Abstract
Background
Fractional exhaled nitric oxide concentration (FeNO) is widely used to support diagnosis and monitoring of bronchial asthma (BA). Tsoukias and George proposed a two‐compartment model (2CM) for assessing the alveolar concentration of NO, referred to as CANO(2CM), while Condorelli et al proposed a model based on the trumpet shape of the airway tree and axial diffusion (TMAD), referred to as CANO(TMAD). In addition, Högman et al proposed non‐linear model, referred to as CANO(non‐linear).
Objective
We examined associations between the expression of inducible nitric oxide synthase (iNOS) mRNA in airway cells (ACs) by bronchoscopy and NO‐parameters calculated by the three methods and identified which of them accurately reflected expression of iNOS mRNA from different airway portions.
Methods
We retrospectively analysed data of 18 patients with stable, mild‐moderate asthma, including 10 steroid‐naïve BA (snBA) patients. Samples were obtained from airway brushings and bronchoalveolar lavage (BAL). Expressions of iNOS protein in tissue samples were evaluated by immunostaining. The iNOS mRNA in ACs was measured by qPCR. NO‐parameters calculated by the three methods above and evaluated whether they were associated with iNOS mRNA in ACs derived from proximal (2nd carina), distal (10‐15th) airways and alveolar regions.
Results
Immunostaining revealed expression of iNOS proteins mainly in epithelial cells in the airways, while it was mainly expressed in macrophages in the alveolar region in the snBA group. The iNOS mRNA expression was increased in both proximal and distal ACs in the snBA group compared with steroid‐treated BA group (stBA). CANO(2CM) negatively associated with FEV 1 (%predicted) and also associated with iNOS mRNA in distal ACs significantly. However, CANO(TMAD) and CANO(non‐linear) showed no correlation with lung function nor iNOS mRNA expression in any portions of ACs.
Conclusions
These results suggested that CANO(2CM) reflected distal airway inflammation in steroid‐naïve asthma.
1. INTRODUCTION
Bronchial asthma is chronic airway inflammatory disease that is mainly dependent on T helper 2 (type 2) cytokines.1 Despite the progress made in the treatment of asthma, problems such as severe refractory asthma or asthma‐related deaths have not yet been resolved.2 Distal airway inflammation is one cause of severe refractory asthma because an inhaled corticosteroid (ICS) may not reach the distal airway, resulting in residual inflammation.3, 4 While it is important to evaluate the extent of distal airway inflammation, it is difficult to do so in the clinical setting. Tissue biopsy by bronchoscopy is thought to be useful for obtaining a direct evaluation, but because it is invasive, it cannot be performed repeatedly. It is already recognized that fractional exhaled nitric oxide (FeNO) is a non‐invasive biomarker of airway inflammation in asthma. The FeNO multi‐flow rate measurement method is considered a non‐invasive distal airway inflammation evaluation method. Tsoukias and George proposed a two‐compartment model (2CM) and Condorelli et al proposed the trumpet model based on the shape of the airway tree and axial diffusion (TMAD) to evaluate alveolar air with nitric oxide (NO) at a steady‐state alveolar concentration.5, 6 In addition, Högman et al7 proposed non‐linear model, referred to as CANO(non‐linear). In this report, we refer to the measure of distal airway NO‐parameters by Tsoukias and George's method as CANO(2CM) and that by Condorelli et al's method as CANO(TMAD) as well as Högman et al's methods as CANO(non‐linear). It has been reported that CANO(TMAD) is negatively associated with FEV1 and FEF25‐75, and it is thought to be associated with the pathogenesis of asthma.8, 9 CANO(TMAD) is modified by CANO(2CM), and it is generally believed that CANO(TMAD) is a precise measure of alveolar NO. However, it remains unclear which NO‐parameters represented the proper region of NO production in distal airway.
We questioned whether CANO(2CM), CANO(TMAD) and CANO(non‐linear) were associated with the expression of iNOS mRNA in the distal airways of asthma patients. Thus, we examined the distribution of iNOS protein by immunostaining different airway components. We then compared the expression of iNOS mRNA in the proximal and distal airway cells (ACs) and total bronchoalveolar lavage (BAL) cells of asthma patients via bronchoscopy. Furthermore, we examined the associations between the expression levels of iNOS mRNA and NO‐parameters including FeNO at 50 mL/s, J'awNO(2CM, TMAD and non‐linear) and CANO(2CM, TMAD and non‐linear).
2. METHODS
2.1. Patients
We conducted a retrospective study of 18 patients with asthma, including 10 with steroid‐naïve asthma (snBA) and eight with steroid‐treated asthma (stBA), who visited the Department of Pulmonary Medicine and Clinical Immunology of Dokkyo Medical University Hospital from June 2009 to March 2014 (Table 1). All patients met the American Thoracic Society criteria for asthma and had a pre‐bronchodilator FEV1 greater than 80% predicted in combination with an FEV1/FVC greater than 70%. The snBA patients had mild symptoms such as cough with wheezing and nocturnal dyspnoea but had not been treated with ICS or oral corticosteroids for at least 6 months. Written‐informed consent was obtained from all participants. This study was approved by the Ethics Committee of Dokkyo University School of Medicine (hop‐m22095).
