Abstract
Introduction
There are concerns that immediate ART initiation (regardless of CD4 count) negatively affects HIV status disclosure, ART adherence and healthcare interactions. We assessed changes in these factors after the ‘Early access to ART for all’ intervention, a universal test‐and‐treat study in Swaziland.
Methods
We recruited two samples of participants between 2014 and 2017. The first group was interviewed before the intervention (control); the second group at the implementation and 6 months thereafter (intervention).
Results
High levels of disclosure to partners (controls and intervention: 94%) and family members (controls: 78%, intervention: 79%) were reported, and high levels of adherence (85% did not miss a dose among the controls, 84% in the intervention group). There were no changes in patients reporting feeling pressured to initiate ART (controls: 10%, intervention: 11%). The quality of interaction with healthcare workers improved after the intervention; healthcare workers explained more often the choice of ART initiation (controls: 88%, intervention: 93%) and the meaning of both CD4 and viral load test results (controls: 15%, intervention: 47%). More patients in the intervention group reported receiving test results (controls: 13%, intervention: 46%). We observed no changes in disclosure, adherence or patient experiences 6 months into the intervention compared to its start.
Conclusion
Our results suggest that both reported adherence and disclosure levels remain high after the introduction of immediate ART in Swaziland. We observed an improvement in the healthcare interactions, possibly due to training at participating facilities, which will be an important element for a successful roll‐out of immediate ART.
Keywords: HIV, antiretroviral therapy, disclosure, adherence, treatment as prevention, Swaziland
Abstract
Objectif
Il y a des craintes que l'initiation de l’ART immédiat (quel que soit la numération des CD4) affecte négativement la divulgation du statut VIH, l'adhésion au traitement et les interactions avec les soins de santé. Nous avons évalué les modifications de ces facteurs après l'intervention «Accès précoce à l’ART pour tous», une étude universelle de dépistage et traitement au Swaziland.
Méthodes
Nous avons recruté deux échantillons de participants entre 2014 et 2017. Le premier groupe a été interviewé avant l'intervention (témoins), le deuxième groupe lors de l'implémentation et six mois après (intervention).
Résultats
Des niveaux élevés de divulgation aux partenaires (témoin et intervention: 94%) et aux membres de la famille (témoins: 78%, intervention: 79%) ont été rapportés, ainsi que des taux élevés d'adhésion (85% n'ont pas oublié une dose chez les témoins, 84% dans le groupe d'intervention). Aucun changement n'a été observé chez les patients déclarant se sentir poussés à commencer l’ART (témoins: 10%, intervention: 11%). La qualité de l'interaction avec les agents de la santé s'est améliorée après l'intervention; les agents de santé expliquent plus souvent le choix de l'initiation de l’ART (témoins: 88%, intervention: 93%) et la signification des résultats des tests de CD4 et de la charge virale (témoins: 15%, intervention: 47%). Plus de patients du groupe d'intervention ont déclaré avoir reçu les résultats des tests (témoins: 13%, intervention: 46%). Nous n'avons observé aucun changement dans la divulgation, l'adhésion ou l'expérience des patients six mois après le début de l'intervention par rapport à son début.
Conclusion
Nos résultats suggèrent que les taux d'adhésion et de divulgation rapportés restent élevés après l'introduction de l’ART immédiat au Swaziland. Nous avons observé une amélioration des interactions avec les soins de santé, probablement due à la formation dispensée dans les établissements participants, ce qui constituera un élément important pour le succès du déploiement de l’ART immédiat.
Keywords: VIH, ART, divulgation, adhésion, traitement préventif, Swaziland
Introduction
International recommendations on the initiation of antiretroviral therapy (ART) included a threshold based on the patient's CD4 count until 2015 1, 2, 3, when WHO recommended initiation of ART on positive HIV diagnosis, regardless of WHO clinical stage or CD4 count 4.
