Abstract
Aim
To investigate the magnitude of executive function deficits and their dependency on gestational age, sex, age at assessment, and year of birth for children born preterm and/or at low birthweight.
Method
PubMed, PsychINFO, Web of Science, and ERIC were searched for studies reporting on executive functions in children born preterm/low birthweight and term controls born in 1990 and later, assessed at a mean age of 4 years or higher. Studies were included if five or more studies reported on the same executive function measures.
Results
Thirty‐five studies (3360 children born preterm/low birthweight, 2812 controls) were included. Children born preterm/low birthweight performed 0.5 standardized mean difference (SMD) lower on working memory and cognitive flexibility and 0.4 SMD lower on inhibition. SMDs for these executive functions did not significantly differ from each other. Meta‐regression showed that heterogeneity in SMDs for working memory and inhibition could not be explained by study differences in gestational age, sex, age at assessment, or year of birth.
Interpretation
Children born preterm/low birthweight since 1990 perform half a SMD below term‐born peers on executive function, which does not seem to improve with more recent advances in medical care or with increasing age.
What this paper adds
Children born preterm/low birthweight perform below term‐born children on core executive functions.
Lower gestational age or male sex are not risk factors for poorer executive functions.
Executive function difficulties in children born preterm/low birthweight remain stable across childhood.
Executive function difficulties are similar for children born recently and children born in earlier eras.
What this paper adds
Children born preterm/low birthweight perform below term‐born children on core executive functions.
Lower gestational age or male sex are not risk factors for poorer executive functions.
Executive function difficulties in children born preterm/low birthweight remain stable across childhood.
Executive function difficulties are similar for children born recently and children born in earlier eras.
This article's abstract has been translated into Spanish and Portuguese.
Follow the links from the http://onlinelibrary.wiley.com/doi/10.1111/dmcn.14213/abstract to view the translations.
Resumen
Déficit en funciones ejecutivas en niños nacidos pretérmino o con bajo peso al nacer: un metaanálisis
Objetivo
Investigar la magnitud del déficit de funciones ejecutivas y su dependencia de la edad gestacional, sexo, edad a la evaluación y año de nacimiento de los niños nacidos pretérmino y/o bajo peso al nacer.
Metodo
Se buscaron en PubMed, PsychINFO, Web of Science y ERIC estudios que reportaran las funciones ejecutivas de los niños nacidos pretérmino y/o bajo peso al nacer y en niños nacidos de término como controles nacidos en 1990 y posterior, evaluados a una edad media de 4 años o más. Los estudios se incluyeron si 5 o más estudios informaban sobre las mismas medidas de la función ejecutiva.
Resultados
Se incluyeron 35 estudios (3360 niños nacidos pretérmino y/o bajo peso al nacer, 2812 controles). Estos niños tuvieron una diferencia media estandarizada (DME) de 0.5 en la memoria de trabajo y la flexibilidad cognitiva y 0.4 en la inhibición de la DME. La DMEs en funciones ejecutivas no tuvieron diferencias significativas entre ellos. La meta‐regresión mostró que la heterogeneidad de las DMEs para el trabajo de memoria y la inhibición no podría explicarse por la diferencia en la edad gestacional, sexo, edad a la evaluación o año de nacimiento.
Interpretacion
Los niños nacidos pretérmino y/o bajo peso al nacer desde 1990 realizan la mitad de un SMD por debajo de sus pares nacidos a término en la función ejecutiva, que no parece mejorar con los avances más recientes en la atención médica o con el aumento de la edad.
Resumo
Deficits da função executiva em crianças nascidas pré‐termo ou com baixo peso ao nascer: uma metanálise
Objetivo
Investigar a magnitude dos déficits da função executiva e sua dependência da idade gestacional, sexo, idade no momento da avaliação e ano de nascimento de crianças pré‐termo e / ou baixo peso ao nascer.
Método
PubMed, PsychINFO, Web of Science e ERIC foram pesquisados para estudos sobre funções executivas em crianças nascidas prematuras / com baixo peso ao nascer e controles a termo, nascidos em 1990 e anos posteriores, avaliados em uma idade média de 4 anos ou mais. Os estudos foram incluídos se 5 ou mais estudos relatassem as mesmas medidas de função executiva.
Resultados
Trinta e cinco estudos (3360 crianças nascidas pré‐termo / baixo peso ao nascer, 2812 controles) foram incluídos. As crianças nascidas pré‐termo / baixo peso ao nascer apresentaram uma diferença média padronizada (DMP) 0,5 menor na memória operacional e na flexibilidade cognitiva e DMP 0,4 menor na inibição. DMPs para essas funções executivas não diferiram significativamente entre si. Meta‐regressão mostrou que a heterogeneidade em DMPs para memória de trabalho e inibição não pode ser explicada pelas diferenças de estudo em idade gestacional, sexo, idade na avaliação ou ano de nascimento.
Interpretação
Crianças nascidas pré‐termo / baixo peso ao nascer desde 1990 realizam metade de um DMP abaixo de pares nascidos a termo em função executiva, o que não parece melhorar com os avanços mais recentes nos cuidados médicos ou com o aumento da idade.
Abbreviations
- DST
Digit Span Task
- SMD
Standardized mean difference
Preterm birth frequently occurs all over the world. Of all live‐born children in the USA in 2015, for instance, 9.6% were born preterm (gestational age <37wks, according to the World Health Organization definition)1 and 8.1% were born with a low birthweight (<2500g, according to the World Health Organization definition).1, 2 Preterm birth and low birthweight co‐occur frequently, with 69.2% of children born preterm also being born with a low birthweight and 49.8% of children born with a low birthweight also being born preterm.3 Preterm birth and/or low birthweight survivors are at high risk of adverse cognitive, academic, and behavioural outcomes.1, 4, 5 Children born preterm/low birthweight have 0.8 standard deviation (SD) lower IQ scores and perform about a 0.5 SD poorer than term‐born peers on mathematics, reading, and spelling tests.4, 5 Also, a two to four times higher risk of being diagnosed with attention‐deficit/hyperactivity disorder than for term‐born peers has been reported.4, 6, 7 A large body of studies has also shown impairments in the so‐called executive functions in children born preterm/low birthweight.8, 9 ‘Executive functions’ is an umbrella term for a set of higher‐order cognitive functions, with core functions including working memory, inhibition, and cognitive flexibility, which allow for top‐down, goal‐directed behaviour.10, 11 Executive functions rely upon lower‐order cognitive processes, such as processing speed, to operate effectively.12
Executive functions are increasingly studied because of their crucial role in the onset of academic and behavioural problems.13, 14 Even as early as the toddler and preschool years, executive functions are predictive of both (pre‐)academic skills and behaviour problems.15, 16 Importantly, executive function deficits have also been shown to be key to the behavioural and academic problems observed in children born preterm/low birthweight.12, 17, 18, 19, 20, 21, 22, 23 Recent research showed that executive function is a substantially better predictor of poor behavioural and academic outcomes in children born preterm/low birthweight than IQ and motor functions.12, 17, 18, 19, 20, 21, 22, 23, 24 For example, measures of executive functions are highly predictive of mathematic and reading abilities, and attention regulation in children born very preterm and/or very low birthweight.12, 17, 22, 23
The initial literature on executive functions in children born preterm/low birthweight was summarized in two meta‐studies published in 2009, reporting a 0.36 standardized mean difference (SMD) for working memory, a 0.25 SMD for inhibition, and a 0.49 to 0.50 SMD for cognitive flexibility between children born preterm/low birthweight and term‐born comparison children.8, 9 Newly published literature on executive functions since the meta‐studies published in 2009 warrants an update of this work. In addition, a meta‐analysis on cognitive function, including executive functions, in children born very preterm was published recently.25 However, this paper only focused on children born at less than 32 weeks of gestation and piled the diverse subdomains within the broad concept of executive functions. Including both newly published studies on executive functions since 2009 and studies into executive functions in children across the entire range of preterm birth (<37wks gestational age) offers the possibility of improving on previous meta‐analytic work by examining the profile of executive function difficulties, i.e. whether specific executive function domains are more severely impaired than others, and whether the magnitude of the effect sizes depend on the degree of preterm birth (gestational age), sex,26 age at assessment,27, 28, 29 and year of birth (as a proxy measure of advances in neonatal care).30, 31 Owing to slightly different inclusion criteria and the large number of studies published on this subject since 2009, only two studies included in this meta‐analysis were included in the 2009 meta‐analyses.32, 33
Using meta‐regression, this quantitative meta‐analysis aimed to aggregate and quantify impairments in executive function domains and assess the impact of gestational age, sex, age at assessment, and year of birth on executive function effect sizes. This unravels the nature and extent of executive function impairments in the population born preterm/low birthweight and contributes to a better understanding of the behavioural and academic problems observed in children born preterm/low birthweight.
