34 (P = 0.018) | ns |
Figure 9.
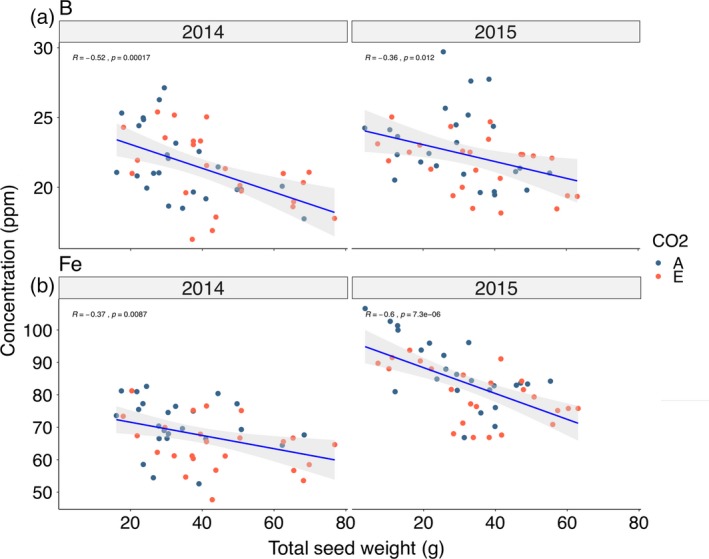
Correlation plots between seed mineral concentration and seed yield (g per 10 plants) at the corresponding canopy position for (a) seed [B] and (b) seed [Fe].
Data points are coded for samples taken from plants grown at ambient and elevated atmospheric CO2 concentration (eCO 2).
Discussion
In the present study, we analyzed the impact of eCO2 and elevated temperature, singly and in combination, on soybean seed yield and seed protein, oil and mineral concentrations. The results showed that eCO2 increased seed yield and reduced the concentrations of a few minerals such as Fe, consistent with many previous studies on nutritional quality for a range of crops (Myers et al., 2014; Broberg et al., 2017; Chaturvedi et al., 2017). Elevated temperature, however, reduced yield and increased the concentration of a range of minerals. In general, elevated temperature counteracted the increase in yield and the reduction of mineral concentrations (where observed), suggesting that the previously reported increase in seed yield and threat to human nutrition associated with eCO2 (McGrath and Lobell, 2013; Loladze, 2014; Myers et al., 2014) may not be realized when combined with elevated temperatures (Ruiz‐Vera et al., 2013; Köhler et al., 2016). These conclusions are discussed in more detail below.
Elevated temperature reduces seed yield differentially through the canopy
As expected (Huxley et al., 1976; Sionit et al., 1987; Gibson and Mullen, 1996; Ruiz‐Vera et al., 2013), the elevated temperature treatment reduced seed yield on both a land area basis (Figure 2) and per plant basis (Figure 3a). However, looking at seed yield as a function of position within the canopy suggested that elevated temperature preferentially reduced yield in the bottom third of the canopy (Figure 3b). This is somewhat counter‐intuitive, as it would be reasonable to assume that elevated temperature generated by infrared heaters (that do not increase air temperature) would primarily have an impact in the upper portion of the canopy. However, the observed result would be consistent with the notion that upper canopy leaves play the primary role in provision of assimilates (reduced‐C in the form of sucrose and reduced‐N in the form of amide amino acids) to support seed development throughout the canopy; when leaf photosynthesis (and hence assimilate supply) is limited by elevated temperature (Ruiz‐Vera et al., 2013), seed development lower in the canopy would be most attenuated. The unexpected impact of the heat treatment on yield of seed produced at the bottom canopy position was observed at both ambient and eCO2.
Elevated temperature, but not eCO2, affects seed protein and oil concentrations
Consistent with earlier reports (Collins and Cartter, 1956; Escalante and Wilcox, 1993a,b; Huber et al., 2016), there was a significant canopy position effect on seed storage product concentration, with seeds at the top of the canopy having higher protein and seeds at the bottom having higher oil concentrations (Figure 4). In general, eCO2 did not have a significant effect on seed protein or oil concentrations, as observed in other studies with soybeans (Myers et al., 2014; Bishop et al., 2015; Jin et al., 2017). In contrast, elevated temperature tended to reduce seed protein concentration and increase seed oil concentration, which may be related to the previously reported reduction of leaf photosynthesis at high temperature (Ruiz‐Vera et al., 2013) that could impact seed composition throughout the canopy.