Table 1.
Characteristics of patients
| Patient characteristics | snBA | stBA | P‐value |
|---|---|---|---|
| Gender (M : F) | 9 : 1 | 5 :3 | |
| Smoking (N : E : C) | 1 : 8 : 1 | 2 : 6 :0 | |
| Age (years) | 54 ± 4 | 54 ± 5 | 0.96 |
| FVC (L) | 3.9 ± 0.2 | 3.6 ± 0.3 | 0.41 |
| FEV1 (L) | 2.8 ± 0.2 | 2.8 ± 0.3 | 0.98 |
| FEV1/FVC | 73 ± 4 | 80 ± 3 | 0.19 |
| FEV1 (% predicted) | 86 ± 3 | 93 ± 7 | 0.35 |
| FEF25‐75 (% predicted) | 53 ± 7 | 75 ± 11 | 0.09 |
| FEF25 (% predicted) | 45 ± 7 | 65 ± 3 | 0.16 |
| IgE (IU) | 272 ± 79 | 155 ± 64 | 0.32 |
| Eosinophlis (/μL) | 473 ± 87 | 162 ± 44 | 0.01* |
| FeNO at 50 mL/s (ppb) | 111 ± 27 | 50 ± 8 | 0.06 |
| J'awNO(2CM) (nL/s) | 6.0 ± 1.2 | 2.5 ± 1.3 | 0.07 |
| J'awNO(TMAD) (nL/s) | 10.2 ± 2.1 | 4.3 ± 2.3 | 0.07 |
| J'awNO(non‐linear) (nL/s) | 9.4 ± 2.6 | 3.0 ± 2.7 | 0.11 |
| CANO(2CM) (ppb) | 13.4 ± 2.3 | 6.1 ± 2.6 | 0.05 |
| CANO(TMAD) (ppb) | 5.7 ± 2 | 3.7 ± 2 | 0.46 |
| CANO(non‐linear) (ppb) | 9.8 ± 3 | 4.5 ± 3 | 0.17 |
| Cell counts (×105/mL) | |||
| Distal airway cells | 5.0 ± 1.1 | 5.0 ± 1.4 | 0.96 |
| Proximal airway cells | 4.0 ± 1.1 | 4.3 ± 1.5 | 0.88 |
| BAL cells | 0.9 ± 0.2 | 1.1 ± 0.2 | 0.31 |
| BALF | |||
| BALF volume (mL) | 80 ± 4 | 84 ± 5 | 0.50 |
| BALF recovery rate (%) | 53 ± 3 | 56 ± 3 | 0.50 |
| Macrophages (%) | 89 ± 3 | 90 ± 2 | 0.78 |
| Neutrophils (%) | 0.3 ± 0.1 | 0.6 ± 0.2 | 0.15 |
| Eosinophils (%) | 0.5 ± 0.2 | 1.1 ± 1.0 | 0.34 |
| Lymphocytes (%) | 11 ± 3 | 8.6 ± 2 | 0.62 |
| CD4/8 ratio | 2.3 ± 0.4 | 2.6 ± 0.6 | 0.66 |
2.2. FeNO measurement
FeNO was measured via a chemiluminescence analyser NOA 280i (Sievers Instruments, Boulder, CO). FeNO at a flow rate of 50 mL/s was measured three times following the ATS/ERS guidelines,10 and the mean value was calculated. Additionally, NO‐parameters at different flow rates of 100, 150 and 200 mL/s were measured three times each, and the mean values were calculated among the patients.
2.3. Calculation of the NO‐parameters
We calculated the NO‐parameters using the three methods of the proximal airways as for J'awNO and distal‐alveolar region as for CANO, respectively. CANO(2CM) was measured according to the 2CM method; specifically, the reciprocal of the flow rate at 100, 150 and 200 mL/s plotted on the x‐axis and NO concentrations plotted on the y‐axis were obtained by a linear equation, with the y‐intercept and the slope giving estimates of the alveolar region NO and proximal airway NO (J'awNO), respectively.5 When we looked at the associations, J'awNO (2CM) and J'awNO (TMAD) were exactly same, so we presented J'awNO (2CM and TMAD) in this study. According to a previous report, CANO(TMAD) was estimated using a model developed by Condorelli et al6 that incorporated the trumpet‐shaped airways and axial diffusion rather than simply assuming that the lung was comprised of two separate regions with a rigid airway compartment and a well‐mixed expansible compartment. The following equation was used:
CANO(TMAD) = slope−intercept/740.
The slope and intercept of this equation were determined from linear regression after plotting the NO outputs as a function of expiratory flow. CANO(TMAD) values below zero were assigned a value of zero. In addition, J'awNO(non‐linear) and CANO(non‐linear) values were calculated using spreadsheet downloaded from manuscript written by Högman.7
2.4. Bronchoscopy
Bronchial brushings were performed with a standard sterile single‐sheathed nylon cytology brush (Olympus T‐260; Olympus, Tokyo, Japan). A total of four brushings were performed in the proximal and distal airways. The proximal ACs were obtained from the second or third carina visible through the bronchoscope. The distal ACs were obtained from the airways about 1 to 2 cm away from the pleura—equivalent to the airway of the 10th to 15th branch of Weibel's model—with a diameter of less than 2 mm, that is, the so‐called distal airways.11 Transbronchial lung biopsy, endobronchial biopsy, and BAL were subsequently performed. Total BAL cells were counted, cell fractionation was performed, and the RNA was extracted.
