Abstract
Background and Objectives
Rare but potentially life‐threatening hypersensitivity reactions can occur during the administration of intravenous iron. To provide guidance to healthcare professionals caring for adults receiving intravenous iron, a panel of 10 Canadian clinical experts developed a practical algorithm for the identification and management of hypersensitivity reactions to intravenous iron.
Materials and methods
A systematic search of PubMed to February 2018 was performed. Articles related to hypersensitivity reactions were selected for review. The algorithm was developed during a 1‐day live meeting based on the literature review and clinical expertise where evidence was lacking. The algorithm was then refined through an iterative process involving a web‐based platform and virtual meetings.
Results
The algorithm provides guidance to healthcare professionals in preparing for and administering IV iron, as well as recognizing and managing hypersensitivity reactions to intravenous iron. Considerations for re‐challenging patients who have experienced prior reactions are provided.
Conclusion
Healthcare professionals who are involved in the care of patients receiving intravenous iron should be trained to anticipate, recognize and manage hypersensitivity reactions to intravenous iron to optimize patient care.
Keywords: anaphylaxis, consensus, hypersensitivity reactions, intravenous iron
Introduction
Intravenous (IV) iron is used for the treatment of iron deficiency anaemia when oral iron is inappropriate, ineffective or poorly tolerated 1, 2. Hypersensitivity reactions can occur during iron infusion; such reactions are rare but can be life‐threatening. These reactions can manifest with different signs and symptoms, and range in severity from mild self‐limited findings to severe anaphylactic shock 1, 2, 3. In order to provide guidance in the management of hypersensitivity reactions to IV iron to healthcare professionals (HCPs) caring for adults receiving IV iron, we sought to develop a practical algorithm for the identification and management of hypersensitivity reactions to IV iron.
Materials and methods
Development of Algorithm
With the objective of reviewing the literature and developing a practical algorithm for the management of hypersensitivity reactions to IV iron, we formed a multidisciplinary Canadian consensus group. The consensus group included seven physicians with expertise in the areas of haematology, transfusion medicine, allergy and immunology, gastroenterology and nephrology, as well as a pharmacist, nurse manager and nurse practitioner. The panel members were identified through their involvement in developing protocols for IV iron administration and/or hypersensitivity management at their respective institutions, through publications or policy work related to IV iron or hypersensitivity management, or recommendations from other panel members as being clinical leaders with expertise in this area. Diverse representation from stakeholders involved in IV iron administration was sought in order to provide a multidisciplinary perspective.
We searched the PubMed database to February 2018 using the following search terms: “(intravenous OR parenteral) AND (iron OR ferric OR ferrous) AND (Fishbane OR hypersensitivity OR hypersensitive OR anaphylaxis OR anaphylactic OR anaphylactoid)” and limited the results to humans. The chair of the consensus group reviewed the identified studies and selected articles for inclusion. Articles were included if adult patients received IV iron, and any of the following data were reported: incidence of hypersensitivity reactions, signs and symptoms of hypersensitivity reactions and frequency following administration of IV iron, or a management strategy when a reaction occurred was outlined. Additional articles were identified through review of the references of included studies and/or personal records.
The first draft of the management algorithm was developed based on the identified articles and the clinical experience of the panel members during a 1‐day live meeting held in April 2018. In the course of this meeting, the literature was reviewed and the panel discussed how to incorporate these data into the algorithm. The product monographs of currently available IV iron products 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, institutional and nursing protocols for IV iron administration and monitoring, and expert recommendations and systematic reviews on the management of anaphylaxis and general hypersensitivity reactions 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 were reviewed. The panel members’ personal clinical experience with IV iron administration and management of hypersensitivity reactions were also taken into consideration.
The algorithm was refined through an iterative process, using a web‐based platform (Google Docs) and a web‐based teleconference held in June 2018. The draft algorithm and manuscript were uploaded to the web‐based platform, and panel members were asked to provide comments for both documents. These changes were discussed during the web‐based teleconference and applied to the next draft of the algorithm. The process was then repeated, with the panel being asked to explicitly indicate their agreement or disagreement with the proposed changes. Finally, all members of the consensus group voted in order to indicate agreement with the final version of the algorithm to achieve consensus.
Terminology
The term ‘hypersensitivity reactions’ was used to cover all types of reactions to IV iron, regardless of severity, timing or pathophysiology.
