Summary
Background
Candida auris is a globally emerging yeast, causing severe infections in patients with underlying diseases. This yeast is responsible for several outbreaks within healthcare facilities, where it can be found on hospital surfaces and patient care devices. Spread from these fomites may be prevented by improving the decontamination of hospital surfaces. UV‐C decontamination may constitute an effective adjunct to routine room cleaning.
Objectives
Our aim was to investigate the effect of different UV‐C exposure times and distance in killing C auris, using strains from different countries.
Methods
Candida auris was seeded on glass slides and exposed to UV‐C for 5, 10, 20 and 30 minutes at 2 and 4 m.
Results
A maximal effect of C auris killing was found after 30 minutes of UV‐C exposure at 2 m. With half the time or twice the distance, the efficacy strongly diminished to ~10 and ~50 fold, respectively. At suboptimal exposure times and distances, the C auris strains from Japan/Korea were more sensitive to UV‐C killing than C auris strains originating from Venezuela, Spain and India.
Conclusions
Altogether, UV‐C exposure times and distance are the most critical parameters to kill C auris, while strain variations of C auris also determine UV‐C efficacy. Future studies should aim to determine the effect and place of UV‐C on surface decontamination in hospital setting.
Keywords: Candida auris, decontamination, distance, exposure time, outbreak, ultraviolet‐C, yeast
1. INTRODUCTION
Candida auris is a globally emerging yeast, only recognised in the last 10 years causing severe infections.1 This yeast has been reported to cause hospital outbreaks in various healthcare facilities across the globe.2, 3, 4, 5 Skin colonisation and inanimate surface contamination in close vicinity of infected and colonised patients is likely an important factor in patient to patient transmission.6, 7 Rapid identification of C auris, skin decolonisation of patients, and decontamination of hospital surfaces are essential steps in controlling C auris outbreaks.
Although there are no established guidelines for decontaminating surfaces contaminated by C auris, healthcare organisations have issued different recommendations. The Center for Disease Control and Prevention (CDC) suggests the use of Environmental Protection Agency (EPA)‐registered hospital grade disinfectant effective against Clostridium difficile spores, while Public Health England (PHE) suggests the use of hypochloride, possibly in conjunction with other products.8 These recommendations have been based on an increasing number of studies, which demonstrated that such agents are indeed effective in vitro as well as during clinical care.3, 7, 9, 10, 11, 12 In addition, in vitro studies demonstrated the effectiveness of hydrogen peroxide vapour.7, 9, 10
Another approach in the decontamination of hospital surfaces is the use of mobile ultraviolet‐C (UV‐C) devices, which are broadly applied in some countries.13 PHE also recommends UV‐C as a potential adjunct in controlling the spread of C auris, although there is no clinical evidence for its efficacy. A single in vitro study by Cadnum et al14 demonstrated that 10 or 30 minutes UV‐C led to a 40 to 1 x 106‐fold reduction, respectively, in colony‐forming units, demonstrating the importance of time as an essential factor in UV‐C efficacy. The samples were placed 1.5 m from the UV‐C device. In most hospital rooms, the radius is larger than 1.5 m, requiring the UV‐C device to be relocated after a first exposure cycle in order to cover the whole room.14 To determine the effect of distance on C auris decontamination with UV‐C, we implemented a sample distance of 2 and 4 m, which better reflects the clinical situation. In addition, we tested the effect of UV‐C exposure time and the UV‐C sensitivity of C auris strains from different worldwide clades and origins.
2. MATERIALS AND METHODS
2.1. Candida auris and Candida albicans strains
In the present study, clinical C auris strains originating from Venezuela (n = 3),15 Spain (n = 3),4 India (n = 3)16 and Japan/Korea (KCTC 17809, KCTC 17810 and JCM 15448) were used, along with C albicans strains ATCC 90028, ATCC 10231 and ATCC 24433.
2.2. UV‐C decontamination device
The UV‐360 Room Sanitiser (UltraViolet Devices, Inc., Valencia, CA) device was used. It is a four‐wheeled unit containing four vertically placed maximum output UV Germicidal lamps that are 158 cm tall and emit light of predominantly 254 nm in 360° (Figure 1). The system also contains four motion sensors, which abort the cycle if someone enters the room during use.
Figure 1.