Concerns have been raised that early initiation of ART might be associated with lower levels of treatment adherence. These concerns are based on studies where more treatment interruptions and an increased risk of loss to follow‐up was observed in people who started ART at a higher baseline CD4 count 5, 6. People with higher CD4 count were less likely to start ART when offered, primarily because they reported feeling healthy 7, 8. However, a study in Uganda challenged these concerns and found that individuals starting ART with a higher baseline CD4 cell count were equally adherent as those starting with lower CD4 counts 8. A systematic review did not find evidence to conclude that baseline CD4 count has an effect on ART adherence 9. Qualitative research identified barriers to starting ART regardless of CD4 count, such as feeling healthy, fear of side effects and stigma 10, 11. In particular, early initiation of ART also entails a fear of unintended disclosure, from visible side‐effects or being seen accessing HIV services 10. Factors that positively impact ART adherence are, among others, increased levels of disclosure of HIV status 12, 13, 14, better communication with healthcare workers 13, and reduced HIV‐related stigma 15, which is expected to decrease after an increased ART coverage 16.
Swaziland (statutory name of the country at time of study, current statutory name is Eswatini) is a country with a high HIV prevalence with 27% of adults HIV positive in 2016 17. Based on international guidelines, the Swaziland National AIDS Program (SNAP) has changed national recommendations from an initiation threshold of CD4 count < 200 cells/mm3 in 2003 to < 500 cells/mm3 in 2015 and to universal test and treat in October 2016 18, 19. ART coverage among diagnosed HIV positive adults was 87% in 2016 17.
The consortium Maximising antiretroviral therapy for better health and zero new infections (MaxART) implemented an ‘Early access to ART for all’ intervention in selected public facilities in Hhohho region, Swaziland. This meant that all HIV positive adults received ART, regardless of WHO clinical stage and CD4 count 18.
The objective of this study was to assess whether the implementation of an ‘Early access to ART for all’ intervention changed patterns of disclosure, treatment adherence and healthcare interactions. We hypothesised that (1) starting ART early could have an effect on disclosure. The effect could be an increase in disclosure as patients starting ART early would benefit from reduced transmission and therefore treatment could be seen as a protection against transmission. However, patients starting early ART may be less likely to disclose due to the lack of symptoms, and therefore a reduced need to do so while avoiding HIV‐related stigma; and (2) patients starting ART early are less likely to adhere to treatment compared to patients initiating ART as per standard of care, because they might feel healthier.
Methods
Setting
The MaxART study is a multidisciplinary implementation study in Hhohho Region, Swaziland assessing the impact of ‘Early Access to ART for All’ (EAAA) using clinical, social science and economic outcomes. This study is a social science sub‐study of the MaxART implementation study 18. The MaxART study protocol is described in detail elsewhere 18. Briefly, the MaxART study implemented an EAAA intervention for all HIV positive, ART naïve individuals attending one of the participating facilities. The study used a stepped‐wedge design, where facilities transitioned from control (national ART guidelines) to intervention (EAAA). The MaxART study took place in 14 facilities, whereas this sub‐study used nine of those, purposefully selected to include larger and smaller facilities and based on previous research in these facilities 19.
Participants
For this study we conducted interviews at three time points with MaxART study participants, one before the intervention and two during. The control group, interviewed at the first time point, consisted of a random sample of HIV‐positive patients who initiated ART less than 12 months prior to the interview in a facility included in the MaxART study, where the intervention was not yet implemented (i.e. these patients were treated following the national ART guidelines, with an ART initiation threshold of 350 cells/mm3). The intervention group was interviewed at two time points. At the first time point during the intervention (t0), the group consisted of patients recruited randomly after the facility had been implementing the EAAA strategy for at least 3 months. Patients were eligible for interviews if they had a CD4 count above 350, WHO clinical stage 1 or 2 and were willing to be contacted 6 months later for a follow‐up interview. These respondents were interviewed at a second time point during the intervention, which composed the intervention t1 group.
Sample size
We calculated a required sample size of 380 HIV positive patients per survey round to be able to detect a 5% change in prevalence of disclosure or self‐reported adherence between two interview rounds, with a power of 90% and a significance level of 0.05, accounting for a design effect of 1.5 due to clustering of the data, caused by recruitment in clinics.
Data collection
Trained Swazi research assistants collected data using a semi‐structured questionnaire in siSwati. The questionnaire was adopted from the WHO's manual HIV Testing, Treatment and Prevention: Generic Tools for Operational Research 20 and translated into siSwati by two native siSwati speakers. The questionnaire was pre‐tested before finalisation. The questionnaire included both close and open‐ended questions and was administered face‐to‐face. Respondents’ answers were entered in siSwati on the paper‐based questionnaire by the research assistants.
