Summary
Lymphoid organs guarantee productive immune cell interactions through the establishment of distinct microenvironmental niches that are built by fibroblastic reticular cells (FRC). These specialized immune‐interacting fibroblasts coordinate the migration and positioning of lymphoid and myeloid cells in lymphoid organs and provide essential survival and differentiation factors during homeostasis and immune activation. In this review, we will outline the current knowledge on FRC functions in secondary lymphoid organs such as lymph nodes, spleen and Peyer's patches and will discuss how FRCs contribute to the regulation of immune processes in fat‐associated lymphoid clusters. Moreover, recent evidence indicates that FRC critically impact immune regulatory processes, for example, through cytokine deprivation during immune activation or through fostering the induction of regulatory T cells. Finally, we highlight how different FRC subsets integrate innate immunological signals and molecular cues from immune cells to fulfill their function as nexus between innate and adaptive immune responses.
Keywords: B cells, fibroblastic reticular cells, innate immunological sensing, mesenchymal stromal cells, T cells
1. FIBROBLASTIC RETICULAR CELLS UNDERPIN THE STRUCTURE OF LYMPHOID ORGANS
The activation of adaptive immune responses depends on the interaction of professional antigen‐presenting cells (APC) with T and B cells in specialized compartments of lymphoid organs. Secondary lymphoid organs (SLO) are strategically positioned at routes of pathogen invasion and thereby increase the likelihood of lymphocytes to encounter their cognate antigens at a particular location and during a certain time window. Lymph nodes are found at convergence points of afferent lymph vessels and surveil extracellular fluids from separate areas of peripheral tissue. Other classical SLO such as Peyer′s patches are located right below the area of the intestinal surface they surveil, while the splenic white pulp provides specialized niches for the development of immune responses against blood‐borne pathogens.1 Nonclassical SLO such as fat‐associated lymphoid clusters (FALC) serve as surveillance hubs of the body cavities by sampling the fluids that are secreted by mesothelial cells.2 Transient lymphoid aggregates that display T‐ and B‐cell zone segregation and that appear in inflamed tissues are referred to as tertiary lymphoid structures (TLS, also known as tertiary lymphoid organs).3 Fluid flow in lymphoid organs is sustained by endothelial stromal cells with blood endothelial cells facilitating the delivery of oxygen and nutrients via blood vessels and lymphatic endothelial cells granting drainage of extracellular liquids via lymphatic vessels. The structural integrity of all lymphoid organs is determined by fibroblastic stromal cells that build, for example, the capsule of lymph nodes or the spleen. In addition, specialized immune‐interacting fibroblasts, generally termed fibroblastic reticular cells (FRC), form the scaffold structures that underpin the distinct microenvironments required for efficient immune cell interactions (Figure 1). FRC crucially contribute to the functioning of the immune system through the secretion of homeostatic chemokines to coordinate the interaction between APC and lymphocytes and provide growth and survival factors that nurture both innate and adaptive lymphocytes.4, 5 Moreover, FRC are equipped with a large range of pattern recognition receptors6, 7, 8 that play a crucial role in the control innate immune reactions.
Figure 1.
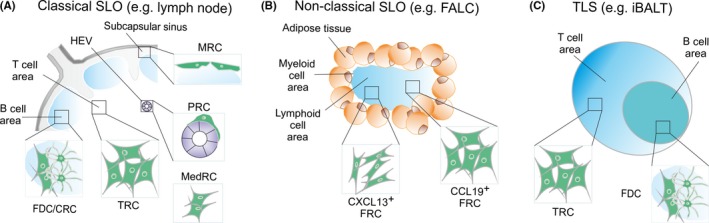
Lymphoid organs are underpinned by distinct fibroblastic reticular cell (FRC) subsets. (A) The structural segregation of classical secondary lymphoid organs (SLO) such as lymph nodes is granted by FRC that form dedicated microenvironmental niches. Marginal reticular cells are underlying the subcapsular sinus floor and form a bridge to the B‐cell follicle. The area densely populated by T cells harbors T‐cell zone reticular cells (TCR), while perivascular reticular cells from concentric layers around high endothelial venules and other blood vessels. Within the B‐cell follicle, follicular dendritic cells (FDC) and CXCL12‐producing reticular cells (CRC) regulate B‐cell trafficking and the germinal center reaction. The medullary region is rich in lymphatic vessels that are surrounded by medullary reticular cells (medRC). (B) Nonclassical SLO such as fat‐associated lymphoid clusters (FALC) lack dedicated subcompartments and are underpinned by CCL19‐ and/or CXCL13‐expressing FRC. (C) Tertiary lymphoid structures (TLS) form in the vicinity of blood vessels under inflammatory conditions, for example in the lungs as inducible bronchus‐associated lymphoid tissue (iBALT). Structural segregation of TLS with T‐cell and B‐cell areas can occur during long‐lasting inflammation with mature TLS harboring TCR‐like and FDC‐like reticular cells
The detection of pathogen‐associated molecular signatures by germline‐encoded receptors is a key determinant for the immune system to distinguish harmful molecules of foreign origin from inoccuous self‐molecules.9, 10 Sensing of microbial products by pattern recognition receptors expressed by APC such as dendritic cells and macrophages has been considered as a key step for the activation of the adaptive immune system.9, 11 However, recent studies have revealed that FRC in classical and nonclassical SLO actively shape adaptive immune responses through the integration of innate immunological signals.8, 12 In this review, we will highlight the ability of FRC to generate tissue‐specific microenvironmental niches that orchestrate complex immunological processes and discuss recent work that has revealed the role of FRC as an important nexus of innate and adaptive immune responses.
2. DIVERSITY OF FIBROBLASTIC RETICULAR CELLS IN LYMPHOID ORGANS
The most commonly used distinction of endothelial and fibroblastic stromal cells of SLO relies on the expression of the endothelial marker CD31 (platelet endothelial cell adhesion molecule‐1, PECAM‐1) and the fibroblast marker podoplanin (PDPN).13 While PDPN expression captures the majority of FRC in lymph nodes, the expression of this marker in the spleen is restricted mainly to FRC in the T‐cell zone.14 Moreover, PDPN is a surface molecule that is expressed by fibroblasts in nonlymphoid tissues and in tumors15 rendering this marker unsuitable to specifically track lymphoid tissue FRC. In contrast, the promoter activity of the FRC signature genes Ccl19 and Cxcl13 is well‐suited to capture the complexity of the immune‐interacting fibroblasts in SLO.16, 17 Indeed, the Ccl19‐Cre model facilitates targeting of FRC in all relevant microenvironments in lymph nodes,16, 18, 19 in Peyer's patches12 and in the white pulp of the spleen.20 Likewise, the Cxcl13‐Cre/tdTomato transgene targets the majority of FRC in all SLO.17 The combination of such advanced transgenic mouse models with single‐cell RNA‐seq‐based analyses of lymph node7, 21 and splenic white pulp22 FRC will enable a series of novel studies to further explore the functional complexity of FRC in lymphoid organs.
2.1. The many shapes of FRC in classical secondary lymphoid organs
While the differentiation trajectories of splenic white pulp FRC from perivascular progenitors have been delineated recently using Ccl19 promoter‐based cell fate mapping22 and lineage tracing,20 the origin of lymph node FRC has not yet been fully elucidated. Nevertheless, the aggregation of Ccl19‐Cre+ and Cxcl13‐Cre+ cells in the vicinity of blood vessels of the lymph node anlage16, 17 strongly suggests that lymph node FRC originate from myofibroblastic progenitors in the perivascular space. It appears that these precursor cells are able to generate the various FRC subsets that underpin the major compartments of the lymph node (Figure 1A).