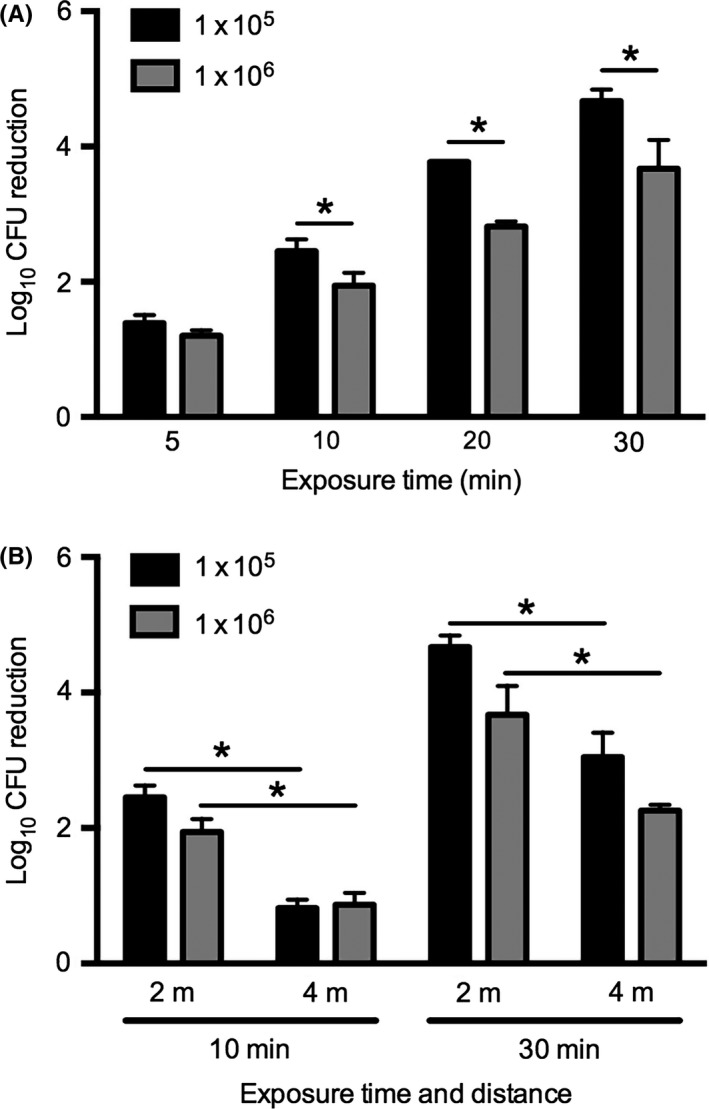
The effect of UV‐C exposure time and distance on killing Candida auris. A C auris isolate was seeded on multitest slide glasses at a density of 1 x 105 or 1 x 106 CFU per well. Subsequently, glasses were exposed for 5, 10, 20 or 30 min to UV‐C at a distance of 2 m (A) or for 10 and 30 min at 2 and 4 m (B). Significant differences (P < 0.05) are indicated with an asterisk
2.3. Killing Candida yeasts with UV‐C
Strains were suspended in 500 μL phosphate‐buffered saline (PBS, Diasorin Molecular LCC, CA), and yeast quantity was determined using the Genesys 20 Spectrophotometer (ThermoSpectronic, UK). Subsequently, 10 μL of suspension with 1 x 105 or 1 x 106 colony‐forming units (CFU) was spread to cover 50 mm2 wells of multitest glass slides (MP Biomedicals, LCC, Illkirch, France). These were placed in petri dishes and allowed to air dry for 30‐40 minutes in incubator at 35°C. Subsequently, the multitest slides were transferred to a standard laboratory room (3 × 5 m) and placed on a table with a standard height of 90 cm at a distance of 2 or 4 m from the UV‐360 Room Sanitiser. Thus, the samples were perpendicular (horizontal) to the UV‐C source. The glass slides with Candida cells were directly exposed to a UV‐C cycle of 5, 10, 20 or 30 minutes or incubated for the same time without exposure to UV‐C. After exposure, yeast were suspended in 100 μL PBS for 1 minute and the suspension was further diluted in PBS. Finally, 100 μL of the diluted suspension was transferred to Sabouraud dextrose agar plates (Tritium Microbiologie BV, Eindhoven, The Netherlands) and incubated at 35°C for 24‐48 hours for colony count determination. Log reductions were calculated in comparison with unexposed samples. Experiments were performed in triplicate.
