Abstract
To date, no reports of hypersensitivity reactions (HSRs) among nonsteroidal anti‐inflammatory drugs (NSAIDs) according to cyclo‐oxygenase (COX) selectivity and chemical groups have been published in a single study. The present study assessed the reporting frequency of HSRs for NSAIDs based on their relative inhibitory potency toward COX enzymes and chemical groups, including the presence/absence of a functional sulfonamide group, in strata observed 5 years after market authorization. A case/noncase study was performed among individual case safety reports (ICSRs) with NSAIDs as suspected drugs in VigiBase, the WHO spontaneous reporting database. Cases were ICSRs mentioning angioedema and anaphylactic/anaphylactoid shock conditions, while noncases were ICSRs without HSRs. NSAIDs were categorized into (i) NSAIDs with high COX‐2 selectivity (coxibs), (ii) noncoxib NSAIDs with COX‐2 preference, (iii) NSAIDs with poor selectivity, or (iv) NSAIDs with unknown selectivity. Chemical groups were defined based on the Anatomical Therapeutic Chemical classification system and the presence/absence of a functional sulfonamide group. Reporting odds ratios (RORs) and 95% confidence intervals (95% CIs) were calculated using logistic regression analysis. We identified 13 229 cases and 106 444 noncases. In the first 5 years after marketing, poor‐selectivity NSAIDs and acetic acid derivatives were associated with the highest ROR of HSRs (age‐ and sex‐adjusted ROR 2.12, 95% CI 1.98–2.28; and ROR 2.21, 95% CI 1.83–2.66, respectively) compared with coxibs, and sulfonamide NSAIDs were associated with the highest ROR of HSRs compared with nonsulfonamide NSAIDs (age‐ and sex‐adjusted ROR 1.38, 95% CI 1.29–1.47). After the first 5 years of marketing, most of the RORs returned to approximately 1.
Keywords: a sulfonamide functional group, chemical groups, cyclo‐oxygenase selectivity, hypersensitivity reactions, nonsteroidal anti‐inflammatory drugs, spontaneous reporting
Introduction
Nonsteroidal anti‐inflammatory drugs (NSAIDs) are the drugs most often reported to be associated with adverse drug reactions (ADRs) 1, 2. This group of drugs may cause various types of hypersensitivity reactions (HSRs) and be classified into either allergic reactions or nonallergic reactions with similar symptoms, that is, pseudoallergic (anaphylactoid reactions) 3, 4.
Both clinical trials and observational studies have shown that cyclo‐oxygenase (COX) selectivity and NSAID chemical groups are associated with differences in sensitizing capacities and risk of hypersensitivity. Selective COX‐2 inhibitors were associated with a lower hospitalization risk for angioedema compared with nonselective NSAIDs 5 and well tolerated by patients with angioedema and urticaria attributable to the administration of nonselective NSAIDs 6, 7, 8, 9, 10. A pharmacovigilance study in the Netherlands showed that among all chemical groups of NSAIDs, propionic acid derivatives (PADs) and acetic acid derivatives (AADs) were associated with a higher risk of anaphylaxis compared with nonuse 11. The presence of a sulfonamide functional group in the chemical structure of NSAIDs is also suspected to influence the risk of HSRs 12.
Many studies have investigated the association between NSAIDs and risk of HSRs. These studies differed in study design and definitions of exposure, and none of them evaluated the above‐mentioned exposure classifications in a single study. Therefore, the present study was aimed to evaluate the association between NSAIDs, classified by their COX selectivity and chemical groups, including the presence of a sulfonamide functional group, and HSRs as reported in VigiBase using a single‐study design.
Materials and Methods
Setting
The study used coded fields from the WHO global individual case safety report (ICSR) database, VigiBase, which stores reports on suspected ADRs submitted since 1968. In 1978, VigiBase was transferred from the WHO to the Uppsala Monitoring Centre (UMC) in Sweden. Currently, >150 countries are members of the WHO Programme for International Drug Monitoring and contribute reports from their respective national ADR reporting system.