Method
Our (unpublished) protocol was performed according to Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.34
Search strategy
A literature search was performed by one author (CAvH) in PubMed, PsychINFO, Web of Science, and ERIC, on 16th January 2017, using search terms concerning the birth status (e.g. preterm, low birthweight, small for gestational age), executive function measures (working memory, inhibition, cognitive flexibility), and age group (child, adolescent, teenager, young adult, adult, middle aged). An experienced librarian was consulted for construction of the search terms, which are provided in (Appendix S1).
Study selection
Inclusion criteria for study selection were as follows: (1) the study included participants born preterm (<37wks gestation) and/or with low birthweight (<2500g) and a comparison group of term‐born, typical‐birthweight participants (>37wks gestation and >2500g); (2) the mean age at assessment of the participants was at least 4 years (studies with younger participants were not included in this meta‐analysis as executive functions cannot be reliably assessed before the age of 4y);35 (3) the year of birth of the participants was 1990 or later (i.e. after the introduction of antenatal steroids and surfactant supplementation); (4) the study reported administration of working memory, inhibition, and/or cognitive flexibility tasks; and (5) the study was published in an English‐language, peer‐reviewed journal.
There are many different tasks available to measure executive functions and some executive function tasks may have been used in only one or two studies. Meta‐analytic procedures can be applied to a small number of studies; however, the results obtained might then be unstable.36 Therefore, to maximize the robustness of findings, only executive function tasks reported in at least five papers, as was done in a previous meta‐analysis,8 were included. Papers were thorougly checked for overlapping cohorts of children born preterm/low birthweight. If multiple papers reported on overlapping cohorts of children born preterm/low birthweight on the same executive function domain, the study with the most complete data for that domain was selected. When multiple papers reported on overlapping cohorts of children born preterm/low birthweight but the papers differed in the executive function domains described, all these papers were included in analyses. If it was not clear whether cohorts were overlapping, the authors of the studies were contacted. Screening of titles and abstracts and assessment of full‐text articles was performed by two authors (CAvH, CSHAM).
Measures
This meta‐analysis reports the results for the following executive function tasks; results for each of these tasks were reported in at least five papers.
Working memory
Working memory is the ability to hold information in mind and actively manipulate this information.10 Working memory comprises a verbal and a visual–spatial subsystem. For both subsystems, tasks were reported in at least five papers. Both the visual–spatial and verbal working memory tasks reported below have been shown to activate brain areas that are important for working memory.37, 38, 39
Visual–spatial working memory
In the Cambridge Neuropsychological Test Automated Battery Spatial Working Memory task,40 a number of coloured boxes are shown on a screen. Children were asked to find a yellow token in these boxes without selecting boxes that have already been found to be empty or revisiting boxes that have already been found to contain a token. Raw scores were used in the analyses.
Verbal working memory
In the Digit Span Task (DST),41, 42, 43 children are asked to repeat a number of digits, first in the same order and then in reverse order. The reverse part measures verbal working memory. When the DST reverse score was not available, the DST total score was used instead. For the reversed DST, either raw scores or scaled scores (mean 10 [SD 3]) were used (as indicated in Table SI, online supporting information). For the DST total, scaled scores were used (mean 100 [SD 15]).
In the Letter Number Sequencing task,42, 43 the test administrator reads a sequence of numbers and letters out loud and asks the child to repeat the numbers in ascending order, followed by the letters in alphabetical order. For Letter Number Sequencing, scaled scores were used (mean 10 [SD 3]).
When DST and Letter Number Sequencing scores were not available but the Working Memory Index (i.e. DST, Letter Number Sequencing, and Arithmetic subtests of the fourth and fifth editions of the Wechsler Intelligence Scales for Children)42, 43 was, the Working Memory Index was used instead (scaled scores, mean 100 [SD 15]).
Inhibition
Inhibition is the ability to deliberately inhibit a prepotent response or stop an ongoing response (response inhibition) or suppress disruption by competing responses (interference control).44 For response inhibition and interference control, tasks were reported in at least five papers. Both the response inhibition and interference control tasks reported below (or tasks very similar to those) have been shown to activate brain areas that are important for inhibition.45, 46, 47, 48, 49 For all tasks, raw scores were used in analyses.
Response inhibition
In the Go/No‐Go task, children have to press a button in case of a go‐trial and have to withhold from responding in case of a no‐go trial.50, 51
In the Test of Everyday Attention for Children Opposite Worlds task,52 children have to read aloud a series of numbers twice. In the ‘same world’ condition they read the numbers aloud as they appear; in the ‘opposite world’ condition they are asked to say the opposite of each digit (i.e. if they read ‘1’, they need to respond by saying ‘2’ and vice versa).
Interference control
In the Test of Everyday Attention for Children Sky Search task,52 children are asked to find and circle target spaceships as quickly as possible on a sheet filled with similar but not exactly the same distractor spaceships.
Cognitive flexibility
Cognitive flexibility is the ability to shift between multiple tasks or mental sets.10 For all tasks, raw scores were used in analyses.