2.4. Statistical analysis
A 1‐ or 2‐way ANOVA with Bonferroni's multiple comparisons test was used to compare CFU of non‐exposed isolates and mean log reductions, respectively. Data were analysed using GraphPad Prism 6.2 (GraphPad Software, Inc., San Diego, CA).
3. RESULTS
To determine the effect of UV‐C on the decontamination of surfaces with C auris, we first investigated whether UV‐C exposure time and C auris seeding density affected UV‐C efficacy using a single C auris strain. After seeding C auris on glass well plates at two different densities, these were placed at 2 m exposure distance from the UV‐C device and exposed for 5, 10, 20 and 30 minutes. A strong time‐dependent reduction of C auris CFU was observed, which was significantly different (P < 0.05) for all time points for each density (Figure 2A). At 10, 20 and 30 minutes, CFU reduction was significantly higher in C auris inoculated at a density of 1 x 105 as compared to those seeded at 1 x 106 CFU (Figure 2A). Subsequently, the effect of distance was studied. Increasing the distance to the UV‐C source from 2 to 4 m strongly reduced the efficacy of UV‐C to kill C auris (Figure 2B).
Figure 2.
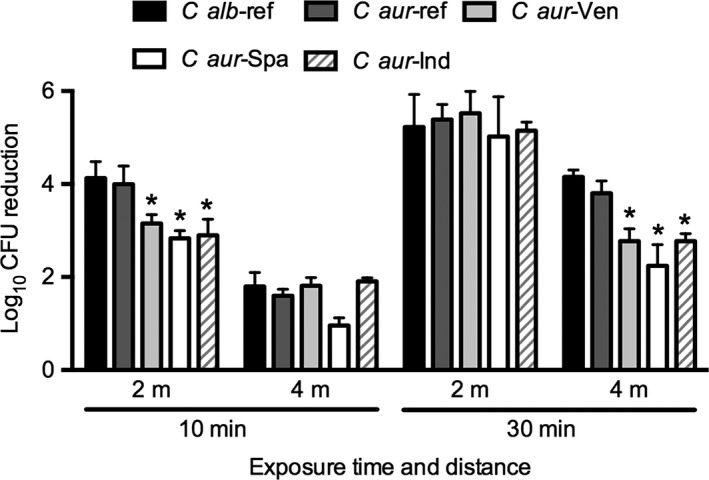
UV‐C efficacy in killing Candida auris strains from different countries. Candida albicans and C auris isolates from different countries were seeded on multitest slide glasses at a density of 1 x 105 CFU per well. Glasses were exposed for 10 or 30 min to UV‐C at a distance of 2 or 4 m. Significant differences (P < 0.05) as compared to C auris reference strains are indicated with an asterisk. Ref, reference; Ven, Venezuela; Spa, Spain; Ind, india
Additionally, we investigated the sensitivity of C auris strains originating from different countries to UV‐C and compared this to the sensitivity of C albicans reference strains. For this experiment, we used the low density of 1 x 105, as this count better reflects the clinical situation. With a UV‐C exposure time of 10 minutes at 2‐metre distance, we found a decreased efficacy of UV‐C to kill C auris strains originating from Venezuela, Spain and India as compared to the C auris strains from Japan/Korea. The latter C auris strains were similar to C albicans (Figure 3). Similar results were obtained for the UV‐C exposure time of 30 minutes at a distance of 4 m, while for the other exposure conditions there were no significant differences among the strains (Figure 3).
Figure 3.