Information from contributing countries is heterogeneous, but several items must be included: patient demographics (age, sex, and reporting countries), ADRs (onset, duration, and causality assessment), suspected drugs (route of administration, dates of first intake and discontinuation, dosing regimen, and co‐medications), and administrative data (type of report, reporters, and source). Unfortunately, such information is often missing. Duplication is detected by checking case identifiers, manual inspection of case series, and specific statistical algorithms. The reported drugs are encoded using the WHO Drug Dictionary Enhanced, which uses the WHO Anatomical Therapeutic Chemical (ATC) classification. ADRs are encoded in the WHO Adverse Reaction Terminology (WHO‐ART) and the Medical Dictionary for Regulatory Activities (MedDRA) in parallel. The MedDRA is used to assess medical issues involving a system, organ, or etiology using its hierarchical structure or through the distinctive features of Standardized MedDRA Queries (SMQs) 13, 14. By June 2016, >13 million ICSRs were recorded.
Design
We performed a case/noncase study using data from 1978 to June 2016. We nested our study among ICSRs with an NSAID as a suspected drug. Only individual NSAIDs with first market approval after 1977 were included. Information on the date of market approval for an individual NSAID was obtained from the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the Pharmaceutical and Medical Devices Agency (PMDA), Japan. ICSRs with missing information on age, sex, or date of ADR occurrence were excluded.
Definitions of cases and noncases
Hypersensitivity reaction cases were defined as ICSRs for angioedema and anaphylactic/anaphylactoid shock conditions using relevant SMQ codes, including 40 and 9 narrow scopes of MedDRA preferred terms (PTs), respectively (Table S1). The narrow scope refers to most likely HSRs that give a high specificity. Noncases were ICSRs without HSRs with an NSAID as a suspected drug. As each NSAID‐associated ICSR contributed only once, a single ICSR with multiple ADRs was considered a case if one of these ADRs was a HSR and as a noncase if none of these was a HSR.
Drugs of interest
Nonsteroidal anti‐inflammatory drugs were categorized based on both COX selectivity and chemical groups. The COX selectivity was defined based on their relative inhibitory potency toward COX enzymes as the ratios of 80% inhibitory concentration (IC80) against COX‐2 and COX‐1 enzymes 15, 16, 17, 18, 19, 20, 21. The 50% inhibitory concentration (IC50) values are often used when comparing the potencies of NSAIDs against COX‐1 and COX‐2 with the following assumptions: The inhibitory curves should be preferably parallel and NSAIDs produce a 50% or lower reduction in prostanoid formation at therapeutic doses. However, as IC50 does not meet these assumptions, IC80 is a more valid approach to compare the potency of NSAIDs 15. It includes the following: (i) coxibs, that is, NSAIDs based on the Anatomical Therapeutic Chemical (ATC) classification system that inhibit both COX‐2 and COX‐1 with high selectivity toward COX‐2 by five times or more; (ii) noncoxib NSAIDs based on ATC classification that inhibit COX‐2 and COX‐1 with high selectivity toward COX‐2 by five times or more; (iii) NSAIDs that inhibit both COX‐2 and COX‐1 with selectivity toward COX‐2 enzyme by five times or less (poor selectivity); and (iv) NSAIDs without information available on COX inhibitory potency. Individual NSAIDs under each category are shown in Table S2. Chemical groups of NSAIDs were categorized based on the ATC classification, that is, butylpyrazolidines, AADs, oxicams, PADs, fenamates, coxibs, and other NSAIDs (i.e., NSAIDs that are not classified elsewhere). Based on the presence of a sulfonamide functional group, NSAIDs were categorized into (i) sulfonamide NSAIDs (i.e., oxicam group, celecoxib, valdecoxib, polmacoxib, parecoxib, and nimesulide) and (ii) nonsulfonamide NSAIDs (i.e., acetic acid and propionic acid derivatives, butylpyrazolidine and fenamate groups, rofecoxib, etoricoxib, lumiracoxib, nabumetone, niflumic acid, azapropazone, and proquazone). Concomitant use of two or more NSAIDs was excluded.
