Abstract
Lenvatinib is a novel multikinase inhibitor that has recently shown antitumor activity against hepatocellular carcinoma (HCC) in a phase III trial. We report the case of a woman in whom lenvatinib showed long‐term antitumor activity, and in whom computed tomography (CT) scans revealed a series of suggestive radiological changes on the intratumor vascularity. A 68‐year‐old woman with hepatitis C virus‐related liver disease presented with multiple HCCs. Following previous therapy, including six sessions of transcatheter arterial chemoembolization, we introduced lenvatinib monotherapy. Lenvatinib could rapidly cause hypovascularity in the main hypervascular target lesion, and portal vein tumor thrombosis also became undetectable 11 months after the initiation of lenvatinib. These radiological changes suggested that lenvatinib could exert not only anti‐angiogenic activity but also direct antitumoral effect. Of note, CT scans during lenvatinib treatment revealed the target lesion as a low‐density area in the early arterial phase, whereas scans during drug interruption due to proteinuria showed that the lesion was enhanced in the arterial phase. Finally, near‐complete response could be achieved as the best response. We successfully managed various adverse events including proteinuria and hypertension, and the patient was able to continue this lenvatinib therapy for more than 4 years with well‐controlled general condition. We report the first case of a patient with HCC in whom lenvatinib monotherapy demonstrated long‐term antitumor activity. Suggestive radiological changes reflecting intratumor vascularity as presented here should be considered in patients receiving lenvatinib for HCC.
Keywords: hepatocellular carcinoma, intratumoral vascularity, lenvatinib, modified RECIST, molecular targeting therapy, response
Introduction
Hepatocellular carcinoma (HCC) is the second most lethal malignancy worldwide,1, 2, 3 and the prognosis of advanced HCC is especially dismal.4 Although sorafenib has been reported to prolong overall survival (OS) among patients with unresectable HCC, its antitumor effect is limited;5, 6 less than 5% of HCC patients achieved a partial response (PR) in the SHARP trial,5 and the objective response rate of sorafenib has also been limited in clinical practice.7, 8 Numerous phase III trials of molecular targeting agents for HCC have been undertaken, but most have failed to reach their primary end‐point,9, 10, 11 and sorafenib has been the only first‐line systemic therapy approved for HCC in the past 8 years.
E7080 (lenvatinib) is an oral multikinase inhibitor targeting kinases including vascular endothelial growth factor receptor (VEGF) 1–3, fibroblast growth factor receptor (FGFR) 1–4, platelet‐derived growth factor receptor‐α, RET, and KIT.12, 13 Recently, a phase II, single‐arm, open‐label multicenter study reported a high response rate to lenvatinib monotherapy in patients with unresectable HCC, with an acceptable safety profile.14 In a multicenter, randomized, open‐label, phase III trial (REFLECT), lenvatinib was compared with sorafenib and showed the treatment effect on OS by statistical confirmation of non‐inferiority.15 This novel anti‐angiogenesis multikinase inhibitor is thus now expected to be introduced into clinical practice as a first‐line systemic chemotherapy for advanced HCC, along with sorafenib.16, 17, 18
We herein report the initial case of a female patient with advanced HCC in whom lenvatinib monotherapy showed long‐term antitumor activity in a phase II study. She achieved a near‐complete response (CR) and remained alive for more than 5 years after the initiation of lenvatinib. We present her clinical course and the radiological results suggestive of changes in intratumoral vascularity during lenvatinib monotherapy.
Case Report
The patient was a 68‐year‐old woman with hepatitis C virus‐related HCC. She had a past medical history of hypertension and spinal canal stenosis. She was a non‐smoker and social drinker. She had been diagnosed with solitary HCC located in S8 by abdominal ultrasound sonography and computed tomography (CT) scan 5 years previously. The patient's HCC was a radiologically typical solid nodule and eradicated following radiofrequency ablation (RFA) therapy. However, multinodular intrahepatic recurrences with portal vein invasion (P8) were identified approximately 2 years after RFA, and six transcatheter arterial chemoembolization (TACE) therapies were enforced. She was refractory to TACE, and therefore participated in the phase II trial of lenvatinib monotherapy, after providing written informed consent.
