Abstract
Although partial thickness burns are the most frequently reported burn injuries, there is no consensus on the optimal treatment. The objective of this study was to compare the clinical effectiveness and scar quality of Flaminal® Forte to silver sulfadiazine (Flamazine®) in the treatment of partial thickness burns. In this two‐arm open label multicenter randomized controlled trial, adult patients with acute partial thickness burns and an affected total body surface area of less than 30% were randomized between Flaminal® Forte and Flamazine® and followed for 12 months. Dressing changes in the Flamazine® group were performed daily, and in the Flaminal® group during the first 3 days post burn and thereafter every other day until complete wound healing or surgery. Forty‐one patients were randomly allocated to Flaminal® Forte and 48 patients to Flamazine®. The primary outcome was time to wound healing, which did not differ between the groups: median 18 days with Flaminal® Forte (range 8–49 days) versus 16 days with Flamazine® (range 7–48 days; p = 0.24). Regarding the secondary outcomes during hospital admission, there were no statistically significant differences between the groups concerning need for surgery, pain scores, pruritus, or pain‐related and anticipatory anxiety. More patients in the Flaminal® group developed wound colonization (78% versus 32%, p < 0.001), but the treatment groups did not differ regarding the incidence of local infections and use of systemic antibiotics. In terms of scar quality, no statistically significant differences between both treatment groups were found regarding subjective scar assessment (Patient and Observer Scar Assessment Scale (POSAS)), scar melanin and pigmentation (DermaSpectrometer®), and scar elasticity and maximal extension (Cutometer®) during 12 month postburn. In conclusion, time to wound healing did not differ, but the use of Flaminal® Forte seemed favorable because less dressing changes are needed which lowers the burden of wound care.
Abbreviations
- BSPAS
The Burn Specific Pain Anxiety Scale
- LDI
Laser Doppler Imaging
- POSAS
Patient and Observer Scar Assessment Scale
- SSD
Silver sulfadiazine
- TBSA
Total body surface area
- Ue
Scar elasticity
- Uf
Scar maximal extension
- VAT
Visual Analogue Thermometer
Although various treatment modalities are available for partial thickness burns none of these are generally accepted as standard or optimal care.1 Since decades, silver sulfadiazine (SSD), such as Flamazine®, has been used for treatment of partial thickness burns.1, 2, 3, 4, 5 The widespread use of SSD may be explained by its broad antimicrobial effect in vitro.4, 6, 7 However, a Cochrane review of clinical studies showed that SSD does not prevent wound infection better than nonsilver containing comparators.8 Several studies have also shown considerable disadvantages of SSD despite its popularity. SSD is highly toxic to the wound bed, forms a pseudoeschar that can lead to bacterial proliferation and impaired wound assessment, requires daily dressing changes and is consistently associated with poorer wound healing of partial thickness burns compared to nonsilver treatments.1, 3, 9, 10, 11
To overcome the limitations of SSD, various local therapies have been developed. Several systematic reviews showed that in more than half of the studies that wound healing time was shorter with viscous dressings (e.g. Flammacerium®, honey based wound dressings, Silvazine®), solid dressings (e.g. Acticoat®, Aquacell®, Mepitel®, Biobrane®, and Trancyte®) and biologicals dressings (e.g. Xenoderm, Amnion) compared with SSD.1, 9, 12, 13, 14 However, only studies with honey based wound dressings showed consistently better results for wound infection compared with SSD.13 In general, solid dressings needed less dressing changes, while their application was found to be more difficult in some anatomical locations compared to SSD.12 These results should be interpreted in light of the paucity of high‐quality evidence, high risk of bias, limited number of included patients and unclear role of sponsorship in the majority of the included clinical trials. Therefore, no firm conclusion regarding the effectiveness of the studied local treatments of partial thickness burns can be drawn based on these systematic reviews.
In recent years, Flaminal® Forte (Flen Pharma, Kontich, Belgium) used for the treatment of burn wounds, has gained popularity, in particular because Flaminal® Forte does not requires daily dressing change. Flaminal® Forte is composed of hydrated alginate polymers with a biologic enzyme system that is based on glucose oxidase and lactoperoxidase stabilized by guaiacol. Due to its composition, Flaminal® Forte is expected to have an antimicrobial and continuous debriding effect.15, 16, 17 in vitro studies have shown that Flaminal® Forte is not toxic to keratinocytes and fibroblasts,15, 18 and that it reduces wound colonization by a wide range of Gram‐negative and Gram‐positive micro‐organisms.15, 18 However, one retrospective clinical study found significantly more bacterial growth in partial thickness burns when treated with Flaminal® compared to SSD.19 Furthermore, two retrospective studies showed faster wound healing when partial thickness burns were treated with Flaminal® compared to SSD.19, 20
To the best of our knowledge, there is a paucity of evidence for Flaminal® Forte in the treatment of partial thickness burns. Available evidence is based on retrospective studies with a limited number of studied patients and relevant outcomes. Despite the limitation of these studies, Flaminal® Forte might have advantages such as faster wound healing and less dressing changes compared to Flamazine®, while the preventing effect on wound colonization and infection remains unclear.
Therefore, we performed a multicenter randomized controlled clinical trial in which the clinical effects, quality of life and cost‐effectiveness of Flaminal® Forte and Flamazine® in the treatment of partial thickness burns were compared. This first part of the paper reports on the clinical effectiveness and scar quality of Flaminal® Forte and Flamazine® during the clinical treatment phase of partial thickness burns with a follow‐up of 12 months.
MATERIALS AND METHODS
Study design and randomization
In this investigator‐initiated, open label, multicenter, randomized controlled trial (RCT) we compared the clinical effectiveness of Flaminal® Forte versus Flamazine® in the treatment of partial thickness burns. An extensive description of the study protocol was published previously.21 The results are reported following the Consolidated Standards of Reporting Trials (CONSORT) guidelines.22 The study was conducted in compliance with the ethical rules for human experimentation that are stated in the 1975 Declaration of Helsinki and approved by the Medical Research Ethics Committee Noord‐Holland (NL43671.094.13). The study was registered in the European Clinical Trials Database (EudraCT number: 2013‐000901‐21) and the Netherlands Trial Registry (trial number 4486).
Patients
Patients were enrolled in this study from February 2014 until September 2015 in two burn centers in the Netherlands (Red Cross Hospital, Beverwijk and Maasstad Hospital, Rotterdam). In these burn centers, both Flaminal® and Flamazine® are already commonly used for treating partial thickness burns. Patients were eligible for the study if they had partial thickness burns of minimally 1% affected total body surface area (TBSA) based on clinical evaluation and Laser Doppler Imaging (possibly in combination with full thickness burns); were admitted to the hospital within 48 hours of the burn injury; were mentally competent or temporary incompetent (because of sedation and/or intubation) and provided written informed consent. The exclusion criteria were age < 18 years; TBSA of >30%; burns caused by chemicals, electricity or radiation; if local therapy had already started; or if the treating physician expected that the patient would not comply with the study protocol.