Impact of eCO2 and elevated temperature on seed minerals
Consistent with previous reports, elevated CO2 reduced the concentration of Fe and Zn significantly (with the exception of Zn in 2014). No other elements were significantly impacted by eCO2, although the general trend was to reduce mineral concentrations, consistent with earlier reports (McGrath and Lobell, 2013; Loladze, 2014; Myers et al., 2014; Weigel, 2014). In contrast, elevated temperature significantly affected the concentrations of Fe, Zn and a number of minerals not affected by eCO2, generally having the opposite effect of eCO2. These changes suggest that there is a linkage between the increased seed yield and decreased seed mineral concentrations in eCO2, and that elevated temperature reverses both effects. Collectively, these results suggest that the previously reported effects of increasing atmospheric [CO2] on seed mineral concentration may not be realized in future climates where eCO2 occurs with other environmental changes such as elevated temperature. Interestingly, the observed effects of elevated temperature and eCO2 on seed minerals do not correlate with the mode of mineral delivery and uptake into the plant; i.e. both treatments affected minerals that move by mass flow and are therefore affected by transpiration (e.g. Ca, Mg, and S), as well as those with diffusion to the root as the main mechanism (e.g. K, P, Mn, Zn, Cu and Fe; Oliver and Barber, 1966; Oliveira et al., 2010).
To accumulate in seeds, minerals need to be available in the soil water; presented to the root surface by mass flow or diffusion; taken up into the root; distributed within the plant; and finally partitioned to the developing seeds. Limitations at any of these steps could result in changes in seed mineral concentrations when the number of seeds produced is altered and seed size does not change (as was observed in this study). For example, a mineral present in limiting or fixed amounts may be reduced in concentration in seeds when the number of seeds produced is increased (e.g. at eCO2), and conversely increased in concentration when the number of seeds produced is decreased (e.g. at high temperature). In such a case, seed mineral concentration would be negatively correlated with seed yield, reflecting enrichment when yield was reduced and dilution when growth was increased. Indeed, this was observed for seed [B] and [Fe] in both years of the study, and in 1 year of study for seed [Ca] and [Mg]. Exactly which step in the process of mineral accumulation in the developing seed is limiting or highly regulated may vary with the mineral, and will be an important focus for future research. Fundamentally, this study suggests that the effects of climate change will be variable on nutrient and caloric levels, and our current knowledge is insufficient to predict the results.
The unusual response of B to eCO2 and elevated temperature
Boron is an essential micronutrient and, while the functions of this micronutrient are not fully understood, it is recognized that B may impact various aspects of plant metabolism and transport (Pilbeam and Kirkby, 1983) and cell wall composition (O'Neill et al., 2004). In terms of transport, B is present at neutral pH primarily as boric acid, which can be transported along with the borate anion (Woods, 1996). However, B can be more readily transported as a B‐polyol complex (Brown and Shelp, 1997). It is thought that species that produce polyols (e.g. sugar alcohols such as sorbitol and mannitol) may display greater B mobility within the plant, in particular in the phloem, and may explain the large variation in B mobility among species that has been noted. One intriguing possibility is that at elevated temperature, soybean leaves produce more sugar alcohols compared with ambient temperature, which could result in the increased seed [B] observed. This speculative mechanism would also be consistent with increased seed [B] at the top rather than bottom of the canopy (Figure 8). This is clearly an area for further study.