We subdivided reactions based on timing into acute reactions (i.e. those occurring during and up to 30 min after the conclusion of IV iron administration) and delayed reactions (i.e. those occurring more than 30 min after IV iron has been administered). The 30‐min time frame was based on IV iron product monographs and existing recommendations advising that patients be monitored for at least 30 minutes following administration 1, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17. We further categorized reactions according to their clinical presentation: anaphylaxis, defined as an acute, potentially life‐threatening systemic reaction involving two or more organ systems (respiratory and one of the following: skin, gastrointestinal or cardiovascular) or either hypotension or angio‐oedema of the tongue/airway alone; Fishbane reaction, an acute but non‐life‐threatening combination of symptoms typically characterized by transient flushing and tightness or pain in the chest and back; and isolated symptoms, non‐life‐threatening symptoms limited to one organ system excluding respiratory symptoms (e.g. urticaria).
Sources of support
The meetings and the assistance of a medical writer were funded by an unrestricted grant from Pfizer Canada. The funding source had no role in drafting, editing or approving the algorithm.
Results
The PubMed search identified 226 articles, 26 of which were considered to be potentially relevant following abstract screening. Following full review, 15 articles were excluded, leaving 11 articles included in the final review: one guidance document on managing hypersensitivity reactions to IV iron 1, one consensus report on iron management in chronic kidney disease 28, three reviews of hypersensitivity reactions 2, 29, 30, two articles assessing incidence and comparative risks 31, 32, and four articles describing case reports of signs, symptoms and managing reactions 33, 34, 35, 36. Review of the references of these articles, as well as personal records of the panel, identified an additional eight articles: one review of hypersensitivity reactions 37; two analyses of the rates of reaction with IV iron based on observational data 38, 39; four articles describing case reports of signs, symptoms and managing reactions 40, 41, 42, 43; and the European Medicines Agency (EMA)'s assessment report for iron‐containing IV medicinal products, a comprehensive review of available safety data on IV iron, including post‐marketing incident reports 3.
Based on the available evidence and clinical expertise, a management algorithm was developed (Fig. 1). The algorithm was created by considering IV iron administration in three sections: pre‐infusion considerations, infusion details and management of reactions, and post‐reaction considerations.
Figure 1.
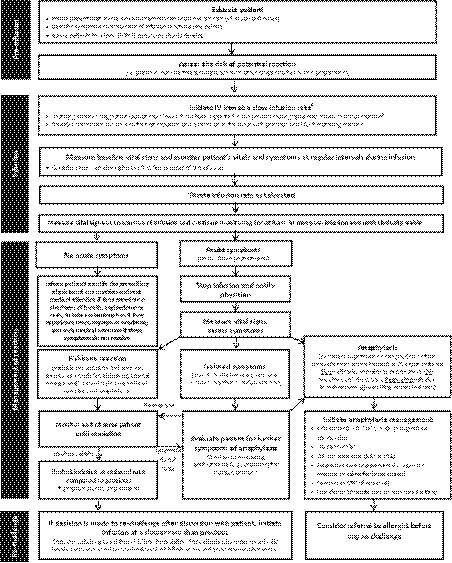
Hypersensitivity reaction management algorithm for intravenous iron administration. aInfusions should be conducted at a site where personnel and resuscitative interventions are immediately available for treatment of severe hypersensitivity reactions. bRefer to the product monograph for recommended rates of infusion. cHypotension defined as a drop of 30 mmHg SBP from baseline or SBP < 90 mmHg. CPR, cardiopulmonary resuscitation; CV, cardiovascular; ED, emergency department; GI, gastrointestinal; HCP, healthcare professional; IM, intramuscular; IV, intravenous; SBP, systolic blood pressure
Upon final voting, the panel members all approved the final version of the management algorithm.
Discussion
In selected patients, IV iron should be considered an alternative to red blood cell transfusion for the treatment of severe iron deficiency anaemia, and it can also be used in the perioperative setting to optimize haemoglobin levels in anticipation of blood loss with surgery. The risk of acute serious adverse events and death from IV iron administration is almost negligible compared to transfusion; serious adverse events with transfusion are estimated at 1 in 21 413 44. In comparison, the risk of anaphylaxis (serious adverse event) associated with IV iron is estimated at less than 1 in 200 000 29.
Pre‐infusion preparation
Infusion location and available resources
Intravenous iron infusions should be administered at a site where trained personnel and appropriate resuscitative equipment are immediately available for treatment of severe hypersensitivity reactions (anaphylaxis) 3. Table 1 lists basic equipment and medications recommended to be readily available for the management of anaphylaxis, adapted from the World Allergy Organization (WAO) guidelines for the assessment and management of anaphylaxis 18.
Table 1.
Suggested basic equipment for anaphylaxis kita
| Medication |
|---|
| First‐line medication |
|
| Second‐line medication |
|
| Equipment and supplies |
|
IM, intramuscular; IV, intravenous; WAO, World Allergy Organization.
Adapted from WAO anaphylaxis guidelines 18.