Study procedure and randomization
Either the local investigator or the on‐call burn physician/ ‐surgical resident informed the eligible patients about the study and randomized the participants after they had provided informed consent. If a patient was temporarily incompetent, a legal representative of the patient was informed about the study and provided informed consent. In these cases, informed consent was obtained from the patient as soon as possible. If these patients did not confirm the consent provided by their legal representative, they were withdrawn from the study. Their collected study data was deleted and the allocated treatment was continued as usual care.
Patients were randomly assigned to treatment with either Flamazine® or Flaminal® Forte, using the online randomization program TenALEA (Trans European Network for Clinical Trials Services). The randomization was stratified by center and used variably sized blocks in a 1:1 ratio. The patients and medical staff who provided the burn wound care could not be blinded because both treatments can be recognized by their appearance. Also, the observers could not be blinded because they were involved in the clinical care of the participants.
Interventions
The patients received treatment with either Flaminal® Forte (Glucose oxidase‐Lactoperoxidase Guaiacol complex of 50 g in 5.5% alginogel) manufactured by Flen Pharma, Belgum, or Flamazine® (containing silver sulfadiazine 10 mg/g in hydrophilic crème base) manufactured by Sinclair Pharmaceuticals, Surrey, United Kingdom.
Treatment with Flaminal® Forte consisted of cleaning and rinsing the burn wound with Prontosan® (containing 0.1% Polyaminopropyl Biguanide (Polihexanide), Betaine Surfactant and purified water) manufactured by B. Braun, Switzerland. Thereafter, a sufficiently thick layer (4–5 mm) of Flaminal® Forte was applied on a nonadhesive dressing and applied on the burn wound. A net bandage was used to keep the dressing in place. Dressings were changed daily during the first 3 days post burn and thereafter every other day until complete wound healing or surgery.
Treatment with Flamazine® also started with cleaning and rinsing the burn wound with Prontosan®, followed by application of Flamazine® on the burn wound and coverage with a net bandage to keep the dressing in place. This procedure was repeated once every 24 hours until the sixth day post burn. Thereafter, Furacine Soluble Dressing (Furacine 2 mg/g ointment) was applied on the burn wound on the even postburn days and Flamazine® on the odd postburn days until complete wound healing or operation. The alternation of treatment in this study arm was justified because of the cytotoxicity of the silver particles in Flamazine® in the wound bed when used continuously.
In case of wound colonization or infection, the treatment with either Flaminal® Forte or Flamazine® was changed to the relevant treatment based on the results of the wound culture. Treatment of colonized wounds required daily dressing changes, which could influence the number of daily dressing changes in both treatment groups. Need for split skin graft was evaluated between 10 and 14 days postburn. Partial thickness burn wounds that were not expected to heal within 21 days, were excised and skin grafted, as this leads to a lower risk of hypertrophic scar formations.23, 24 This treatment strategy is standard approach of treatment of partial thickness burns at the Dutch Burn Centres. After discharge, patients in both groups were treated in an outpatient setting according to the local protocol.
Baseline characteristics and outcome measures
The following baseline parameters were collected for both study arms: age, gender, wound etiology, bacterial contamination at admission, location and type of the wound, TBSA and co‐morbidities. The burn depth of the study area was accurately determined on day 2–5 post burn by clinical assessment and Laser Doppler Imaging (LDI), using a MoorLDI2‐Burn Imager™ (Moor Instruments, UK) and based on predefined criteria.21 Studies demonstrated that LDI has an accuracy of 95% in combination with clinical estimation, for assessing burn wound depth.25, 26
The primary outcome was time to wound healing, defined as the number of days until complete (defined as >95%) reepithelialization of the study area, as judged by two experienced burn specialists during each dressing change. Secondary outcomes were: The need for operation, performed between 10 and 14 days postburn if the burn wound was not expected to heal; percentage TBSA of the study area that was covered with skin graft; postsurgical complications; number of dressing changes; length of hospital stay; wound colonization; wound infection; use of systemic antibiotics; pain; anxiety; and pruritus. A wound swab was taken from the study area at admission and twice weekly. Infection was defined as a combination of skin redness, pain, swelling, tenderness, warmth, fever, or pus draining from the wound in presence or absence of wound colonization (established by wound culture). Pain of the study area was assessed every day in the evening (background pain) and before and during dressing change (procedural pain) using a Visual Analogue Thermometer (VAT) on a scale from 0 (no pain) to 10 (worst imaginable pain). Pruritus was assessed daily in the evening during hospital admission by use of a VAT on a scale from 0 (no pruritus) to 10 (worst imaginable pruritus).27 The Burn Specific Pain Anxiety Scale (BSPAS) was used to assess pain‐related and anticipatory anxiety in burn patients on the day of discharge.28, 29 BSPAS consists of a nine‐item self‐report scale from 0 (not at all) to 100 (the worst imaginable way).
Scar quality
The scar quality of the study area was assessed at 3, 6, and 12 months postburn in the outpatient clinic using different measurement instruments. First, the Patient and Observer Scar Assessment Scale (POSAS) was used on a scale from 1 (resembles normal skin) to 10 (worst imaginable scar). The POSAS is a reliable and validated scar assessment scale, which is designed to evaluate scars by both professionals and patients. The questionnaire consists of two separate six‐item scales: the Patient Scar Assessment Scale (patient scale) and the Observer Scar Assessment Scale (observer scale). The six items scored by the patient are pain, itching, color, stiffness, thickness, and irregularity. The six items scored by the observer are vascularization, pigmentation, thickness, relief, pliability, and surface area.30, 31
Second, the DermaSpectrometer® (Cortex Technology, Hadsund, Denmark) was used to measure the scar erythema (color) and melanin (pigmentation). It is a validated instrument to measure scar vascularization (erythema) and pigmentation (melanin) by a narrow band simple reflectance meter. Results were calculated as absolute difference between scar tissue and the nonaffected skin.32 Finally, scar elasticity (Ue) and maximal extension (Uf) in mm were measured with the Cutometer® (Courage & Khazaka GmbH, Cologne, Germany). Cutometer® is a validated instrument to measure the vertical deformation of the skin in millimeters when the skin is pulled by means of a controlled vacuum into a circular aperture. Results represent the ratio between scar tissue and nonaffected skin.33
Sample size calculation
Based on a retrospective study of 70 patients with partial thickness burns,20 we expected wound healing in 11 days on average with Flamazine® and 6 days on average with Flaminal® (pooled standard deviation 7.5 days). To identify such a clinically relevant difference regarding time to complete epithelialization between the treatment arms (with 80% power and alpha 5%), it was calculated that 41 patients per arm were needed. Assuming a 10% attrition rate, the sample size was fixed at 45 patients in each arm.