Data collection for the control group took place from September 2014 to September 2015. For the intervention t0 group, data collection took place from April 2015 to June 2017. The follow‐up (t1), took place from January 2016 to May 2017.
Data were entered in Excel. The open‐ended answers were entered in siSwati, and translated into English by the research assistant who carried out the interview. Data entry and translation was cross‐checked by a second research assistant, and any inconsistencies were discussed and checked by a third member of the team before the database was finalised. Records were anonymised and labelled as control, intervention t0 and intervention t1. We linked records between intervention t0 and t1 after three approaches: (i) on study identifier (68%); when that was not available (ii) on local identifier (25%); when that was not available (iii) on an algorithm that took into account gender, birth year, religion, facility, education and marital status (7%). In the follow‐up round (t1), 19 questionnaires could not be matched using any of the three criteria and are therefore not included in the final analysis.
Data analysis
We obtained socio‐demographic information as well as information on the primary outcomes disclosure and adherence, and the secondary outcomes of healthcare interactions. Disclosure was assessed via questions on keeping HIV status a secret, with whom the respondent shared their HIV status, and whether their HIV status has been disclosed as a consequence of visiting healthcare services or taking ART. Adherence was a self‐reported measure assessing if the respondent missed a dose of ART in the last 30 days. For patient experiences of HIV‐testing, the respondents were asked if they felt ill when they went for HIV testing. For patient experiences of ART initiation, they were asked if they felt ill (generally unwell) when initiating ART, if they felt pressured to start ART, and what the main reason was to start ART as an open‐ended question. Healthcare interaction was assessed by asking respondents what the healthcare workers usually asked, explained and reported, both at start of ART and at refill visits. Age was calculated based on reported birth and interview dates. Education was categorised into no formal education, primary, secondary, higher and other. Marital status was categorised into married, not married but in a relationship and not in a relationship. Religion was categorised into mainstream Christian, Evangelical (born‐again churches), African Healing Churches, other and none. The categorisation for religion and marital status was decided upon in consultation with anthropologists in the study team who were familiar with the local context. The wealth index was created based on a principal component analysis of eight variables assessing the presence of household assets. The resulting wealth score was used to divide the respondents in the control group in wealth quintiles. The cut‐off values of the quintiles in the control group were used to divide the respondents in the intervention t0 and t1 group into five groups based on their wealth score. The answers from the open‐ended question were classified in themes and the most reported are cited. Frequencies are compared using Pearson's chi squared test and Fisher's exact test, where appropriate. We did not take into account clustering of participants in the health facilities, given the emphasis on frequencies rather than effect size. For comparisons between before and during intervention on an aggregated level, we used the control and intervention t0 group, for comparisons over time during the intervention on patient level, we used the intervention t0 and t1, but only including the respondents who were followed up. We used Stata 14.2 for all analyses. The two‐sided α‐value was set at 0.05.
Ethical review
The MaxART implementation study and this social science sub‐study were approved by the Swaziland National Health Research Review Board in July 2014 (Reference Number: MH/599C/FWA 000 15267). Individuals were included in the MaxART study after giving informed oral consent and they provided additional written consent to be interviewed for this sub‐study.
Results
The recruitment of participants in the control and intervention group is described in Figure 1. In the control group we achieved the planned sample size, however, in the intervention group we achieved only 63% of the planned sample size, due to delays in recruitment. The participants’ characteristics are described in Table 1. The majority of the sample was female. The mean age was slightly higher in the intervention group (38 years) compared with the control group (35 years). Most participants had either no education or finished only primary education. The socio‐economic distribution was skewed towards the lower groups in the intervention groups compared with the control (24.4% in the poorest group in the controls vs. 31.3% in the intervention group).
Figure 1.