Note: uni‐dose epinephrine auto‐injectors may be included instead of IM epinephrine and a 22‐gauge needle; IM epinephrine should not be given intravenously.
Assessing risk of reaction
Hypersensitivity reactions to IV iron are estimated to occur with a prevalence of less than 0·1% 31. Anaphylactic reactions are even less frequent, with an incidence of less than one in 200 000 when high‐molecular‐weight iron dextran is excluded 29. Given the higher rates of anaphylaxis with high‐molecular‐weight iron dextran, it is recommended that alternate IV iron preparations be utilized first line. Several factors have been identified to increase the risk and/or severity of hypersensitivity reactions to IV iron (Table 2) 3, 27. Patients should be evaluated for these risk factors, and appropriate precautions should be implemented for patients considered at higher risk (see below).
Table 2.
Risk factors for hypersensitivity reactions to intravenous iron
| Factors increasing risk of reaction 3 | Factors increasing severity of reaction 27, a |
|---|---|
|
|
ACE, angiotensin‐converting enzyme; IV, intravenous.
Factors increasing severity of anaphylactic reactions in general – not specific to IV iron.
Patient and healthcare provider education
Education regarding the prevalence of hypersensitivity reactions to IV iron should be provided to patients and HCPs to help alleviate anxiety, which is recognized to increase the severity of an anaphylactic reaction 27. Other information that the panel felt may be helpful to provide to patients includes the indication, duration of time required for infusion and post‐infusion monitoring, symptoms of hypersensitivity reactions and when to notify an HCP, reassurance that the patient will be monitored, contact information and online references. Patients should also be informed of the symptoms of a potential delayed reaction and be provided instruction in the event such symptoms develop (Fig. 1).
Administration of infusion
Intravenous Iron formulations
A variety of IV iron formulations exist, with availability varying across countries (Table 3) 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17. The management of hypersensitivity reactions is the same regardless of which IV iron preparation is used, although rates of reactions are higher with high‐molecular‐weight iron dextran than other preparations 1, 29, 38, 39. Current product monographs for IV iron products available in Canada may be accessed through the Health Canada Drug Product Database (available at: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp) 45.
Table 3.
Intravenous iron formulations
| Characteristic | Ferric carboxymaltose (Ferinject, Injectafer) 4, 5 | Ferumoxytol (Feraheme, Rienso) 6, 7 | Iron isomaltoside 1000 (Monofer, Monoferric) 8, 9 | Iron sucrose (Venofer) 10, 11, 12 | LMW iron dextran (CosmoFer, DexIron, INFeD) 13, 14, 15 | Sodium ferric gluconate (Ferrlecit) 16, 17 |
|---|---|---|---|---|---|---|
| Availability | Europe, USA | Europe, USA | Canada, Europe | Canada, Europe, USA | Canada, Europe, USA | Canada, USA |
| Indicationa | Iron deficiency when oral iron cannot be used | Iron deficiency anaemia in adults with CKD | Iron deficiency anaemia when oral iron cannot be used (or rapid iron supply required – Europe only) | Iron deficiency in CKD (or when oral iron cannot be used or rapid iron supply required – Europe only) | Iron deficiency when oral iron cannot be used (or rapid iron supply required – Europe only) | Iron deficiency in haemodialysis patients receiving erythropoietin therapy |
| Carbohydrate shell | Carboxymaltose | Polyglucose sorbitol | Isomaltoside | Sucrose | Dextran | Gluconate |
| Molecular weight (kDa) | 150 | 750 | 150 | 43 | 400 | 289–440 |
| Volume distribution (L) | 3 | 3·16 | N/A | ~3 | N/A | 6 |
| Test dose required | No | No | No | No | In Canada/USA only | No |
| Maximum single doseb | 750 mg (USA)1000 mg (Europe) | 510 mg | 1500 mg | 500 mg | 100 mg (Canada/USA)20 mg/kg (Europe) | 125 mg |
| Infusion rate or timeb | ≥15 min | Injection at ≤1 ml/s (≤30 mg/s) | ≥15 min (≤1000 mg, Europe); ≥20 min (≤1000 mg, Canada); ≥30 min (>1000 mg) | 8 min (50 mg); 15 min (100 mg); 30 min (200 mg); 1·5 h (300 mg); 2·5 h (400 mg) | First 25 mg over15 min, remaining dose at < 100 ml/30 min; total dose infusion over 4–6 h | 1 h |
Specific indications vary across jurisdictions – refer to relevant product information. Off‐label use of certain products is not uncommon, but should be done in the context of full disclosure to the patient.
Maximum single dose and minimum infusion time as per product information, which may vary across jurisdictions.