Statistical analysis
The data analysis was performed according to the intention‐to‐treat principle using IBM SPSS Statistics for Windows, version 22 (IBM Corp., Armonk, NY). The baseline patient characteristics were described as mean ± standard deviation for normally distributed continuous variables, as median (range) for skewed continuous variables, and as number (proportion) for categorical variables. The difference in time to complete reepithelialization was compared in both treatment groups and analyzed with Kaplan–Meier curves and log rank test. To correct for potentially confounding variables, a multivariable Cox regression analysis was performed to confirm the primary analysis.
The secondary clinical and patient‐reported outcomes on specific follow‐up moments was compared between the treatment groups using a two‐sided t‐test or Mann–Whitney test for continuous data, and a two‐sided Chi‐square test or Fisher's exact test for categorical data. Repeatedly measured study parameters (pain, pruritus, and scar quality) were analyzed using a linear mixed model with treatment as fixed effect and patient as random effect. To check for effect‐modification of the treatment differences by time, an interaction term (treatment*time) was added in the models. In the analyses a p‐value <0.05 was considered statistically significant.
RESULTS
Inclusion and baseline characteristics
From February 2014 until September 2015, 135 patients were eligible for the study, of whom 90 were randomized (Figure 1). Twelve patients were withdrawn from the study within 2 weeks after randomization for the following reasons: Five patients who had been intubated due to inhalation injury did not confirm the consent provided by their legal representative after detubation, two patients did not sufficiently speak the Dutch language, two patients lived outside of the Netherlands and could therefore not take part in the follow‐up, two patients had TBSA of >30% after reassessment of the wound during admission and one patient received other treatment than the allocated study treatment. The Medical Research Ethics Committee gave permission to randomize 12 more patients to replace the withdrawn patients and meet the required sample size. Eventually, 90 patients were included in the study, of whom 42 were randomized for treatment with Flaminal® Forte and 48 for treatment with Flamazine®. The imbalance in patient numbers between the study groups was caused by the additional inclusion of 12 patients replacing the patients who were excluded after randomization. A major protocol violation occurred in one patient who was randomized for Flaminal® Forte but crossed over to treatment with Flamazine® because of high pain levels with Flaminal® Forte during dressing changes.
Figure 1.
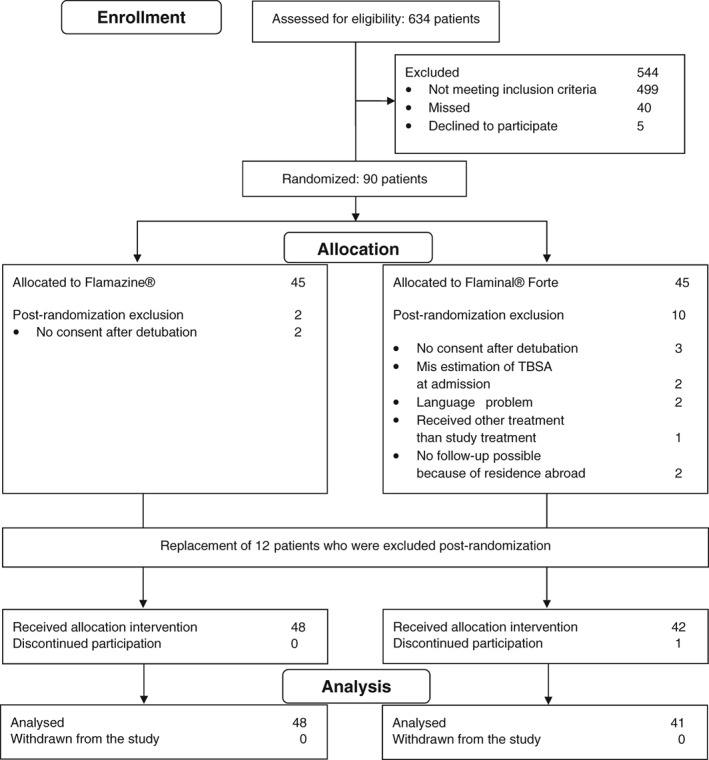
Flowchart of patients.
The baseline characteristics of the analyzed patients are presented in Table 1. The patients in the Flaminal group were on average 7.6 years older compared with the Flamazine® group. The treatment groups were comparable regarding gender, percentage TBSA of the study area, trauma mechanism, anatomical location of the study area, comorbidity and wound colonization at admission.
Table 1.
Baseline characteristics
| Characteristic | Flaminal (n = 41) | Flamazine® (n = 48) |
|---|---|---|
| Age in years, mean (SD) | 50.2 (15.4) | 42.6 (16.2) |
| Male gender, n (%) | 32 (78) | 39 (81) |
| Smoking, n (%) | 12 (29) | 16 (34) |
| %TBSA study area, median (range) | ||
|
3 (0.75–10) | 3 (0.5–16) |
|
1 (0–9) | 1 (0–4) |
|
0.5 (0–3.5) | 0.8 (0–7) |
|
0.25 (0–4) | 0.18 (0–15) |
| On ventilation, n (%) | 6 (15) | 8 (17) |
| Duration in days, median (range) | 3 (1–19) | 3.5 (1–10) |
| Trauma mechanism, n (%) | ||
|
4 (10) | 7 (15) |
|
20 (49) | 21 (44) |
|
12 (29) | 16 (33) |
|
2 (5) | 4 (8) |
|
3 (7) | 0 (0) |
| Location of study area, n (%) | ||
|
1 (2) | 1 (2) |
|
10 (24) | 6 (13) |
|
6 (15) | 2 (4) |
|
16 (39) | 24 (50) |
|
8 (20) | 15 (31) |
| Comorbidity, n (%) | ||
|
2 (5) | 3 (6) |
|
8 (20) | 3 (6) |
|
0 (0) | 1 (2) |
|
2 (5) | 1 (2) |
|
6 (15) | 2 (4) |
|
2 (5) | 0 (0) |
| Colonization on admission, n (%) | 4 (10) | 8 (17) |
According to the protocol, dressing changes were less often performed during hospital admission in the Flaminal® group compared to the Flamazine® group (p < 0.0001): while the dressings of the patients in the Flamazine® group were changed every day, the dressings of the patients in the Flaminal group were changed on median 85% of the days admitted in hospital (range 52–100%).