The lymph fluid from peripheral tissues that arrives to the lymph node via the afferent lymph vessels is drained through the subcapsular sinus which is lined by lymphatic endothelial cells and, beneath the subcapsular sinus floor, by several layers of marginal reticular cells (MRC).23 These cells can be distinguished from other FRC subsets in lymph nodes by the expression of mucosal vascular addressin cell adhesion molecule 1 (MAdCAM‐1), receptor activator of nuclear factor kappa‐Β ligand (RANKL, TNFSF11), and CXCL13.23 In the spleen, MRC support immune responsiveness by supporting the capture and delivery of antigens from the marginal zone to B‐cell follicles.24 The expression of RANKL by Ccl19‐Cre+ lymph node MRC has been shown to be critical for the integrity of lymphatic endothelial cells in the subcapsular sinus25 indicating that MRC actively participate in shaping the cellular environment of the lymph node. Likewise, splenic white pulp MRC impact the phenotype of their interacting immune cell partners. For example, Notch 2‐driven differentiation of marginal zone B cells and of Esam+ dendritic cells requires the expression of the Notch ligand Delta‐like (DL)1 in splenic Ccl19‐Cre+ cells,26 while RANK has been shown to maintain myeloid cell populations in the marginal zone to secure initiation of antiviral immune responses.27 As an extension from the MRC layer unfolds the conduit system through the lymph node parenchyma as a fibrillary network formed by FRC.28 Conduits both in lymph nodes and in the splenic white pulp are ensheathed by FRC and consist of a collagen‐rich core that is surrounded by a microfibrillar zone and a basement membrane.29, 30 The conduit system conveys low‐molecular‐weight substances such as chemokines and antigens from the lymph node subcapsular sinus through the T‐cell area toward high endothelial venules.30 A recent report revealed that IgM transiently gains access to the luminal side of the lymph node conduit system to facilitate rapid export of early‐response IgM antibodies out of the lymph node parenchyma.31 In sum, MRC of lymph nodes and the splenic white pulp shape the border region of lymphoid tissues through the dedicated interaction with immune cells and other stromal cells and by maintaining communication channels for rapid distribution of immunologically relevant information. It will be important to fully exploit existing and novel reticular cell targeting approaches (such as the CollagenVI‐Cre model32) to characterize the function of MRC in Peyer's patches.
T‐cell zone reticular cells (TRC), ie, the FRC that co‐localize predominantly with T cells and dendritic cells, express PDPN and the signature chemokines CCL19 and CCL21.12, 13, 14, 22 It is important to reiterate that PDPN expression in lymph nodes can be found in almost all FRC subsets and that high PDPN expression is associated with maturation into fully immunocompetent FRC,16 while PDPN expression both in Peyer's patches and in the splenic white pulp is mainly found in TRC.12, 22 Both T cells and dendritic cells are in close contact with TRC and move along their projections within the T‐cell area.33 The mobility of dendritic cells is boosted by the ligation of the C‐type lectin‐like receptor 2 (CLEC‐2) with PDPN on TRC.34 Moreover, lymph node TRC are thought to function as the major source of the cytokine IL‐7 to promote T‐cell homeostasis.13 However, since IL‐7 can also be produced by lymphatic endothelial cells within lymph nodes35 and in the afferent lymphatics,36 the specific function of TRC‐derived IL‐7 for T‐cell sustenance and activation remains to be demonstrated.
B‐cell follicles harbor distinct FRC subsets that produce CXCL13, the B cell‐attracting chemokine that binds to CXCR5.37 As mentioned above, CXCL13‐expressing MRC extend into the B cell follicle, while the most prominent reticular cell subset that underpins the B‐cell area has been named follicular dendritic cell (FDC).38 Due to their dendritic morphology, FDC have been assumed to be related to conventional dendritic cells, ie, to originate from bone marrow progenitors. Only recently, the progenitor cell of FDC has been revealed as perivascular myofibroblast that is characterized by the expression of platelet‐derived growth factor receptor β (Pdgfrb, CD140b).39 FDC in the B cell follicle can be identified by the expression the complement receptors CD21 and CD35 and can be found both in primary and secondary follicles.40 The main functions of FDC are the retention and presentation of particulate antigen on their surface to B cells using a broad range of FCγ‐receptors (ie, CD16, CD32)41 and the promotion of affinity maturation and somatic hypermutation of B cells in the germinal center reaction.42 FDC and probably other FRC subsets43 in the B‐cell follicle control the survival of B cells via B cell activation factor (BAFF) or transmembrane activator and CAML interactor (TACI).44 It has been suggested that the dark zone of the germinal center harbors a subset of CXCL12‐expressing reticular cells (CRC) that express PDPN and can be lineage‐traced using the Ccl19‐Cre and CD21‐Cre model.18 However, a unique phenotype of CRC could not be revealed using single‐cell RNA‐seq analysis7 indicating that the phenotypical distinction of FRC/FDC subsets underpinning the germinal center is still unclear.
A main fraction of FRC express markers that are indicative for their perivascular location such as Itga7 (integrin α7), Pdgfrb (CD140b), and Acta2 (αSMA) in lymph nodes6, 7 and Ly6a (Sca‐1), Pdgfra (CD140a), and Vcam1 (CD106) in the spleen.22 It is likely that the perivascular reticular cell (PRC) fraction harbors the adult progenitor of all FRC subsets.22, 39 Other regions of the lymph node such as the deep cortical area appear to harbor a subset of FRC that is characterized by the expression of CCL21a, CXCL12, and LepR.19 This area of the lymph node is occupied by T cells, dendritic cells, and B cells suggesting that FRC acquire distinct phenotypical properties when they interact with multiple cell types. Indeed, FRC attain yet other properties when they co‐localize in medullary cords with macrophages, NK cells, and plasma cells.19 In this location, medullary reticular cells (medRC) express high levels of CXCL12, IL‐6, and BAFF and facilitate thereby the formation of dedicated niches for plasma cells.45 Single‐cell RNA‐seq analysis has confirmed the existence of at least two FRC subsets that localize in the medullary region indicating that medRC also promote the maintenance of NK cells in this region.7 Clearly, further studies are required to unveil the molecular properties and function of FRC subsets not only in the lymph node B‐cell niches but also in the different microenvironments of classical SLO.
2.2. Limited FRC heterogeneity in nonclassical SLO and TLS
While the formation of classical SLO, ie, lymph nodes, splenic white pulp and Peyer's patches, is fully dependent on the presence of the lymphotoxin‐β receptor,46 the generation of nonclassical SLO (eg, FALC) or TLS (eg, inducible bronchus‐associated lymphoid tissue [BALT]) is largely independent of this pathway.2 For example, the formation of FALC requires the activation of stromal cells via the production of inflammatory cytokines such as the tumor necrosis factor (TNF), which are induced through the presence of microbiota in the intestine.47 Interestingly, the highly activated milieu of the intestinal lamina propria does not provide sufficient cytokine‐mediated stimulation to override the dependence of cryptopatch and isolated follicle formation on lymphotoxin‐β receptor signaling,48 indicating that the pathways employed in the generation of nonclassical SLOs are organ‐dependent. Likewise, TLS, which are locally inducible leukocytic aggregates that form in chronically inflamed nonlymphoid tissues,49 can form in different organs in a context‐dependent manner through triggering of inflammatory circuits involving IL‐17, IL‐6, IL‐1β, and/or IL‐22.50, 51, 52, 53 In terms of structural organization and FRC content, both nonclassical SLO (Figure 1B) and TLS (Figure 1C) exhibit a reduced complexity when compared to the classical SLO. We will focus our review here on FALC and inducible BALT as examples of nonclassical SLOs and TLS, respectively, to highlight the few knowns and many unknowns of FRC biology in these compartments.