At the start of lenvatinib, her vital signs were within normal ranges and her Eastern Cooperative Oncology Group performance status (PS) was 0. Her body height and body weight were 149.5 cm and 55 kg, respectively. Laboratory tests showed an α‐fetoprotein concentration of 5.3 ng/mL (normal range, <10 ng/mL) and an increased des‐γ‐carboxy prothrombin (DCP) concentration of 254 mAU/mL (normal range, <39 mAU/mL). Serum aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and γ‐glutamyl transpeptidase levels were also elevated (Table 1). Her liver function was Child–Pugh grade A (5 points).
Table 1.
Local laboratory data before the initiation of lenvatinib in a 68‐year‐old woman with unresectable advanced hepatocellular carcinoma with portal vein invasion
| Variables | Value | Unit | Variables | Value | Unit |
|---|---|---|---|---|---|
| WBC | 5470 | /μL | Ch‐E | 329 | IU/L |
| RBC | 463 | 104/μL | Na | 141 | mEq/L |
| Hemoglobin | 13.8 | g/dL | K | 3.8 | mEq/L |
| Platelet | 20.0 | 104/μL | Cl | 105 | mEq/L |
| ALT | 68 | IU/L | BUN | 13.5 | mg/dL |
| AST | 56 | IU/L | Creatinine | 0.42 | mg/dL |
| ALP | 629 | IU/L | Glucose | 95 | mg/dL |
| γGTP | 143 | IU/L | CRP | <0.2 | mg/dL |
| LDH | 239 | IU/L | PT | 70 | % |
| TP | 8.4 | g/dL | AFP | 5.3 | ng/mL |
| Albumin | 4.3 | g/dL | AFP‐L3 | 0 | % |
| T‐BIL | 0.5 | mg/dL | DCP | 254 | mAU/mL |
| D‐BIL | 0.1 | mg/dL |
γGTP, γ‐glutamyl transpeptidase; AFP, α‐fetoprotein; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen, Ch‐E, cholinesterase; CRP, C‐reactive protein; D‐BIL, direct bilirubin; DCP, des‐γ‐carboxy prothrombin; LDH, lactate dehydrogenase; PT, prothrombin time; RBC, red blood cell; T‐BIL, total‐bilirubin; TP, total protein; WBC, white blood cell.
A dynamic CT scan showed a typical hypervascular HCC. A typical HCC lesion 29 mm in diameter was located adjacent to the previously ablated area in S8 (Fig. 1), and this nodule was considered as the target lesion for lenvatinib therapy. Computed tomography findings also showed tumor invasion into the anterior branch of the right portal vein (VP2) (Fig. 1a,b). Liver biopsy prior to the initiation of lenvatinib therapy revealed moderately differentiated HCC (Fig. 2).
Figure 1.
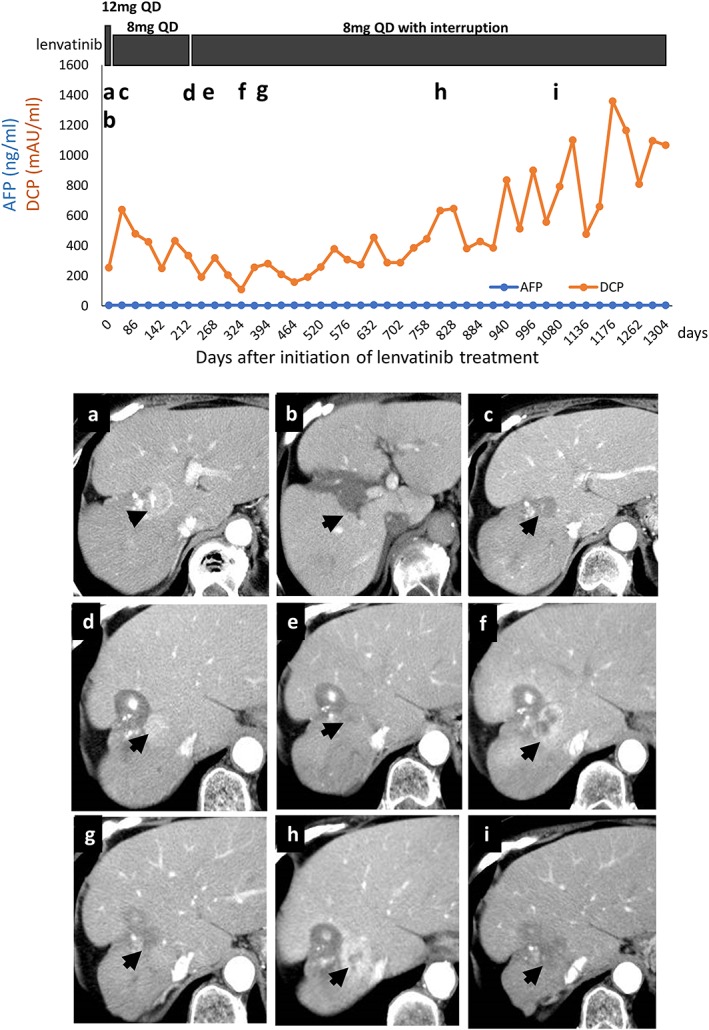