Data analyses
We calculated reporting odds ratios (RORs) as a measure of disproportionality. Analogous to a case–control study, a ROR was calculated by dividing the exposure odds among cases divided by the exposure odds in noncases 22. To minimize the effect of underreporting of ADRs, we considered time after market approval of a NSAID in the analyses. Even though the Weber effect is described as ADR reports that peak at the second year after drug approval, other studies showed that ADR reports still increase up to 5 years 23, 24. We calculated RORs in three strata of 5 years after market approval to ensure that time after market approval did indeed contribute to ROR differences. Any NSAID‐associated ICSRs that were reported after this period were excluded. We stratified our analyses to the most reported HSRs (urticaria, angioedema, and anaphylactic shock), age, sex, and reporting countries. Reports from the United States were analyzed separately because these reports (i) were submitted by healthcare professionals (such as physicians, pharmacists, nurses, and others) as well as consumers (such as patients, family members, lawyers, and others), while from non‐US countries reports were submitted mostly by healthcare professionals, (ii) also come from other countries because of industry reports with head offices located in the United States, and (iii) contributed to the highest proportion of reports in VigiBase. Coxibs were the reference group in the analyses based on COX selectivity and chemical groups, and nonsulfonamide NSAIDs were the reference group for sulfonamide NSAIDs. If the proportion of individual NSAIDs that caused HSRs was >2%, these NSAIDs were further analyzed. Rofecoxib was used as the reference group because it had the most favorable tolerability for patients intolerant of NSAIDs 25, 26. As rofecoxib was approved in 1999 and then withdrawn in 2004, RORs for individual NSAIDs were calculated only for 5 years after marketing. Crude and age‐ and sex‐adjusted RORs and 95% confidence intervals (95% CIs) were determined using logistic regression analysis. The statistical package SPSS version 24 was used to perform data analyses, and P values <0.05 were considered statistically significant.
Sensitivity analysis
In sensitivity analyses, to test the robustness of our main findings, a broad scope of PT SMQs of HSRs was used to calculate the RORs. The broad scope includes terms that are often less likely to represent the outcome of interest, but gives high sensitivity, including 52 and 21 broad scopes for angioedema and anaphylactic/anaphylactoid shock conditions, respectively (Table S2).
Results
By June 2016, VigiBase contained 394 957 ICSRs where an NSAID was a suspected drug. After excluding ICSRs reported before 1978, those without information on sex, age, or onset date, NSAIDs without information on the market authorization, NSAIDs with >15 years after market approval, and concomitant use of two or more NSAIDs, 119 673 ICSRs remained, including 13 229 HSR cases and 106 444 non‐HSR cases. The inclusion flow diagram is presented in Figure 1. The mean age of the cases was lower than that of the noncases (47.8 vs. 57.8 years). Most of the cases and noncases were aged <60 years (75 vs. 52.2%) and female (69.4 vs. 64%). The most significant number of NSAID‐associated ICSRs originated from the United States (29.2%), followed by the United Kingdom (12.2%), Thailand (7.6%), South Korea (5.9%), and Singapore (4.9%). Among these countries, a NSAID as a suspected drug associated with HSRs was found mostly in Thailand. When ignoring the date of market authorization, NSAIDs with poor selectivity were more likely to be a suspected drug among cases (58.3%), while coxibs were more likely to be suspected drugs among noncases (47.5%). According to chemical groups, coxibs were more likely to be suspected drugs among both cases and noncases (27.9 and 47.5%, respectively). In addition, nonsulfonamide NSAIDs were more likely to be suspected drugs among both cases and noncases (61.4 and 62.4%, respectively). Heterogeneity was found in the proportion of cases and noncases for the reporting countries and NSAIDs according to either COX selectivity or chemical groups (P < 0.05). The characteristics of ICSRs are shown in Table 1.
Figure 1.