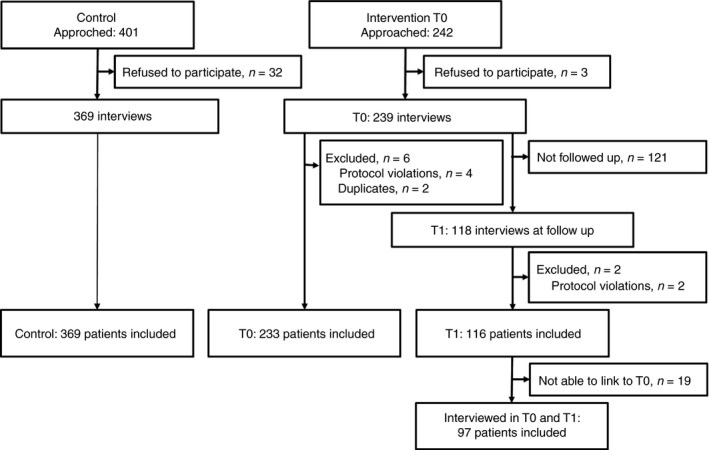
Recruitment flowchart.
Table 1.
Characteristics of study participants
| Control | Intervention t0 | Intervention t0 (who are followed up) | |
|---|---|---|---|
| n/N (%) | n/N (%) | n/N (%) | |
| N | 369 (100) | 233 (100) | 97 (100) |
| Age: mean (SD) | 35 (10.5) | 38 (11.8) | 39 (10.8) |
| Sex: female | 282/369 (76.4) | 182/233 (78.1) | 74/97 (76.3) |
| Education | |||
| No formal education | 51/369 (13.8) | 49/233 (21.0) | 19/97 (19.6) |
| Primary | 133/369 (36.0) | 76/233 (32.6) | 29/97 (29.9) |
| Secondary | 102/369 (27.6) | 73/233 (31.3) | 31/97 (32.0) |
| Higher | 76/369 (20.6) | 32/233 (13.7) | 17/97 (17.5) |
| Other | 7/369 (1.9) | 3/233 (1.3) | 1/97 (1.0) |
| Marital status | |||
| Married | 212/369 (57.5) | 127/232 (54.7) | 54/96 (56.3) |
| Relationship, not married | 117/369 (31.7) | 63/232 (27.2) | 26/96 (27.1) |
| Not in a relationship | 40/369 (10.8) | 42/232 (18.1) | 16/96 (16.7) |
| Religion | |||
| Evangelical | 110/369 (29.8) | 74/233 (31.8) | 34/97 (35.1) |
| Mainstream Christian | 21/369 (5.7) | 12/233 (5.2) | 3/97 (3.1) |
| African Healing Churches | 196/369 (53.1) | 125/233 (53.7) | 52/97 (53.6) |
| Other | 5/369 (1.4) | 2/233 (0.9) | 0 |
| None | 37/369 (10.0) | 20/233 (8.6) | 8/97 (8.3) |
| Wealth index | |||
| Poorest | 90/369 (24.4) | 73/233 (31.3) | 28/97 (28.9) |
| Poor | 59/369 (16.0) | 24/233 (10.3) | 10/97 (10.3) |
| Middle | 73/369 (19.8) | 32/233 (13.7) | 15/97 (15.5) |
| Rich | 78/369 (21.1) | 64/233 (27.5) | 33/97 (34.0) |
| Richest | 69/369 (18.7) | 40/233 (17.2) | 11/97 (11.3) |
| Missed a meal | |||
| Never | 272/369 (73.7) | 147/232 (63.4) | 60/96 (62.5) |
| Sometimes | 68/369 (18.4) | 50/232 (21.6) | 24/96 (25.0) |
| Always or often | 29/369 (7.9) | 35/232 (15.1) | 12/96 (12.5) |
| Travel time to facility | |||
| Mean (SD) | 57 (53.8) | 59 (54.9) | |
| <60 | 229/368 (62.2) | 132/232 (56.9) | |
| >60 | 139/368 (37.8) | 100/232 (43.1) | |
| Time since HIV positive, years | |||
| Mean (SD) | 2.5 (3.1) | 3.5 (3.6) | |
| Time on ART, months | |||
| Mean (SD) | 5.4 (3.1) | 7.0 (3.4) | |
SD, standard deviation.