In the first part of the Trail Making Test/Trails Preschool Revised,53, 54, 55 children are asked to connect numbers in the correct order (1–2–3). In the second part, children are asked to connect numbers and letters in the correct order (1–A–2–B). The Trails Preschool Revised Test is a version of the Trail Making Test adapted for the use in younger children. In the first part, children are asked to connect dogs in order of increasing size. In the second part, children are asked to alternate connecting dogs and bones in order of increasing size.
Data extraction
Data on working memory, inhibition, and/or cognitive flexibility and the moderators gestational age, sex, age at assessment, and year of birth were extracted from the studies by one author (CAvH) and entered in the database. A second person, not involved in the design and writing of this meta‐analysis, independently confirmed the data extracted from the studies. If necessary, authors were contacted for additional data. If studies reported on subgroups of children born preterm/low birthweight, subgroup data were pooled using the formulas 56 Pooled data were used in subsequent analyses.
Study quality
Study quality was assessed with an adapted version (i.e. maximum of 7 points) of the Newcastle–Ottowa Scale for cohort studies and were assessed independently by two authors (CAvH, CSHAM).57 Inconsistencies between raters were discussed and consensus was reached for all studies.
Statistical analyses
Comprehensive Meta‐Analysis V3.0 (Biostat Inc., Englewood, NJ, USA) was used to perform this meta‐analysis. Hedges’ g was used as measure for the SMDs in executive function between children born preterm/low birthweight and controls. Hedges’ g corrects for the bias in Cohen's d, which becomes increasingly more apparent in smaller sample sizes.58 A SMD of 0.2 translates into a small effect, a SMD of 0.5 is a medium effect, and a SMD of 0.8 is a large effect.59
Random effects meta‐analyses were used to calculate SMDs and to investigate whether SMDs differed significantly between executive function tasks, executive function subdomains, and executive function domains.
To rule out any dependency of data, data for one executive function task or for one executive function subdomain per study were entered in the analysis. The selection was based on maximizing the number of studies per executive function task or per executive function subdomain. In case no significant differences in SMDs for the diverse executive function tasks or for the diverse executive function subdomains were found, in subsequent analyses the mean SMD aggregated across all executive function tasks assessing a subdomain or aggregated across executive function subdomains was used respectively.
Variation in SMDs that were used to calculate a mean SMD across studies was tested using Cochran's Q. The percentage of variation across studies due to heterogeneity rather than chance was expressed at I 2, with 30% to 60% representing moderate heterogeneity; 50% to 90% representing substantial heterogeneity; and 75% to 100% representing considerable heterogeneity.60
Random‐effects meta‐regressions were performed to explore whether heterogeneity in SMDs between studies was explained by between study differences in gestational age, sex, age at assessment, and year of birth of the children born preterm/low birthweight. Meta‐regressions were only performed for those executive function domains of which more than 10 studies were included in the analyses. Associations between study quality and SMDs were assessed with random‐effects meta‐regressions. Publication bias was assessed by Egger tests.
Results
In total, 2079 articles were retrieved from PubMed, 538 articles from PsychINFO, and 2109 articles from Web of Science. After the removal of duplicates, 3030 articles remained. After initial screening of titles and abstracts, 475 full‐text articles were assessed. Of all articles that fulfilled the inclusion criteria, executive function tasks used were checked to extract which tasks had been reported on in at least five papers. All articles not reporting on those tasks were excluded. Furthermore, the remaining articles were checked for overlapping cohorts. The selection process is depicted in detail in (Figure S1). A total of 45 studies met all the inclusion criteria. Executive function data were provided by 35 of these studies (3360 children born preterm/low birthweight and 2812 term‐born controls) either in the study paper or after a request for additional data sent to the authors.18, 21, 24, 32, 33, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89 The characteristics and main study results are given in (Tables SI, SII, and SIII, online supporting information; working memory, inhibition, and cognitive flexibility respectively). A total of 25 studies reported data on working memory (2272 children born preterm/low birthweight and 2021 term‐born controls), 13 studies reported data on inhibition (1258 children born preterm/low birthweight and 984 term‐born controls), and five studies reported data on cognitive flexibility (287 children born preterm/low birthweight and 307 term‐born controls). Eight studies reported data on more than one executive function domain. Study quality ranged between 3 and 7, with a mean of 5.7 (SD 1.2). Overall, study quality was considered fair to good (see Tables SI, SII, and SIII).
SMDs for executive functions
Based on the pooled analysis, children born preterm/low birthweight performed 0.52 SMD (95% confidence interval [CI] 0.65–0.38) lower than controls on working memory (Fig. 1), 0.39 SMD (95% CI 0.55–0.23) lower than controls on inhibition (Fig. 2), and 0.51 SMD (95% CI 0.72–0.31) lower than controls on cognitive flexibility (Fig. 3). SMDs between domains did not differ significantly (Q=1.19, p=0.55). Substantial heterogeneity among studies was found for working memory (I 2=70.22, p<0.001) and inhibition (I 2=68.2, p<0.001). SMDs for the different tasks for verbal working memory and response inhibition did not differ significantly (Q=0.73 [p=0.87] and Q=0.34 [p=0.56] respectively). Also, SMDs did not differ significantly between the subdomains verbal and spatial working memory, or between the subdomains response inhibition and interference control (Q=0.38 [p=0.54] and Q=1.1 [p=0.30] respectively).
Figure 1.
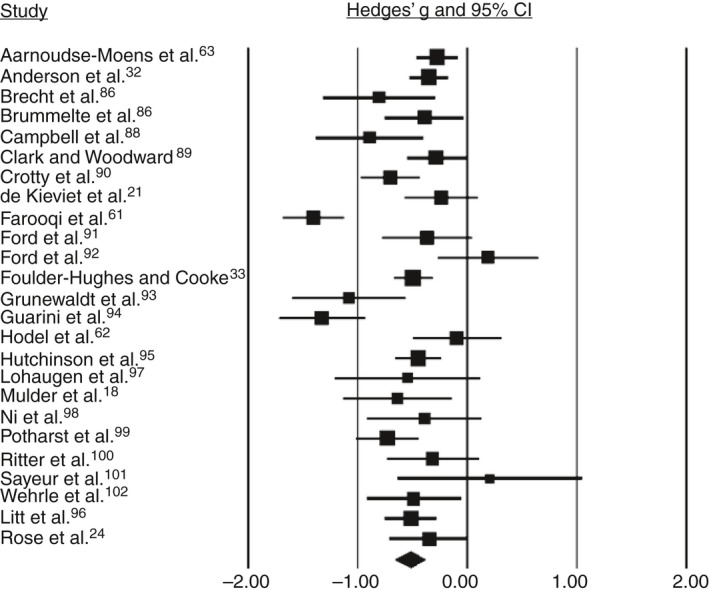
Forest plot of the studies on working memory. CI, confidence interval.
Figure 2.