Primary outcome: wound healing
The median time to wound healing in the Flaminal® group was 18 days (range 8–49 days) compared with 16 days (range 7–48 days, Mann–Whitney test p = 0.24) in the Flamazine® group. Figure 2 shows the Kaplan–Meier curves of time to wound healing for the Flaminal® group and the Flamazine® group (log‐rank test, p = 0.44). Given that the patients in the Flaminal group were on average more than 7 years older, a Cox proportional hazards model was performed to adjust for age, showing no difference in time to wound healing (hazard ratio 0.89 for Flaminal compared to SSD, 95% confidence interval [CI] 0.58–1.35, p = 0.58). In the model, age was not associated with time to wound healing (hazard ratio per one‐year increase 0.99, 95% CI 0.98–1.00, p = 0.19). Furthermore, no difference was found between the treatment groups with respect to time to wound healing of the nonoperated study area.
Figure 2.
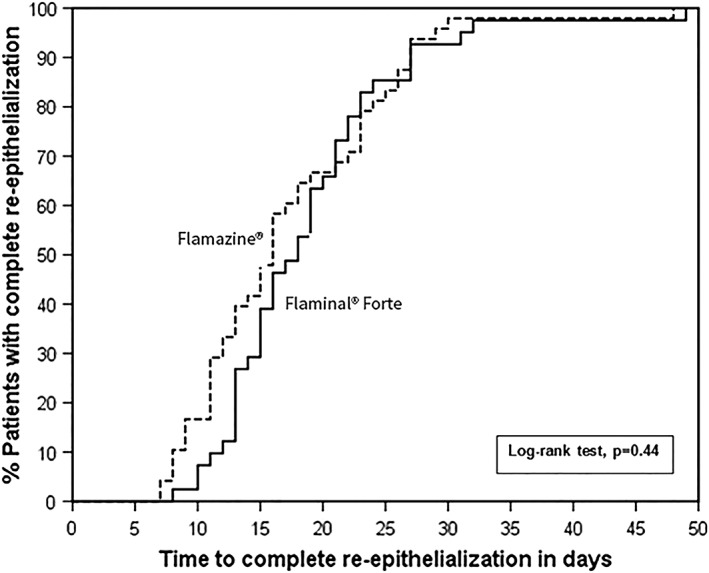
Kaplan–Meier curves for time to wound healing of partial thickness burn in the Flaminal® Forte and Flamazine® group.
Surgical outcomes
No difference was found between the treatment groups regarding need for operation, percentage of the study area covered with skin graft, complications after surgery and length of hospital stay (Table 2).
Table 2.
Outcome measures—intention‐to‐treat analyze
| Outcome measure | Flaminal® Forte (n = 41) | Flamazine® (n = 48) | p |
|---|---|---|---|
| Time to wound healing (days)*, median (range) | 18 (8–49) | 16 (7–48) | 0.24† |
| Time to wound healing of nonoperated study area, median (range) | 14.5 (8–27) | 11 (7–29) | 0.07† |
| Length of hospital stay, median (range) | 16 (1–33) | 17 (2–102) | 0.79† |
| Need for operation, n (%) | 21 (51) | 24 (50) | 0.91⊥ |
| %TBSA of study area covered with skin graft, median (range) | 1.5 (0–5) | 0.9 (0–6) | 0.20† |
| Complication after surgery, n | 3/21 | 4/24 | (not tested) |
|
1/21 | 0/24 | |
|
1/21 | 0/24 | |
|
1/21 | 3/24 | |
|
0/21 | 1/24 | |
|
0/21 | 1/24 | |
|
0/21 | 1/24 |
Defined as reepithelialization >95%.
Mann–Whitney test.
Chi‐square test.
Wound colonization and infection
At admission, four patients in the Flaminal® group and eight in the Flamazine® group already had colonized burn wounds. Of the initially not colonized wounds, 29 (78%) in the Flaminal group developed wound colonization during admission compared to 13 (33%) in the Flamazine® group (p < 0.0001; Table 3). The number of days until wound colonization did not differ between treatment groups, nor did the local infection rate and the use of systemic antibiotics between the treatment groups (Table 3). The microbiology of the colonized burn wounds is described in Table 3. The studied burn wounds were mainly colonized by Gram+ microorganisms, mostly Staphylococcus aureus.
Table 3.
Wound colonization and infection
| Outcome measure | Flaminal® Forte (n = 41) | Flamazine® (n = 48) | p |
|---|---|---|---|
| Colonization of study area, n (%)* | 29/37 (78) | 13/40 (33) | <0.0001† |
| Time to colonization of study area in days, median (range) | 5 (2–11) | 4 (2–19) | 0.36⊥ |
| Species, n | (not tested) | ||
| Gram+ | |||
|
3 | 1 | |
|
1 | 0 | |
|
2 | 0 | |
|
24 | 9 | |
| Gram− | |||
|
1 | 0 | |
|
0 | 1 | |
|
3 | 0 | |
|
0 | 1 | |
|
0 | 1 | |
|
2 | 0 | |
| Infection of study area, n (%) | 4/41 (10) | 1/48 (2) | 0.18¶ |
| Use of systemic antibiotics, n (%) | 0/4 | 0/1 | (not tested) |
Wounds which were colonized at admission were excluded.
Chi‐square test.
Mann–Whitney test.
Fisher's exact test.
Pain, anticipatory anxiety, and pruritus
Pain before and during dressing changes decreased significantly over time during hospital admission in both treatment groups (Figure 3A and B). In the model, the mean decrease in pain score before dressing change was 0.10 points per day (95% CI 0.08–0.12, p < 0.0001) and the mean decrease in pain score during dressing change was 0.13 points per day (95% CI 0.11–0.15, p < 0.0001). No difference in procedural pain was seen for the Flaminal® group compared to the Flamazine® group for pain before dressing change (mean difference 0.10, 95% CI −0.56 to 0.77, p = 0.76), nor for pain during dressing change (mean difference 0.26, 95% CI −0.45 to 0.97, p = 0.47). Scores for background pain (measured in the evening) also decreased over time during hospital admission by an average of 0.07 points per day (95% CI 0.05–0.09, p < 0.0001), but did not differ between the treatment groups (p = 0.89; Figure 3C).
Figure 3.