Comparing disclosure before and during the intervention, a high proportion of participants (94%) reported to have disclosed their HIV status to their partner at both time points (Table 2). Disclosure to family showed similar proportions in control and intervention group (78% in the control group and 79% in the intervention t0 group). Disclosure outside the family was rare, independently of the timing of ART initiation. The disclosure patterns did not change over time during the intervention. The proportion of people worried about non‐intended disclosure after visits to healthcare services or taking ART differed between control (14%) and intervention group (20%).
Table 2.
Disclosure and adherence
| Control | Intervention (t0) | Intervention (t0) | Intervention (t1) | |||
|---|---|---|---|---|---|---|
| n/N (%) | n/N (%) | P | n/N (%) | n/N (%) | P | |
| N | 369 (100) | 233 (100) | 97 (100) | 97 (100) | ||
| Disclosure | ||||||
| Keeps HIV status a secret from most people | 237/367 (64.6) | 159/232 (68.5) | 0.319 | 71/96 (74.0) | 60/96 (62.5) | 0.088 |
| HIV results shared with anyone outside the health clinic | 364/369 (98.6) | 229 (98.7) | 0.949 | 94/96 (97.9) | 97/97 (100) | 0.153 |
| Shared with Partner | 314/335 (93.7) | 182/194 (93.8) | 0.970 | 76/83 (91.6) | 79/85 (92.9) | 0.739 |
| Shared with Family | 284/364 (78.0) | 180/229 (78.6) | 0.867 | 68/96 (70.8) | 75/97 (77.3) | 0.428 |
| Shared with Other | 44/364 (12.1) | 37/229 (16.2) | 0.160 | 16/96 (16.7) | 16/97 (16.5) | 0.922 |
| Worried that visiting HTC services or taking ART may disclose HIV status | 34/368 (9.2) | 37/229 (16.0) | 0.012 | 19/95 (20.0) | 16/96 (16.7) | 0.552 |
| Attending HIV services or taking ART has caused disclosure HIV status | 52/365 (14.3) | 46/228 (20.2) | 0.059 | 22/92 (23.9) | 20/97 (20.6) | 0.586 |
| Adherence | ||||||
| Dose missed in the last 30 days | 54/369 (14.6) | 38/232 (16.4) | 0.563 | 12/95 (12.3) | 10/96 (10.3) | 0.632 |
Reported treatment adherence did not change with the introduction of early ART, nor over time during the intervention. However, of those that felt ill when starting ART, 20.0% (34/170) reported missing a dose, whereas of those that did not feel ill 12.5% (58/431) reported missing a dose (P = 0.045).
The proportion of respondents feeling ill when testing was higher in the control group (36.4%) than in the intervention t0 group (28.1%) (Table 2). The proportion of respondents feeling ill when initiating ART was higher in the control group (32.5%) compared to the intervention group (21.5%).
An increased proportion of respondents was told that it was their own choice to start ART, and both the CD4 count and the viral load test were explained more often in the intervention (from 64.6% in t0 to 75.0% in t1 for the CD4 count and from 19.2% in t0 to 52.6% in t1 for the viral load test) compared to the control group (Table 3). At ART refill, the introduction of the intervention was associated with a significant increase in participants who reported that they were given the CD4 count and viral load test results, namely from 14.0% to 48.9% for viral load test results and from 68.8% to 76.3% for CD4 count results. Over time during the intervention, the number of participants reporting that they received the CD4 count and viral load test results increased, from 44.2% to 67.0% for viral load, and from 75.5% to 84.5% for CD4 count results. There was no change in the proportion of respondents feeling pressured to start ART.
Table 3.