Concluding remarks
The reduction in a broad range of nutritionally important minerals in soybean seeds as a result of growth at eCO2 (McGrath and Lobell, 2013; Loladze, 2014; Myers et al., 2014) was generally confirmed in the present study and, conversely, the increase in seed mineral concentrations by growth at elevated temperature (at ambient CO2) was shown. In general, growth at eCO2 plus elevated temperature restored seed yield and mineral concentrations to nearly those observed at current ambient conditions, calling into question the predictive value of studies looking only at eCO2 to identify threats to human nutrition. The general inverse relationship between mineral concentration and seed yield per plant as affected by eCO2 and elevated temperature suggests that dilution by increased growth (at eCO2) or enrichment by reduced growth (at elevated temperature) may be general factors impacting seed mineral concentrations. A key mechanism behind these responses can be related to rates of water movement through the plants. Elevated CO2 causes a decrease in stomatal conductance (Bernacchi et al., 2006), which is shown to lead to lower evapotranspiration rates (Bernacchi et al., 2007). Elevated temperature, however, drives a higher VPD and leads to higher evapotranspiration (Kimball, 2005). The impact of elevated CO2 and warmer temperature on evapotranspiration could potentially explain the opposite responses of the treatments on nutritional quality, including the key interaction between treatment and canopy position on seed quality. Alternatively, a ‘dilution’ of nutrients by elevated CO2 has been proposed as another potential mechanism driving the decrease in nutrition in crops (Chaturvedi et al., 2017), and may contribute to our observed responses. Warmer soils associated with the heating treatment (Black et al., 2017) may also increase the rate at which soil nutrients are taken up by roots through a variety of mechanistic chemical, physical and biological responses to temperature (Pregitzer and King, 2005). It is likely that the observed responses are driven by a complex interaction involving plant and soil responses to the treatments, necessitating more research to fully refine the underlying mechanisms.
It remains to be determined how broadly applicable our results are outside of the single cultivar of soybeans used in this experiment. Other cultivars and species may respond differently to either or both stresses, with unknown effects on the nutritional components. We are also unable to predict whether breeding programs conducted in these predicted conditions will be able to ameliorate the loss of nutritional content while maintaining the yield gains.
Another noteworthy point is that seed composition of protein, oil and some minerals varies with node position at which the seeds developed, and confirms our earlier study (Huber et al., 2016). Moreover, environmental factors that affect composition tend to impact seed produced at all positions within the canopy, even though the effects on yield are not necessarily uniform across the canopy. The largest exception to this generalization was seed [B], which was strikingly increased in seeds at the top of the canopy when plants were grown at elevated temperature. As discussed above, the potential for altered metabolism at elevated temperature resulting in enhanced B mobility within the plant is an attractive hypothesis for further testing.
Experimental procedures
Plant material and growth conditions
An indeterminant variety of soybean [Glycine max (L.) Merr. cv. ‘Thorne’] was grown in a complete block design (n = 4) in the Soybean Temperature by Free‐Air CO2 Enrichment (Soy‐T‐FACE) experiment at the SoyFACE field site near Urbana‐Champaign, Illinois, USA (40°2′30.49″N, 88°13′58.80″W, 230 m above sea level) during 2014 and 2015 growing seasons. The experiment consisted of four blocks, each containing one ambient and one eCO2 plot, and within each plot there was an unheated and a heated sub‐plot. Seeds were planted by hand at 5‐cm intervals in 38‐cm rows. Eight 11‐m‐long rows were planted in each of the eight plots (four rows of the wild‐type ‘Thorne’ used in this study, and the other four of a transgenic soybean line that was used for another experiment). Characteristics of the site and details of agricultural practices can be found in previous SoyFACE studies (Ainsworth et al., 2004; Bernacchi et al., 2006).
The ambient [CO2] plot was at approximately 400 μmol mol−1, and the elevated one at approximately 600 μmol mol−1, using FACE fumigation technology (Miglietta et al., 2001). The heated sub‐plots were each equipped with an infrared heater array, as described in detail previously (Ruiz‐Vera et al., 2013), installed at 1.0–1.2 m above the canopy on a telescoping mast system (Ruiz‐Vera et al., 2015). Using a PID feedback control system we warmed the crop canopy to a target elevation of +3.5°C above that of the canopy temperature in the unheated sub‐plot. The target temperature increase was based on the low‐response model projections for surface temperature in the Midwest in 2050 (Rowlands et al., 2012). During the day (06:00 hours to 18:00 hours) and with rainy days excluded, mean temperature differences between the sub‐plots were between 0.5 and 1.0°C lower than the target set point (Table 1), resulting in an average temperature increase of +2.7°C. During the night the average temperature difference was +3.4°C. The heated sub‐plot diameter was 3.5 m, resulting in an effective heated sub‐plot area of 9.6 m2. Canopy temperature in each sub‐plot was measured by infra‐red radiometers (SI‐111; Apogee Instruments) connected to data‐loggers CR1000 (Micrologger, Campbell Scientific, Logan, UT, USA). Canopy temperature measurements were collected every 5 sec to control the heater output, averaged every 10 min, and these values were stored. For more information, see Köhler et al. (2016). In the figures, the treatments are referred to as ‘Ac’ (Ambient [CO2], control temperature), ‘Ah’ (Ambient [CO2], heat), ‘Ec’ (Elevated [CO2], control temperature) and ‘Eh’ (Elevated [CO2] + heat).