FALC are located beneath the mesothelium and are surrounded by adipose tissues. A clear structural segregation of lymphocytes is not recognizable with a dense cluster of B cells being intermingled with CD4+ T cells and CD11b+ myeloid cells.54, 55 The main B cell population within FALC are B1 B cells that patrol body cavities and are the source of natural, low‐affinity immunoglobulin M (IgM) antibodies that bind to pathogenic bacteria.56 FALC also contain innate lymphoid cells (ILC), particularly type 2 ILCs and NKT cells.47, 55 The production of CCL19 and CCL21 by FALC FRC most likely mediates the attraction and retention of naive T cells.54 PDPN‐expressing FRC that underpin FALC structures are highlighted by the Ccl19‐Cre transgene, express PDPN and occupy perivascular niches with a surface marker profile that resembles splenic PRC (ie, PDGFRα+VCAM‐1+ICAM‐1+).8 Although these cells do not display the general phenotype of MRC or FDC, FALC FRC have been shown to produce the B cell‐attractant CXCL13.57 Hence, it appears that the somewhat random mixture of T and B cells in FALC is due to a high versatility of FALC FRC which permits attraction and interaction with B cells, T cells, and myeloid cells. Clearly, FALC FRC—and probably as well the FRC underpinning intestinal isolated lymphoid follicles—can steer both innate and adaptive immune responses without forming distinct microenvironments such as germinal centers.
The formation of TLS is frequently associated with chronic inflammatory and autoimmune diseases.3, 58 Importantly, in the context of cancer, the presence of TLS correlates with improved survival in a growing list of human cancers including breast cancer,59 lung cancer,60 oral squamous cell61 and Merkel cell carcinomas,62 and melanoma.63 Hence, it is tempting to speculate that TLS serve as inducible and transient outposts of the immune system to locally cope with ongoing immunological threats. Moreover, it appears that the coordination of immune cell interaction within these structures relies on organizational principles that are comparable to those in the classical SLO (Figure 1C). During tumor formation, TLS undergo a maturation process that has been suggested to start with the segregation of T‐cell and B‐cell areas in the perivascular space and progresses by the appearance of germinal centers.64, 65 The subsequent development of germinal centers in tumor TLS is accompanied by the appearance of CXCL13‐producing, CD21+ FDC networks both in human colorectal cancer65 and in squamous cell carcinoma of the lung.64 The presence of chemokine‐secreting FRC that underpin TLS has been demonstrated in a variety of models of chronic organ inflammation.52, 53, 66 The formation of inducible BALT in the lung has revealed that the activation of the innate immune system via lipopolysaccharide instillation can drive the formation of local TLS in an IL‐17‐dependent fashion,50 while this cytokine is not necessary to induce BALT formation following intranasal infection with a propagation‐deficient virus.67 Nevertheless, viral infection appears to be sufficient to induce highly organized BALT structures that contain B‐cell follicles underpinned by a network of CXCL13‐expressing FDC as well as CXCL12‐producing, yet undefined, reticular stromal cells.66 Furthermore, the bacterial infection with Pseudomonas aeruginosa triggers BALT formation in a TLR‐dependent manner leading to the emergence of CXCL12+ reticular cells that is dependent on γδ T cell‐derived IL‐17, while FDC fail to develop under these conditions.66 Overall, the emerging view is that remodeling and maturation of immune‐stimulating FRC is one of the initiating events in the establishment of an immune‐competent niche capable of recruiting and retaining disease‐relevant lymphocytes in TLS.3 Hence, targeted modulation of FRC differentiation processes within TLS may lead to treatment modalities that either attenuate TLS formation during chronic inflammatory diseases or foster the development of such immune‐activating structures in malignant diseases.
3. IMMUNE CELL‐FRC INTERACTIONS
The main function of secondary lymphoid organs is to preempt68 and to deal with1 the encounter of microbial agents and tumor cells. Pathogens and other antigenic material arrive at the antigen‐sampling zone of SLO, eg, the subcapsular sinus region of lymph nodes, where dedicated macrophage/dendritic cell populations take up and transfer antigen to B cells.69, 70 Consequently, disruption of the subcapsular sinus structure results in defective immune responses during secondary infection in mice.71 The cellular infrastructure of the murine splenic marginal zone functions in the same manner and efficiently retains infectious agents.72 Interestingly, it appears that the function of antigen capture and innate immunological sensing in the marginal zone of murine spleens is assigned to CD169‐positive macrophages and/or dendritic cells,69, 72 while in the human spleen MAdCAM1‐positive MRC operate as coordinators of immune cell interaction and drivers of subsequent immune activation.24 Antigens are further dispersed in the lymph node through the lymphatic sinuses that pervade the lymph node parenchyma, and are taken up by distinct dendritic cell subsets for the delivery to CD8+ or CD4+ T cells.73 The interaction of dendritic cells and T cells depends on the infrastructure provided by TRC74 and is regulated by TRC‐derived factors such as CCL2175 or lysophosphatidic acid.76 The efficient interaction of T and B cells at the T‐B border during viral infection depends on the presence of BAFF‐producing FRC.44 Other FRC subsets contribute during the subsequent steps of B‐cell activation in lymph nodes including antigen presentation in the germinal center,42 regulating B‐cell migration during the germinal center reaction,18, 42 and establishing plasma cell competent microenvironments in the medulla.45 FRC can perform these multiple functions because they are able to integrate a variety of signals through sensing of innate immunological stimuli and the differentiation of cellular signals in their immediate environment (Figure 2A). In the following sections, we will summarize how FRC detect and process pathogen‐derived innate immunological signals and illustrate the molecular pathways employed by FRC to regulate adaptive immune responses in lymph nodes, Peyer's patches and FALC.
Figure 2.