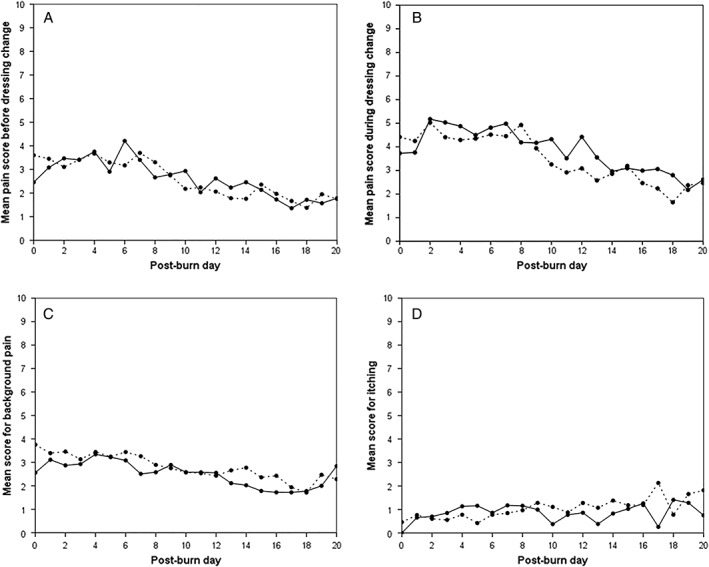
Mean scores for (A) pain before dressing change, (B) pain during dressing change, (C) background pain and (D) pruritus of the study area in the Flaminal group (solid line) and Flamazine® group (dotted line). Scores are presented up to 20 days postburn; scores thereafter are not shown as these were considered too variable due to the small numbers of observations.
Pain‐related and anticipatory anxiety during admission was comparable in the treatment groups: the median BSPAS score in the Flaminal® group was 35 (range 0–78) compared with 26 (range 0–82) in the Flamazine® group (Mann–Whitney test p = 0.45).
The scores for pruritus of the study area increased slightly over time during hospital admission by on average 0.02 points per day (95% CI 0.01–0.04, p = 0.004; Figure 3D). No difference in scores for itching was found between the treatment groups (p = 0.52).
Scar quality
Results on subjective and objective scar quality are shown in Table 4. POSAS general impression score for both patient and observer score showed statistically significant decrease during the first 12 months postburn (p < 0.0001), while no statistically significant difference was found between both treatment groups during the first 12 months postburn (POSAS patient general impression p = 0.32; POSAS observer general impression score p = 0.73). A complete overview of POSAS individual items for patients and observers are shown in Supplement A.
Table 4.
Subjective and objective scar assessment
| Flaminal® Forte | Flamazine® | ||||||
|---|---|---|---|---|---|---|---|
| No. (valid) | Median | Range | No. (valid) | Median | Range | p * | |
| Subjective scar assessment | |||||||
| POSAS patient score† | |||||||
| General impression | |||||||
| 3 months post burn | 35 | 5 | 1–10 | 42 | 4 | 1–10 | 0.70 |
| 6 months post burn | 34 | 4 | 1–10 | 41 | 3 | 1–10 | 0.30 |
| 12 months post burn | 35 | 3 | 1–10 | 38 | 2 | 1–10 | 0.09 |
| POSAS observer score⊥ | |||||||
| General impression | |||||||
| 3 months post burn | 35 | 5 | 1–10 | 42 | 4 | 1–10 | 0.70 |
| 6 months post burn | 34 | 4 | 1–10 | 41 | 3 | 1–10 | 0.30 |
| 12 months post burn | 35 | 3 | 1–10 | 38 | 2 | 1–10 | 0.09 |
| Objective scar assessment | |||||||
| Scar color (erythema)¶ , †† | |||||||
| 3 months post burn | 35 | 11.0 | 0.24–27.9 | 42 | 9.5 | 0.66–37.1 | 0.65 |
| 6 months post burn | 35 | 5.8 | 0–28.3 | 41 | 5.3 | 0.43–27.7 | 0.37 |
| 12 months post burn | 35 | 3.2 | 0.07–17.4 | 35 | 3.3 | 0.5–10.5 | 0.24 |
| Scar pigmentation (melanin)** , †† | |||||||
| 3 months post burn | 35 | 6.7 | 0.3–28.5 | 42 | 8.0 | 0.1–25.0 | 0.53 |
| 6 months post burn | 35 | 3.3 | 0.4–15.0 | 35 | 4.2 | 0.07–12.8 | 0.84 |
| 12 months post burn | 39 | 3.7 | 0–17.4 | 39 | 2.6 | 0.3–18.4 | 0.59 |
| Scar extension (Uf)⊥⊥ , *** | |||||||
| 3 months post burn | 35 | 0.70 | 0.35–1.58 | 40 | 0.70 | 1.40–1.60 | 0.86 |
| 6 months post burn | 35 | 0.73 | 0.20–1.28 | 41 | 0.74 | 0.06–1.31 | 0.86 |
| 12 months post burn | 35 | 0.84 | 0.29–1.35 | 40 | 0.79 | 0.47–1.60 | 0.75 |
| Scar elasticity (Ue)¶¶ , *** | |||||||
| 3 months post burn | 35 | 0.62 | 0.22–1.36 | 35 | 0.60 | 0.20–1.94 | 0.50 |
| 6 months post burn | 35 | 0.62 | 0.09–1.27 | 41 | 0.60 | 0.35–1.33 | 0.86 |
| 12 months post burn | 35 | 0.78 | 0.19–1.35 | 40 | 0.70 | 0.36–1.57 | 0.71 |
Mann–Whitney U test.
Patient and Observer Scar Assessment Scale (POSAS) general impression score provided by the patient.
Patient and Observer Scar Assessment Scale (POSAS) general impression score provided by the observer.
Scar color (Erythema) obtained by the DermaSpectrometer®.
Scar pigmentation (Melanin) obtained by the DermaSpectrometer®.
Values were calculated as absolute difference between scar tissue and the nonaffected skin.
Scar extension results (Uf) obtained by the Cutometer®.
Scar elasticity (Ue) obtained by the Cutometer®.
Values represent the ratio between scar tissue and nonaffected skin.
The absolute difference between scar tissue and the nonaffected skin for erythema and melanin, as assessed by the DermaSpectrometer®, showed a statistically significant decrease (p < 0.0001) during the first 12 months postburn. However, no statistically significant difference was found between both treatment groups in respect to erythema (p = 0.68) or melanin (p = 0.97).
The ratio between scar tissue an nonaffected skin for maximal scar extension (Uf) and scar elasticity (Ue), as assessed by Cutometer®, showed a statistically significant decrease during the first 12 months postburn (p < 0.00001). No statistically significant difference was found between both treatment groups in respect to Uf (p = 0.97) or Uf (p = 0.90) during the first 12 months postburn.