CKD, chronic kidney disease; LMW, low molecular weight; N/A, information not available in product monograph; USA, United States of America.
Test doses
Based on data from post‐marketing reports, the EMA concluded that a successful test dose may give false assurance to HCPs administering IV iron 3, and therefore, test doses should not be used unless stated as mandatory in the product monograph. In Europe, no IV iron product requires a test dose, whereas in North America, a test dose is required for certain formulations (Table 3) 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17.
Infusion rate
Manufacturer‐ and administrative body‐recommended infusion rates vary across IV iron preparations and jurisdictions 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17. During administration, HCPs should be familiar with the approved maximal dose, dilution volume and infusion speed for each IV iron formulation. Although there is a paucity of data comparing the risk of hypersensitivity reactions with slower compared to faster infusion rates, it is recognized that more rapid infusion rates increase the risk of a hypersensitivity reaction. It is hypothesized that rapid exposure to IV iron activates the complement system, promoting anaphylatoxin production and thus triggering a hypersensitivity reaction 1, 28. Panel members’ clinical experience also noted that hypersensitivity reactions are more likely to occur with faster infusion rates, and these rates may be in accordance with approved product monographs. In patients with identified risk factors for hypersensitivity reactions, or in those receiving a first dose, the panel concurred that the infusion should be initiated at a slow infusion rate to minimize the risk of a reaction. In accordance with existing guidance documents that suggest initiating infusions at low rates (<50% of recommended rate), increasing it after a few minutes if no infusion reaction occurs 1, 28, we also recommend using an initially slow infusion rate, particularly during the first infusion. While infusion times will vary depending on the IV iron formulation, dilution volume and institutional protocol, we suggest a minimum infusion time of between 15 min and approximately one hour for a first infusion based on expert opinion. As hypersensitivity reactions have been reported to be most likely to occur within the first 10 minutes of the infusion 3, if no reactions occur at the slow infusion rate, consideration may then be given to increasing the infusion rate as tolerated, as suggested by other guidance documents 1, 28. The available personnel and administrative regulations at individual institutions should also be considered when determining infusion rates.
Dosage
In addition to increasing the infusion time, the panel also noted that lower doses of IV iron are associated with lower rates of hypersensitivity reactions. Thus, in selected patients with identified risk factors for hypersensitivity reactions or previous isolated symptoms, a lower dose may be considered based on expert opinion.
Monitoring
Vital signs should be measured at baseline and the patient closely monitored for the first few minutes of the infusion as hypersensitivity reactions have been reported to be most likely to occur within the first 10 min of the infusion 3. After the first 10 min, we suggest that patients should be monitored at regular intervals, such as mid‐way through the infusion or sooner. Vital signs should be measured again at the conclusion of the infusion and the patient monitored for at least 30 min post‐infusion (Fig. 1).
Management of hypersensitivity reactions
If acute symptoms of a reaction occur, the infusion should be stopped, the responsible physician notified and the patient's vital signs and symptoms assessed to determine the type of reaction.
Anaphylaxis
Anaphylaxis is a clinical diagnosis. The probability of anaphylaxis is high when any one of three criteria are fulfilled: (i) acute onset of skin and/or mucosal tissue manifestations (e.g. urticaria, itching, flushing, swollen lips/tongue/uvula) with one of the following: respiratory compromise (e.g. dyspnoea, wheeze–bronchospasm, stridor, reduced peak expiratory flow, hypoxaemia) or hypotension/end‐organ dysfunction; (ii) following exposure to a likely allergen, at least two of the following: skin–mucosal tissue involvement, respiratory compromise, hypotension/end‐organ dysfunction or gastrointestinal symptoms; or (iii) development of hypotension (drop of 30 mmHg systolic blood pressure [SBP] from baseline or SBP < 90 mmHg) following exposure to a known allergen 18. If anaphylaxis is suspected based on presenting symptoms (Table 4), 0·3–0·5 ml of 1:1000 (1 mg/ml) intramuscular (IM) epinephrine should be administered immediately as early use of epinephrine has been shown to reduce the risk of hospitalization and death 18, 19, 30. No serious adverse effects are associated with the correct dose of IM epinephrine 18, 19. While IV epinephrine is also effective, the risk of overdose and thus serious adverse effects, including ventricular arrhythmias, hypertensive crisis and pulmonary oedema, is higher with the IV route of administration 18, 19. We thus recommend that only IM epinephrine be included in infusion centre anaphylactic kits and that HCPs administering IV iron be trained in its use. Once IM epinephrine has been administered, further steps for the basic management of anaphylaxis, as outlined in the WAO guidelines for the assessment and management of anaphylaxis, should be followed (Table 5) 18.
Table 4.