Harvest and seed yield determination
After full maturity and dry‐down were complete, 10 plants were harvested by hand (stems were cut with scissors at about 2.5 cm above the ground), wrapped in burlap bags and air‐dried at 27°C in a drying room. The individual plants were cut into three equal parts (bottom, middle and top sections), and the pods from the three sections threshed separately and the seeds weighed. The three sections are hereafter referred to as bottom, middle and top canopy positions.
Protein and oil analysis
Protein and oil were measured with an Infratech 1241 Grain Analyzer (FOSS Analytical AB, Höganäs, Sweden), which is a true Near‐Infrared Transmission instrument that generates a spectrum from 850 to 1050 nm via the monochrome light source and mobile grating system. A 50‐ml seed sample was used that allowed for 10 subsample readings reported on a 13% moisture basis.
Mineral analysis
Six seeds from each sample were selected at random from the seed yield samples for mineral analysis as described in Ziegler et al. (2013). In brief, a single seed from each main stem canopy position was weighed using a custom‐built seed‐weighing robot, and then digested in concentrated nitric acid before loading onto an Elan ICP‐MS. Internal standards were used to control for differences in dilution and sample injection. Custom scripts were used to correct for internal standards and correct for sample weight.
Statistical analysis
Data were analyzed using a randomly blocked mixed‐model analysis of variance (PROC MIXED, SAS 9.4) taking into account the split‐plot design of the experiment. The analyses were performed separately for individual years. The model for the seed yield, protein and oil data from the harvest of individual plants and for the elemental concentrations included the fixed factors, [CO2] (ambient, elevated), temperature (control, heated), and position in the canopy (bottom, middle or top). The model for the bulk seed yield included only the fixed factors, [CO2] (ambient, elevated) and temperature (control, heated). Block was included as a random factor in all five models. For the analysis of the elemental concentrations, the results from the individually analyzed six seeds per sample were averaged for each canopy layer within each sub‐plot. Significant differences between least square means for a priori determined comparisons were analyzed using post hoc tests (LSMEANS). The probability for statistical significance was set at P < 0.1 a priori to reduce the possibility of type II errors. All data and analysis scripts used in the analysis are available at http://www.ionomicshub.org/home/PiiMS/fileDownload?file=52.
Conflict of interest
The authors declare no conflicts of interest.
Supporting information
Figure S1. Variation in single seed weight as a function of canopy position (bottom, middle or top third of the main stem) where the seeds were produced. A.Control, ambient CO2, control temperature; A.Hot, ambient CO2, heated + 3.5°C; E.Control, elevated CO2, control temperature; E.Hot, elevated CO2, heated + 3.5°C.
Figure S2. Correlation plots between single seed weight and seed yield for the corresponding canopy position. Results from 2014 and 2015 were combined, and data points are color coded according to (a) ambient versus elevated temperature; (b) canopy position; and (c) ambient versus elevated CO2. The correlations were not statistically significant (P = 0.28) and simply document that changes in yield were primarily driven by variation in number of seeds produced.
Acknowledgements
This work was supported by the USDA‐Agricultural Research Service. This material is based upon work that is supported by the National Institute of Food and Agriculture, US Department of Agriculture, under award number 2014‐67013‐21783. The authors thank Greg Ziegler for skilled technical assistance in the mineral analysis, and Jennifer Barrett and Amber Wolf for assistance with figure creation.
This article has been contributed to by US Government employees and their work is in the public domain in the USA.
Contributor Information
Carl J. Bernacchi, Email: carl.bernacchi@ars.usda.gov.
Ivan R. Baxter, Email: ibaxter@danforthcenter.org.