Patient experiences of testing and ART initiation
| Control | Intervention (t0) | Intervention (t0) | Intervention (t1) | |||
|---|---|---|---|---|---|---|
| n/N (%) | n/N (%) | P | n/N (%) | n/N (%) | P | |
| N | 369 (100) | 233 (100) | 97 (100) | 97 (100) | ||
| Feeling ill when testing | 134/368 (36.4) | 72/224 (30.9) | 0.165 | – | – | |
| Feeling ill when starting ART | 120/369 (32.5) | 50/233 (21.5) | 0.003 | – | – | |
| Felt pressured to start ART | 38/368 (10.3) | 25/233 (10.7) | 0.875 | – | – | |
| At ART initiation | ||||||
| Explain the meaning of the CD4 count results and the viral load test | 49/333 (14.7) | 94/202 (46.5) | <0.001 | – | – | |
| Explain it was your choice to start ART | 321/366 (87.7) | 217/233 (93.1) | 0.032 | – | – | |
| Gave you time to ask questions | 309/364 (84.9) | 201/231 (87.0) | 0.471 | – | – | |
| At refill | ||||||
| Asks if you have any problems with taking ART | 294/369 (79.7) | 185/231 (80.1) | 0.903 | 78/96 (81.3) | 69/97 (71.1) | 0.099 |
| Gives you back CD4 results and viral load test results | 45/349 (12.9) | 102/225 (45.3) | <0.001 | 38/95 (40.0) | 61/97 (62.9) | 0.002 |
| Gives you time to ask questions | 295/369 (80.0) | 174/231 (75.3) | 0.182 | 70/95 (73.7) | 67/97 (69.1) | 0.480 |
Discussion
We found no changes in patterns of disclosure and treatment adherence after the EAAA intervention, with both being reported by a very high percentage of patients at each of the interview rounds. Respondents did not report more pressure to start ART after the intervention was introduced, indicating that, even though all HIV‐positive individuals were eligible to start ART, initiation remained a choice.
The literature reports mixed results from studies on treatment adherence in patients starting ART at higher baseline CD4 count, yet our results are in line with the study by Jain et al. 8 that challenged the concerns on treatment adherence in this patient group, as reported by Adakun et al. 5. It should be noted that this latter study took place before the WHO guideline changed. We found that more people worried that visiting HIV testing and counselling (HTC) services or taking ART may disclose their HIV status after the intervention, which is in line with a qualitative study in Kenya that cited fear of unintended disclosure as a barrier to early initiation of ART 10.
Feeling healthy was reported as a reason for delayed ART initiation in a qualitative sub‐study carried out in the MaxART program 21. In our sub‐study, only 21.5% of those who started ART in the intervention group reported feeling ill at the time of ART initiation, which shows that the majority of patients start ART despite feeling healthy. The most cited reasons to start ART in the intervention group are wanting to avoid becoming sick or being explained that one can start ART regardless of health status or CD4 count. In addition, those that did not feel ill at ART initiation reported being more adherent than those that did feel ill, which indicates that feeling healthy was not a barrier to adherence for the majority of participants in this study.
A qualitative MaxART study on healthcare providers’ perspectives of early ART revealed that health providers were motivating clients to start ART early in order to avoid severe illness and becoming ‘bed ridden’ 22. Avoiding visibly apparent illness was described as particularly advantageous by providers because it meant that clients could evade disclosing their HIV status to friends and neighbours. Whilst we reported high levels of disclosure to partners and family members, only a small percentage (12.1% in the control group and 16.2% in the intervention group) reported having disclosed to someone other than partner or family. This confirms qualitative insights from MaxART which revealed that disclosure was generally reserved for one's immediate family, and few people reported disclosure to non‐family members 21.
With regard to healthcare interactions we found that the communication between patient and healthcare provider at the initiation of ART improved after the intervention. Test results were more widely shared with the patients after the intervention due to a wider access to point of care CD4‐cell count machines and routine viral load monitoring. In contrast, fewer patients were asked about problems with taking ART over time. At intervention t1, clinical mentors worked on improving the logistics around viral load tests and how the results were explained to patients, which probably contributed to the improvement in giving back test results in intervention t1, but the increased time dedicated to giving back the results might have led to less time for the other topics. Another explanation might be that the healthcare workers assume that problems such as side effects related to taking ART will arise shortly after the initiation and to a lesser extent later on, and therefore less time is spent on inquiring about problems with ART as time passes.
This study compared the respondents before and after intervention, but also aimed to follow‐up the respondents after the intervention, to assess if the results were sustained. One of the limitations is that the data were collected using face‐to‐face interviews, which might have introduced a social desirability bias. Treatment adherence was self‐reported, however, literature suggest that self‐reported treatment adherence is, in general, a reliable measure 23, 24, 25, 26, 27. Using the same methodology in both comparison groups makes that true differences should not be affected. Linking between intervention t0 and t1 was based on personal identifying number, which was not always recorded, resulting in the use of different matching strategies. This might have led to false matching results. With the absence of the initial study number being due to operational issues, rather than patient characteristics, we feel that this potential miss‐matches do not influence the validity of our results.