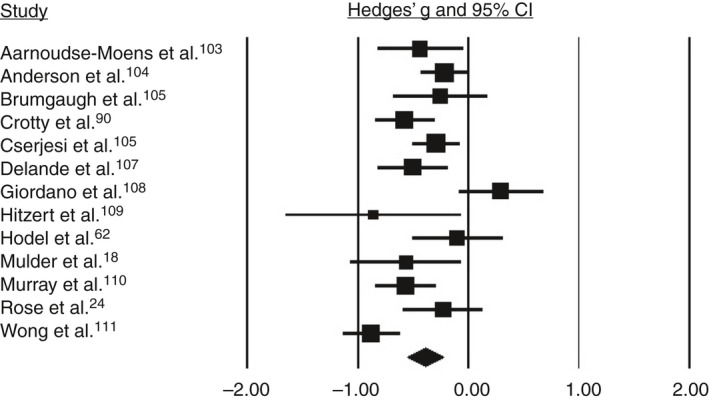
Forest plot of the studies on inhibition. CI, confidence interval.
Figure 3.
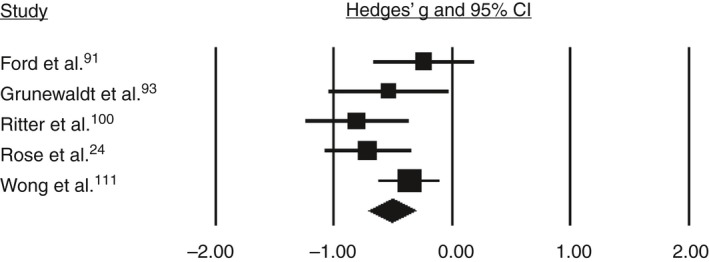
Forest plot for studies on cognitive flexibility. CI, confidence interval.
Meta‐regression analyses
Meta‐regression analyses were carried out for working memory and inhibition, as only five studies reported data on cognitive flexibility. In Table 1, the range of each moderator (gestational age, sex, age at assessment, year of birth, and study quality) is depicted for working memory and inhibition separately. Meta‐regression analyses performed for working memory showed a significant relationship with gestational age (β=0.07; 95% CI 0.01–0.13; R 2=0.15, p=0.02). Visual inspection of the scatterplots, however, indicated that this result relied on two studies featuring children at the extreme ends of the gestational age distribution (i.e. 24.4wks and 35.6 wks respectively) (Fig. 4a).61, 62 Rerunning analyses without these two studies yielded a non‐significant relationship between gestational age and working memory (β=0.03; 95% CI –0.05 to 0.11; R 2=0.00, p=0.48 (Fig. 4b). No significant relationships with working memory SMDs were found for sex, age at assessment, and year of birth. None of the moderators were significantly associated with SMDs for inhibition. Study quality was not significantly associated with SMDs for working memory or inhibition.
Table 1.
Moderator ranges for the working memory and inhibition subdomains
| Executive function domain | Gestational age range (wks) | Percentage of males (%) | Age range at assessment (y:mo) | Year of birth (range) | Study quality (range) |
|---|---|---|---|---|---|
| Working memory | 24.4–35.6 | 31–65 | 4:6–14:10 | 1991–2007 | 4–7 |
| Inhibition | 26.0–35.8 | 45–65 | 4:6–11:2 | 1996–2011 | 3–7 |
Figure 4.
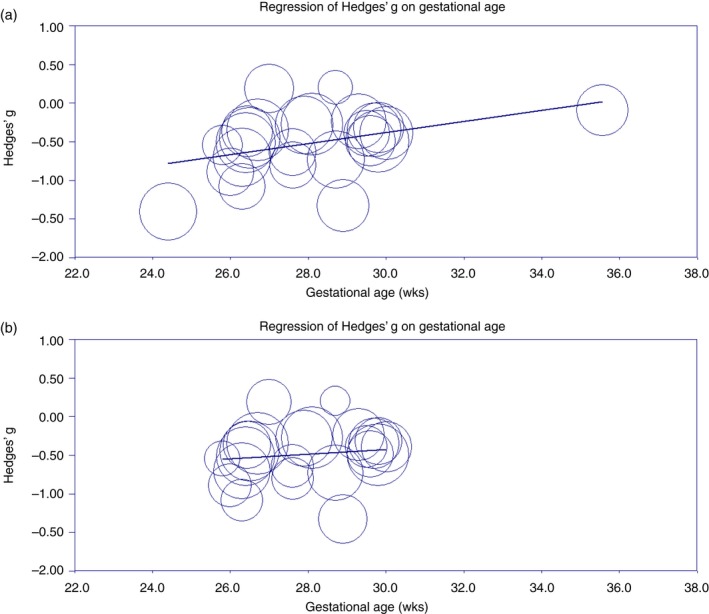
(a) Meta‐regression of gestational age on working memory. (b) Meta‐regression of gestational age on working memory after excluding two outliers with gestational ages of 24.4 and 35.6 weeks respectively. [Colour figure can be viewed at http://www.wileyonlinelibrary.com/].
Publication bias
Egger's tests were non‐significant for all three executive function domains, as well as for subdomains within working memory and inhibition, indicating that there is no evidence for publication bias.
Discussion
This meta‐analysis aggregated the literature on the three core executive functions (working memory, inhibition, and cognitive flexibility), in children born preterm/low birthweight after the introduction of antenatal steroids and surfactant supplementation. Results show that, compared with term‐born peers, children born preterm/low birthweight perform 0.5 SMD lower on working memory and cognitive flexibility measures (medium effect) and 0.4 SMD lower on inhibition measures (small‐to‐medium effect). Analysis indicated no significant differences between the SMDs for working memory, cognitive flexibility, and inhibition measures, indicating that all three executive functions seem to be affected to a similar degree. There was significant heterogeneity in effect sizes for working memory and inhibition. Heterogeneity in working memory, but not inhibition, could partly be explained by study differences in gestational age; however, this effect was driven by two studies at the extreme ends of the gestational age range. Heterogeneity could not be explained by study differences in sex, age at assessment, or year of birth.
In the literature, executive function deficits have been described to be proportional to decreasing gestational age.8, 9, 63, 90 The magnitude of the difficulties in working memory as observed in our meta‐analysis was dependent on gestational age; however, we are cautious in interpreting this result as it was driven by two studies featuring children at the extreme ends of the gestational age range, and with these studies removed the gestational age of included studies only ranged between 26 and 30 weeks. Also, for inhibition, there were only a small number of studies investigating the ends of the gestational age range. To be able to draw robust conclusions on whether there is an effect of gestational age on executive function, more studies in children born extremely preterm (<26wks gestational age) and moderate‐to‐late preterm (32–37wks gestational age) are clearly needed. In our analysis, executive function difficulties were not related to male sex, even though male sex is a risk factor for multiple medical adverse outcomes.26 This suggests that male as well as female children born preterm/low birthweight are at substantial risk for poor executive function.
There is debate about whether executive function deficits in children born preterm/low birthweight represent a stable deficit, a deficit that increases during development (growing into deficit), or a delay in maturation in which children ‘catch up’ over time.27, 28, 29 In our meta‐analysis, there was no significant association between age at assessment and the SMDs for both working memory and inhibition, suggesting that difficulties in these areas in children born preterm/low birthweight are stable and do not deteriorate or diminish as these children grow older. It should be noted that in our meta‐analysis age at assessment ranged between 4 years 6 months and 14 years 10 months for working memory studies, and between 4 years 6 months and 11 years 2 months for the inhibition studies. There might be catch‐up in these executive functions after this age, but at least until primary school age (inhibition) or secondary school age (working memory), we found no evidence for this.