2.5. Immunostaining
Transbronchial lung biopsy and endobronchial biopsy specimens from patients were fixed in formalin. Serial 4‐μm sections were immunostained using a rabbit polyclonal antibody against iNOS (1:500) (Abcam, Cambridge, MA) with Dako EnVision™ FLEX Mini Kit High pH detection system including secondary anti‐rabbit antibody for detection. Data were collected using an all‐in‐one fluorescence microscope (BZ‐X700; Keyence Co., Tokyo, Japan).
2.6. Quantitative real‐time PCR
The expression levels of iNOS mRNA in ACs and total BAL cells were determined by quantitative real‐time PCR (qRT‐PCR) as described previously.12 First‐strand cDNA was synthesized by PrimeScript RT Reagent Kit (Takara Bio, Shiga, Japan) with both oligo(dT) primer and random hexamers. Reverse transcription was performed with a TaKaRa PCR Thermal Cycler MP (TP3000, Takara Bio). The following sequences were used for iNOS and GAPDH. iNOS: forward primer, 5′‐GCACGGCAACACATTGAA‐3′; reverse primer, 5′‐TGAGGTTCTGAAGGCCTAAATC‐3′; GAPDH: forward primer, 5′‐GCACCGTCAAGGCTGAGAAC‐3′; reverse primer, 5′‐TGGTGAAGACGCCAGTGGA‐3′.
Diluted first‐strand cDNA product (4 μL) was used for amplification in a 25‐μL reaction solution containing 12.5 μL SYBR Premix Ex Taq II (Takara Bio) and 1 μL of each primer. DNA was amplified for 40 cycles of denaturation at 95°C for 5 seconds and annealed at 60°C for 30 seconds with the Takara Thermal Cycler Dice (TP900; Takara Bio). The data generated from each PCR reaction were analysed using Thermal Cycler Dice Real Time System version 4.2 (Takara Bio). The specificity of the reactions was determined by melting curve analysis. The relative expression of each gene of interest and GAPDH were calculated by the ΔΔCt method.
2.7. Statistical analysis
Variables were checked for normality of distribution. Since most of the data were not normally distributed, the analysis was performed using nonparametric tests. The Kruskal–Wallis version of the Wilcoxon rank sum test was used to compare overall differences among the groups (the overall P‐value). When the overall P‐value was <0.05, intergroup comparisons were conducted using the Wilcoxon test for multiple comparisons. Spearman's rank correlation was calculated to assess the correlation among NO‐parameters, lung function parameters and iNOS mRNA. In Tables 2 and 3, conservative level of significance was not applied because these analyses have done for each hypothesis of the association. JMP version 10 (SAS Institute, Cary, NC) was used in all analyses.
Table 2.
Correlations pulmonary function test between NO‐parameters in snBA
| NO‐parameters | Spearmman’ρ | P‐value | |
|---|---|---|---|
| FeNO at 50 mL/s | FVC | ‐0.26 | 0.48 |
| FeNO at 50 mL/s | FEV1 | ‐0.36 | 0.30 |
| FeNO at 50 mL/s | FEV1(% predicted) | ‐0.27 | 0.44 |
| FeNO at 50 mL/s | FEV1/FVC | ‐0.20 | 0.58 |
| FeNO at 50 mL/s | FEF25‐75(% predicted) | ‐0.30 | 0.40 |
| FeNO at 50 mL/s | FEF25(% predicted) | ‐0.31 | 0.38 |
| J'awNO(2CM and TMAD) | FVC | ‐0.41 | 0.24 |
| J'awNO(2CM and TMAD) | FEV1 | ‐0.60 | 0.07 |
| J'awNO(2CM and TMAD) | FEV1(% predicted) | ‐0.55 | 0.10 |
| J'awNO(2CM and TMAD) | FEV1/FVC | ‐0.47 | 0.17 |
| J'awNO(2CM and TMAD) | FEF25‐75(% predicted) | ‐0.64 | < 0.05* |
| J'awNO(2CM and TMAD) | FEF25(% predicted) | ‐0.65 | < 0.05* |
| J'awNO(non‐linear) | FVC | ‐0.54 | 0.13 |
| J'awNO(non‐linear) | FEV1 | ‐0.47 | 0.20 |
| J'awNO(non‐linear) | FEV1(% predicted) | ‐0.40 | 0.28 |
| J'awNO(non‐linear) | FEV1/FVC | ‐0.10 | 0.80 |
| J'awNO(non‐linear) | FEF25‐75(% predicted) | ‐0.33 | 0.38 |
| J'awNO(non‐linear) | FEF25(% predicted) | ‐0.28 | 0.46 |
| CANO(2CM) | FVC | ‐0.54 | 0.11 |
| CANO(2CM) | FEV1 | ‐0.45 | 0.19 |
| CANO(2CM) | FEV1(% predicted) | ‐0.64 | < 0.05* |
| CANO(2CM) | FEV1/FVC | ‐0.03 | 0.93 |
| CANO(2CM) | FEF25‐75(% predicted) | ‐0.30 | 0.40 |
| CANO(2CM) | FEF25(% predicted) | ‐0.47 | 0.17 |
| CANO(TMAD) | FVC | ‐0.12 | 0.74 |
| CANO(TMAD) | FEV1 | 0.19 | 0.60 |
| CANO(TMAD) | FEV1(% predicted) | ‐0.34 | 0.33 |
| CANO(TMAD) | FEV1/FVC | 0.42 | 0.23 |
| CANO(TMAD) | FEF25‐75(% predicted) | 0.21 | 0.56 |
| CANO(TMAD) | FEF25(% predicted) | ‐0.01 | 0.97 |
| CANO(non‐linear) | FVC | 0.28 | 0.43 |
| CANO(non‐linear) | FEV1 | 0.41 | 0.24 |
| CANO(non‐linear) | FEV1(% predicted) | 0.02 | 0.97 |
| CANO(non‐linear) | FEV1/FVC | 0.38 | 0.28 |
| CANO(non‐linear) | FEF25‐75(% predicted) | 0.38 | 0.27 |
| CANO(non‐linear) | FEF25(% predicted) | 0.27 | 0.44 |
Table 3.