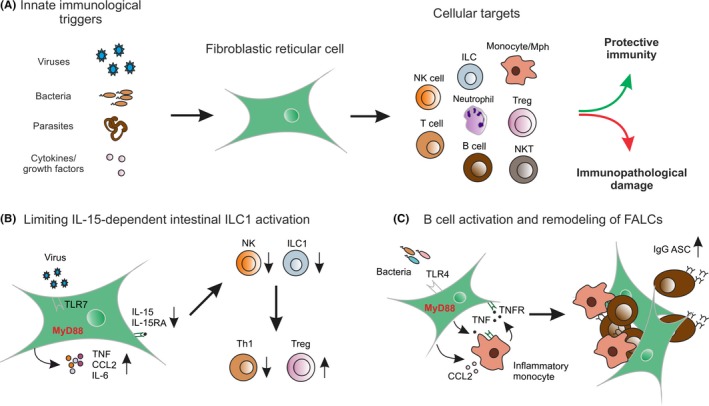
Fibroblastic reticular cells (FRC) coordinate innate and adaptive immune responses in lymphoid organs. (A) FRC recognize pathogen‐associated molecular patterns and integrate cellular signals from immune cells such as cytokines and growth factors. A broad variety of immune cells have been shown to receive crucial signals from FRC to steers the outcome of immune responses. FRC are key decision‐making cells that foster protective immunity while minimizing immunopathological damage. (B) FRC of Peyer's patches and mesenteric lymph nodes can recognize viral infection via Toll‐like receptor 7 (TLR7) and activate other immune cells through inflammatory cytokines. Concomitantly, MyD88‐dependent IL‐15 production is tuned down leading to attenuated type 1 innate lymphoid cell (ILC1) and natural killer (NK) cell activation. Shifting the balance between interferon‐γ‐producing Th1 and regulatory T cell (Treg) differentiation prevents immunopathological sequelae in the intestinal lamina propria.12 (C) Immunological sensing of bacterial products via TLR4 leads to MyD88‐dependent induction of CCL2 with subsequent recruitment of inflammatory monocytes. A TNF‐driven amplification of both FRC and monocyte activation drives the expansion and remodeling of FALC that grants optimal conditions for the generation of IgG antibody‐secreting B cells (ASC)8
3.1. Innate immunological sensing by FRC
FRC can directly recognize pathogens and their immune‐activating signals using various pattern recognition receptors and particular sets of immune‐activating molecules.6, 7, 77 The activation of in vitro cultivated FRC from SLO12 or FALC8 with various TLR ligands including lipopolysaccharide, poly(I:C), and zymosan leads to upregulation of adhesion molecules ICAM‐1 and VCAM‐1 and the secretion of inflammatory mediators such as CCL2, IL‐6, and TNF. In vivo, lymph node FRC react rapidly to systemic application of lipopolysaccharide with the activation of antigen presentation and type 1 interferon (IFN) pathways and alterations in the generation of extracellular matrix proteins including matrix metalloproteinase‐9, periostin, collagen type VI, and laminin α2.6 Direct ligation of TLR4 on FDC by lipopolysaccharide leads to increased expression of adhesion molecules and promotes the production of antigen‐specific antibodies when FDC are co‐cultured with B cells in vitro.78 Moreover, the activation of FDC by oxidized phospholipids, which function as endogenous TLR4 ligands, can foster the germinal center reaction by promoting higher rates of class‐switch recombination and somatic hypermutation in B cells.79 The formyl peptide receptor 2 that binds microbial products derived from Escherichia coli or Listeria, interacts with the endogenous ligand LL‐37 to enhance CXCL13 and BAFF production by FDC and thereby promotes B‐cell proliferation.80
Viruses can directly infect FRC as demonstrated for the lymphocytic choriomeningitis virus in mice14, 81 and human viruses including Chikungunya virus82 or Ebola virus.83 Complex immune cell interactions are triggered when intracellular viral RNA is recognized by FRC via TLR712 (Figure 2B). The transcriptomic analysis of lymph node FRCs after subcutaneous infection with herpes simplex virus‐1 (HSV‐1) revealed a pronounced activation of type‐I IFN pathway in FRC.77 Virus‐induced inflammation results in FRC proliferation and the induction of a substantial remodeling of the FRC landscape.35, 77 However, excessive activation of TLR7 ligands, eg, through internalization of ribonucleotide proteins complexes via CD21, can result in type 1 IFN production by FDC which supports the long‐lasting maintenance of the germinal center response and sustained production of antinuclear antibodies with perpetuation of a lupus‐like disease in mice.84 Hence, FRC activation through innate immunological pathways requires dedicated control mechanisms to avoid immunopathological sequela.
Innate immunological recognition circuits are integrated intracellularly via particular molecular switches such as the myeloid differentiation primary response 88 (MYD88) protein85 and can be amplified by cellular receptors such as the type‐I IFN receptor (IFNAR).86 During infection with the murine cytomegalovirus, blockade of the type‐I IFN pathway leads to a change in viral tropism with a shift from subcapsular macrophages to MRC as the main target cells. The elevated infection rate of MRC leads to the activation and recruitment of NK cells, which efficiently reduce the viral burden in the subcapsular sinus but consequently destroy the reticular cell network of lymph nodes leading to systemic distribution of the virus.87 Overall, it appears that IFNAR signaling in the stromal cell compartment is important to contain viral replication in a broad range of experimental models. However, whether and to which extent IFNAR signaling in FRC directly contributes to the control of a viral infection has not been determined yet.
Further FRC innate activation signals can be derived from immune cells that populate their particular microenvironmental niches. For example, in murine FALC, both FRC and hematopoietic cells attracted by FRC can serve as source for TNF8 (Figure 2C). Likewise, human tonsillar FRC respond in vitro to TNF exposure with increased expression of adhesion molecules and enhanced production of inflammatory cytokines.88 Further consequences of innate immunological sensing by FRC include the production of T cell‐activating factors such as IL‐33, which is produced by splenic PDPN+ FRC during vaccination with a recombinant viral vector.89 Single‐cell RNA‐seq analysis has revealed a population of FRC in naive lymph nodes that express high levels of Cxcl9.7 It appears that CXCL9 is mainly provided by stromal cells while CXCL10 is mainly express by murine myeloid cells DC following immunization of mice with dendritic cells that are pulsed with ovalbumin protein and activated with lipopolysaccharide and poly(I:C).90 In sum, FRC actively participate in the earliest phases of developing immune responses through their function as recipients of innate immunological signals and as coordinators of subsequent immune reactions through autocrine and paracrine signal amplification.
3.2. FRC control adaptive immune responses in lymph nodes and Peyer's patches
Molecules of the TNF receptor superfamily are not only crucial for the formation of lymphoid organs2, 46, 68, 91 but also profoundly impact the maturation of myofibroblastic progenitors into fully immunocompetent FRC. For example, genetic ablation of lymphotoxin‐β receptor expression on Ccl19‐Cre+ FRC leads to defective FRC maturation with reduced expression of key molecules such as Ccl19, Ccl21, and Il7, that precipitates high susceptibility to viral infection due to impaired activation of T cells.16 While the lymphotoxin‐β receptor appears to affect the maturation of all FRC subsets in lymph nodes, other molecules from the TNF receptor superfamily appear to be required for subset specification. For example, FDC differentiation depends on TNF produced by B cells,92 while CD30 contributes to proper B‐ and T‐cell zone segregation that is associated with reduced PDPN expression on yet undefined FRC subsets.93 The intricate regulatory circuits of FRC‐immune cell interaction that found the basis of protective immune responses is highlighted in a recent study on intestinal Listeria monocytogenes infection in mice.94 Listeria infection induces intestinal epithelial cell proliferation and depletion of goblet cells, while CX3CR1+ myeloid cells in Peyer's patches produce IL‐23 and thereby activate IL‐17‐secreting γδ T cells. The resulting IL‐11 production by PDPN‐expressing cells in Peyer's patches facilitates the activation of enterocytes and limits intestinal villus invasion by Listeria.94 However, such protective FRC‐immune cell interactions may precipitate immunopathological consequences such as a decreased thickness of the mucus barrier that eventually fosters intestinal inflammation.94 Thus, FRC can function both as activating and regulating cellular entities during immune responses.
The ability of FRC to negatively impact T‐cell responses has been first noted by the Turley group who demonstrated that FRC in lymph nodes can present peripheral tissue antigens to T cells and thereby attenuate self‐reactivity.95 It has been proposed that the generation of nitric oxide by lymph nodes FRC regulates the expansion and activity of T cells.96, 97 However, since nitric oxide can be produced by other lymph node stromal cells such as lymphatic endothelial cells,96 it remains to be determined, for example, through FRC‐specific ablation of the inducible nitric oxide synthase gene, to which extent FRC regulate T‐cell activity through this mechanism. It is also possible that FRC regulate global immune responsiveness by impacting regulatory T‐cell differentiation. It is generally assumed that dendritic cells are the main cell population that control the differentiation pathway of CD4+ T cells toward the FoxP3+ regulatory T‐cell phenotype.98, 99 However, a recent study suggests that FRC in mesenteric lymph nodes can modulate resident dendritic cells via a bone morphogenic protein‐2‐dependent pathway to foster the induction of regulatory T cells.21 Further studies are warranted to elaborate such direct and indirect pathways of FRC‐dependent immune regulation.