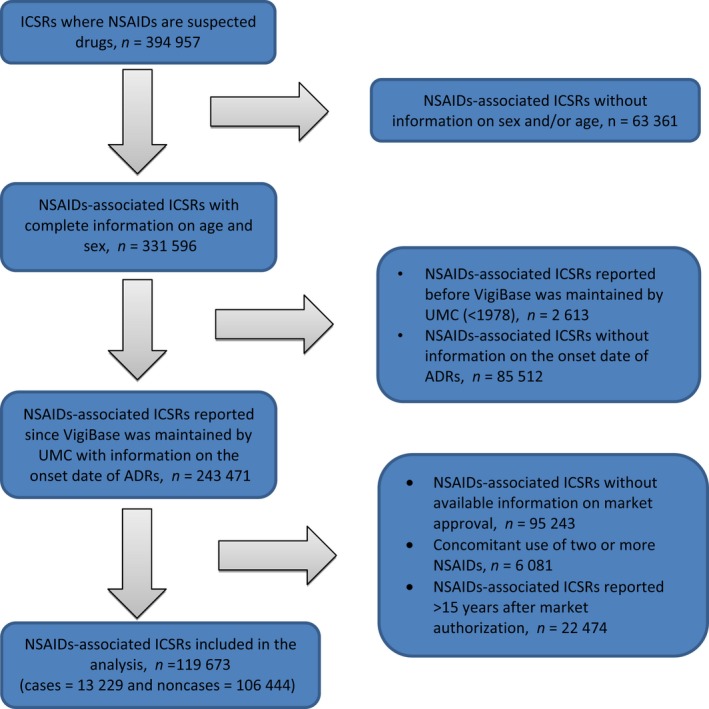
Flowchart describing the inclusion of ICSRs. ADRs, adverse drug reactions; ICSRs, individual case safety reports; NSAIDs, nonsteroidal anti‐inflammatory drugs; UMC, Uppsala Monitoring Centre.
Table 1.
Characteristics of ICSRs where NSAIDs are a suspected drug with hypersensitivity reactions (cases) and without hypersensitivity reactions (noncases)
| Cases (n = 13 229) | Noncases (n = 106 444) | P value | |
|---|---|---|---|
| Age, years (mean ± SD) | 47.8 ± 17.6 | 57.8 ± 18.2 | 0.000* |
| Adults (<60 years old), n (%) | 9 926 (75.0) | 55 544 (52.2) | 0.000* |
| Elderly (≥60 years old), n (%) | 3 303 (25.0) | 50 900 (47.8) | |
| Sex | |||
| Female, n (%) | 9 180 (69.4) | 68 149 (64.0) | 0.000* |
| Male, n (%) | 4 049 (30.6) | 38 295 (36.0) | |
| Reporting NSAID use according to COX selectivity | |||
| Coxibs, n (%) | 3 689 (27.9) | 50 596 (47.5) | 0.000* |
| NSAIDs with poor selectivity, n (%) | 7 706 (58.3) | 41.123 (38.6) | |
| Noncoxib NSAIDs with COX‐2 preference, n (%) | 1 741 (13.2) | 13 680 (12.9) | |
| Unknown potency, n (%) | 93 (0.7) | 1 045 (1.0) | |
| Reporting NSAID use according to chemical groups | |||
| Coxibs, n (%) | 3 689 (27.9) | 50 596 (47.5) | 0.000* |
| Oxicams, n (%) | 3 006 (22.7) | 20 290 (19.1) | |
| Acetic acid derivatives and related substances, n (%) | 2 754 (20.8) | 16 389 (15.4) | |
| Fenamates, n (%) | 2 427 (18.3) | 6 820 (6.4) | |
| Propionic acid derivatives, n (%) | 669 (5.1) | 5 405 (5.1) | |
| Butylpyrazolidines, n (%) | 132 (1.0) | 1 096 (1.0) | |
| Other NSAIDs, n (%) | 552 (4.2) | 5 848 (5.5) | |
| Reporting NSAID use according to the presence/absence of sulfonamide group | |||
| Nonsulfonamide NSAIDs, n (%) | 8 124 (61.4) | 66 380 (62.4) | 0.033* |
| Sulfonamide NSAIDs, n (%) | 5 105 (38.6) | 40 064 (37.6) | |
| Reporting countries | |||
| Thailand, n (%) | 3 268 (24.7) | 5 812 (5.5) | 0.000* |
| United States, n (%) | 2 210 (16.7) | 32 779 (30.8) | |
| Singapore, n (%) | 1 496 (11.3) | 4 388 (4.1) | |
| Great Britain, n (%) | 971 (7.3) | 13 634 (12.8) | |
| South Korea, n (%) | 905 (6.8) | 6 176 (5.8) | |
| Other countries, n (%) | 4 379 (33.1) | 43 655 (41.0) |
COX, cyclo‐oxygenase; ICSRs, individual case safety reports; NSAIDs, nonsteroidal anti‐inflammatory drugs.
Statistically significant (P < 0.05).