Top panel, clinical course of a 68‐year‐old woman with unresectable advanced hepatocellular carcinoma (HCC) with portal vein invasion. Bottom panels, dynamic computed tomography (CT) scan findings. (a) Target lesion in S8, considered a typical HCC with enhancement in the arterial phase, prior to lenvatinib initiation. (b) Target nodule is shown as a defect lesion in the portal phase before treatment. (c) CT scan 1 month after initiation of lenvatinib shows decreased vascularity of the target lesion in the arterial phase. (d) CT scan at the second assessment shows re‐increase of intratumoral enhancement of the target lesion in the arterial phase. (e) CT scan 6 months after the initiation of lenvatinib, during therapy, shows that tumor enhancement had decreased again. (f) CT scan 1 year after the initiation of lenvatinib, during therapy interruption, shows that intratumoral enhancement had increased again, but the maximum tumor diameter had not changed. (g) CT scan 18 months after the initiation of lenvatinib. (h) CT scan 26 months after the initiation of lenvatinib CT scans. (i) CT scan finding 3 years after the initiation of lenvatinib shows maintenance of decreased intratumoral vascularity. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 2.
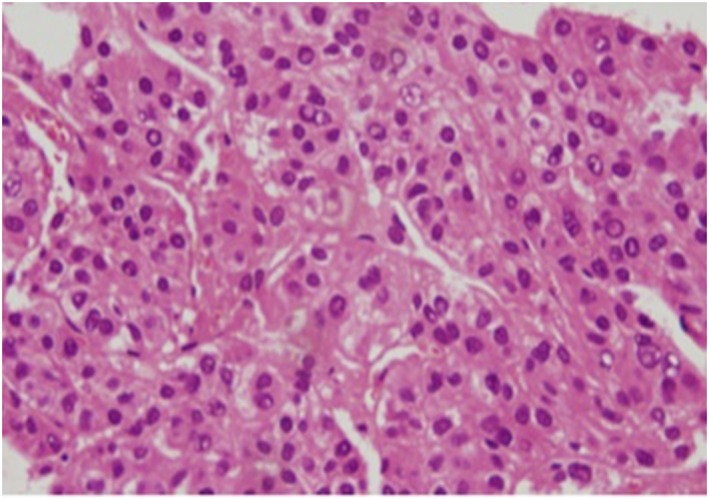
Histopathological examination of liver biopsy sample before initiation of lenvatinib (hematoxylin–eosin staining, ×200) in a 68‐year‐old woman with unresectable advanced hepatocellular carcinoma (HCC) with portal vein invasion. The image shows moderately differentiated HCC tumor cells. [Color figure can be viewed at http://wileyonlinelibrary.com]
Based on the phase II study protocol, lenvatinib 12 mg was given once daily (QD), but lenvatinib was interrupted due to grade 3 thrombocytopenia after 2 weeks. After recovering from thrombocytopenia, lenvatinib was restarted at a dose of 8 mg QD (level 1 dose reduction) and no remarkable adverse events were found for 3 months. Computed tomography findings at the first evaluation (week 8) showed markedly decreased intratumoral enhancement in the early arterial phase suggesting decreased vascularity, although the maximum diameter of the target lesion had not changed compared with its pretreatment tumor size (Fig. 1c). The therapeutic effect was determined to be PR as assessed by modified Response Evaluation Criteria in Solid Tumors (mRECIST) and stable disease as assessed by conventional RECIST 1.1.