Innate lymphoid cells (ILC) regulate immune responsiveness by bridging innate and adaptive immunity. In contrast to T and B cells, ILC lack rearranged antigen receptors and their development and activation is therefore mainly steered via soluble factors and their receptors. Since ILC are particularly abundant at mucosal sites, they are considered as the main cell population that maintains tissue integrity and homeostasis via innate immune mechanisms.100 ILC accumulation at mucosal surfaces relies on the provision of survival factors such as IL‐7 and IL‐15 that can be produced by FRC13, 101 However, ILC also reside in SLO where they are involved in the regulation of adaptive immunity.102, 103 Recently, it has been shown that group 1 ILC are localized in the T cell zones of Peyer's patches and that Ccl19‐Cre+ FRC generate an essential niche for these cells through the provision of IL‐15.12 However, IL‐15 production by PDPN+ FRC in Peyer's patches is rapidly abrogated under excessive inflammatory conditions such as infection with a cytopathic virus. Importantly, the swift cessation of IL‐15 production by FRC in Peyer's patches and mesenteric lymph nodes is dependent on MyD88 signaling which prevents an overshooting activation of NK1.1+ ILC and immunopathological overstimulation of IFN‐γ‐producing Th1 cells (Figure 2B). As a consequence, unrestrained Peyer's patch FRC lacking MyD88 expression permit the rapid clearance of a cytopathic viral infection through boosted ILC1 and Th1 responses which is accompanied by impaired intestinal integrity, bacterial dysbiosis, and chronical intestinal inflammation.12 This study shows that FRC in lymph nodes and Peyer's patches can act as immune rheostats through the regulation of group 1 ILC activity. It will be important to further elaborate the mechanisms that grant FRC in lymphoid organs control over innate and adaptive immune responses.
3.3. FRC‐dependent immune responses in FALC
In case of a breach of pathogenic microorganisms into one of the body cavities, protective immunity needs to be mounted swiftly to prevent harm to the internal organs. The adipose tissue underlying the mesothelial surface of the pericardial, pleural and peritoneal cavities harbors variable numbers of FALC.104 In the omentum, a mesothelium‐covered tissue flap that connects stomach, pancreas, colon, and spleen, FALC have been shown to collect fluids and particles from the peritoneal cavity.105 The uptake of inflammation‐inducing substances from the peritoneal cavity such as lipopolysaccharide106 or the lipid‐based antigen zymosan47 induces an increase of omental FALC size and numbers. It has been shown that opsonization of bacterial antigens by natural, low‐affinity IgM antibodies generated by peritoneal B1 B cells can promote the uptake and elimination of bacteria by myeloid cells.107 Moreover, the presence of viral particles in the peritoneal cavity increases the cellularity and size of FALC with concomitant attraction of macrophages from the peritoneal cavity to these structures.108 Other immune cells in the peritoneal cavity such as B‐2 B cells109 or neutrophils110 reach FALC via the blood vasculature. Although FALC lack the compartmentalization observed in classical SLO, their structural foundations support the rapid generation of both T‐ and B‐cell immune responses. For example, intraperitoneal application of antigens such as ovalbumin leads to the initial activation of antigen‐specific CD4+ and CD8+ T cells in omental FALC.54 Similarly, T‐dependent and T‐independent B‐cell responses are generated first in FALC following intraperitoneal antigen application.47 The role of FRC in the initiation of adaptive immune responses in FALC has only recently been clarified. It appears that CXCL13‐expressing FRC in FALC not only support the attraction and retention of B cells,54, 57 but that Ccl19‐Cre+ FALC FRC can directly sense the presence of microbial products via TLR4 and initiate a MyD88‐dependent immune‐amplifying cascade8 (Figure 2C). Following exposure to TLR2 and TLR4 ligands FALC FRC secrete inflammatory cytokines and chemokines including CCL2 to attract CCR2+ inflammatory monocytes from the circulation.8 Inflammatory monocytes establish a crucial crosstalk with FALC FRC that leads to the rapid growth and remodeling of FALC. TNF functions as the main factor that regulates the reciprocal communication between FRC and the myeloid cells and eventually facilitates the generation of humoral immunity within FALC8, 47 (Figure 2C). Interestingly, although FALC lack FDC, the microenvironmental remodeling provided by FRC is sufficient to promote germinal center‐like B‐cell responses with class‐switch recombination and a discrete somatic hypermutation.47, 54 Currently, it remains unknown whether CXCL13+ and CCL19+ FRC represent two distinct cell types or whether a variable expression of the two chemokines represents different functional states. It will be possible to clarify this question by using appropriate cell fate mapping models to track the lineage commitments of FRC subsets within nonclassical SLO.
Beyond their prominent function in the initiation and coordination of innate and adaptive immune responses in FALC, FRC present in visceral adipose tissues may also contribute to the maintenance of immune homeostasis and the regulation of the immune‐suppressive environment of adipose tissues under steady‐state conditions. Adipose tissues, including the omental fat favor the accumulation of IL‐10‐producing B cells111 and regulatory T cells.112 Regulatory T cells in adipose tissues are characterized by high expression of the IL‐33 receptor ST2,112 with IL‐33 being one of the main tissue factors that impacts the development and maintenance of regulatory T cells in this compartment.113 Since IL‐33 is mainly produced by FRC‐like cells in FALC,114 it is tempting to speculate that this circuit not only controls B1 B‐cell activation and local IgM production during lung infection and inflammation,114 but that FALC FRC equilibrate immune‐activating and ‐suppressive circuits in these tissues. The regulation of physiological functions by FRC‐derived IL‐33 in the adipose tissue may even extend to other body functions such a thermogenesis.115 Further studies will be required to better understand the molecular processes underlying FRC‐dependent immune activation in FALC. Moreover, it will be important to dissect the relation of FALC FRC to other PDPN expression fibroblasts in visceral adipose tissues and to elaborate the mechanisms employed by FRC that contribute to the maintenance of tissue homeostasis.
4. CONCLUDING REMARKS
The translation of innate immune signals into activating or regulating processes that steer adaptive immune responses has been regarded as one of the main roles of professional APC such as dendritic cells. FRC as dedicated immune‐interacting fibroblasts have now entered the stage to be recognized as cells that crucially contribute to the decision‐making within the immune system. The many functions of FRC are accomplished through the generation of specific microenvironmental niches for various types of immune cells. Distinct subsets of FRC form these niches to support immune cell migration, survival, and differentiation. Interestingly, it seems that particular genetic programs are imprinted in the immune‐interacting fibroblasts depending on their anatomical location. The main differentiation switches of FRC differentiation have been identified with the lymphotoxin‐β receptor representing a necessary “signal 1” for FRC maturation in classical SLO.16 However, further research needs to dissect the intercellular communication pathways between FRCs and the various immune cells that impact the differentiation trajectories of FRC in lymphoid and nonlymphoid organs. Indeed, it appears that the intestinal lamina propria of patients suffering from inflammatory bowel disease harbors at least one mesenchymal stromal cell population that closely resembles lymphoid organ FRC and is highlighted by the expression of IL‐33 and Lysyl oxidases.116 Thus, understanding the mechanisms that govern FRC differentiation in SLO and TLS will, for example, open novel avenues to target drugable FRC differentiation pathways in TLS that form during human chronic inflammatory diseases such as rheumatoid arthritis117, 118 or Sjören's syndrome.119
A major challenge that should to be addressed in the future is the lineage relationship of FRC with other mesenchymal cell types such as adipocytes, chondrocytes, or regular tissue fibroblasts. The definition of mesenchymal cell types needs to be coupled to their development origin and their function within a tissue. Hence, a combination of single‐cell transcriptome analysis and faithful in vivo lineage tracing needs to be employed120 to obtain consistent definitions of the cell types that are currently covered by the broad terms “stromal cells” or “mesenchymal cells”.
ACKNOWLEDGEMENTS
We thank Dr. Natalia Pikor for critical reading of the manuscript. This study received financial support from the Swiss National Science Foundation (grants 166500 and 177208 to BL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Perez‐Shibayama C, Gil‐Cruz C, Ludewig B. Fibroblastic reticular cells at the nexus of innate and adaptive immune responses. Immunol Rev. 2019;289:31–41. 10.1111/imr.12748
This article is part of a series of reviews covering Global positioning by Chemokines and other Mediators appearing in Volume 289 of Immunological Reviews.