Correlations between NO‐parameters and iNOS mRNA expressions
| Subjects | Nitric oxide | iNOS mRNA | Spearmman’ρ | P‐value |
|---|---|---|---|---|
| stBA (n = 8) | FeNO at 50 mL/s | Proximal ACs | 0.60 | 0.12 |
| FeNO at 50 mL/s | Distal ACs | 0.60 | 0.12 | |
| FeNO at 50 mL/s | Total BAL cells | 0.49 | 0.22 | |
| J'awNO(2CM and TMAD) | Proximal ACs | 0.62 | 0.10 | |
| J'awNO(2CM and TMAD) | Distal ACs | 0.24 | 0.57 | |
| J'awNO(2CM and TMAD) | Total BAL cells | 0.06 | 0.89 | |
| J'awNO(non‐linear) | Proximal ACs | 0.67 | 0.07 | |
| J'awNO(non‐linear) | Distal ACs | 0.48 | 0.23 | |
| J'awNO(non‐linear) | Total BAL cells | 0.41 | 0.32 | |
| CANO(2CM) | Proximal ACs | ‐0.29 | 0.49 | |
| CANO(2CM) | Distal ACs | ‐0.02 | 0.96 | |
| CANO(2CM) | Total BAL cells | 0.52 | 0.19 | |
| CANO(TMAD) | Proximal ACs | ‐0.27 | 0.52 | |
| CANO(TMAD) | Distal ACs | ‐0.05 | 0.91 | |
| CANO(TMAD) | Total BAL cells | 0.36 | 0.39 | |
| CANO(non‐linear) | Proximal ACs | ‐0.69 | 0.06 | |
| CANO(non‐linear) | Distal ACs | ‐0.64 | 0.09 | |
| CANO(non‐linear) | Total BAL cells | ‐0.42 | 0.30 | |
| snBA (n = 10) | FeNO at 50 mL/s | Proximal ACs | ‐0.02 | 0.96 |
| FeNO at 50 mL/s | Distal ACs | 0.49 | 0.15 | |
| FeNO at 50 mL/s | Total BAL cells | 0.25 | 0.49 | |
| J'awNO(2CM and TMAD) | Proximal ACs | 0.18 | 0.63 | |
| J'awNO(2CM and TMAD) | Distal ACs | 0.50 | 0.14 | |
| J'awNO(2CM and TMAD) | Total BAL cells | 0.45 | 0.19 | |
| J'awNO(non‐linear) | Proximal ACs | 0.03 | 0.93 | |
| J'awNO(non‐linear) | Distal ACs | 0.28 | 0.46 | |
| J'awNO(non‐linear) | Total BAL cells | 0.02 | 0.97 | |
| CANO(2CM) | Proximal ACs | 0.41 | 0.24 | |
| CANO(2CM) | Distal ACs | 0.83 | <0.01* | |
| CANO(2CM) | Total BAL cells | 0.20 | 0.58 | |
| CANO(TMAD) | Proximal ACs | 0.26 | 0.47 | |
| CANO(TMAD) | Distal ACs | 0.45 | 0.19 | |
| CANO(TMAD) | Total BAL cells | ‐0.24 | 0.50 | |
| CANO(non‐linear) | Proximal ACs | ‐0.10 | 0.78 | |
| CANO(non‐linear) | Distal ACs | ‐0.13 | 0.73 | |
| CANO(non‐linear) | Total BAL cells | 0.07 | 0.85 |
3. RESULTS
3.1. Patient characteristics and clinical parameters
Table 1 shows the characteristics of the patients in this study. Briefly, there were nine male patients in the snBA group and five in the stBA group. The snBA group comprised eight ex‐smokers, one never smoker, and one current smoker and the stBA group comprised six ex‐smokers and two never smokers. Mean age was 54 years in both groups. The lower values of FEV1/FVC, FEF25‐75(% predicted) and FEF25(% predicted) were observed in the snBA group, but there were no significant differences compared with the stBA group in the pulmonary function test. Mean serum IgE levels were 272 and 155 IU/mL and peripheral blood eosinophil counts were 473 and 162, respectively, but these values were not significantly different. Mean values of FeNO (at 50 mL/s), J'awNO (2CM), J'awNO (TMAD) and J'awNO(non‐linear) in the snBA and stBA groups were 111and 50 ppb, 6.0 and 2.5 nL/s, 10.2 and 4.3 nL/s, and 9.4 and 3.0 nL/s, respectively. CANO(2CM) measurements were marginally higher in the snBA group than in the stBA group (P = 0.05). However, there was no significant difference in CANO(TAMD) and CANO(non‐linear) between snBA and stBA groups. Mean BALF volumes were 80 mL and 84 mL and recovery rates were 53% and 56% in the snBA and stBA groups, respectively. There was no significant difference in cell fractions between the snBA and stBA groups.