Symptoms of anaphylaxisa
| At least one of the following: |
|---|
|
| OR | |||
|---|---|---|---|
| Involvement of at least two organ systems: | |||
| Skin/mucosal tissue | Cardiovascular system | Respiratory system | Gastrointestinal system |
|
|
|
|
Table 5.
Basic management of anaphylaxis in adultsa
| Preliminary steps: |
|---|
|
| Steps to perform promptly and simultaneously: |
|
| Steps to perform at any time, when indicated: |
|
| Steps to perform at frequent and regular intervals: |
|
CPR, cardiopulmonary resuscitation; IM, intramuscular; IV, intravenous; WAO, World Allergy Organization.
Adapted from WAO anaphylaxis guidelines 18.
Corticosteroids are commonly used in anaphylaxis management 20; however, a systematic review found no evidence of the effectiveness of corticosteroids in anaphylaxis 21. Because corticosteroids have short‐term effects and may be beneficial for some patients, corticosteroids along with other adjunctive therapies may be considered after epinephrine administration in patients with anaphylaxis 22.
Isolated symptoms
For patients experiencing an isolated symptom of a hypersensitivity reaction, such as IV site irritation, urticaria, nausea, diarrhoea or abdominal pain, but not meeting the criteria for anaphylaxis, we recommend stopping the IV iron infusion, evaluating the patient for anaphylaxis and monitoring the patient until symptoms abate (Fig. 1). The use of antihistamines (H1 receptor antagonists) may be considered for symptomatic relief in patients suffering from isolated urticaria 23. First‐generation histamine H1 receptor antagonists (e.g. diphenhydramine and chlorpheniramine) can cause somnolence, diaphoresis, hypotension and tachycardia, which can exacerbate the presentation. Furthermore, these symptoms can then ostensibly be attributed to the administered IV iron. Second‐generation antihistamines (e.g. cetirizine and loratadine) or third‐generation antihistamines (e.g. desloratadine and fexofenadine) are preferred given the non‐sedating effects compared to first‐generation agents 23, 24, 25. A Cochrane systematic review evaluating H2 receptor antagonists for urticaria was limited by lack of precision and limitations in the studies reporting a measure of urticarial relief 26. While H2 receptor antagonists may be considered for treatment of urticaria, the data limitations suggest these may be considered only if the IV route is required and oral H1 antihistamines cannot be administered 22. Regardless of whether antihistamines are administered, patients should be monitored until the symptoms have resolved, at which point a discussion with the patient regarding whether to resume the infusion at a slower rate than was initially used may be initiated (Fig. 1).
Fishbane reactions
Fishbane reactions have been described in association with IV iron administration as acute, non‐life‐threatening reactions characterized by transient symptoms including facial flushing, truncal myalgia or chest tightness, and/or joint pains 1, 32. While the presentation and symptom severity are highly variable, no symptoms of anaphylaxis are present; specifically, Fishbane reactions are not associated with systemic hypotension, mucosal involvement (periorbital oedema) or involvement of other organ systems (e.g. respiratory stridor and gastrointestinal pain). Fishbane reactions are believed to be triggered by labile iron, rather than being immunoglobulin E‐ or complement‐mediated 32. As such, Fishbane reactions resolve without intervention following cessation of the IV iron infusion and do not usually reoccur upon re‐challenge 1. The recommended management of a Fishbane reaction is thus similar to that of an isolated symptom (Fig. 1), although antihistamines should not be used as they may potentiate hypotension and exacerbate the reaction 1, 37.
Delayed reactions
Delayed anaphylaxis, occurring more than 30 min after the conclusion of the infusion, is extremely rare 3. Prior to being discharged after the infusion is complete, patients should be advised to inform the prescribing physician of any delayed reactions and to seek medical assistance if they experience shortness of breath, angio‐oedema or rash within the next 24 h (Fig. 1). We also recommend informing patients about more common delayed symptoms, such as fever, myalgia, arthralgia or headache 30. These latter symptoms may be treated with acetaminophen and monitored. Non‐steroidal anti‐inflammatory drugs should be avoided as they may serve as augmenting factors and worsen the reaction 46.
Documentation
Reactions to IV iron, including the type of IV iron preparation and details regarding the nature of the reaction, should be reported to the appropriate national pharmacovigilance system.
Re‐challenging patients with previous hypersensitivity reaction
Patients with previous non‐life‐threatening reaction
Patients who have experienced a previous hypersensitivity reaction to IV iron are at increased risk of experiencing another reaction 3. In addition, anxiety arising from the previous reaction may contribute to increased vigilance and severity of a future reaction 27. Thus, we suggest including patients in the discussion regarding whether a re‐challenge to IV iron should be considered. The decision to re‐challenge should be based on the severity and nature of the previous reaction, patient preference, individual characteristics and medical necessity.