UV‐360 Room Sanitiser (UltraViolet Devices, Inc., Valencia, CA)
4. DISCUSSION
The present study demonstrates that C auris can be effectively killed by UV‐C, although the density of C auris, the time of UV‐C exposure and the distance to the UV source strongly influenced the effectivity of UV‐C treatment. With a 10‐fold higher concentration of C auris, the effectivity of UV‐C treatment diminished around 10‐fold when exposed for 20 or 30 minutes UV‐C radiation. Time and distance were even more important parameters, as with a 2‐fold increase in time or decrease in distance, respectively, and ~10‐ and ~50‐fold reductions in CFU were found. A maximal effect of UV‐C was reached with 30 minutes exposures with UV‐C and when surfaces were at 2 m of the UV‐C device. Cadnum et al14 also demonstrated that 30 minutes UV‐C exposure is required to reach a maximal effect of C auris reduction while placing the specimen at 1.5 m from the UV‐C device. A recent report from Ponnachan et al17 found that 15 minutes of exposure killed all C auris. It has to be noted though that in the latter study the standard sample distance to UV‐C light was only 1 m, while the samples were directly facing the UV‐C lamp. Facing samples in parallel directly to the UV‐C source led to a >10‐fold higher effectivity in killing methicillin‐resistant Staphylococcus aureus (MRSA) and a ~5‐fold higher effectivity to kill Clostridium difficile, as compared to placing them horizontally to the UV‐C lamp.18 As the minimum distance of sample to UV‐C source in our study was 2 m, while the samples were placed horizontally to the UV‐C source, the previous studies14, 17 are largely in agreement with our findings regarding the effect of time and distance in killing C auris with UV‐C.
The report of Cadnum et al14 found that the sensitivity of C auris for UV‐C in a laboratory setting appeared to be similar to C difficile. A 10 minutes UV‐C cycle at 1.5 m was not sufficient to kill all C auris nor all C difficile, which was in contrast to MRSA that was effectively killed under these conditions. The effect of UV‐C on C auris killing has—to our knowledge—not yet been investigated in the clinical setting, while there have been different hospital and patient studies on the effect of UV‐C on C difficile surface contamination and hospital‐acquired infection rates. In agreement with the relative resistance for UV‐C in the laboratory setting, the contamination of high‐touch surfaces with pulsed xenon UV was less efficient on C difficile as compared to MRSA.19 Interestingly, several large patient studies investigating the effect of UV‐C on hospital‐acquired C difficile infection rate demonstrated that the application of two UV‐C cycles in the patient room and one in the bathroom with 5 or 6 minutes per cycle reduced hospital‐acquired infection rate for C difficile by 41% (P = 0.01)20 and 17% (P = 0.02).21 Thus, despite the fact that the laboratory settings suggest that a total UV‐C exposure time of 10 minutes is not sufficient to kill all C difficile, in the clinical setting a total UV‐C exposure time of 10‐12 minutes leads to reduced C difficile infection rates. Moreover, the effect of UV‐C on hospital‐acquired infection rates for MRSA, which is much more sensitive to UV‐C than C difficile, seemed equally or even less efficient in several studies.20, 21, 22 Thus, despite differences in UV‐C sensitivity to kill pathogens in the laboratory or clinical setting, the actual hospital‐acquired infection rate might differ, demonstrating the importance to identify the effect of UV‐C on hospital‐acquired C auris infection rates.
Altogether, our findings demonstrate that, with a longer cycle time, and at an optimised distance, C auris can successfully be decontaminated by UV‐C. Future studies in hospitals struggling with endemic C auris presence should further investigate the effect of UV‐C under routine conditions in clinical practice.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Acquisition, analysis and interpretation of the data: TG, AC, JM, AV. Statistical Analysis: TG. Drafted the manuscript: TG. Reviewed and modified the manuscript: TG, AC, JM, AV.