Within 5 years after market approval, NSAIDs with poor COX selectivity as suspected drugs were associated with the highest ROR of HSRs (age‐ and sex‐adjusted ROR 2.12, 95% CI 1.98–2.28) compared with coxibs. Of NSAIDs with unknown selectivity, no ICSRs were reported within 10 years after market approval (Figure 2 a and Table S3a). As suspected drugs, out of all the NSAID chemical groups, AADs, fenamates, and PADs were associated with the highest RORs (age‐ and sex‐adjusted ROR 3.07, 95% CI 2.83–3.33; ROR 2.21, 95% CI 1.83–2.66; and ROR 1.93, 95% CI 1.69–2.19) compared with coxibs (Figure 2 b and Table S3b), and sulfonamide NSAIDs were associated with a higher ROR (age‐ and sex‐adjusted ROR 1.38, 95% CI 1.29–1.47) compared with nonsulfonamide NSAIDs (Figure 2 c and Table S3c). After the first 5 years of marketing, most of the RORs returned to approximately 1. Among individual NSAIDs, tolmetin, zomepirac, and loxoprofen were associated with the highest RORs (age‐ and sex‐adjusted ROR 12.83, 95% CI 10.53–15.64; ROR 11.26, 95% CI 9.89–12.81; and ROR 5.73, 95% CI 4.64–7.09) as suspected drugs compared with rofecoxib (Table S3d).
Figure 2.
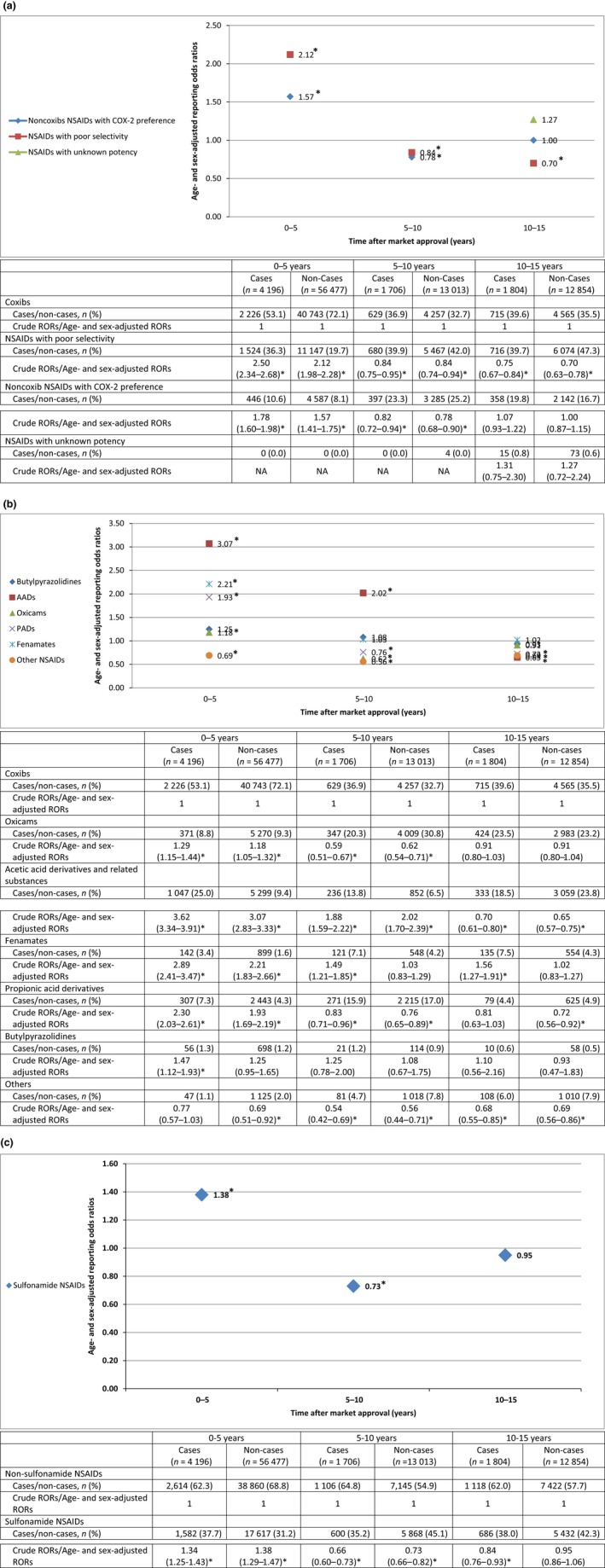
- AADs, acetic acid derivatives and related substances; COX‐2, cyclo‐oxygenase‐2; NA, not applicable; NSAIDs, nonsteroidal anti‐inflammatory drugs; PADs, propionic acid derivatives; RORs, reporting odds ratios.