DISCUSSION
This study is the first randomized controlled trial comparing the clinical effectiveness of Flaminal® Forte with Flamazine® in the treatment of partial thickness burns. No statistically significant or clinically relevant differences were found between the interventions with respect to the wound healing. Furthermore, the need for surgery, pain during dressing changes, pain‐related and anticipatory anxiety or pruritus did not differ significantly between the treatment groups. In the Flaminal® group, there were twice as many wound colonizations during treatment than in the Flamazine® group. Although the incidence of wound infection seemed higher in the Flaminal® group, the difference was not statistically significant. Noteworthy, patients treated with Flaminal® Forte required less dressing changes than the patients treated with Flamazine®.
Interestingly, time to wound healing was not significantly different between both treatment groups. This finding is in contrast with previous retrospective studies that described a better wound healing of partial thickness burns that were treated with Flaminal® Forte in comparison with SSD.19, 20 Selection bias in these retrospective studies may have contributed to this finding. In the current study, the alternated treatment strategy with Furacine Soluble Dressing from 6th post burn day in the Flamazine® group may have minimized the cytotoxicity of the silver particles in the SSD on the wound bed. Silver is highly toxic to keratinocytes and fibroblasts in vitro.3, 10, 11, 15 In effect, this treatment strategy may have limited the poor wound healing that is often seen in burn wounds treated with SSD for a longer period of time.3, 9, 12, 34 This use of Flamazine®/Furacine Soluble Dressing may have resulted in no difference in time to wound healing between both treatments. Overall, rapid wound healing is vital, because delayed wound healing time is found to be a risk factor for worse scar quality.23, 24, 35 Cubison et al. concluded that the risk of developing a hypertrophic scar was high when the wound healing took more than 21 days.23 A recent study found that the scar quality worsens with an increase in time to wound healing, as measured by the Vancouver Scar Scale (VSS).35
Besides a comparable time to wound healing, the treatment groups also did not differ regarding the need for surgery and size of the study area that required skin grafting. From a clinical perspective, this means that both treatments equally reduce the number of operations of the deep partial thickness burns that are most likely not to heal spontaneously. At the Dutch Burn Centers burn wounds are grafted when no wound healing is expected within 21 days postburn to minimize the risk of hypertrophic scar formation. This is likely the reason for the high percentage of grafted burn wounds in the current study. The favorable results on scar quality in the current study support this approach. However, this treatment strategy might also have confounded results on wound healing.
Dressing changes in both treatment groups were applied according to the manufacturer recommendations. Therefore, number of dressing changes was not an outcome in this study. However, it is essential to have more insight into dressing changes and its effect on the patient because burn wound pain is most intense during dressing changes (procedural pain).36, 37 Procedural pain is recognized to be a multidimensional experience that often induces significant anxiety and distress in burn patients.38 The management of this type of burn pain is challenging for burn specialists, especially in absence of a consensus on treatment strategy.39 Therefore, less dressing changes could contribute to minimize burn wound pain, anxiety and distress. In the current study, dressing changes were less often performed during hospital admission in the Flaminal® group compared to the Flamazine® group (p < 0.0001): while the dressings of the patients in the Flamazine® group were changed every day, the dressings of the patients in the Flaminal group were changed on median 85% of the days admitted in hospital (range 52–100%). As a result, patients in the Flaminal® group had less moments of procedural pain compared to the patients in the Flamazine® group during hospital admission. Despite the higher incidence of wound colonization in the Flaminal® group, no significant differences in the incidence of wound infection, use of systemic antibiotics or quality of wound healing were observed compared with the Flamazine® group. This observation is in line with a previous retrospective study by Hoeksema et al.19 There are several explanations for this finding. First, wound colonization alone, in the absence of tissue damage, may not delay the wound healing process.40 Studies indicated that subinfective levels of bacteria may even be required for the formation of granulation tissue and collagen formation to accelerate the wound healing process.41, 42 However, a transient stage from wound colonization to critical colonization or wound infection is likely to result in delayed wound healing.40 This theory supports our results as no difference in incidence of wound infection and time to wound healing was found between the treatment groups. Second, the continuous debridement effect of Flaminal® Forte may reduce the bacterial load in the presence of wound colonization. However, this theory was not studied in the present study and should be examined in future studies. Third, wound colonization in our study was treated based on the results of the wound culture. This may have prevented a higher incidence of wound infection and, consequently, have prevented a delayed wound healing in colonized burn wounds in this study. Fourth, one might speculate that less wound colonization in the Flamazine® group could be explained by the alternated treatment strategy in the Flamazine® group from the 6th postburn day. However, the median time to first wound colonization in the SSD group was 4 days (range 2–19). On the other hand, the statistical power of the study was insufficient to ascertain a statistically significant difference in the incidence of wound infection between the treatment groups.
In terms of scar quality, no statistical differences were found between both treatment groups. The POSAS score by both patient and observer were low and decreased during a follow‐up of 12 months. In line with these findings, the melanin and the erythema indices measured by DermaSpectrometer® and scar elasticity and maximal extension measured by Cutometer® were also improved during follow‐up of 12 months, which corresponds with improvement of scar quality in both treatment groups. This finding is important because scar formation negatively impacts quality of life not only in terms of physical limitations and appearance but also in terms of psychological problems including social anxiety, depression, posttraumatic stress and poor body image.43, 44, 45
The current study has some limitations. First, randomization would ideally have been performed after LDI for an optimal evaluation of the burn wound depth of the study area. However, in order to get reliable results LDI has to be performed between 2 and 5 days post burn.25, 26 Local treatment could not be started before LDI was performed if randomization was performed after LDI. Consequently, burn wounds that are untreated before performing LDI are prone to delayed wound healing. Alternatively, when a local treatment other than Flammazine® or Flaminal® Forte was started before LDI, a bias was introduced to the study which may have affected the wound healing time. Moreover, the current study was designed to evaluate our daily clinical practice for the treatment of partial thickness burns in two of the three Dutch Burn Centres. In both centers local treatment is started directly after admission. Second, results were not stratified for superficial and deep partial thickness burns, because the study area was often partial thickness burns with different depth. This distinction is important because some authors postulate that standard operative treatment for the deep partial thickness burns minimizes poor scar quality, although, there is no consensus in the literature regarding timing and type of the operation, debridement technique, use of skin substitutes or application of growth factors and other humoral agents to enhance wound healing.46, 47, 48, 49 Spontaneous wound healing of deep partial thickness burns is still possible because of the surviving keratinocytes and epidermal stem cells in the remaining dermis layer.50 Nevertheless, the reepithelization of deep partial thickness burns is significantly prolonged and associated with poor scar quality when treated conservatively for more than 21 days.23, 24, 51 Therefore, in the current study partial thickness burns were operated (split skin graft) when the wound healing took more than 21 days. Moreover, the distribution of superficial, intermediate and deep partial thickness wounds was similar in the treatment groups, so we believe that the presence of deep partial thickness burns did not affect the conclusions of our study. Third, it was not possible to blind the patients and clinicians because of the characteristic appearance of both treatments. Fourth, the exclusion of psychiatric patients and children makes the sample not entirely representative. Therefore, the findings of this study should be extrapolated to psychiatric and pediatric burn patients with caution. Finally, the lack of power for our study outcome wound colonization as mentioned above.