References
- Ainsworth, E.A. and Long, S.P. (2005) What have we learned from 15 years of free‐air CO2 enrichment (FACE)? A meta‐analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2 . New Phytol. 165, 351–371. [DOI] [PubMed] [Google Scholar]
- Ainsworth, E.A. and Rogers, A. (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ. 30, 258–270. [DOI] [PubMed] [Google Scholar]
- Ainsworth, E.A. , Rogers, A. , Nelson, R. and Long, S.P. (2004) Testing the “source–sink” hypothesis of down‐regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max . Agric. For. Meteorol. 122, 85–94. [Google Scholar]
- Baxter, I. (2009) Ionomics: studying the social network of mineral nutrients. Curr. Opin. Plant Biol. 12, 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacchi, C.J. , Morgan, P.B. , Ort, D.R. and Long, S.P. (2005) The growth of soybean under free air [CO2] enrichment (FACE) stimulates photosynthesis while decreasing in vivo Rubisco capacity. Planta 220, 434–446. [DOI] [PubMed] [Google Scholar]
- Bernacchi, C.J. , Leakey, A.D.B. , Heady, L.E. , Morgan, P.B. , Dohleman, F.G. , McGrath, J.M. , Gillespie, K.M. , Wittig, V.E. , Rogers, A. and Long, S.P. (2006) Hourly and seasonal variation in photosynthesis and stomatal conductance of soybean grown at future CO2 and ozone concentrations for 3 years under fully open‐air field conditions. Plant Cell Environ. 29, 2077–2090. [DOI] [PubMed] [Google Scholar]
- Bernacchi, C.J. , Kimball, B.A. , Quarles, D.R. , Long, S.P. and Ort, D.R. (2007) Decreases in stomatal conductance of soybean under open‐air elevation of [CO2] are closely coupled with decreases in ecosystem evapotranspiration. Plant Physiol. 143, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, K.A. , Betzelberger, A.M. , Long, S.P. and Ainsworth, E.A. (2015) Is there potential to adapt soybean (Glycine max Merr.) to future [CO2]? An analysis of the yield response of 18 genotypes in free‐air CO2 enrichment. Plant Cell Environ. 38, 1765–1774. [DOI] [PubMed] [Google Scholar]
- Black, C.K. , Davis, S.C. , Hudiburg, T.W. , Bernacchi, C.J. and DeLucia, E.H. (2017) Elevated CO2 and temperature increase soil C losses from a soybean‐maize ecosystem. Glob. Chang. Biol. 23, 435–445. [DOI] [PubMed] [Google Scholar]
- Broberg, M.C. , Högy, P. and Pleijel, H. (2017) CO2‐induced changes in wheat grain composition: meta‐analysis and response functions. Agronomy 7, 32. [Google Scholar]
- Brown, P.H. and Shelp, B.J. (1997) Boron mobility in plants. Plant Soil 193, 85–101. [Google Scholar]
- Chaturvedi, A.K. , Bahuguna, R.N. , Pal, M. , Shah, D. , Maurya, S. and Jagadish, K.S.V. (2017) Elevated CO2 and heat stress interactions affect grain yield, quality and mineral nutrient composition in rice under field conditions. Field Crops Res. 206, 149–157. [Google Scholar]
- Collins, F.I. and Cartter, J.L. (1956) Variability in chemical composition of seed from different portions of the soybean plant. Agron. J. 48, 216–219. [Google Scholar]
- Collins, M. , Knutti, R. , Arblaster, J.M. (2013) Long‐term climate change: Projections, commitments and irreversibility. Climate change 2013: The physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change(Stocker, T.F., Qin, D., Plattner, G.‐K. et al, eds). Cambridge, UK: Cambridge University Press, pp. 1029–1136. [Google Scholar]
- Escalante, E.E. and Wilcox, J.R. (1993a) Variation in seed protein among nodes of determinate and indeterminate soybean near‐isolines. Crop Sci. 33, 1166–1168. [Google Scholar]
- Escalante, E.E. and Wilcox, J.R. (1993b) Variation in seed protein among nodes of normal‐protein and high‐protein soybean genotypes. Crop Sci. 33, 1164–1166. [Google Scholar]
- Gibson, L.R. and Mullen, R.E. (1996) Influence of day and night temperature on soybean seed yield. Crop Sci. 36, 98–104. [Google Scholar]