Comparing the respondents interviewed before and during the intervention, the mean time since HIV positive diagnosis is longer after the intervention was introduced, which means respondents recruited in intervention groups had more time to disclose. However, since the proportion of people who disclosed is already high, namely 94% disclosed to partner in the control group, the difference in time since HIV positive diagnosis probably did not have much effect. We managed to follow‐up 43% of respondents 6 months after the first interview, which might have introduced a selection bias. We tried to correct this using analysis‐weights based on sex, age, wealth index, religion, marital status, education, religion and recruitment facility of the respondent. However, this resulted in unstable weights due to the limited numbers and did not allow us to reach valid estimates. Hence, we present unweighted estimates that might suffer from bias, if the loss to follow‐up is associated with the outcome, which could be the case for adherence. In this case, we may have overestimated the proportion that was adherent.
Conclusion
This study challenges the concerns that early initiation of ART would lead to less adherence, less disclosure or more pressure to start ART. We found that the intervention improved the interaction with healthcare workers, this might be due to training and mentoring of healthcare workers and will be important for a successful roll‐out of immediate ART.
Acknowledgements
We thank Anita Hardon for her contributions to the design and her institutional support to this study, Christopher Pell for his supervisory assistance, Nombulelo Simelane for her support with data collection during the early stages of the study, Nelisiwe Masilela and other research assistants for their support during the end stages of data collection. We also thank the MaxART partners, EAAA study team, healthcare providers and patients at EAAA study sites for their cooperation during the study. The MaxART consortium received support of the Dutch Postcode Lottery in the Netherlands, the Embassy of the Kingdom of the Netherlands in South Africa/Mozambique, British Colombia Centre of Excellence in HIV/AIDS in Canada, Mylan and Médecins Sans Frontières. The findings and analysis presented are those of the authors and do not reflect those of any funders.
References
- 1. World Health Organization . Scaling Up Antiretroviral Therapy in Resource Limited Settings: Guidelines for a Public Health Approach [Internet], 2002. (Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00019048-200203000-00012) [PubMed]
- 2. World Health Organization . Antiretroviral therapy for HIV infection in adults and adolescents [Internet], 2010. (Available from: http://www.who.int/hiv/pub/arv/adult2010/en/index.html) [PubMed]
- 3. World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach [Internet]. WHO Guidelines, 2013. (Available from: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf) [PubMed]
- 4. World Health Organization . Guideline on When To Start Antiretroviral Therapy and on Pre‐Exposure Prophylaxis for Hiv [Internet]. World Health Organization, 2015. (Available from: http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/) [PubMed]
- 5. Adakun SA, Siedner MJ, Muzoora C et al Higher baseline CD4 cell count predicts treatment interruptions and persistent Viremia in patients initiating ARVs in rural Uganda. JAIDS J Acquir Immune Defic Syndr 2013: 62: 317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grimsrud A, Cornell M, Schomaker M et al CD4 count at antiretroviral therapy initiation and the risk of loss to follow‐up: results from a multicentre cohort study. J Epidemiol Community Health 2016: 70: 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Geng EH, Bwana MB, Muyindike W et al Failure to initiate antiretroviral therapy, loss to follow‐up and mortality among HIV‐infected patients during the pre‐ART period in Uganda. JAIDS J Acquir Immune Defic Syndr 2013: 63: e64–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jain V, Byonanebye DM, Amanyire G et al Successful antiretroviral therapy delivery and retention in care among asymptomatic individuals with high CD4 + T‐cell counts above 350 cells/μl in rural Uganda. AIDS 2014: 28: 2241–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bock P, James A, Nikuze A et al Baseline CD4 count and adherence to antiretroviral therapy. JAIDS J Acquir Immune Defic Syndr 2016: 73: 514–521. [DOI] [PubMed] [Google Scholar]