3.2. Correlation of NO‐parameters with respiratory function parameters
Table 2 shows the correlation between NO‐parameters including FeNO at 50 mL/s, J'awNO(2CM, TMAD and non‐linear) and CANO(2CM, TMAD and non‐linear) in the snBA group. J'awNO(2CM, TMAD) negatively correlated with FEF25‐75(% predicted) and FEF25(% predicted) significantly (P < 0.05). CANO(2CM) negatively correlated with FEV1(% predicted) significantly (P < 0.05).
3.3. Distribution of iNOS protein expression
Figure 1 shows the immunostained representative proximal, distal airways and alveolar region biopsy specimens from an snBA patient, stained with isotype control IgG (A, B, and C) and anti‐iNOS antibody (D, E and F). The upper row shows the proximal airway, the middle row shows the distal airway, and the lower row shows the alveolar region. The bronchial epithelial cells (BECs) and inflammatory cells were stained with iNOS protein in the proximal and distal airway lumens. In the alveolar region, alveolar macrophages (AMs) were strongly and alveolar epithelial cells (AECs) weakly stained with iNOS protein.
Figure 1.
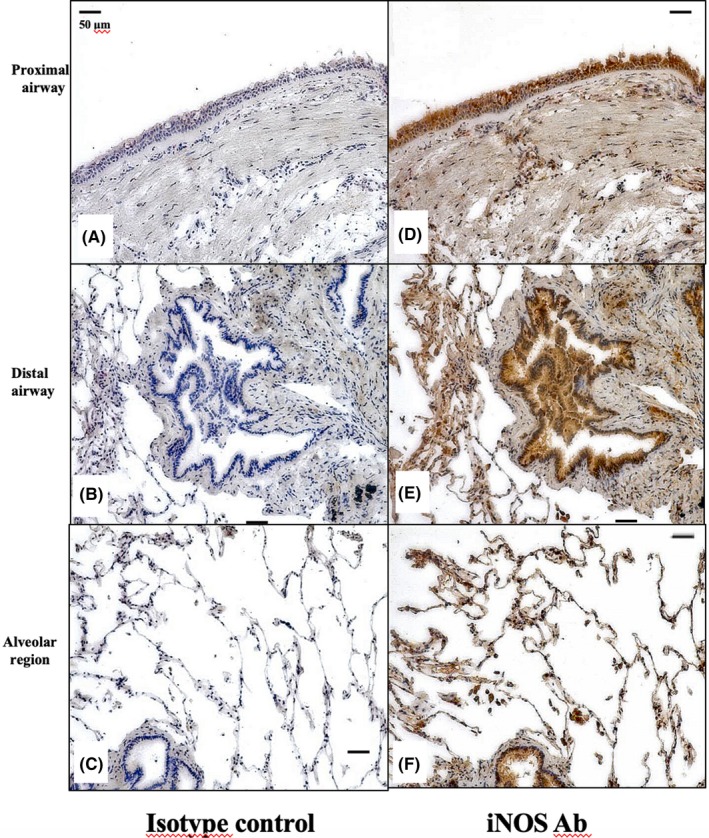
Distribution of iNOS protein expression in the snBA group by immunostaining. (A‐C), Proximal, distal airway and alveolar region samples from the snBA group stained with isotype IgG. (D‐F), Proximal, distal airway and alveolar region samples from the snBA group stained with anti‐iNOS antibody. Antibodies were 1:500 diluted and the magnifications were ×200. iNOS protein was strongly expressed in BECs and AMs, and weakly expressed in AECs
3.4. Expression of iNOS mRNA in total BAL cells and distal and proximal ACs
The expression of iNOS mRNA was detected in total BAL cells and distal and proximal ACs from stBA and snBA patients by qRT‐PCR. In the snBA group, the expression of iNOS mRNA was significantly higher in both the distal and proximal ACs than in the total BAL cells (P < 0.01). There were no significant differences between the distal and proximal ACs. There were no significant differences among total BAL cells, distal ACs and proximal ACs in stBA group. Comparing the stBA and snBA groups, the expression of iNOS mRNA in both distal and proximal ACs was significantly higher in the snBA group than in the stBA group (P < 0.01). However, there was no significant difference in the total BAL cells between the groups, (Figure 2).
Figure 2.