- Hatfield, J.L. , Boote, K.J. , Kimball, B.A. , Ziska, L.H. , Izaurralde, R.C. , Ort, D. , Thomson, A.M. and Wolfe, D. (2011) Climate impacts on agriculture: implications for crop production. Agron. J. 103, 351–370. [Google Scholar]
- Hesketh, J.D. , Myhre, D.L. and Willey, C.R. (1973) Temperature control of time intervals between vegetative and reproductive events in soybeans. Crop Sci. 13, 250–254. [Google Scholar]
- Huber, S.C. , Li, K. , Nelson, R.L. , Ulanov, A.V. , DeMuro, C.M. and Baxter, I. (2016) Canopy position has a profound effect on soybean seed composition. PeerJ 4, e2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain, M.Z. , VanLoocke, A. , Siebers, M.H. , Ruiz‐Vera, U.M. , Markelz, R.J.C. , Leakey, A.D.B. , Ort, D.R. and Bernacchi, C.J. (2013) Future carbon dioxide concentration decreases canopy evapotranspiration and soil water depletion by field‐grown maize. Glob. Chang. Biol. 19(5), 1572–1584. [DOI] [PubMed] [Google Scholar]
- Huxley, P.A. , Summerfield, R.J. and Hughes, A.P. (1976) Growth and development of soyabean cv. TK5 as affected by tropical daylengths, day/night temperatures and nitrogen nutrition. Ann. Appl. Biol. 82, 117–133. [Google Scholar]
- Jin, J. , Li, Y. , Liu, X. , Wang, G. , Tang, C. , Yu, Z. , Wang, X. and Herbert, S.J. (2017) Elevated CO2 alters distribution of nodal leaf area and enhances nitrogen uptake contributing to yield increase of soybean cultivars grown in Mollisols. PLoS One 12, e0176688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball, B.A. (2005) Theory and performance of an infrared heater for ecosystem warming. Glob. Chang. Biol. 11, 2041–2056. [Google Scholar]
- Köhler, I.H. , Ruiz‐Vera, U.M. , VanLoocke, A. , Thomey, M.L. , Clemente, T. , Long, S.P. , Ort, D.R. and Bernacchi, C.J. (2016) Expression of cyanobacterial FBP/SBPase in soybean prevents yield depression under future climate conditions. J. Exp. Bot. 68, 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leakey, A.D. , Ainsworth, E.A. , Bernacchi, C.J. , Rogers, A. , Long, S.P. and Ort, D.R. (2009) Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J. Exp. Bot. 60, 2859–2876. [DOI] [PubMed] [Google Scholar]
- Loladze, I. (2014) Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. Elife 3, e02245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, S.P. , Ainsworth, E.A. , Rogers, A. and Ort, D.R. (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu. Rev. Plant Biol. 55, 591–628. [DOI] [PubMed] [Google Scholar]
- Marschner, H. (1995) Mineral Nutrition of Higher Plants, 2nd edn London: Academic Press. [Google Scholar]
- McGrath, J.M. and Lobell, D.B. (2013) Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO2 concentrations. Plant Cell Environ. 36, 697–705. [DOI] [PubMed] [Google Scholar]
- Miglietta, F. , Peressotti, A. , Vaccari, F.P. , Zaldei, A. , DeAngelis, P. and Scarascia‐Mugnozza, G. (2001) Free‐air CO2 enrichment (FACE) of a poplar plantation: the POPFACE fumigation system. New Phytologist., 150, 465–476. [Google Scholar]
- Myers, S.S. , Zanobetti, A. , Kloog, I. et al. (2014) Increasing CO2 threatens human nutrition. Nature 510, 139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, E.M.M. , Ruiz, H.A. , Alvarez, V.H. , Ferreira, P.A. , Costa, F.O. and Almeida, I.C.C. (2010) Nutrient supply by mass flow and diffusion to maize plants in response to soil aggregate size and water potential. Rev. Bras. Cienc. Solo 34, 317–327. [Google Scholar]
- Oliver, S. and Barber, S.A. (1966) An evaluation of mechanisms governing supply of Ca, Mg, K, and Na to soybean roots (Glycine max). Soil Sci. Soc. Am. J. 30, 82–86. [Google Scholar]
- O'Neill, M.A. , Ishii, T. , Albersheim, P. and Darvill, A.G. (2004) Rhamnogalacturonan II: structure and function of a borate cross‐linked cell wall pectic polysaccharide. Annu. Rev. Plant Biol. 55, 109–139. [DOI] [PubMed] [Google Scholar]
- Pilbeam, D.J. and Kirkby, E.A. (1983) The physiological role of boron in plants. J. Plant Nutr. 6, 563–582. [Google Scholar]