REFERENCES
- 1. Junt T, Scandella E, Ludewig B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat Rev Immunol. 2008;8(10):764‐775. [DOI] [PubMed] [Google Scholar]
- 2. Randall TD, Mebius RE. The development and function of mucosal lymphoid tissues: a balancing act with micro‐organisms. Mucosal Immunol. 2014;7(3):455‐466. [DOI] [PubMed] [Google Scholar]
- 3. Buckley CD, Barone F, Nayar S, Benezech C, Caamano J. Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu Rev Immunol. 2015;33:715‐745. [DOI] [PubMed] [Google Scholar]
- 4. Chang JE, Turley SJ. Stromal infrastructure of the lymph node and coordination of immunity. Trends Immunol. 2015;36(1):30‐39. [DOI] [PubMed] [Google Scholar]
- 5. Fletcher AL, Acton SE, Knoblich K. Lymph node fibroblastic reticular cells in health and disease. Nat Rev Immunol. 2015;15(6):350‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malhotra D, Fletcher AL, Astarita J, et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol. 2012;13(5):499‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodda LB, Lu E, Bennett ML, et al. Single‐cell RNA sequencing of lymph node stromal cells reveals niche‐associated heterogeneity. Immunity. 2018;48(5):1014‐1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perez‐Shibayama C, Gil‐Cruz C, Cheng HW, et al. Fibroblastic reticular cells initiate immune responses in visceral adipose tissues and secure peritoneal immunity. Sci Immunol. 2018;3:eaar4539. [DOI] [PubMed] [Google Scholar]
- 9. Janeway CA. Approaching the asymptote? Evolution and revolution in immunobiology. Cold Spring Harb Symp Quant Biol. 1989;54:1‐13. [DOI] [PubMed] [Google Scholar]
- 10. Medzhitov R, Janeway CA Jr.. How does the immune system distinguish self from nonself? Semin Immunol. 2000;12(3):185‐188. [DOI] [PubMed] [Google Scholar]
- 11. Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271‐296. [DOI] [PubMed] [Google Scholar]
- 12. Gil‐Cruz C, Perez‐Shibayama C, Onder L, et al. Fibroblastic reticular cells regulate intestinal inflammation via IL‐15‐mediated control of group 1 ILCs. Nat Immunol. 2016;17(12):1388‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Link A, Vogt TK, Favre S, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8(11):1255‐1265. [DOI] [PubMed] [Google Scholar]
- 14. Scandella E, Bolinger B, Lattmann E, et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue‐inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9(6):667‐675. [DOI] [PubMed] [Google Scholar]
- 15. Buechler MB, Turley SJ. A short field guide to fibroblast function in immunity. Semin Immunol. 2018;35:48‐58. [DOI] [PubMed] [Google Scholar]
- 16. Chai Q, Onder L, Scandella E, et al. Maturation of lymph node fibroblastic reticular cells from myofibroblastic precursors is critical for antiviral immunity. Immunity. 2013;38(5):1013‐1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Onder L, Morbe U, Pikor N, et al. Lymphatic endothelial cells control initiation of lymph node organogenesis. Immunity. 2017;47(1):80‐92. [DOI] [PubMed] [Google Scholar]
- 18. Rodda LB, Bannard O, Ludewig B, Nagasawa T, Cyster JG. Phenotypic and morphological properties of germinal center dark zone Cxcl12‐expressing reticular cells. J Immunol. 2015;195(10):4781‐4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takeuchi A, Ozawa M, Kanda Y, et al. A distinct subset of fibroblastic stromal cells constitutes the cortex‐medulla boundary subcompartment of the lymph node. Front Immunol. 2018;9:2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schaeuble K, Britschgi MR, Scarpellino L, et al. Perivascular fibroblasts of the developing spleen act as LTalpha1beta2‐dependent precursors of both T and B zone organizer cells. Cell Rep. 2017;21(9):2500‐2514. [DOI] [PubMed] [Google Scholar]
- 21. Pezoldt J, Pasztoi M, Zou M, et al. Neonatally imprinted stromal cell subsets induce tolerogenic dendritic cells in mesenteric lymph nodes. Nat Commun. 2018;9(1):3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng HW, Onder L, Novkovic M, et al. Origin and differentiation trajectories of fibroblastic reticular cells in the splenic white pulp SSRN; 2018. [DOI] [PMC free article] [PubMed]
- 23. Katakai T, Suto H, Sugai M, et al. Organizer‐like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol. 2008;181(9):6189‐6200. [DOI] [PubMed] [Google Scholar]
- 24. Magri G, Miyajima M, Bascones S, et al. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat Immunol. 2014;15(4):354‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cordeiro OG, Chypre M, Brouard N, et al. Integrin‐alpha IIb identifies murine lymph node lymphatic endothelial cells responsive to RANKL. PLoS ONE. 2016;11(3):e0151848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fasnacht N, Huang HY, Koch U, et al. Specific fibroblastic niches in secondary lymphoid organs orchestrate distinct Notch‐regulated immune responses. J Exp Med. 2014;211(11):2265‐2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Habbeddine M, Verthuy C, Rastoin O, et al. Receptor activator of NF‐kappaB orchestrates activation of antiviral memory CD8 T cells in the spleen marginal zone. Cell Rep. 2017;21(9):2515‐2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph‐borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192(10):1425‐1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nolte MA, Belien JA, Schadee‐Eestermans I, et al. A conduit system distributes chemokines and small blood‐borne molecules through the splenic white pulp. J Exp Med. 2003;198(3):505‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph‐borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192(10):1425‐1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thierry GR, Kuka M, De Giovanni M, et al. The conduit system exports locally secreted IgM from lymph nodes. J Exp Med. 2018;215(12):2972‐2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prados A, Kollias G, Koliaraki V. CollagenVI‐Cre mice: a new tool to target stromal cells in secondary lymphoid organs. Sci Rep. 2016;6:33027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bajenoff M, Egen JG, Koo LY, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25(6):989‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Acton SE, Astarita JL, Malhotra D, et al. Podoplanin‐rich stromal networks induce dendritic cell motility via activation of the C‐type lectin receptor CLEC‐2. Immunity. 2012;37(2):276‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Onder L, Narang P, Scandella E, et al. IL‐7‐producing stromal cells are critical for lymph node remodeling. Blood. 2012;120(24):4675‐4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iolyeva M, Aebischer D, Proulx ST, et al. Interleukin‐7 is produced by afferent lymphatic vessels and supports lymphatic drainage. Blood. 2013;122(13):2271‐2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87(6):1037‐1047. [DOI] [PubMed] [Google Scholar]
- 38. Tew JG, Phipps RP, Mandel TE. The maintenance and regulation of the humoral immune response: persisting antigen and the role of follicular antigen‐binding dendritic cells as accessory cells. Immunol Rev. 1980;53:175‐201. [DOI] [PubMed] [Google Scholar]
- 39. Krautler NJ, Kana V, Kranich J, et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell. 2012;150(1):194‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aguzzi A, Kranich J, Krautler NJ. Follicular dendritic cells: origin, phenotype, and function in health and disease. Trends Immunol. 2014;35(3):105‐113. [DOI] [PubMed] [Google Scholar]