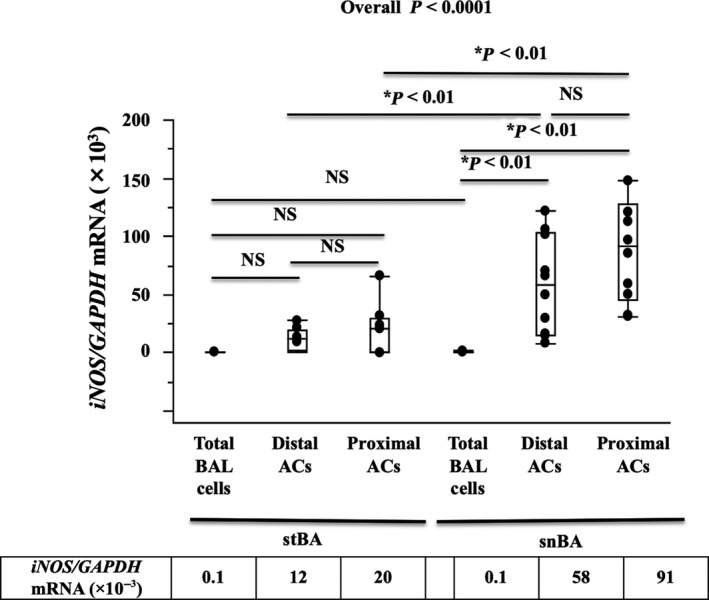
Expression of iNOS mRNA in Airway cells (ACs) and total BAL cells. Expression of iNOS mRNA detected in ACs and total BAL cells. In the snBA group, iNOS mRNA expression in ACs, including distal and proximal airway, were significantly higher than in total BAL cells (P < 0.01). The expression of iNOS mRNA was higher in snBA airway ACs than in stBA ACs (P < 0 .01). There was no significant difference in iNOS mRNA expression in the total BAL cells between the stBA and snBA groups. *P < 0.01, **P < 0.05. A small table shows median values of iNOS mRNA expression
3.5. Correlation of NO‐parameters with iNOS mRNA expression
There was no correlation between any NO‐parameters and the expression of iNOS mRNA in the stBA group. In the snBA group, only CANO(2CM) correlated with the expression of iNOS mRNA in distal ACs (r = 0.83, P < 0.01). On the other hand, CANO(non‐linear) and CANO(TMAD) did not correlated iNOS mRNA expression from any airway portions, in Table 3.
4. DISCUSSION
In this study, we showed that the expression of iNOS protein was mainly in BECs in the airway, and in macrophages in the alveolar region of snBA patients. However, quantitative RT‐PCR revealed that the expression of iNOS mRNA was significantly higher in the ACs than in total BAL cells. The CANO(2CM) results significantly correlated with iNOS mRNA expression in ACs from the distal ACs. On the other hand, CANO(TMAD) and CANO(non‐linear) was not associated with the expression of iNOS mRNA from any airway portions. To the best of our knowledge, this is the first study to demonstrate that NO‐parameters including FeNO at 50 mL/s, J'awNO and CANO and non‐linear model methods are significantly associated with the expression of iNOS mRNA in selectively collected ACs from the proximal and distal airways and alveolar regions, respectively, in adult snBA patients. In addition, there were no correlations between NO‐parameters and the expression of iNOS mRNA in stBA patients.
The measurement of FeNO is widely used in clinical practice and recognized as an important tool that supports the diagnosis and monitoring of asthma. The main pathogenic route of bronchial asthma is type 2 inflammation, characterized by the expression of type 2 related markers, including periostin, serpinB2 and CACL1, as well as eosinophil infiltration in the airway lumens.1
Numerous studies on FeNO and asthma have already been reported. An increase in FeNO measurement has been observed in the presence of symptoms, and airway NO production correlates with airway obstruction in asthma. Mahut et al examined whether the control and severity of asthma could be evaluated using CANO(TMAD) in a sample size of 200 asthmatics. CANO(TMAD) correlated inversely with FEF25‐75; thus, it was suggested that CANO(TMAD) has some association with distal airway flow limitation, but there was no correlation between CANO(TMAD) and asthma control or severity.13 Fujisawa et al8 showed that CANO(TMAD) correlated with FEF25‐75 and FEF50, and reported that CANO(TMAD) was an index of distal airway obstruction in stable asthma. Matsumoto et al. reported that CANO(TMAD) is significantly associated with pre‐bronchodilator reactance, reactance at low frequency at 5 Hz (Xrs5), integrated area of low‐frequency Xrs (AX), and resistance at 5 Hz‐20 Hz (R5‐R20). Furthermore, they showed that CANO(TMAD) levels correlate with the bronchodilator reversibility of FEV1 and FEF 25–75.14 Kobayashi et al9 compared CANO(2CM) and CANO(TMAD) and showed that the former correlates with FEV1/FVC, FEF25‐75 and sputum eosinophil counts and the latter with FEF25‐75. They concluded that CANO(TMAD) is a specific marker of distal airway pathology. Based on these reports, CANO(TMAD) reflects the pathology of the distal airways in asthma. Our study was different from other reports, CANO(TMAD) did not correlated with airway obstruction but CANO(2CM) was negatively associated with FEV1(%predicted) significantly (P < 0.05) in snBA.