de Groot T, Chowdhary A, Meis JF, Voss A. Killing of Candida auris by UV‐C: Importance of exposure time and distance. Mycoses. 2019;62:408–412. 10.1111/myc.12903
REFERENCES
- 1. Saris K, Meis JF, Voss A. Candida auris . Curr Opin Infect Dis. 2018;31(4):334‐340. [DOI] [PubMed] [Google Scholar]
- 2. Eyre DW, Sheppard AE, Madder H, et al. A Candida auris outbreak and its control in an intensive care setting. N Engl J Med. 2018;379(14):1322‐1331. [DOI] [PubMed] [Google Scholar]
- 3. Schelenz S, Hagen F, Rhodes JL, et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control. 2016;5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruiz‐Gaitan A, Moret AM, Tasias‐Pitarch M, et al. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses. 2018;61(7):498‐505. [DOI] [PubMed] [Google Scholar]
- 5. Chow NAGL, Gade L, Tsay SV, et al. Multiple introductions and subsequent transmission of multidrug‐resistant Candida auris in the USA: a molecular epidemiological survey. Lancet Infect Dis. 2018;18:30597‐30598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chowdhary A, Voss A, Meis JF. Multidrug‐resistant Candida auris: ‘new kid on the block’ in hospital‐associated infections? J Hosp Infect. 2016;94(3):209‐212. [DOI] [PubMed] [Google Scholar]
- 7. Biswal M, Rudramurthy SM, Jain N, et al. Controlling a possible outbreak of Candida auris infection: lessons learnt from multiple interventions. J Hosp Infect. 2017;97(4):363‐370. [DOI] [PubMed] [Google Scholar]
- 8. Ku TSN, Walraven CJ, Lee SA. Candida auris: disinfectants and implications for infection control. Front Microbiol. 2018;9:726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cadnum JL, Shaikh AA, Piedrahita CT, et al. Effectiveness of disinfectants against Candida auris and other Candida species. Infect Control Hosp Epidemiol. 2017;38(10):1240‐1243. [DOI] [PubMed] [Google Scholar]
- 10. Abdolrasouli A, Armstrong‐James D, Ryan L, Schelenz S. In vitro efficacy of disinfectants utilised for skin decolonisation and environmental decontamination during a hospital outbreak with Candida auris . Mycoses. 2017;60(11):758‐763. [DOI] [PubMed] [Google Scholar]
- 11. Moore G, Schelenz S, Borman AM, Johnson EM, Brown CS. Yeasticidal activity of chemical disinfectants and antiseptics against Candida auris . J Hosp Infect. 2017;97(4):371‐375. [DOI] [PubMed] [Google Scholar]
- 12. Kean R, Sherry L, Townsend E, et al. Surface disinfection challenges for Candida auris: an in‐vitro study. J Hosp Infect. 2018;98(4):433‐436. [DOI] [PubMed] [Google Scholar]
- 13. Health Quality Ontario . Portable ultraviolet light surface‐disinfecting devices for prevention of hospital‐acquired infections: a health technology assessment. Ont Health Technol Assess Ser. 2018;18(1):405‐73. [PMC free article] [PubMed] [Google Scholar]
- 14. Cadnum JL, Shaikh AA, Piedrahita CT, et al. Relative resistance of the emerging fungal pathogen Candida auris and other Candida species to killing by ultraviolet light. Infect Control Hosp Epidemiol. 2018;39(1):94‐96. [DOI] [PubMed] [Google Scholar]
- 15. Calvo B, Melo AS, Perozo‐Mena A, et al. First report of Candida auris in America: clinical and microbiological aspects of 18 episodes of candidemia. J Infect. 2016;73(4):369‐374. [DOI] [PubMed] [Google Scholar]
- 16. Chowdhary A, Sharma C, Duggal S, et al. New clonal strain of Candida auris, Delhi, India. Emerg Infect Dis. 2013;19(10):1670‐1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ponnachan P, Vinod V, Pullanhi U, et al. Antifungal activity of octenidine dihydrochloride and ultraviolet‐C light against multidrug‐resistant Candida auris . J Hosp Infect. 2018. 10.1016/j.jhin.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 18. Cadnum JL, Tomas ME, Sankar T, et al. Effect of variation in test methods on performance of ultraviolet‐C radiation room decontamination. Infect Control Hosp Epidemiol. 2016;37(5):555‐560. [DOI] [PubMed] [Google Scholar]
- 19. Nerandzic MM, Cadnum JL, Pultz MJ, Donskey CJ. Evaluation of an automated ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare‐associated pathogens in hospital rooms. BMC Infect Dis 2010;10:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vianna PG, Dale CR Jr, Simmons S, Stibich M, Licitra CM. Impact of pulsed xenon ultraviolet light on hospital‐acquired infection rates in a community hospital. Am J Infect Control. 2016;44(3):299‐303. [DOI] [PubMed] [Google Scholar]
- 21. Haas JP, Menz J, Dusza S, Montecalvo MA. Implementation and impact of ultraviolet environmental disinfection in an acute care setting. Am J Infect Control. 2014;42(6):586‐590. [DOI] [PubMed] [Google Scholar]
- 22. Napolitano NA, Mahapatra T, Tang W. The effectiveness of UV‐C radiation for facility‐wide environmental disinfection to reduce health care‐acquired infections. Am J Infect Control. 2015;43(12):1342‐1346. [DOI] [PubMed] [Google Scholar]