Year of birth of children born preterm/low birthweight was not a factor of relevance for the size of the executive function difficulties. This finding suggests that for working memory and inhibition, outcomes are not improving with advances in medical care. Burnett et al.65 have recently investigated whether executive function outcomes of children born extremely preterm (<28wks gestational age) have improved by comparing three cohorts born respectively in 1991 to 1992, 1997, and 2005. They found that the outcomes of the three cohorts did not improve and that some outcome measures were even deteriorating. Although the study of Burnett et al. relied on a questionnaire to assess executive function, their outcomes on year of birth for working memory and inhibition are in accordance with the results of our meta‐regression analyses.92 To ensure that gestational age‐related survival bias did not explain the lack of executive function improvement in more recent years, we examined the relationship between mean gestational age and year of birth for the studies included in our meta‐analysis (data available upon request). No significant relationship was found, suggesting that studies reporting on more recent cohorts of children did not contain a larger number of children at the lower end of the gestational age range.
Preterm/low birthweight birth is associated with substantial reductions in cognitive outcomes, as measured by IQ,4 deficits in academic performance,4, 5 and children born preterm/low birthweight have a two to four times higher risk of receiving a diagnosis of attention‐deficit/hyperactivity disorder than term‐born peers.6, 7 Importantly, deficits in executive function may underlie these adverse outcomes in children born preterm/low birthweight. The neuroanatomical sequels of preterm/low birthweight support the idea that executive function might be crucially involved in the adverse outcome of children born preterm/low birthweight. Executive function is highly dependent on white matter network integrity, which is often compromised after preterm birth.93, 94, 95, 96 Furthermore, research has shown that hypoxic‐ischaemic events lead to damage in the striatum and its connections with the prefrontal cortex.97 These brain structures are known to be very important for executive functions,98, 99, 100, 101 and children born preterm/low birthweight are vulnerable to damage in these brain areas as repeated hypoxic‐ischaemic events are common in these children.97
Early interventions are warranted to improve outcomes for children born preterm/low birthweight. There is evidence that effects of a computerized executive function training programme do not generalize to other functions than the trained executive function.102, 103, 104 However, this literature is based on solely training working memory, while other executive functions, such as inhibitory control and cognitive flexibility, are also impaired in this population and may benefit from computerized interventions. Given the fact that executive function remains a vulnerable area of cognitive function in the population born preterm, future studies should be conducted on which type of interventions may be effective to diminish the encountered difficulties in executive function.103, 105, 106, 107, 108, 109, 110
Limitations
First, because of the limited number of studies on executive function in children born moderate‐to‐late preterm/low birthweight (i.e. >32wks gestational age), analyses for working memory included only one study within this gestational age range. Second, not enough studies presented data on cognitive flexibility to perform meta‐regressions. Third, we were not able to obtain executive function data for 10 of the 45 studies that met all inclusion criteria and could not include these studies in our analyses. Of those 10 studies, three reported on inhibition and three reported on working memory in children and adolescents with a mean gestational age above 30 weeks. Lastly, unwelcome and potentially biasing heterogeneity could be introduced when the different instruments that are summarized with meta‐analytic techniques are, in fact, not all measuring the same construct.111 Therefore, we excluded executive function tasks that were used in less than five papers and we analysed the executive function tasks separately at first. However, as there were no significant differences in effect sizes between the separate executive function tasks within one executive function domain, and as there is empirical evidence that similar brain regions are activated by these tasks, we combined the effect sizes of the tasks into aggregated effect sizes for each specific executive function domain.
Conclusion
Children born preterm/low birthweight since the 1990s perform poorer than term‐born peers on the three core executive function of working memory, inhibition, and cognitive flexibility, and none of these three core executive functions are more severely affected than the other. The magnitude of executive function difficulties was not associated with gestational age, and male sex was not a specific risk factor for poor executive function. Executive function difficulties remained persistent during transition to adolescence and did not improve with more recent year of birth. Given that executive function deficits are associated with worse academic performance at school age, executive functions should be assessed at early schoolage in children born preterm/low birthweight to initiate early intervention targeted at improving these executive functions.
Supporting information
Appendix S1: Search strategy.
Table SI: Details on studies included for working memory
Table SII: Details on studies included for inhibition
Table SIII: Details on studies included for cognitive flexibility
Figure S1: Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flowchart of the study selection procedure.
Acknowledgements
This work was supported by the Stichting Kinderpostzegels Nederland, Cornelia Stichting, Stichting Zabawas and Elise Mathilde Stichting. The authors have stated that they had no interests that might be perceived as posing a conflict or bias.
References
- 1. Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health 2013; 10(Suppl. 1): S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: final data for 2015. Natl Vital Stat Rep 2017; 66: 1. [PubMed] [Google Scholar]
- 3. Savitz DA, Ananth CV, Berkowitz GS, Lapinski R. Concordance among measures of pregnancy outcome based on fetal size and duration of gestation. Am J Epidemiol 2000; 151: 627–33. [DOI] [PubMed] [Google Scholar]
- 4. Allotey J, Zamora J, Cheong‐See F, et al. Cognitive, motor, behavioural and academic performances of children born preterm: a meta‐analysis and systematic review involving 64 061 children. BJOG 2018; 125: 16–25. [DOI] [PubMed] [Google Scholar]
- 5. Twilhaar ES, de Kieviet JF, Aarnoudse‐Moens CS, van Elburg RM, Oosterlaan J. Academic performance of children born preterm: a meta‐analysis and meta‐regression. Arch Dis Child Fetal Neonatal Ed 2018; 103: F322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aylward GP. Update on neurodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr 2014; 35: 392–3. [DOI] [PubMed] [Google Scholar]
- 7. Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school‐aged children who were born preterm: a meta‐analysis. JAMA 2002; 288: 728–37. [DOI] [PubMed] [Google Scholar]
- 8. Aarnoudse‐Moens CS, Weisglas‐Kuperus N, van Goudoever JB, Oosterlaan J. Meta‐analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 2009; 124: 717–28. [DOI] [PubMed] [Google Scholar]
- 9. Mulder H, Pitchford NJ, Hagger MS, Marlow N. Development of executive function and attention in preterm children: a systematic review. Dev Neuropsychol 2009; 34: 393–421. [DOI] [PubMed] [Google Scholar]
- 10. Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol 2000; 41: 49–100. [DOI] [PubMed] [Google Scholar]
- 11. Lezak MD. Neuropsychological Assessment (2nd Edition). New York: Oxford University Press, 1983. [Google Scholar]