FeNO increases in individuals with asthma and reflects the level of airway inflammation.15, 16 Ichinose et al17 reported that asthmatic individuals have higher eosinophil counts and bradykinin concentrations in induced sputum in addition to higher FeNO levels. Nitric oxide and L‐citrulline are generated from L‐arginine by nitric oxide synthase in vivo.18, 19 It is known that iNOS is particularly important in airway inflammation in asthma, and the stimulation of cytokines such as IL‐13 increases iNOS, which results in increased NO production. We and other researchers have reported that iNOS mRNA, nitrite and NO are induced by IL‐13 stimulation in vitro from primary cultured BECs.20, 21 Based on these reports, iNOS mRNA expression reflects NO production in asthma and the expression of iNOS mRNA is thought to be an adequate index to evaluate airway inflammation in asthma.
Distal airway inflammation is one of the causes of severe refractory asthma3, 4, 22 and is assessed by three calculations (2CM, TMAD and non‐linear).5, 6, 7 However, few reports have compared a histological study of the distal airways and distal airway nitric oxide levels. Recently, the iNOS levels of both bronchial and alveolar tissues have been increased in uncontrolled asthma. However, although they showed that iNOS levels in BAL macrophages are not reflected by CANO(2CM), they did not investigate CANO(TMAD) or CANO(non‐linear).23 It is difficult to clarify whether AMs or AECs are the main cell sources of iNOS. In an animal model investigation, nitrites/nitrates were produced by AMs as well as AECs. However, both production of nitrite/nitrates and iNOS expression seemed to be higher in AECs.24
We have conducted the first study into the association between the expression of iNOS mRNA derived from different airway components and NO‐parameters. It is recognized that TMAD gives a more precise estimate of NO levels in the alveolar region than 2CM.6 CANO(TMAD) is mainly used to assess asthmatic distal airway dysfunction and its importance in determining the pathophysiology of the distal airways has been highlighted.8, 9, 14 Based on these reports, we hypothesized that CANO(TMAD) and CANO(non‐linear) are better indexes of inflammation from the distal airways to the alveolar region in asthmatic patients; hence, we decided to conduct this study. Unexpectedly, CANO(TMAD) and CANO(non‐linear) indicated no significant correlation with the expression of iNOS mRNA in distal ACs, while CANO(2CM) correlated with distal iNOS mRNA expression significantly. Although, CANO(2CM) was thought to be a spurious parameter including alveolar NO production and an indefinite NO concentration derived from diffusion from airway compartment. However, we cannot ignore CANO(2CM) since only CANO(2CM) correlated with iNOS mRNA in distal ACs. CANO(2CM) is presumed to be able to serve as a marker of distal airway inflammation.
This study has some limitations. First, it involved only a small number of cases. The snBA group had active inflammation in their airways, and it was difficult to collect airway samples, so there were few samples from snBA patients. Second, we could not examine airway hypersensitivity or reversibility in all cases. We opted to use bronchoscopy because of the need to histologically investigate and promptly collect ACs and to avoid the risk of an asthma attack induced by an examination of airway hypersensitivity. Moreover, we wanted to avoid delays for the initiation of ICS treatment, which was started after bronchoscopy. All patients had clinical and pathological features compatible with asthma, and we thought that the lack of examination of airway sensitivity did not interfere with the asthma diagnosis. Third, BALF reflected not only the alveolar tissues but also the distal airways. A bronchoscope with a diameter of around 4.9 mm was wedged into the airway. Some components derived from the airway that was smaller than 4.9 mm in diameter may have been extracted. However, among the total BAL cells, 90% were macrophages and a few were airway epithelial cells, and BAL samples were thought to originate mainly from the alveolar region. Regarding statistics, the confounding of each association could not be considered because of the univariate analysis in the present study. Further large sample study was warranted to conduct multivariable analysis to consider confounding factors. Despite these limitations, this study is important because it shows the expression of iNOS mRNA in cells from different components of the airway in association with NO‐parameters.
5. CONCLUSIONS
This study indicated that iNOS mRNA express in mainly airway epithelial cells and CANO(2CM) correlated with iNOS mRNA expression in distal airway epithelial cells. CANO(2CM) may reflect distal airway inflammation of steroid‐naïve asthma.
CONFLICT OF INTEREST
The authors declares no conflict of interest.
AUTHOR CONTRIBUTION
YS, KC, YH, NU, MM, RK, YN, TW, TS, RA, YS and AT carried out sampling BECs and PCR studies, and drafted the manuscript. YS, KC and YH carried out the immunoassays. YS, KC, YS and YI participated in the design of the study and performed the statistical analysis. YS, KC and YI conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
We thank Reiko Komura and Kazumi Okazaki, who measured FeNO. We also appreciated Dr. Toshimi Sairenchi for considerable help about statistics.