- *Statistically significant (P < 0.05).
RORs stratified by individual HSRs, reporting countries, sex, and age
The RORs of most frequently reported HSRs (urticaria, angioedema, and anaphylactic shock) were similar to the composite of all HSRs for NSAIDs based on either COX‐2 selectivity, chemical groups, or individual NSAIDs, except for ROR of anaphylactic shock for sulfonamide NSAIDs. Within 5 years after marketing, sulfonamide NSAIDs were associated with a similar ROR of anaphylactic shock compared with nonsulfonamide NSAIDs (Tables S4a–d, S5a–d and S6a–d). Different reporting countries were associated with different RORs for NSAID use based on both COX selectivity and the presence or absence of a sulfonamide group, but not on chemical groups or individual NSAIDs. For non‐US reports, noncoxib NSAIDs with COX‐2 preference and NSAIDs with poor selectivity were associated with similar RORs, but for US reports, these groups were associated with higher RORs compared with coxibs. For non‐US reports, sulfonamide NSAIDs were associated with a higher ROR, but for US reports, this group was associated with a similar ROR compared with nonsulfonamide NSAIDs (Table S7a–d). Finally, differences in age and sex generally were not associated with differences in the RORs (Tables S8a–d and S9a–d).
Sensitivity analyses
Using a broad scope of HSRs to assess the RORs for NSAIDs, the main findings showed similar results (Table S10a–d).
Discussion
Our results show that COX selectivity and chemical groups of NSAIDs, as well as time after market authorization, contribute to the differences in the reporting of HSRs for NSAID use. In the first 5 years after marketing, as suspected drugs, NSAIDs with poor COX selectivity were associated with the highest ROR compared with coxibs. This group was also associated with the highest ROR of urticaria and angioedema compared with coxibs. As suspected drugs, of all chemical groups, AADs, fenamates, and PADs when compared with coxibs, and sulfonamide NSAIDs when compared with nonsulfonamide NSAIDs, were associated with higher RORs. Among individual NSAIDs, tolmetin and zomepirac (AADs) and loxoprofen (a PAD) were associated with the highest RORs compared with rofecoxib. Our sensitivity analyses supported these findings.
Our findings are in line with previous studies. The risk of hospitalization for angioedema was higher for nonselective NSAIDs compared with selective COX‐2 inhibitors 5. Celecoxib (a sulfonamide NSAID) had a higher risk of urticaria compared with rofecoxib (a nonsulfonamide NSAID), although both drugs belong to the coxib group 12. In addition, zomepirac was associated with a high risk of allergy and anaphylaxis compared with other NSAIDs 27, 28.
Both nonallergic and allergic mechanisms might trigger NSAID‐associated HSRs. The inhibition of COX‐1 enzyme alters eicosanoid biosynthesis, leading to a disruption of the arachidonic acid pathway. This induces an imbalance of prostaglandins and cysteinyl leukotriene that is responsible for the clinical features of HSRs 29, 30. The presence of an N1 heterocyclic ring and an arylamine group at the N4 position in a sulfonamide group is expected to be responsible for HSRs in sulfonamide chemotherapeutics. This structure triggers mast cells to release IgE 31, 32. However, as most sulfonamide NSAIDs do not contain this structure, the risk of HSRs probably involves other factors such as the effect of other chemical structures in the sulfonamide group and the involvement of non‐type I immune responses. Parent sulfonamide or its reactive metabolite can cause tissue damage or stimulate cellular or humoral immunity, such as T cells, to initiate an immune response toward haptenation or antigen. HSRs might also more likely occur among those with atopy 31, 33.