- 12. Aarnoudse‐Moens CS, Weisglas‐Kuperus N, Duivenvoorden HJ, van Goudoever JB, Oosterlaan J. Executive function and IQ predict mathematical and attention problems in very preterm children. PLoS ONE 2013; 8: e55994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bull R, Scerif G. Executive functioning as a predictor of children's mathematics ability: inhibition, switching, and working memory. Dev Neuropsychol 2001; 19: 273–93. [DOI] [PubMed] [Google Scholar]
- 14. Chiappe P, Hasher L, Siegel LS. Working memory, inhibitory control, and reading disability. Mem Cognit 2000; 28: 8–17. [DOI] [PubMed] [Google Scholar]
- 15. Espy KA, McDiarmid MM, Cwik MF, Stalets MM, Hamby A, Senn TE. The contribution of executive functions to emergent mathematic skills in preschool children. Dev Neuropsychol 2004; 26: 465–86. [DOI] [PubMed] [Google Scholar]
- 16. Mulder H, Hoofs H, Verhagen J, van der Veen I, Leseman PP. Psychometric properties and convergent and predictive validity of an executive function test battery for two‐year‐olds. Front Psychol 2014; 5: 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mulder H, Pitchford NJ, Marlow N. Processing speed and working memory underlie academic attainment in very preterm children. Arch Dis Child Fetal Neonatal Ed 2010; 95: F267–72. [DOI] [PubMed] [Google Scholar]
- 18. Mulder H, Pitchford NJ, Marlow N. Processing speed mediates executive function difficulties in very preterm children in middle childhood. J Int Neuropsychol Soc 2011; 17: 445–54. [DOI] [PubMed] [Google Scholar]
- 19. Nadeau L, Boivin M, Tessier R, Lefebvre F, Robaey P. Mediators of behavioral problems in 7‐year‐old children born after 24 to 28 weeks of gestation. J Dev Behav Pediatr 2001; 22: 1–10. [DOI] [PubMed] [Google Scholar]
- 20. Jaekel J, Wolke D. Preterm birth and dyscalculia. J Pediatr 2014; 164: 1327–32. [DOI] [PubMed] [Google Scholar]
- 21. de Kieviet JF, van Elburg RM, Lafeber HN, Oosterlaan J. Attention problems of very preterm children compared with age‐matched term controls at school‐age. J Pediatr 2012; 161: 824–9. [DOI] [PubMed] [Google Scholar]
- 22. Jaekel J, Eryigit‐Madzwamuse S, Wolke D. Preterm toddlers’ inhibitory control abilities predict attention regulation and academic achievement at age 8 years. J Pediatr 2016; 169: e81. [DOI] [PubMed] [Google Scholar]
- 23. Mulder H, Pitchford NJ, Marlow N. Inattentive behaviour is associated with poor working memory and slow processing speed in very pre‐term children in middle childhood. Br J Educ Psychol 2011; 81: 147–60. [DOI] [PubMed] [Google Scholar]
- 24. Rose SA, Feldman JF, Jankowski JJ. Modeling a cascade of effects: the role of speed and executive functioning in preterm/full‐term differences in academic achievement. Dev Sci 2011; 14: 1161–75. [DOI] [PubMed] [Google Scholar]
- 25. Brydges CR, Landes JK, Reid CL, Campbell C, French N, Anderson M. Cognitive outcomes in children and adolescents born very preterm: a meta‐analysis. Dev Med Child Neurol 2018; 60: 452–68. [DOI] [PubMed] [Google Scholar]
- 26. Stevenson DK, Verter J, Fanaroff AA, et al. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Arch Dis Child Fetal Neonatal Ed 2000; 83: F182–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baron IS, Weiss BA, Litman FR, Ahronovich MD, Baker R. Latent mean differences in executive function in at‐risk preterm children: the delay‐deficit dilemma. Neuropsychology 2014; 28: 541–51. [DOI] [PubMed] [Google Scholar]
- 28. Baron IS, Weiss BA, Baker R, et al. Subtle adverse effects of late preterm birth: a cautionary note. Neuropsychology 2014; 28: 11–8. [DOI] [PubMed] [Google Scholar]
- 29. Ritter BC, Nelle M, Perrig W, Steinlin M, Everts R. Executive functions of children born very preterm – deficit or delay? Eur J Pediatr 2013; 172: 473–83. [DOI] [PubMed] [Google Scholar]
- 30. Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ 2012; 345: e7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Healy P, Fallon A. Developments in neonatal care and nursing responses. Br J Nurs 2014; 23: 21–4. [DOI] [PubMed] [Google Scholar]
- 32. Anderson PJ, Doyle LW, Victorian Infant Collaborative Study Group . Executive functioning in school‐aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics 2004; 114: 50–7. [DOI] [PubMed] [Google Scholar]
- 33. Foulder‐Hughes LA, Cooke RW. Motor, cognitive, and behavioural disorders in children born very preterm. Dev Med Child Neurol 2003; 45: 97–103. [PubMed] [Google Scholar]
- 34. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Diamond A. Executive functions. Annu Rev Psychol 2013; 64: 135–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosenthal R. Writing meta‐analytic reviews. Psychol Bull 1995; 118: 183. [Google Scholar]
- 37. Haut MW, Kuwabara H, Leach S, Arias RG. Neural activation during performance of number‐letter sequencing. Appl Neuropsychol 2000; 7: 237–42. [DOI] [PubMed] [Google Scholar]
- 38. Li R, Qin W, Zhang Y, Jiang T, Yu C. The neuronal correlates of digits backward are revealed by voxel‐based morphometry and resting‐state functional connectivity analyses. PLoS ONE 2012; 7: e31877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Luciana M, Nelson CA. The functional emergence of prefrontally‐guided working memory systems in four‐ to eight‐year‐old children. Neuropsychologia 1998; 36: 273–93. [DOI] [PubMed] [Google Scholar]
- 40. Fray PJ, Robbins T, Sahakian B. Neuorpsychiatyric applications of CANTAB. Int J Geriatr Psychiatry 1996; 11: 329–36. [Google Scholar]
- 41. Wechsler D. The Wechsler Intelligence Scale for Children, 3rd ed San Antionio, TX: The Psychological Corporation, 1991. [Google Scholar]
- 42. Wechsler D. The Wechsler Intelligence Scale for Children, 4th ed London: Pearson, 2003. [Google Scholar]
- 43. Wechsler D. Wechsler Intelligence Scale for Children, 5th ed Bloomington, IN: Pearson, 2014. [Google Scholar]
- 44. Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 1997; 121: 65–94. [DOI] [PubMed] [Google Scholar]
- 45. Hazeltine E, Poldrack R, Gabrieli JD. Neural activation during response competition. J Cogn Neurosci 2000; 12(Suppl. 2): 118–29. [DOI] [PubMed] [Google Scholar]
- 46. Marsh R, Zhu H, Schultz RT, et al. A developmental fMRI study of self‐regulatory control. Hum Brain Mapp 2006; 27: 848–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Montgomery DE, Koeltzow TE. A review of the day–night task: the Stroop paradigm and interference control in young children. Dev Rev 2010; 30: 308–30. [Google Scholar]
- 48. Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta‐analysis of two response inhibition tasks. NeuroImage 2011; 56: 1655–65. [DOI] [PubMed] [Google Scholar]
- 49. Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry 2002; 41: 1231–8. [DOI] [PubMed] [Google Scholar]
- 50. Casey BJ, Trainor RJ, Orendi JL, et al. A developmental functional MRI study of prefrontal activation during performance of a go‐no‐go task. J Cogn Neurosci 1997; 9: 835–47. [DOI] [PubMed] [Google Scholar]
- 51. Berwid OG, Curko Kera EA, Marks DJ, Santra A, Bender HA, Halperin JM. Sustained attention and response inhibition in young children at risk for Attention Deficit/Hyperactivity Disorder. J Child Psychol Psychiatry 2005; 46: 1219–29. [DOI] [PubMed] [Google Scholar]
- 52. Manly T, Robertson IH, Anderson V, Nimmo‐Smith I. Test of Everyday Attention for Children (TEA‐Ch). London: Harcourt Assessment, 1990. [DOI] [PubMed] [Google Scholar]
- 53. Orchinik LJ, Taylor HG, Espy KA, et al. Cognitive outcomes for extremely preterm/extremely low birth weight children in kindergarten. J Int Neuropsychol Soc 2011; 17: 1067–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reitan RM, Wolfson D. The Halstead‐Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson, AZ: Neuropsychology Press, 1993. [Google Scholar]
- 55. Delis DC, Kaplan E, Kramer J. Delis Kaplan Executive Function System. San Antonio, TX: Psychological Corporation, 2001. [Google Scholar]
- 56. Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd Edition). Hillsdale, NJ: Lawrence Erlbaum, 1988. [Google Scholar]
- 57. Wells GA, Shea B, O'Connell D, et al. The Newcastle‐Ottowa Scale (NOS) for assessing quality of nonrandomised studies in meta‐analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 6th February 2019).