- 41. Allen CD, Okada T, Cyster JG. Germinal‐center organization and cellular dynamics. Immunity. 2007;27(2):190‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang X, Cho B, Suzuki K, et al. Follicular dendritic cells help establish follicle identity and promote B cell retention in germinal centers. J Exp Med. 2011;208(12):2497‐2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang Y, Tech L, George LA, et al. Plasma cell output from germinal centers is regulated by signals from Tfh and stromal cells. J Exp Med. 2018;215(4):1227‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cremasco V, Woodruff MC, Onder L, et al. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat Immunol. 2014;15(10):973‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang HY, Rivas‐Caicedo A, Renevey F, et al. Identification of a new subset of lymph node stromal cells involved in regulating plasma cell homeostasis. Proc Natl Acad Sci USA. 2018;115(29):E6826‐E6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Randall TD, Carragher DM, Rangel‐Moreno J. Development of secondary lymphoid organs. Annu Rev Immunol. 2008;26:627‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Benezech C, Luu NT, Walker JA, et al. Inflammation‐induced formation of fat‐associated lymphoid clusters. Nat Immunol. 2015;16(8):819‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Taylor RT, Lugering A, Newell KA, Williams IR. Intestinal cryptopatch formation in mice requires lymphotoxin alpha and the lymphotoxin beta receptor. J Immunol. 2004;173(12):7183‐7189. [DOI] [PubMed] [Google Scholar]
- 49. Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid‐like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014;14(7):447‐462. [DOI] [PubMed] [Google Scholar]
- 50. Rangel‐Moreno J, Carragher DM, Garcia‐Hernandez dlL, et al. The development of inducible bronchus‐associated lymphoid tissue depends on IL‐17. Nat Immunol. 2011;12(7):639‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kuroda E, Ozasa K, Temizoz B, et al. Inhaled fine particles induce alveolar macrophage death and interleukin‐1alpha release to promote inducible bronchus‐associated lymphoid tissue formation. Immunity. 2016;45(6):1299‐1310. [DOI] [PubMed] [Google Scholar]
- 52. Barone F, Nayar S, Campos J, et al. IL‐22 regulates lymphoid chemokine production and assembly of tertiary lymphoid organs. Proc Natl Acad Sci USA. 2015;112(35):11024‐11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pikor NB, Astarita JL, Summers‐Deluca L, et al. Integration of Th17‐ and lymphotoxin‐derived signals initiates meningeal‐resident stromal cell remodeling to propagate neuroinflammation. Immunity. 2015;43(6):1160‐1173. [DOI] [PubMed] [Google Scholar]
- 54. Rangel‐Moreno J, Moyron‐Quiroz JE, Carragher DM, et al. Omental milky spots develop in the absence of lymphoid tissue‐inducer cells and support B and T cell responses to peritoneal antigens. Immunity. 2009;30(5):731‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue‐associated c‐Kit(+)Sca‐1(+) lymphoid cells. Nature. 2010;463(7280):540‐544. [DOI] [PubMed] [Google Scholar]
- 56. Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595‐621. [DOI] [PubMed] [Google Scholar]
- 57. Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16(1):67‐76. [DOI] [PubMed] [Google Scholar]
- 58. Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012;33(6):297‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Martinet L, Filleron T, Le Guellec S, Rochaix P, Garrido I, Girard JP. High endothelial venule blood vessels for tumor‐infiltrating lymphocytes are associated with lymphotoxin beta‐producing dendritic cells in human breast cancer. J Immunol. 2013;191(4):2001‐2008. [DOI] [PubMed] [Google Scholar]
- 60. Germain C, Gnjatic S, Tamzalit F, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189(7):832‐844. [DOI] [PubMed] [Google Scholar]
- 61. Wirsing AM, Rikardsen OG, Steigen SE, Uhlin‐Hansen L, Hadler‐Olsen E. Characterisation and prognostic value of tertiary lymphoid structures in oral squamous cell carcinoma. BMC Clin Pathol. 2014;14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Behr DS, Peitsch WK, Hametner C, et al. Prognostic value of immune cell infiltration, tertiary lymphoid structures and PD‐L1 expression in Merkel cell carcinomas. Int J Clin Exp Pathol. 2014;7(11):7610‐7621. [PMC free article] [PubMed] [Google Scholar]
- 63. Martinet L, Le Guellec S, Filleron T, et al. High endothelial venules (HEVs) in human melanoma lesions: major gateways for tumor‐infiltrating lymphocytes. Oncoimmunology. 2012;1(6):829‐839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Silina K, Soltermann A, Attar FM, et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res. 2018;78(5):1308‐1320. [DOI] [PubMed] [Google Scholar]
- 65. Posch F, Silina K, Leibl S, et al. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology. 2018;7(2):e1378844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fleige H, Ravens S, Moschovakis GL, et al. IL‐17‐induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J Exp Med. 2014;211(4):643‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fleige H, Haas JD, Stahl FR, Willenzon S, Prinz I, Forster R. Induction of BALT in the absence of IL‐17. Nat Immunol. 2011;13(1):1. [DOI] [PubMed] [Google Scholar]
- 68. Eberl G. From induced to programmed lymphoid tissues: the long road to preempt pathogens. Trends Immunol. 2007;28(10):423‐428. [DOI] [PubMed] [Google Scholar]
- 69. Junt T, Moseman EA, Iannacone M, et al. Subcapsular sinus macrophages in lymph nodes clear lymph‐borne viruses and present them to antiviral B cells. Nature. 2007;450(7166):110‐114. [DOI] [PubMed] [Google Scholar]
- 70. Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage‐rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27(1):160‐171. [DOI] [PubMed] [Google Scholar]
- 71. Gaya M, Castello A, Montaner B, et al. Inflammation‐induced disruption of SCS macrophages impairs B cell responses to secondary infection. Science. 2015;347(6222):667‐672. [DOI] [PubMed] [Google Scholar]
- 72. Honke N, Shaabani N, Cadeddu G, et al. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat Immunol. 2011;13(1):51‐57. [DOI] [PubMed] [Google Scholar]
- 73. Gerner MY, Casey KA, Kastenmuller W, Germain RN. Dendritic cell and antigen dispersal landscapes regulate T cell immunity. J Exp Med. 2017;214(10):3105‐3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Novkovic M, Onder L, Cupovic J, et al. Topological small‐world organization of the fibroblastic reticular cell network determines lymph node functionality. PLoS Biol. 2016;14(7):e1002515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Soudja SM, Henri S, Mello M, et al. Disrupted lymph node and splenic stroma in mice with induced inflammatory melanomas is associated with impaired recruitment of T and dendritic cells. PLoS ONE. 2011;6(7):e22639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Takeda A, Kobayashi D, Aoi K, et al. Fibroblastic reticular cell‐derived lysophosphatidic acid regulates confined intranodal T‐cell motility. eLife. 2016;5:e10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gregory JL, Walter A, Alexandre YO, et al. Infection programs sustained lymphoid stromal cell responses and shapes lymph node remodeling upon secondary challenge. Cell Rep. 2017;18(2):406‐418. [DOI] [PubMed] [Google Scholar]
- 78. El Shikh ME, El Sayed RM, Wu Y, Szakal AK, Tew JG. TLR4 on follicular dendritic cells: an activation pathway that promotes accessory activity. J Immunol. 2007;179(7):4444‐4450. [DOI] [PubMed] [Google Scholar]
- 79. Garin A, Meyer‐Hermann M, Contie M, et al. Toll‐like receptor 4 signaling by follicular dendritic cells is pivotal for germinal center onset and affinity maturation. Immunity. 2010;33(1):84‐95. [DOI] [PubMed] [Google Scholar]
- 80. Kim SH, Kim YN, Jang YS. Cutting edge: LL‐37‐mediated formyl peptide receptor‐2 signaling in follicular dendritic cells contributes to B cell activation in peyer's patch germinal centers. J Immunol. 2017;198(2):629‐633. [DOI] [PubMed] [Google Scholar]