In conclusion, there was no statistically significant or clinically relevant difference in wound healing between Flaminal® Forte and Flamazine® in the treatment of partial thickness wounds. Nevertheless, Flaminal® Forte seemed favorable because of less dressing changes and therefore lower burden of wound care. More studies are needed to conform these findings.
Supporting information
Table S1 POSAS scores provided by the patients and observers
ACKNOWLEDGMENTS
The authors sincerely thank the following people for their dedicated contribution to this study: M.E. van Baar, PhD, D. Baas, PhD, J. Dokter, M.D., PhD, K.L.M. Gardien, MD, H. Goei, MD, M. Jaspers, MD, PhD, I.M.M.H Oen, MD, D.T. Roodbergen, MD, C.M. Stekelenburg, MD, PhD, F. R. H. Tempelman, MD, N.R.N. Trommel, M.B.A. van der Wal, MD, PhD, and A.F.P. M. Vloemans, MD, PhD.
Source of Funding: This work was supported by the Dutch Burns Foundation (grant number 12.109).
Conflict of Interest: None of the authors have any potential financial conflicts of interest to disclose.
REFERENCES
- 1. Wasiak J, Cleland H, Campbell F, Spinks A. Dressings for superficial and partial thickness burns. Cochrane Database Syst Rev 2013; 3: CD002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fox CL Jr. Topical therapy and the development of silver sulfadiazine. Surg Gynecol Obstet 1983; 157: 82–8. [PubMed] [Google Scholar]
- 3. Hussain S, Ferguson C. Best evidence topic report. Silver sulphadiazine cream in burns. Emerg Med J: EMJ 2006; 23: 929–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bowler PG, Jones SA, Walker M, Parsons D. Microbicidal properties of a silver‐containing hydrofiber dressing against a variety of burn wound pathogens. J Burn Care Rehabil 2004; 25: 192–6. [DOI] [PubMed] [Google Scholar]
- 5. Hermans MH. Results of a survey on the use of different treatment options for partial and full thickness burns. Burns 1998; 24: 539–51. [DOI] [PubMed] [Google Scholar]
- 6. Ip M, Lui SL, Poon VK, Lung I, Burd A. Antimicrobial activities of silver dressings: an in vitro comparison. J Med Microbiol 2006; 55: 59–63. [DOI] [PubMed] [Google Scholar]
- 7. Nadworny PL, Wang J, Tredget EE, Burrell RE. Anti‐inflammatory activity of nanocrystalline silver in a porcine contact dermatitis model. Nanomedicine 2008; 4: 241–51. [DOI] [PubMed] [Google Scholar]
- 8. Storm‐Versloot MN, Vos CG, Ubbink DT, Vermeulen H. Topical silver for preventing wound infection. Cochrane Database Syst Rev 2010; 3: CD006478. [DOI] [PubMed] [Google Scholar]
- 9. Aziz Z, Abu SF, Chong NJ. A systematic review of silver‐containing dressings and topical silver agents (used with dressings) for burn wounds. Burns 2012; 38: 307–18. [DOI] [PubMed] [Google Scholar]
- 10. Lee AR, Moon HK. Effect of topically applied silver sulfadiazine on fibroblast cell proliferation and biomechanical properties of the wound. Arch Pharm Res 2003; 26: 855–60. [DOI] [PubMed] [Google Scholar]
- 11. Poon VK, Burd A. In vitro cytotoxity of silver: implication for clinical wound care. Burns 2004; 30: 140–7. [DOI] [PubMed] [Google Scholar]
- 12. Heyneman A, Hoeksema H, Vandekerckhove D, Pirayesh A, Monstrey S. The role of silver sulphadiazine in the conservative treatment of partial thickness burn wounds: a systematic review. Burns 2016; 42: 1377–86. [DOI] [PubMed] [Google Scholar]
- 13. Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns 2017; 43: 50–7. [DOI] [PubMed] [Google Scholar]
- 14. Rashaan ZM, Krijnen P, Klamer RR, Schipper IB, Dekkers OM, Breederveld RS. Nonsilver treatment vs. silver sulfadiazine in treatment of partial‐thickness burn wounds in children: a systematic review and meta‐analysis. Wound Repair Regen 2014; 22: 473–82. [DOI] [PubMed] [Google Scholar]
- 15. De Smet K, van den Plas D, Lens D, Sollie P. Pre‐clinical evaluation of a new antimicrobial enzyme for the control of wound bioburden. Wounds 2009; 21: 65–73. [PubMed] [Google Scholar]
- 16. Thomas SS, Lawrence JC, Thomas A. Evaluation of hydrocolloids and topical medication in minor burns. J Wound Care 1995; 4: 218–20. [DOI] [PubMed] [Google Scholar]
- 17. White R. Flaminal: a novel approach to wound bioburden control. Wounds 2006; 2: 64–9. [Google Scholar]
- 18. Vandenbulcke K, Horvat LI, De MM, Slegers G, Beele H. Evaluation of the antibacterial activity and toxicity of 2 new hydrogels: a pilot study. Int J Low Extrem Wounds 2006; 5: 109–14. [DOI] [PubMed] [Google Scholar]
- 19. Hoeksema H, Vandekerckhove D, Verbelen J, Heyneman A, Monstrey S. A comparative study of 1% silver sulphadiazine (Flammazine) versus an enzyme alginogel (Flaminal) in the treatment of partial thickness burns. Burns 2013; 39: 1234–41. [DOI] [PubMed] [Google Scholar]
- 20. Kyriopoulos K, Van den Plas D, Papadopoulos O, Zapandioti P, Tsoutsos D. The use of a new wound alginogel for the treatment of partial‐thickness hand burns. Wounds 2010; 22: 161–4. [PubMed] [Google Scholar]
- 21. Rashaan ZM, Krijnen P, van den Akker‐van Marle ME, van Baar ME, Vloemans AF, Dokter J, et al. Clinical effectiveness, quality of life and cost‐effectiveness of Flaminal(R) versus Flamazine(R) in the treatment of partial thickness burns: study protocol for a randomized controlled trial. Trials 2016; 17: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg 2012; 10: 28–55. [DOI] [PubMed] [Google Scholar]
- 23. Cubison TC, Pape SA, Parkhouse N. Evidence for the link between healing time and the development of hypertrophic scars (HTS) in paediatric burns due to scald injury. Burns 2006; 32: 992–9. [DOI] [PubMed] [Google Scholar]