- 58. Hedges LV. Distribution theory for Glass's estimator of effect size and related estimators. J Educ Behav Stat 1981; 6: 107–28. [Google Scholar]
- 59. Cohen J. A power primer. Psychol Bull 1992; 112: 155–9. [DOI] [PubMed] [Google Scholar]
- 60. The Cochrane Collaboration . Cochrane handbook for systematic reviews of interventions. Available at: https://handbook-5-1.cochrane.org (accessed 6th February 2019).
- 61. Farooqi A, Adamsson M, Serenius F, Hägglöf B. Executive functioning and learning skills of adolescent children born at fewer than 26 weeks of gestation. PLoS ONE 2016; 11: e0151819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hodel AS, Brumbaugh JE, Morris AR, Thomas KM. Hot executive function following moderate‐to‐late preterm birth: altered delay discounting at 4 years of age. Dev Sci 2016; 19: 221–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Aarnoudse‐Moens CS, Duivenvoorden HJ, Weisglas‐Kuperus N, Van Goudoever JB, Oosterlaan J. The profile of executive function in very preterm children at 4 to 12 years. Dev Med Child Neurol 2012; 54: 247–53. [DOI] [PubMed] [Google Scholar]
- 64. Brecht KF, Goelz R, Bevot A, Krägeloh‐Mann I, Wilke M, Lidzba K. Postnatal human cytomegalovirus infection in preterm infants has long‐term neuropsychological sequelae. J Pediatr 2015; 166: 834–9. [DOI] [PubMed] [Google Scholar]
- 65. Brummelte S, Chau CM, Cepeda IL, et al. Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain‐related stress. Psychoneuroendocrinology 2015; 51: 151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Campbell C, Horlin C, Reid C, et al. How do you think she feels? Vulnerability in empathy and the role of attention in school‐aged children born extremely preterm. Br J Dev Psychol 2015; 33: 312–23. [DOI] [PubMed] [Google Scholar]
- 67. Clark CA, Woodward LJ. Neonatal cerebral abnormalities and later verbal and visuospatial working memory abilities of children born very preterm. Dev Neuropsychol 2010; 35: 622–42. [DOI] [PubMed] [Google Scholar]
- 68. Crotty KC, Ahronovich MD, Baron IS, Baker R, Erickson K, Litman FR. Neuropsychological and behavioral effects of postnatal dexamethasone in extremely low birth weight preterm children at early school age. J Perinatol 2012; 32: 139–46. [DOI] [PubMed] [Google Scholar]
- 69. Ford RM, Neulinger K, O'Callaghan M, Mohay H, Gray P, Shum D. Executive function in 7‐9‐year‐old children born extremely preterm or with extremely low birth weight: effects of biomedical history, age at assessment, and socioeconomic status. Arch Clin Neuropsychol 2011; 26: 632–44. [DOI] [PubMed] [Google Scholar]
- 70. Ford RM, Griffiths S, Neulinger K, Andrews G, Shum DH, Gray PH. Impaired prospective memory but intact episodic memory in intellectually average 7‐ to 9‐year‐olds born very preterm and/or very low birth weight. Child Neuropsychol 2017; 23: 954–79. [DOI] [PubMed] [Google Scholar]
- 71. Grunewaldt KH, Fjørtoft T, Bjuland KJ, et al. Follow‐up at age 10 years in ELBW children – functional outcome, brain morphology and results from motor assessments in infancy. Early Hum Dev 2014; 90: 571–8. [DOI] [PubMed] [Google Scholar]
- 72. Guarini A, Marini A, Savini S, Alessandroni R, Faldella G, Sansavini A. Linguistic features in children born very preterm at preschool age. Dev Med Child Neurol 2016; 58: 949–56. [DOI] [PubMed] [Google Scholar]
- 73. Hutchinson EA, De Luca CR, Doyle LW, Roberts G, Anderson PJ, Victorian Infant Collaborative Study Group . School‐age outcomes of extremely preterm or extremely low birth weight children. Pediatrics 2013; 131: e1053–61. [DOI] [PubMed] [Google Scholar]
- 74. Litt JS, Gerry Taylor H, Margevicius S, Schluchter M, Andreias L, Hack M. Academic achievement of adolescents born with extremely low birth weight. Acta Paediatr 2012; 101: 1240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Løhaugen GC, Antonsen I, Håberg A, et al. Computerized working memory training improves function in adolescents born at extremely low birth weight. J Pediatr 2011; 158: 555–61. [DOI] [PubMed] [Google Scholar]
- 76. Ni TL, Huang CC, Guo NW. Executive function deficit in preschool children born very low birth weight with normal early development. Early Hum Dev 2011; 87: 137–41. [DOI] [PubMed] [Google Scholar]
- 77. Potharst ES, van Wassenaer‐Leemhuis AG, Houtzager BA, et al. Perinatal risk factors for neurocognitive impairments in preschool children born very preterm. Dev Med Child Neurol 2013; 55: 178–84. [DOI] [PubMed] [Google Scholar]
- 78. Ritter BC, Perrig W, Steinlin M, Everts R. Cognitive and behavioral aspects of executive functions in children born very preterm. Child Neuropsychol 2014; 20: 129–44. [DOI] [PubMed] [Google Scholar]
- 79. Sayeur MS, Vannasing P, Tremblay E, et al. Visual development and neuropsychological profile in preterm children from 6 months to school age. J Child Neurol 2015; 30: 1159–73. [DOI] [PubMed] [Google Scholar]
- 80. Wehrle FM, Kaufmann L, Benz LD, et al. Very preterm adolescents show impaired performance with increasing demands in executive function tasks. Early Hum Dev. 2016; 92: 37–43. [DOI] [PubMed] [Google Scholar]
- 81. Aarnoudse‐Moens CS, Smidts DP, Oosterlaan J, Duivenvoorden HJ, Weisglas‐Kuperus N. Executive function in very preterm children at early school age. J Abnorm Child Psychol 2009; 37: 981–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Anderson PJ, De Luca CR, Hutchinson E, et al. Attention problems in a representative sample of extremely preterm/extremely low birth weight children. Dev Neuropsychol 2011; 36: 57–73. [DOI] [PubMed] [Google Scholar]
- 83. Brumbaugh JE, Hodel AS, Thomas KM. The impact of late preterm birth on executive function at preschool age. Am J Perinatol 2014; 31: 305–14. [DOI] [PubMed] [Google Scholar]
- 84. Cserjesi R, Van Braeckel KN, Butcher PR, et al. Functioning of 7‐year‐old children born at 32 to 35 weeks’ gestational age. Pediatrics 2012; 130: e838–46. [DOI] [PubMed] [Google Scholar]