However, the patient developed grade 2 proteinuria and lenvatinib therapy was interrupted by the investigator. A second evaluation of the therapeutic effect of lenvatinib was carried out by dynamic CT scan during the drug interruption, and showed unexpected apparent enhancement over the target tumor in the early arterial phase, suggesting increased intratumoral vascularity (Fig. 1d).
The patient's proteinuria improved after 1 week of observation. Lenvatinib 8 mg QD was therefore restarted, as per the phase II study protocol. The next CT scan revealed that the early enhancement of the target lesion had decreased again. At 11 months after the initiation of lenvatinib therapy, portal vein tumor thrombosis was undetectable and there was no sign of vascularization of the main tumor, together with a rapid decrease of DCP level (Fig. 1e). The intratumoral enhancement of the target lesion in the early arterial phase was remarkably decreased, however the best therapeutic effect evaluated by mRECIST was PR. At 12 months after the initiation of lenvatinib, the patient developed acute cholecystitis and interrupted lenvatinib, and a subsequent CT scan showed re‐enhancement of the target lesion (Fig. 1f). Lenvatinib was restarted again after her cholecystitis improved following antibiotic treatment, and the next CT evaluation showed that the target lesion had again become avascular (Fig. 1g). Subsequent CT scans during lenvatinib treatment revealed the target lesion as a low‐density area in the early arterial phase; scans during drug interruption due to proteinuria showed that the lesion was enhanced in the arterial phase (Fig. 1h,i). Lenvatinib 8 mg QD was continued except for the interruption for 1 week to manage the grade 2 proteinuria, and the patient was able to continue this lenvatinib therapy for more than 4 years, with well‐controlled general condition. She did not experience any appetite loss, fatigue, abnormality of thyroid function, or hand–foot syndrome during the observation period. Stable disease was also able to be maintained, and importantly her liver function was maintained with Child–Pugh grade A throughout the lenvatinib treatment. The OS of this subject was 71 months. In summary, lenvatinib monotherapy showed a long‐term antitumor effect during the clinical course of this patient with unresectable advanced HCC with portal vein invasion.
Discussion
To the best of our knowledge, this is the first case report detailing the long‐term antitumor effect of lenvatinib for advanced HCC. The observed adverse events, including thrombocytopenia and proteinuria, were well controlled, and long‐term lenvatinib treatment was achieved with no deterioration of PS. Lenvatinib has been reported to inhibit VEGF1–3, FGFR1–4, platelet‐derived growth factor receptor‐α, RET, and KIT, showing both anti‐angiogenic and direct antitumor activities.16, 17, 18, 19 In this case, the vascularity of the intrahepatic hypervascular nodule was lost in the early phase of dynamic CT, soon after treatment with lenvatinib. In addition, portal vein invasion (VP2) and occlusion of the anterior branch of the right portal vein disappeared completely 11 months after initiation of lenvatinib. The former therapeutic effect indicated the anti‐angiogenic activity of lenvatinib, whereas the latter indicated its direct antitumoral effect.
Typical HCC lesions are characterized radiologically by a tumor blush in the early‐phase scan and as a defect area in the late‐phase scan on dynamic CT or magnetic resonance imaging.20 Considering this radiological nature of typical HCC, mRECIST was proposed in 2010.21 The significant characteristics of this new criterion involve evaluating the intratumor vascularity of the target lesions; that is, hypervascular lesions in the early arterial phase in mRECIST are regarded as viable lesions, and the longest axis of the enhanced area in the arterial phase is the subject for evaluation.21 Considering this, the disappearance of intratumor vascularity of the target lesion after anti‐angiogenesis therapy might indicate CR or PR, even if the maximum diameter of the target lesion remains unchanged compared with baseline. According to this criterion, the current case might be considered to have achieved near‐CR as the best tumor response.