- Pregitzer, K.S. and King, J.S. (2005) Effects of soil temperature on nutrient uptake In Nutrient Acquisition by Plants: An Ecological Perspective (BassiriRad H., ed.). Berlin: Springer, pp. 277–310. [Google Scholar]
- Rowlands, D.J. , Frame, D.J. , Ackerley, D. et al. (2012) Broad range of 2050 warming from an observationally constrained large climate model ensemble. Nat. Geosci. 5, 256–260. [Google Scholar]
- Ruiz‐Vera, U.M. , Siebers, M. , Gray, S.B. , Drag, D.W. , Rosenthal, D.M. , Kimball, B.A. , Ort, D.R. and Bernacchi, C.J. (2013) Global warming can negate the expected CO2 stimulation in photosynthesis and productivity for soybean grown in the Midwestern United States. Plant Physiol. 162, 410–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Vera, U.M. , Siebers, M.H. , Drag, D.W. , Ort, D.R. and Bernacchi, C.J. (2015) Canopy warming caused photosynthetic acclimation and reduced seed yield in maize grown at ambient and elevated [CO2]. Glob. Chang. Biol. 21, 4237–4249. [DOI] [PubMed] [Google Scholar]
- Siebers, M.H. , Yendrek, C.R. , Drag, D. , Locke, A.M. , Rios Acosta, L. , Leakey, A.D. , Ainsworth, E.A. , Bernacchi, C.J. and Ort, D.R. (2015) Heat waves imposed during early pod development in soybean (Glycine max) cause significant yield loss despite a rapid recovery from oxidative stress. Glob. Chang. Biol. 21, 3114–3125. [DOI] [PubMed] [Google Scholar]
- Siebers, M.H. , Slattery, R.A. , Yendrek, C.R. , Locke, A.M. , Drag, D. , Ainsworth, E.A. , Bernacchi, C.J. and Ort, D.R. (2017) Simulated heat waves during maize reproductive stages alter reproductive growth but have no lasting effect when applied during vegetative stages. Agric. Ecosyst. Environ. 240, 162–170. [Google Scholar]
- Singh, S.K. , Barnaby, J.Y. , Reddy, V.R. and Sicher, R.C. (2016) Varying response of the concentration and yield of soybean seed mineral elements, carbohydrates, organic acids, amino acids, protein, and oil to phosphorus starvation and CO2 enrichment. Front. Plant Sci. 7, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sionit, N. , Strain, B.R. and Flint, E.P. (1987) Interaction of temperature and CO2 enrichment on soybean: photosynthesis and seed yield. Can. J. Plant Sci. 67, 629–636. [Google Scholar]
- US Department of Health and Human Services (2015) 2015–2020 Dietary Guidelines for Americans. Washington, DC: USDA. [Google Scholar]
- Weigel, H.J. (2014) Plant quality declines as CO2 levels rise. Elife 3, e03233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, W.G. (1996) Review of possible boron speciation relating to its essentiality. J. Trace Elem. Exp. Med. 9, 153–163. [Google Scholar]
- Yamaji, N. and Ma, J.F. (2017) Node‐controlled allocation of mineral elements in Poaceae. Curr. Opin. Plant Biol. 39, 18–24. [DOI] [PubMed] [Google Scholar]
- Ziegler, G. , Terauchi, A. , Becker, A. , Armstrong, P. , Hudson, K. and Baxter, I. (2013) Ionomic screening of field‐grown soybean identifies mutants with altered seed elemental composition. Plant Genome 6, 1–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Variation in single seed weight as a function of canopy position (bottom, middle or top third of the main stem) where the seeds were produced. A.Control, ambient CO2, control temperature; A.Hot, ambient CO2, heated + 3.5°C; E.Control, elevated CO2, control temperature; E.Hot, elevated CO2, heated + 3.5°C.
Figure S2. Correlation plots between single seed weight and seed yield for the corresponding canopy position. Results from 2014 and 2015 were combined, and data points are color coded according to (a) ambient versus elevated temperature; (b) canopy position; and (c) ambient versus elevated CO2. The correlations were not statistically significant (P = 0.28) and simply document that changes in yield were primarily driven by variation in number of seeds produced.