Several other factors might also influence the reporting of ADRs next to time after marketing. First, mild ADRs such as urticaria and ADRs that are already included in the summary of product characteristics (SmPC) are less likely to be reported 34. Second, several NSAIDs have been withdrawn from the market during the observation time. From 1982 to 2004, benoxaprofen, oxyphenbutazone, indoprofen, suprofen, pirprofen, fenclofenac, zomepirac, isoxicam, and rofecoxib were withdrawn because of various serious ADRs 28. Moreover, an individual NSAID is often not marketed in the same year in different countries. For example, celecoxib was approved in 1998 for the US market and in 2000 for the Dutch market. Third, when the WHO Programme for International Drug Monitoring was launched in 1968, only 10 countries participated, but currently, VigiBase contains data from >150 countries 13, 14. Finally, an alert from regulatory authorities or media attention on safety issues might have a direct and substantial impact on reporting and clinical practice, such as a decrease in drug prescription or utilization 35, 36, 37.
For US reports, we found differences in RORs of HSRs for sulfonamide NSAIDs compared with our primary analyses. Differences in reporting habits and ethnicity 38, 39 might explain these differences as well as NSAID sales and consumptions between countries. For example, only celecoxib among coxibs is still available in the US market 40, 41.
Strengths and limitations
The strengths of this study are as follows: First, VigiBase contains a large number of spontaneous reporting data collected from national pharmacovigilance centers worldwide, representing >90% of the global population. Second, VigiBase enables one to study ADRs in a realistic setting 42, such as the use of over‐the‐counter medications that often are not recorded in electronic health record databases. Finally, this system can detect reporting ADRs immediately following market launch 43.
Nonetheless, we need to point to several limitations. First, pharmacovigilance data represent <10% of the actual events leading to problems in selective and underreporting, and external validity 39, 44. Second, we cannot completely neglect that within 5 years after market authorization, ADR reports are still possibly affected by factors such as new drug policies from regulatory bodies or safety issues from media. Third, the quality of reports is variable, causing misclassification of exposure, and outcomes such as mild ADRs are less likely to be reported, as mentioned above. Finally, information on COX selectivity was not available for several NSAIDs. However, the proportion of ICSRs for this group was small for both cases and noncases.
Owing to the heterogeneity of reports and the limited clinical data associated with HSRs, especially medical history, we cannot reach definite conclusions. Nonetheless, based on the strength of the associations, supported by the consistency of the findings in our sensitivity analyses, and mechanistic considerations, we detected a possible association between either COX selectivity and chemical groups of NSAIDs, including the presence of a sulfonamide functional group, and HSRs. Further pharmacoepidemiological studies are needed to confirm these potential associations by using conventional electronic health databases and considering several important potential risk factors. These include allergy‐associated factors such as atopy, genetic profiles, positive HSRs from rechallenging NSAIDs, and familial history, as well as co‐medication with drugs such as corticosteroids and antihistamines, and comorbidities such as autoimmune disorders (e.g., rheumatoid arthritis and systemic lupus erythematosus).
It remains important for all stakeholders to be vigilant during the initial marketing phase of a drug. Healthcare professionals should be aware of the benefits and risks when prescribing NSAIDs for those who are in need, particularly during the first 5 years after market approval. Market authorization holders should report periodic safety update profiles of their products. Similarly, regulatory bodies should be reactive when a new signal appears, and proactive in monitoring for specific and unexpected signals or to follow the initial users of a drug.
Conclusion
According to spontaneous reporting data, COX selectivity and chemical groups of NSAIDs have an impact on the risk of HSRs, including angioedema and urticaria. As suspected drugs, NSAIDs with poor COX selectivity, as well as AADs, were associated with the highest RORs of HSRs compared with coxibs, and sulfonamide NSAIDs had highest RORs of HSRs compared with nonsulfonamide NSAIDs.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Conflicts of interest
The authors declare no conflict of interest.
Caveat statement
Although the data are provided by the UMC, the findings and conclusions are from the authors. The information comes from various sources, and the likelihood that the suspected adverse reaction is drug‐related is not the same in all cases. The findings of this study do not represent the opinion of either the UMC, national pharmacovigilance centers, or WHO.
Abbreviations
- AADs
acetic acid derivatives
- ADRs
adverse drug reactions
- ATC
Anatomical Therapeutic Chemical
- CIs
confidence intervals
- COX
cyclo‐oxygenase
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- HSRs
hypersensitivity reactions
- ICSRs
individual case safety reports
- MedDRA
Medical Dictionary for Regulatory Activities
- NSAIDs
nonsteroidal anti‐inflammatory drugs
- PADs
propionic acid derivatives
- PMDA
Pharmaceutical and Medical Devices Agency
- PTs
preferred terms
- RORs
reporting odds ratios
- SMQs
Standardized MedDRA Queries
- UMC
Uppsala Monitoring Centre
- WHO‐ART
WHO Adverse Reaction Terminology
- WHO
World Health Organization
Supporting information
Table S1. Diagnoses of hypersensitivity reactions in MedDRA browser.