Strategies that have been used to minimize the risk of another reaction include slowing the infusion rate, decreasing the dose, switching to a different IV iron preparation and premedication with corticosteroids, antihistamines and/or acetaminophen 31, 33, 34, 40, 41, 42. There have been case reports of successfully avoiding hypersensitivity reactions in patients with previous reactions to IV iron by re‐challenging the patient with a different IV iron preparation 34, 42. Alternatively, initial low‐dose slow priming, followed by slow dose escalation, referred to as a low reactogenic protocol, has been used successfully to prevent IV iron reactions in patients with a history of other life‐threatening reactions 31, 33, 40, 41. While infusion rates and the timing of escalation may differ in various low reactogenic protocols, all such protocols start the infusion at a very low rate such that 0·001–0·01% of the full dose is infused in the first 5–15 min 29. More recently, desensitization protocols for IV iron administration in individuals with a history of anaphylaxis to IV iron have been published 43. Both low reactogenic and desensitization protocols often include pretreatment with systemic corticosteroids and antihistamines. However, pretreatment alone, without altering the administration protocol, is of questionable efficacy in preventing hypersensitivity reactions to IV iron 31, 35. For instance, in a study of 135 adults who were pretreated with diphenhydramine, cimetidine and dexamethasone before receiving IV iron, 13% of patients experienced hypersensitivity reactions consisting mainly of myalgias and arthralgias 35. Thus, we do not recommend pretreatment alone for most patients being re‐challenged with IV iron. However, based on clinical experience, we suggest considering pretreatment with an antihistamine (either first‐ or second‐generation) in patients who previously experienced isolated mild urticaria upon receiving IV iron.
Patients with previous anaphylaxis
Most patients who experience anaphylaxis following administration of IV iron will not be re‐challenged. However, in cases where re‐challenge is being considered, we suggest referral to an allergist/immunology specialist for further evaluation and potential desensitization. Desensitization protocols should be carried out under the supervision of a specialist experienced in managing anaphylaxis, in an environment where one‐on‐one care by appropriately trained HCPs is possible and resuscitation equipment and supplies are available.
Strengths and limitations
Hypersensitivity reactions to IV iron are extremely rare, and there are few studies that have examined the risk factors for, management of, re‐challenge following hypersensitivity reaction to IV iron. Limitations to our algorithm include the use of a single database for the literature search and article selection done by a single individual, so it is possible that relevant articles were missed. We attempted to minimize this by identifying additional articles for consideration through review of the selected article references and/or personal records of the panel members. There was no formal assessment of the quality and grading of the evidence as most of the available data were observational and would be considered low quality. Many recommendations described in this document are based on the clinical experience of the committee, supported by low‐ or very‐low‐quality evidence. The strengths of this document include the multidisciplinary representation of the panel, summary of the existing literature including guideline recommendations from the WAO that are supported by stronger levels of evidence, and the emphasis on providing practical guidance to HCPs for the prevention, monitoring and management of hypersensitivity reactions to IV iron.
Conclusion
While hypersensitivity reactions can occur with IV iron administration, they are extremely rare. Limited evidence exists to guide the management of patients experiencing such reactions. Nonetheless, due to the potential for a life‐threatening event, it is important that HCPs recognize the signs and symptoms of various types of hypersensitivity reactions and be able to respond rapidly and appropriately. The implementation of protocols and HCP education in this regard can help ensure patient safety and quality of care.
Conflicts of interest
WA has received consulting honoraria from AbbVie, Allergan, Janssen, Pfizer, Merck, Shire and Takeda, and research support from AbbVie and Janssen. WL has received consulting honoraria from Alexion Pharmaceuticals, Leo Pharma, Pfizer and Portola Pharmaceuticals. YL has received consulting honoraria from Pfizer, and research support from Novartis and Octapharma. IN has received consulting honoraria from AbbVie, Janssen and Pfizer. SK, GL, CM, FR, CS and TX have received honoraria from Pfizer.
Funding
Unrestricted grant from Pfizer Canada Inc.
Acknowledgements
This initiative was funded through an unrestricted grant from Pfizer Canada Inc. The authors administered all aspects of the algorithm development, and the funding source had no role in drafting, editing or approving the algorithm. Rebecca Cowan of MedPlan Communications Inc. is thanked for editorial support in the preparation of the manuscript.