- 10. Curran K, Ngure K, Shell‐Duncan B et al ‘If I am given antiretrovirals I will think I am nearing the grave’: Kenyan HIV serodiscordant couples’ attitudes regarding early initiation of antiretroviral therapy. AIDS 2014: 28: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katirayi L, Chouraya C, Kudiabor K et al Lessons learned from the PMTCT program in Swaziland: challenges with accepting lifelong ART for pregnant and lactating women – a qualitative study. BMC Public Health 2016: 16: 1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reda AA, Biadgilign S. Determinants of adherence to antiretroviral therapy among HIV‐infected patients in Africa. AIDS Res Treat 2012: 2012: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heestermans T, Browne JL, Aitken SC, Vervoort SC, Klipstein‐Grobusch K. Determinants of adherence to antiretroviral therapy among HIV‐positive adults in sub‐Saharan Africa: a systematic review. BMJ Glob Heal 2016: 1: e000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horter S, Thabede Z, Dlamini V et al “Life is so easy on ART, once you accept it”: acceptance, denial and linkage to HIV care in Shiselweni, Swaziland. Soc Sci Med 2017: 176: 52–59. [DOI] [PubMed] [Google Scholar]
- 15. Katz IT, Ryu AE, Onuegbu AG et al Impact of HIV‐related stigma on treatment adherence: systematic review and meta‐synthesis. J Int AIDS Soc 2013: 16(3 Suppl 2): 18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan BT, Tsai AC, Siedner MJ. HIV treatment scale‐up and HIV‐related stigma in sub‐Saharan Africa: a longitudinal cross‐country analysis. Am J Public Health 2015: 105: 1581–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ministry of Health, Central Statistics Office . Swaziland HIV Incidence Measurement Survey 2: A Population‐Based HIV Impact Assessment (SHIMS2) 2016‐2017 [Internet], 2017. Available from: http://phia.icap.columbia.edu/wp-content/uploads/2017/11/Swaziland_new.v8.pdf
- 18. Walsh FJ, Bärnighausen T, Delva W et al Impact of early initiation versus national standard of care of antiretroviral therapy in Swaziland's public sector health system: study protocol for a stepped‐wedge randomized trial. Trials 2017: 18: 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vernooij E, Mehlo M, Hardon A, Reis R. Access for all: contextualising HIV treatment as prevention in Swaziland. AIDS Care – Psychol Socio‐Medical Asp AIDS/HIV 2016: 28: 7–13. [DOI] [PubMed] [Google Scholar]
- 20. Obermeyer CM, Pulerwitz J, Carrieri P, Sarna A. HIV Testing Treatment and Prevention: Generic Tools for Operational Research, 2009; 1–66.
- 21. Pell C, Vernooij E, Masilela N, Simelane N, Shabalala F, Reis R. False starts in ‘test and start’: a qualitative study of reasons for delayed antiretroviral therapy in Swaziland. Int Health. 2018; 10(January):78–83. [DOI] [PubMed] [Google Scholar]
- 22. Pell C, Reis R, Dlamini N, Moyer E, Vernooij E. ‘Then her neighbour will not know her status’: how health providers advocate antiretroviral therapy under universal test and treat. Int Health 2018: 11: 36–41. [DOI] [PubMed] [Google Scholar]
- 23. Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self‐report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS 2002: 16: 269–277. [DOI] [PubMed] [Google Scholar]
- 24. Oyugi JH, Byakika‐Tusiime J, Charlebois ED et al Multiple validated measures of adherence indicate high levels of adherence to generic HIV antiretroviral therapy in a resource‐limited setting. J Acquir Immune Defic Syndr 2004: 36: 1100–1102. [DOI] [PubMed] [Google Scholar]
- 25. Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self‐report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav 2006: 10: 227–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nieuwkerk PT, Oort FJ. Self‐reported adherence to antiretroviral therapy for HIV‐1 infection and virologic treatment response: a meta‐analysis. J Acquir Immune Defic Syndr 2005: 38: 445–448. [DOI] [PubMed] [Google Scholar]
- 27. Van Leth F, Kappelhoff BS, Johnson D et al Pharmacokinetic parameters of nevirapine and efavirenz in relation to antiretroviral efficacy. AIDS Res Hum Retroviruses 2006: 22: 232–239. [DOI] [PubMed] [Google Scholar]