- 24. Deitch EA, Wheelahan TM, Rose MP, Clothier J, Cotter J. Hypertrophic burn scars: analysis of variables. J Trauma 1983; 23: 895–8. [PubMed] [Google Scholar]
- 25. Hoeksema H, Van de Sijpe K, Tondu T, Hamdi M, Van LK, Blondeel P, et al. Accuracy of early burn depth assessment by laser Doppler imaging on different days post burn. Burns 2009; 35: 36–45. [DOI] [PubMed] [Google Scholar]
- 26. Pape SA, Baker RD, Wilson D, Hoeksema H, Jeng JC, Spence RJ, et al. Burn wound healing time assessed by laser Doppler imaging (LDI). Part 1: derivation of a dedicated colour code for image interpretation. Burns 2012; 38: 187–94. [DOI] [PubMed] [Google Scholar]
- 27. Phan NQ, Blome C, Fritz F, Gerss J, Reich A, Ebata T, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol 2012; 92: 502–7. [DOI] [PubMed] [Google Scholar]
- 28. Taal LA, Faber AW. The burn specific pain anxiety scale: introduction of a reliable and valid measure. Burns 1997; 23: 147–50. [DOI] [PubMed] [Google Scholar]
- 29. Taal LA, Faber AW, Van Loey NE, Reynders CL, Hofland HW. The abbreviated burn specific pain anxiety scale: a multicenter study. Burns 1999; 25: 493–7. [DOI] [PubMed] [Google Scholar]
- 30. Draaijers LJ, Tempelman FR, Botman YA, Tuinebreijer WE, Middelkoop E, Kreis RW, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg 2004; 113: 1960–5 discussion 6‐7. [DOI] [PubMed] [Google Scholar]
- 31. van de Kar AL, Corion LU, Smeulders MJ, Draaijers LJ, van der Horst CM, van Zuijlen PP. Reliable and feasible evaluation of linear scars by the patient and observer scar assessment scale. Plast Reconstr Surg 2005; 116: 514–22. [DOI] [PubMed] [Google Scholar]
- 32. Draaijers LJ, Botman YA, Tempelman FR, Kreis RW, Middelkoop E, van Zuijlen PP. Skin elasticity meter or subjective evaluation in scars: a reliability assessment. Burns 2004; 30: 109–14. [DOI] [PubMed] [Google Scholar]
- 33. Draaijers LJ, Tempelman FR, Botman YA, Kreis RW, Middelkoop E, van Zuijlen PP. Colour evaluation in scars: tristimulus colorimeter, narrow‐band simple reflectance meter or subjective evaluation? Burns 2004; 30: 103–7. [DOI] [PubMed] [Google Scholar]
- 34. Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing: review of the literature. Burns 2007; 33: 139–48. [DOI] [PubMed] [Google Scholar]
- 35. Finlay V, Burrows S, Burmaz M, Yawary H, Lee J, Edgar DW, et al. Increased burn healing time is associated with higher Vancouver scar scale score. Scars Burns Heal 2017; 3: 2059513117696324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Byers JF, Bridges S, Kijek J, LaBorde P. Burn patients' pain and anxiety experiences. J Burn Care Rehabil 2001; 22: 144–9. [DOI] [PubMed] [Google Scholar]
- 37. Ashburn MA. Burn pain: the management of procedure‐related pain. J Burn Care Rehabil 1995; 16: 365–71. [DOI] [PubMed] [Google Scholar]
- 38. Carrougher GJ, Ptacek JT, Honari S, Schmidt AE, Tininenko JR, Gibran NS, et al. Self‐reports of anxiety in burn‐injured hospitalized adults during routine wound care. J Burn Care Res 2006; 27: 676–81. [DOI] [PubMed] [Google Scholar]
- 39. Richardson P, Mustard L. The management of pain in the burns unit. Burns 2009; 35: 921–36. [DOI] [PubMed] [Google Scholar]
- 40. Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis 2004; 17: 91–6. [DOI] [PubMed] [Google Scholar]
- 41. Laato M, Niinikoski J, Lundberg C, Gerdin B. Inflammatory reaction and blood flow in experimental wounds inoculated with Staphylococcus aureus . Eur Surg Res—Europaische chirurgische Forschung Recherches chirurgicales europeennes 1988; 20: 33–8. [DOI] [PubMed] [Google Scholar]
- 42. Landen NX, Li D, Stahle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci: CMLS 2016; 73: 3861–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Falder S, Browne A, Edgar D, Staples E, Fong J, Rea S, et al. Core outcomes for adult burn survivors: a clinical overview. Burns 2009; 35: 618–41. [DOI] [PubMed] [Google Scholar]
- 44. Stavrou D, Weissman O, Tessone A, Zilinsky I, Holloway S, Boyd J, et al. Health related quality of life in burn patients—a review of the literature. fFunctional outcome after burns: a review. Burns 2006; 32: 1–9. [DOI] [PubMed] [Google Scholar]
- 45. Sveen J, Dyster‐Aas J, Willebrand M. Attentional bias and symptoms of posttraumatic stress disorder one year after burn injury. J Nerv Ment Dis 2009; 197: 850–5. [DOI] [PubMed] [Google Scholar]
- 46. Baxter CR. Management of burn wounds. Dermatol Clin 1993; 11: 709–14. [PubMed] [Google Scholar]
- 47. Yan H, Chen J, Peng X. Recombinant human granulocyte‐macrophage colony‐stimulating factor hydrogel promotes healing of deep partial thickness burn wounds. Burns 2012; 38: 877–81. [DOI] [PubMed] [Google Scholar]
- 48. Kazmierski M, Mankowski P, Jankowski A, Harasymczuk J. Comparison of the results of operative and conservative treatment of deep dermal partial‐thickness scalds in children. Eur J Pediatr Surg 2007; 17: 354–61. [DOI] [PubMed] [Google Scholar]
- 49. Gerlach JC, Johnen C, McCoy E, Brautigam K, Plettig J, Corcos A. Autologous skin cell spray‐transplantation for a deep dermal burn patient in an ambulant treatment room setting. Burns 2011; 37: e19–23. [DOI] [PubMed] [Google Scholar]
- 50. Zacharevskij E, Baranauskas G, Varkalys K, Rimdeika R, Kubilius D. Comparison of non‐surgical methods for the treatment of deep partial thickness skin burns of the hand. Burns 2018; 44: 445–52. [DOI] [PubMed] [Google Scholar]
- 51. Cleland H. Thermal burns—assessment and acute management in the general practice setting. Aust Fam Physician 2012; 41: 372–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 POSAS scores provided by the patients and observers