References
- 1. Rampton D, Folkersen J, Fishbane S, et al: Hypersensitivity reactions to intravenous iron: guidance for risk minimization and management. Haematologica 2014; 99:1671–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Muñoz M, Gómez‐Ramírez S, Bhandari S: The safety of available treatment options for iron‐deficiency anemia. Expert Opin Drug Saf 2018; 17:149–159 [DOI] [PubMed] [Google Scholar]
- 3. European Medicines Agency : Assessment report for: Iron containing intravenous (IV) medicinal products. EMEA/H/A‐31/1322. European Medicines Agency, 2013. [Last accessed: July 15, 2018] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/IV_iron_31/WC500150771.pdf.
- 4. Ferinject (Ferric Carboxymaltose) : Summary of Product Characteristics. [Last accessed: January 17, 2019] Available from: https://www.medicines.org.uk/emc/medicine/5910.
- 5. Injectafer (Ferric Carboxymaltose Injection) : Full Prescribing Information. [Last accessed: January 17, 2019] Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203565s000lbl.pdf.
- 6. Rienso (Ferumoxytol) : Summary of Product Characteristics. [Last accessed: January 17, 2019] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002215/WC500129749.pdf.
- 7. Feraheme (Ferumoxytol) : Injection Full Prescribing Information. [Last accessed: January 17, 2019] Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022180lbl.pdf.
- 8. Monofer (Iron [III] Isomaltoside 1000) : Summary of Product Characteristics. [Last accessed: January 17, 2019] Available from: https://www.medicines.org.uk/emc/product/5676/smpc#PHARMACOLOGICAL_PROPS.
- 9. Monoferric (Iron Isomaltoside 1000 for Injection) : Product Monograph. Kirkland, QC, Pfizer Canada Inc., 2018[Last accessed: January 22, 2019] Available from: https://www.pfizer.ca/sites/g/files/g10050796/f/201810/MONOFERRIC_PM_E_193890_22JUN2018.pdf.
- 10. Venofer (Iron Sucrose Injection) : Product Monograph. Mississauga, ON, Bellco Health Care Inc., 2013[Last accessed: January 17, 2019] Available from: https://pdf.hres.ca/dpd_pm/00018889.PDF.
- 11. Venofer (Iron Sucrose) : Summary of Product Characteristics. [Last accessed: January 17, 2019] Available from: https://www.medicines.org.uk/emc/product/5911/smpc.
- 12. Venofer (Iron Sucrose Injection) : Full Prescribing Information. [Last accessed: January 17, 2019] Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021135s017lbl.pdf.
- 13. DexIron (Iron Dextran Injection) : Product Monograph. Mississauga, ON, Bellco Health Care Inc., 2017[Last accessed: January 17, 2019] Available from: https://pdf.hres.ca/dpd_pm/00037716.PDF.
- 14. CosmoFer (Iron Dextran) : Summary of Product Characteristics. [Last accessed: January 17, 2019] Available from: https://www.medicines.org.uk/emc/product/48/smpc#POSOLOGY.
- 15. INFeD (Iron Dextran Injection) : Full Prescribing Information. [Last accessed: January 17, 2019] Available from: https://www.allergan.com/assets/pdf/infed_pi.
- 16. Ferrlecit (Sodium Ferric Gluconate Complex in Sucrose Injection) : Product Monograph. Laval, QC, sanofi‐aventis Canada Inc., 2016[Last accessed: January 17, 2019] Available from: https://pdf.hres.ca/dpd_pm/00037569.PDF.
- 17. Ferrlecit (Sodium Ferric Gluconate Complex in Sucrose Injection) : Full Prescribing Information. [Last accessed: January 17, 2019] Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020955s013s015lbl.pdf.