From the radiological point of view, the CT changes were considered to correlate with the anti‐angiogenic activity of lenvatinib. In the current case, the hypervascular target lesion became hypovascular after anti‐angiogenesis therapy but became hypervascular again during the drug interruption period. Although the target lesion demonstrated in the CT scan at the first evaluation showed low density in the arterial phase, which seemed to be necrotic, it was still viable, based on the observation of a subsequent re‐increase in tumor vascularity. Although the vascularity of the target lesion increased in the early period, we continued lenvatinib given that mRECIST indicated PR and because the patient's general status was clinically stable. Later, the patient showed nearly complete disappearance of intratumor vascularity by CT with lenvatinib monotherapy, and her disease and general condition remained well controlled for more than 4 years. Although mRECIST seems to be a reasonable tool for evaluating the hemodynamics in typical HCCs, clinicians should bear in mind the possibility that the disappearance of intratumoral enhancement does not always indicate complete necrosis, and viable tissues could remain even in hypovascular lesions after anti‐angiogenetic therapy.
Recently, Hiraoka et al. reported the early therapeutic responses of lenvatinib in real‐world clinical practice.22 They showed the decrease of blood supply 1 month after lenvatinib initiation, similar to the current case. Considering these clinical data, one of the therapeutic characteristics of lenvatinib might be early radiologic change. Although the early decrease of blood supply in our case was considered not to be complete tumor necrosis, our case actually achieved long survival time. It should be confirmed whether these early radiologic changes could be potential biomarkers to predict clinical outcome including OS.
Des‐γ‐carboxy prothrombin could reflect both the tumor viability and focal ischemic effect around the tumor legion.23 In this case, DCP was elevated in the first month and then gradually decreased to nearly normal value 11 months after the initiation of lenvatinib. The first elevation might reflect the focal ischemic effect around the main tumor, and the following decrease should reflect the antitumor effect. After that, the serum DCP level gradually increased during the observation period. During lenvatinib therapy, DCP worked as both a tumor marker and a suggestion of tumor ischemia, leading to the complicated dynamics of serum DCP value. As a result, it was difficult for us to predict the treatment effect of lenvatinib from serum markers. Possible markers to predict the treatment effect are now urgently needed.
Another concern for clinicians might be the possibility of conversion therapy after lenvatinib therapy. Considering its high objective response rate and low influence on liver dysfunction, lenvatinib therapy could achieve downstaging of tumors and lead to conversion therapy with surgical resection. In fact, in the present case, when the main tumor was well controlled and portal vein invasion vanished, the additional surgical resection might realize the “cure” from the tumor. The possibility of conversion surgery during or after lenvatinib therapy should be determined in real‐world clinical practice.
In this case, we considered the scheduled interruption of lenvatinib for 1 week following grade 2 proteinuria and the restart at the same dose. Because the efficacy of lenvatinib is dose‐dependent, the dose should be maintained for long‐term treatment continuation. Therefore, we recommend treatment at the same dose in patients with low‐grade adverse events if the toxicity can be managed. Approval of lenvatinib could provide an additional therapy option for patients with unresectable HCC.16 In the phase III clinical trial, lenvatinib showed not only non‐inferiority to sorafenib in terms of OS as the primary end‐point, but also superiority to sorafenib in terms of progression‐free survival, time to progression, and objective response rate as secondary end‐points.15 Most lenvatinib‐related adverse events, including hypertension and proteinuria, are not life‐threatening and do not have significant impact on quality of life. These lines of evidence suggest that lenvatinib could be an option for first‐line systemic therapy in patients with unresectable HCC. In the current case, we were able to manage the recurring proteinuria and continue lenvatinib long‐term, without deterioration of the patient's physical condition. To obtain the maximum effect from lenvatinib, it is necessary to understand fully the radiological changes occurring during molecular targeting therapy, to evaluate the precise treatment effects and ascertain the best time to switch to other treatment regimens. The suggestive radiological changes observed in the current case could thus help to guide the appropriate clinical use of molecular targeting agents for HCC.
In conclusion, we report the first case of a patient with advanced HCC in whom lenvatinib monotherapy showed long‐term antitumor activity. Clinicians should be aware of the suggestive radiological changes reflecting intratumor vascularity during treatment with the novel anti‐angiogenesis agent lenvatinib in patients with advanced HCC.
Acknowledgments
The authors would like to thank all the medical staff involved with the care of this patient. We thank Takashi Hisai and Noriyuki Koyama, Eisai Co., Ltd. for scientific discussions.