- 81. Mueller SN, Matloubian M, Clemens DM, et al. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc Natl Acad Sci USA. 2007;104(39):15430‐15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Schilte C, Couderc T, Chretien F, et al. Type I IFN controls chikungunya virus via its action on nonhematopoietic cells. J Exp Med. 2010;207(2):429‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Davis KJ, Anderson AO, Geisbert TW, et al. Pathology of experimental Ebola virus infection in African green monkeys. Involvement of fibroblastic reticular cells. Arch Pathol Lab Med. 1997;121(8):805‐819. [PubMed] [Google Scholar]
- 84. Das A, Heesters BA, Bialas A, et al. Follicular dendritic cell activation by TLR ligands promotes autoreactive B cell responses. Immunity. 2017;46(1):106‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Colonna M. TLR pathways and IFN‐regulatory factors: to each its own. Eur J Immunol. 2007;37(2):306‐309. [DOI] [PubMed] [Google Scholar]
- 86. Barchet W, Cella M, Odermatt B, sselin‐Paturel C, Colonna M, Kalinke U. Virus‐induced interferon alpha production by a dendritic cell subset in the absence of feedback signaling in vivo. J Exp Med. 2002;195(4):507‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Farrell HE, Bruce K, Lawler C, Cardin RD, Davis‐Poynter NJ, Stevenson PG. Type 1 interferons and NK cells limit murine cytomegalovirus escape from the lymph node subcapsular sinus. PLoS Pathog. 2016;12(12):e1006069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bar‐Ephraim YE, Konijn T, Gonultas M, Mebius RE, Reijmers RM. A reproducible method for isolation and in vitro culture of functional human lymphoid stromal cells from tonsils. PLoS ONE. 2016;11(12):e0167555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kallert SM, Darbre S, Bonilla WV, et al. Replicating viral vector platform exploits alarmin signals for potent CD8 + T cell‐mediated tumour immunotherapy. Nat Commun. 2017;8:15327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Groom JR, Richmond J, Murooka TT, et al. CXCR3 chemokine receptor‐ligand interactions in the lymph node optimize CD4 + T helper 1 cell differentiation. Immunity. 2012;37(6):1091‐1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Onder L, Ludewig B. A fresh view on lymph node organogenesis. Trends Immunol. 2018;39(10):775‐787. [DOI] [PubMed] [Google Scholar]
- 92. Tumanov AV, Grivennikov SI, Kruglov AA, et al. Cellular source and molecular form of TNF specify its distinct functions in organization of secondary lymphoid organs. Blood. 2010;116(18):3456‐3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bekiaris V, Withers D, Glanville SH, et al. Role of CD30 in B/T segregation in the spleen. J Immunol. 2007;179(11):7535‐7543. [DOI] [PubMed] [Google Scholar]
- 94. Disson O, Bleriot C, Jacob JM, et al. Peyer's patch myeloid cells infection by Listeria signals through gp38(+) stromal cells and locks intestinal villus invasion. J Exp Med. 2018;215(11):2936‐2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lee JW, Epardaud M, Sun J, et al. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8(2):181‐190. [DOI] [PubMed] [Google Scholar]
- 96. Lukacs‐Kornek V, Malhotra D, Fletcher AL, et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol. 2011;12(11):1096‐1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Siegert S, Huang HY, Yang CY, et al. Fibroblastic reticular cells from lymph nodes attenuate T cell expansion by producing nitric oxide. PLoS ONE. 2011;6(11):e27618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Coombes JL, Siddiqui KR, Arancibia‐Carcamo CV, et al. A functionally specialized population of mucosal CD103 + DCs induces Foxp3 + regulatory T cells via a TGF‐beta and retinoic acid‐dependent mechanism. J Exp Med. 2007;204(8):1757‐1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11(1):7‐13. [DOI] [PubMed] [Google Scholar]
- 100. Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517(7534):293‐301. [DOI] [PubMed] [Google Scholar]
- 101. Cui G, Hara T, Simmons S, et al. Characterization of the IL‐15 niche in primary and secondary lymphoid organs in vivo. Proc Natl Acad Sci USA. 2014;111(5):1915‐1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Withers DR. Innate lymphoid cell regulation of adaptive immunity. Immunology. 2016;149(2):123‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mackley EC, Houston S, Marriott CL, et al. CCR7‐dependent trafficking of RORgamma(+) ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nat Commun. 2015;6:5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cruz‐Migoni S, Caamano J. Fat‐associated lymphoid clusters in inflammation and immunity. Front Immunol. 2016;7:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hodel C. Ultrastructural studies on the absorption of protein markers by the greater omentum. Eur Surg Res. 1970;2(6):435‐449. [DOI] [PubMed] [Google Scholar]
- 106. Ha SA, Tsuji M, Suzuki K, et al. Regulation of B1 cell migration by signals through Toll‐like receptors. J Exp Med. 2006;203(11):2541‐2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Schwartz JT, Barker JH, Long ME, Kaufman J, McCracken J, Allen LA. Natural IgM mediates complement‐dependent uptake of Francisella tularensis by human neutrophils via complement receptors 1 and 3 in nonimmune serum. J Immunol. 2012;189(6):3064‐3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gray KS, Collins CM, Speck SH. Characterization of omental immune aggregates during establishment of a latent gammaherpesvirus infection. PLoS ONE. 2012;7(8):e43196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Berberich S, Dahne S, Schippers A, et al. Differential molecular and anatomical basis for B cell migration into the peritoneal cavity and omental milky spots. J Immunol. 2008;180(4):2196‐2203. [DOI] [PubMed] [Google Scholar]
- 110. Buscher K, Wang H, Zhang X, et al. Protection from septic peritonitis by rapid neutrophil recruitment through omental high endothelial venules. Nat Commun. 2016;7:10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nishimura S, Manabe I, Takaki S, et al. Adipose natural regulatory B cells negatively control adipose tissue inflammation. Cell Metab. 2013;18:759‐766. [DOI] [PubMed] [Google Scholar]
- 112. Bapat SP, Myoung Suh J, Fang S, et al. Depletion of fat‐resident Treg cells prevents age‐associated insulin resistance. Nature. 2015;528(7580):137‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Vasanthakumar A, Moro K, Xin A, et al. The transcriptional regulators IRF4, BATF and IL‐33 orchestrate development and maintenance of adipose tissue‐resident regulatory T cells. Nat Immunol. 2015;16(3):276‐285. [DOI] [PubMed] [Google Scholar]
- 114. Jackson‐Jones LH, Duncan SM, Magalhaes MS, et al. Fat‐associated lymphoid clusters control local IgM secretion during pleural infection and lung inflammation. Nat Commun. 2016;7:12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kohlgruber AC, Gal‐Oz ST, LaMarche NM, et al. gammadelta T cells producing interleukin‐17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat Immunol. 2018;19(5):464‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kinchen J, Chen HH, Parikh K, et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell. 2018;175(2):372‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Shi K, Hayashida K, Kaneko M, et al. Lymphoid chemokine B cell‐attracting chemokine‐1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J Immunol. 2001;166(1):650‐655. [DOI] [PubMed] [Google Scholar]
- 118. Humby F, Bombardieri M, Manzo A, et al. Ectopic lymphoid structures support ongoing production of class‐switched autoantibodies in rheumatoid synovium. PLoS Med. 2009;6(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Barone F, Bombardieri M, Rosado MM, et al. CXCL13, CCL21, and CXCL12 expression in salivary glands of patients with Sjogren's syndrome and MALT lymphoma: association with reactive and malignant areas of lymphoid organization. J Immunol. 2008;180(7):5130‐5140. [DOI] [PubMed] [Google Scholar]
- 120. Kester L, van Oudenaarden A. Single‐cell transcriptomics meets lineage tracing. Cell Stem Cell. 2018;23(2):166‐179. [DOI] [PubMed] [Google Scholar]