- 18. Simons FE, Ardusso LR, Bilò MB, et al: World Allergy Organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J 2011; 4:13–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simons FE, Ebisawa M, Sanchez‐Borges M, et al: 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J 2015; 8:32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. LoVerde D, Iweala OI, Eginlu A, et al: Anaphylaxis. Chest 2018; 153:528–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Choo KJ, Simon E, Sheikh A: Glucocorticoids for the treatment of anaphylaxis: cochrane systematic review. Allergy 2010; 65:1205–1211 [DOI] [PubMed] [Google Scholar]
- 22. Kemp SF, Lockey RF, Simons FE, et al: Epinephrine: the drug of choice for anaphylaxis‐a statement of the World Allergy Organization. World Allergy Organ J 2008; 1(7 Suppl):S18–S26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zuberbier T, Aberer W, Asero R, et al: The EAACI/GA²LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018; 73:1393–1414 [DOI] [PubMed] [Google Scholar]
- 24. Schaefer P: Acute and chronic urticaria: evaluation and treatment. Am Fam Physician 2017; 95:717–724 [PubMed] [Google Scholar]
- 25. Bernstein JA, Lang DM, Khan DA, et al: The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol 2014; 133:1270–1277 [DOI] [PubMed] [Google Scholar]
- 26. Fedorowicz Z, van Zuuren EJ, Hu N: Histamine H2‐receptor antagonists for urticaria. Cochrane Database Syst Rev 2012; 3:CD008596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Worm M, Francuzik W, Renaudin JM, et al: Factors increasing the risk for a severe reaction in anaphylaxis: an analysis of data from The European Anaphylaxis Registry. Allergy 2018; 73:1322–1330 [DOI] [PubMed] [Google Scholar]
- 28. Macdougall IC, Bircher AJ, Eckardt KU, et al: Iron management in chronic kidney disease: conclusions from a “Kidney Disease: improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 2016; 89:28–39 [DOI] [PubMed] [Google Scholar]
- 29. Szebeni J, Fishbane S, Hedenus M, et al: Hypersensitivity to intravenous iron: classification, terminology, mechanisms and management. Br J Pharmacol 2015; 172:5025–5036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bircher AJ, Auerbach M: Hypersensitivity from intravenous iron products. Immunol Allergy Clin North Am 2014; 34:707–723 [DOI] [PubMed] [Google Scholar]
- 31. Wang C, Graham DJ, Kane RC, et al: Comparative risk of anaphylactic reactions associated with intravenous iron products. JAMA 2015; 314:2062–2068 [DOI] [PubMed] [Google Scholar]
- 32. Fishbane S, Ungureanu VD, Maesaka JK, et al: The safety of intravenous iron dextran in hemodialysis patients. Am J Kidney Dis 1996; 28:529–534 [DOI] [PubMed] [Google Scholar]
- 33. Monaghan MS, Glasco G, St John G, et al: Safe administration of iron dextran to a patient who reacted to the test dose. South Med J 1994; 87:1010–1012 [DOI] [PubMed] [Google Scholar]
- 34. Sane R, Baribeault D, Rosenberg CL: Safe administration of iron sucrose in a patient with a previous hypersensitivity reaction to ferric gluconate. Pharmacother 2007; 27:613–615 [DOI] [PubMed] [Google Scholar]
- 35. Barton JC, Barton EH, Bertoli LF, et al: Intravenous iron dextran therapy in patients with iron deficiency and normal renal function who failed to respond to or did not tolerate oral iron supplementation. Am J Med 2000; 109:27–32 [DOI] [PubMed] [Google Scholar]
- 36. Saadeh CE, Srkalovic G: Acute hypersensitivity reaction to ferric gluconate in a premedicated patient. Ann Pharmacother 2005; 39:2124–2127 [DOI] [PubMed] [Google Scholar]
- 37. Auerbach M, Macdougall IC: Safety of intravenous iron formulations: facts and folklore. Blood Transfus 2014; 12:296–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fletes R, Lazarus JM, Gage J, et al: Suspected iron dextran‐related adverse drug events in hemodialysis patients. Am J Kidney Dis 2001; 37:743–749 [DOI] [PubMed] [Google Scholar]
- 39. Chertow GM, Mason PD, Vaage‐Nilsen O, et al: On the relative safety of parenteral iron formulations. Nephrol Dial Transplant 2004; 19:1571–1575 [DOI] [PubMed] [Google Scholar]
- 40. Altman LC, Petersen PE: Successful prevention of an anaphylactoid reaction to iron dextran. Ann Intern Med 1988; 109:346–347 [DOI] [PubMed] [Google Scholar]
- 41. Hickman MA, Bernstein IL, Palascak JE: Successful administration of iron dextran in a patient who experienced a life threatening reaction to intravenous iron dextran. Ann Allergy Asthma Immunol 2000; 84:262–263 [DOI] [PubMed] [Google Scholar]
- 42. Bastani B, Rahman S, Gellens M: Lack of reaction to ferric gluconate in hemodialysis patients with a history of severe reaction to iron dextran. ASAIO J 2002; 48:404–406 [DOI] [PubMed] [Google Scholar]
- 43. Chapman E, Leal D, Alvarez L, et al: Two case reports of desensitization in patients with hypersensitivity to iron. World Allergy Organ J 2017; 10:38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bolton‐Maggs PH, Cohen H: Serious Hazards of Transfusion (SHOT) haemovigilance and progress is improving transfusion safety. Br J Haematol 2013; 163:303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Health Canada : Drug Product Database Online Query. [Last accessed: August 29, 2018] Available from: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp.
- 46. Muñoz‐Cano R, Pascal M, Araujo G, et al: Mechanisms, cofactors, and augmenting factors involved in anaphylaxis. Front Immunol 2017; 8:1193 [DOI] [PMC free article] [PubMed] [Google Scholar]