Sato Y, Chibana K, Horigane Y, et al. Comparison of inducible nitric oxide synthase mRNA expression in different airway portions and association with nitric oxide parameters from patients with asthma. Clin Exp Allergy. 2019;49:582–590. 10.1111/cea.13344
REFERENCES
- 1. Woodruff PG, Modrek B, Choy DF, et al. T‐helper type 2‐driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium‐to‐high‐dose inhaled corticosteroids plus a long‐acting beta2 agonist: a randomised double‐blind placebo‐controlled pivotal phase 2b dose‐ranging trial. Lancet. 2016;388:31‐44. [DOI] [PubMed] [Google Scholar]
- 3. Kraft M, Djukanovic R, Wilson S, Holgate ST, Martin RJ. Alveolar tissue inflammation in asthma. Am J Respir Crit Care Med. 1996;154:1505‐1510. [DOI] [PubMed] [Google Scholar]
- 4. Hamid Q, Song Y, Kotsimbos TC, et al. Inflammation of small airways in asthma. J Allergy Clin Immunol. 1997;100:44‐51. [DOI] [PubMed] [Google Scholar]
- 5. Tsoukias NM, George SC. A two‐compartment model of pulmonary nitric oxide exchange dynamics. J Appl Physiol (1985) 1998;85: 653‐666. [DOI] [PubMed] [Google Scholar]
- 6. Condorelli P, Shin HW, Aledia AS, Silkoff PE, George SC. A simple technique to characterize proximal and peripheral nitric oxide exchange using constant flow exhalations and an axial diffusion model. J Appl Physiol (1985) 2007;102: 417‐425. [DOI] [PubMed] [Google Scholar]
- 7. Hogman M, Thornadtsson A, Liv P, et al. Effects of growth and aging on the reference values of pulmonary nitric oxide dynamics in healthy subjects. J Breath Res. 2017;11:047103. [DOI] [PubMed] [Google Scholar]
- 8. Fujisawa T, Yasui H, Akamatsu T, et al. Alveolar nitric oxide concentration reflects peripheral airway obstruction in stable asthma. Respirology. 2013;18:522‐527. [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi D, Tochino Y, Kanazawa H, et al. Comparison of alveolar nitric oxide concentrations using two different methods for assessing small airways obstruction in asthma. Respirology. 2011;16:862‐868. [DOI] [PubMed] [Google Scholar]
- 10. Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weibel ER. Morphometry of the Human Lung. New York, NY: Academic Press, 1963. [Google Scholar]
- 12. Watanabe T, Chibana K, Shiobara T, et al. Expression of intelectin‐1 in bronchial epithelial cells of asthma is correlated with T‐helper 2 (Type‐2) related parameters and its function. Allergy Asthma Clin Immunol. 2017;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahut B, Trinquart L, Le Bourgeois M, et al. Multicentre trial evaluating alveolar NO fraction as a marker of asthma control and severity. Allergy. 2010;65:636‐644. [DOI] [PubMed] [Google Scholar]
- 14. Matsumoto H, Niimi A, Jinnai M, et al. Association of alveolar nitric oxide levels with pulmonary function and its reversibility in stable asthma. Respiration. 2011;81:311‐317. [DOI] [PubMed] [Google Scholar]
- 15. Kharitonov SA, Yates D, Robbins RA, Logan‐Sinclair R, Shinebourne EA, Barnes PJ. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994;343:133‐135. [DOI] [PubMed] [Google Scholar]
- 16. Barnes PJ, Liew FY. Nitric oxide and asthmatic inflammation. Immunol Today. 1995;16:128‐130. [DOI] [PubMed] [Google Scholar]
- 17. Ichinose M, Takahashi T, Sugiura H, et al. Baseline airway hyperresponsiveness and its reversible component: role of airway inflammation and airway calibre. Eur Respir J. 2000;15:248‐253. [DOI] [PubMed] [Google Scholar]
- 18. Hecker M, Sessa WC, Harris HJ, Anggard EE, Vane JR. The metabolism of L‐arginine and its significance for the biosynthesis of endothelium‐derived relaxing factor: cultured endothelial cells recycle L‐citrulline to L‐arginine. Proc Natl Acad Sci USA. 1990;87:8612‐8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chenais B, Yapo A, Lepoivre M, Tenu JP. High‐performance liquid chromatographic analysis of the unusual pathway of oxidation of L‐arginine to citrulline and nitric oxide in mammalian cells. J Chromatogr. 1991;539:433‐441. [DOI] [PubMed] [Google Scholar]
- 20. Suresh V, Mih JD, George SC. Measurement of IL‐13‐induced iNOS‐derived gas phase nitric oxide in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2007;37:97‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chibana K, Trudeau JB, Mustovich AT, et al. IL‐13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin Exp Allergy. 2008;38:936‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berry M, Hargadon B, Morgan A, et al. Alveolar nitric oxide in adults with asthma: evidence of distal lung inflammation in refractory asthma. Eur Respir J. 2005;25:986‐991. [DOI] [PubMed] [Google Scholar]
- 23. Tufvesson E, Andersson C, Weidner J, Erjefalt JS, Bjermer L. Inducible nitric oxide synthase expression is increased in the alveolar compartment of asthmatic patients. Allergy. 2017;72:627‐635. [DOI] [PubMed] [Google Scholar]
- 24. Warner RL, Paine R 3rd, Christensen PJ, et al. Lung sources and cytokine requirements for in vivo expression of inducible nitric oxide synthase. Am J Respir Cell Mol Biol. 1995;12:649‐661. [DOI] [PubMed] [Google Scholar]