- 85. Delane L, Campbell C, Bayliss DM, et al. Poorer divided attention in children born very preterm can be explained by difficulty with each component task, not the executive requirement to dual‐task. Child Neuropsychol 2017; 23: 510–22. [DOI] [PubMed] [Google Scholar]
- 86. Giordano V, Fuiko R, Leiss U, et al. Differences in attentional functioning between preterm and full‐term children underline the importance of new neuropsychological detection techniques. Acta Paediatr 2017; 106: 601–11. [DOI] [PubMed] [Google Scholar]
- 87. Hitzert MM, Van Braeckel KN, Bos AF, Hunnius S, Geuze RH. Early visual attention in preterm and fullterm infants in relation to cognitive and motor outcomes at school age: an exploratory study. Front Pediatr 2014; 2: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Murray AL, Scratch SE, Thompson DK, et al. Neonatal brain pathology predicts adverse attention and processing speed outcomes in very preterm and/or very low birth weight children. Neuropsychology 2014; 28: 552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wong T, Taylor HG, Klein N, et al. Kindergarten classroom functioning of extremely preterm/extremely low birth weight children. Early Hum Dev 2014; 90: 907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Taylor HG, Minich N, Bangert B, Filipek PA, Hack M. Long‐term neuropsychological outcomes of very low birth weight: associations with early risks for periventricular brain insults. J Int Neuropsychol Soc 2004; 10: 987–1004. [DOI] [PubMed] [Google Scholar]
- 91. Burnett AC, Anderson PJ, Lee KJ, et al. Trends in executive functioning in extremely preterm children across 3 birth eras. Pediatrics 2018; 141: e20171958. [DOI] [PubMed] [Google Scholar]
- 92. Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function: Professional Manual. Lutz, FL: Psychological Assessment Resources, 2000. [Google Scholar]
- 93. Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr 2003; 143: 171–9. [DOI] [PubMed] [Google Scholar]
- 94. Miller SP, Ferriero DM, Leonard C, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr 2005; 147: 609–16. [DOI] [PubMed] [Google Scholar]
- 95. Cheong JL, Thompson DK, Wang HX, et al. Abnormal white matter signal on MR imaging is related to abnormal tissue microstructure. AJNR Am J Neuroradiol 2009; 30: 623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 2009; 8: 110–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lou HC. Etiology and pathogenesis of attention‐deficit hyperactivity disorder (ADHD): significance of prematurity and perinatal hypoxic‐haemodynamic encephalopathy. Acta Paediatr 1996; 85: 1266–71. [DOI] [PubMed] [Google Scholar]
- 98. Stuss DT. Functions of the frontal lobes: relation to executive functions. J Int Neuropsychol Soc 2011; 17: 759–65. [DOI] [PubMed] [Google Scholar]
- 99. Anderson V, Levin HS, Jacobs R. Executive functions after frontal lobe injury: a developmental perspective In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. New York: Oxford University Press, 2002: 504–27. [Google Scholar]
- 100. Hazy TE, Frank MJ, O'Reilly RC. Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philos Trans R Soc London Series B Biol Sci 2007; 362: 1601–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001; 24: 167–202. [DOI] [PubMed] [Google Scholar]
- 102. Anderson PJ, Lee KJ, Roberts G, et al. Long‐term academic functioning following Cogmed working memory training for children born extremely preterm: a randomized controlled trial. J Pediatri 2018; 202: e94. [DOI] [PubMed] [Google Scholar]
- 103. Dunning DL, Holmes J, Gathercole SE. Does working memory training lead to generalized improvements in children with low working memory? A randomized controlled trial Dev Sci 2013; 16: 915–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Spencer‐Smith M, Klingberg T. Correction: benefits of a working memory training program for inattention in daily life: a systematic review and meta‐analysis. PLoS ONE 2016; 11: e0167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Klingberg T, Fernell E, Olesen PJ, et al. Computerized training of working memory in children with ADHD – a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 2005; 44: 177–86. [DOI] [PubMed] [Google Scholar]
- 106. Bergman Nutley S, Soderqvist S, Bryde S, Thorell LB, Humphreys K, Klingberg T. Gains in fluid intelligence after training non‐verbal reasoning in 4‐year‐old children: a controlled, randomized study. Dev Sci 2011; 14: 591–601. [DOI] [PubMed] [Google Scholar]
- 107. Hovik KT, Saunes BK, Aarlien AK, Egeland J. RCT of working memory training in ADHD: long‐term near‐transfer effects. PLoS ONE 2013; 8: e80561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. van der Oord S, Ponsioen AJ, Geurts HM, Ten Brink EL, Prins PJ. A pilot study of the efficacy of a computerized executive functioning remediation training with game elements for children with ADHD in an outpatient setting: outcome on parent‐ and teacher‐rated executive functioning and ADHD behavior. J Atten Disord 2014; 18: 699–712. [DOI] [PubMed] [Google Scholar]
- 109. Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. J Clin Exp Neuropsychol 2002; 24: 781–91. [DOI] [PubMed] [Google Scholar]
- 110. Aarnoudse‐Moens CSH, Twilhaar ES, Oosterlaan J, et al. Executive function computerized training in very preterm‐born children: a pilot study. Games Health 2018; 7: 175–81. [DOI] [PubMed] [Google Scholar]
- 111. Lipsey MW, Wilson DB. Practical Meta‐Analysis. Thousand Oaks, CA: Sage Publications, 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Search strategy.
Table SI: Details on studies included for working memory
Table SII: Details on studies included for inhibition
Table SIII: Details on studies included for cognitive flexibility
Figure S1: Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flowchart of the study selection procedure.