Takeda, H. , Nishijima, N. , Nasu, A. , Komekado, H. , Kita, R. , Kimura, T. , Kudo, M. , and Osaki, Y. (2019) Long‐term antitumor effect of lenvatinib on unresectable hepatocellular carcinoma with portal vein invasion. Hepatol Res, 49: 594–599. 10.1111/hepr.13294.
Conflict of interest: The authors have no conflict of interest.
Financial support: None declared.
References
- 1. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012; 379: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 3. Takeda H, Takai A, Inuzuka T, Marusawa H. Genetic basis of hepatitis virus‐associated hepatocellular carcinoma: linkage between infection, inflammation, and tumorigenesis. J Gastroenterol 2017; 52: 26–38. [DOI] [PubMed] [Google Scholar]
- 4. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999; 19: 329–338. [DOI] [PubMed] [Google Scholar]
- 5. Llovet JM, Ricci S, Mazzaferro V et al Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 6. Cheng AL, Kang YK, Chen Z et al Efficacy and safety of sorafenib in patients in the Asia‐Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double‐blind, placebo‐controlled trial. Lancet Oncol 2009; 10: 25–34. [DOI] [PubMed] [Google Scholar]
- 7. Marrero JA, Kudo M, Venook AP et al Observational registry of sorafenib use in clinical practice across Child‐Pugh subgroups: the GIDEON study. J Hepatol 2016; 65: 1140–1147. [DOI] [PubMed] [Google Scholar]
- 8. Takeda H, Nishikawa H, Osaki Y et al Clinical features associated with radiological response to sorafenib in unresectable hepatocellular carcinoma: a large multicenter study in Japan. Liver Int 2015; 35: 1581–1589. [DOI] [PubMed] [Google Scholar]
- 9. Cainap C, Qin S, Huang WT et al Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol 2015; 33: 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson PJ, Qin S, Park JW et al Brivanib versus sorafenib as first‐line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK‐FL study. J Clin Oncol 2013; 31: 3517–3524. [DOI] [PubMed] [Google Scholar]
- 11. Llovet JM, Hernandez‐Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res 2014; 20: 2072–2079. [DOI] [PubMed] [Google Scholar]
- 12. Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi‐kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA‐MB‐231 via inhibition of vascular endothelial growth factor‐receptor (VEGF‐R) 2 and VEGF‐R3 kinase. Clin Cancer Res 2008; 14: 5459–5465. [DOI] [PubMed] [Google Scholar]
- 13. Tohyama O, Matsui J, Kodama K et al Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res 2014; 2014: 638747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ikeda K, Kudo M, Kawazoe S et al Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol 2017; 52: 512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kudo M, Finn RS, Qin S et al Lenvatinib versus sorafenib in first‐line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non‐inferiority trial. Lancet 2018; 391: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 16. Kudo M. A new era of systemic therapy for hepatocellular carcinoma with regorafenib and lenvatinib. Liver Cancer 2017; 6: 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kudo M. Lenvatinib may drastically change the treatment landscape of hepatocellular carcinoma. Liver Cancer 2018; 7: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eso Y, Marusawa H. Novel approaches for molecular targeted therapy against hepatocellular carcinoma. Hepatol Res 2018; 48: 597–607. [DOI] [PubMed] [Google Scholar]
- 19. Kudo M. Lenvatinib in advanced hepatocellular carcinoma. Liver Cancer 2017; 6: 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayashi M, Matsui O, Ueda K, Kawamori Y, Gabata T, Kadoya M. Progression to hypervascular hepatocellular carcinoma: correlation with intranodular blood supply evaluated with CT during intraarterial injection of contrast material. Radiology 2002; 225: 143–149. [DOI] [PubMed] [Google Scholar]
- 21. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010; 30: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hiraoka A, Kumada T, Kariyama K et al Therapeutic potential of lenvatinib for unresectable hepatocellular carcinoma in clinical practice: multicenter analysis. Hepatol Res 2018. 10.1111/hepr.13243. [DOI] [PubMed] [Google Scholar]
- 23. Ueshima K, Kudo M, Takita M et al Des‐γ‐carboxyprothrombin may be a promising biomarker to determine the therapeutic efficacy of sorafenib for hepatocellular carcinoma. Dig Dis 2011; 29: 321–325. [DOI] [PubMed] [Google Scholar]