Table S2. Categorization of NSAIDs based on COX selectivity as the ratios of inhibitory concentration 80% (IC80) against COX‐2 and COX‐1 enzymes.
Table S3a. Reporting odds ratios of hypersensitivity reactions for any NSAIDs based on cyclooxygenase selectivity.
Table S3b. Reporting odds ratios of hypersensitivity reactions for any NSAIDs based on chemical group.
Table S3c. Reporting odds ratios of hypersensitivity reactions for any NSAIDs according to the presence/absence of sulfonamide group.
Table S3d. Reporting odds ratios of hypersensitivity reactions for individual NSAIDs within 5 years after market approval.
Table S4a. Reporting odds ratios of urticaria for any NSAIDs based on cyclooxygenase selectivity.
Table S4b. Reporting odds ratios of urticaria for any NSAIDs based on chemical group.
Table S4c. Reporting odds ratios of urticaria for any NSAIDs according to the presence/absence of sulfonamide group.
Table S4d. Reporting odds ratios of urticaria for individual NSAIDs within 5 years after market approval.
Table S5a. Reporting odds ratios of angiodema for any NSAIDs based on cyclooxygenase selectivity.
Table S5b. Reporting odds ratios of angioedema for any NSAIDs based on chemical group.
Table S5c. Reporting odds ratios of angioedema for any NSAIDs according to the presence/absence of sulfonamide group.
Table S5d. Reporting odds ratios of angioedema for individual NSAIDs within 5 years after market approval.
Table S6a. Reporting odds ratios of anaphylactic shock for any NSAIDs based on cyclooxygenase selectivity.
Table S6b. Reporting odds ratios of anaphylactic shock for any NSAIDs based on chemical group.
Table S6c. Reporting odds ratios of anaphylactic shock for any NSAIDs according to the presence/absence of sulfonamide group.
Table S6d. Reporting odds ratios of anaphylactic shock for individual NSAIDs within 5 years after market approval.
Table S7a. Reporting odds ratios of hypersensitivity reactions for NSAIDs according to cyclooxygenase selectivity stratified by reporting countries.
Table S7b. Reporting odds ratios of hypersensitivity reactions for any NSAIDs based on chemical group stratified by reporting countries.
Table S7c. Reporting odds ratios of hypersensitivity reactions for any NSAIDs according to the presence/absence of sulfonamide group stratified by reporting countries.
Table S7d. Reporting odds ratios of hypersensitivity reactions for individual NSAIDs within 5 years after market approval stratified by reporting countries.
Table S8a. Reporting odds ratios of hypersensitivity reactions for any NSAIDs based on cyclooxygenase selectivity stratified by age.
Table S8b. Reporting odds ratios of hypersensitivity reactions for any NSAIDs based on chemical group stratified by age.
Table S8c. Reporting odds ratios of hypersensitivity reactions for any NSAIDs according to the presence/absence of sulfonamide group stratified by age group.
Table S8d. Reporting odds ratios of hypersensitivity reactions for individual NSAIDs within 5 years after market approval stratified by age group.
Table S9a. Reporting odds ratios of hypersensitivity reactions for any NSAIDs based on cyclooxygenase selectivity stratified by sex.
Table S9b. Reporting odds ratios of hypersensitivity reactions for any NSAIDs based on chemical group stratified by sex.
Table S9c. Reporting odds ratios of hypersensitivity reactions for any NSAIDs according to the presence/absence of sulfonamide group stratified by sex.
Table S9d. Reporting odds ratios of hypersensitivity reactions for individual NSAIDs within 5 years after market approval stratified by sex.
Table S10a. Reporting odds ratios of hypersensitivity reactions using broad scope definition for any NSAIDs based on cyclooxygenase selectivity.
Table S10b. Reporting odds ratios of hypersensitivity reactions using broad scope definition for any NSAIDs based on chemical group.
Table S10c. Reporting odds ratios of hypersensitivity reactions using broad scope definition for any NSAIDs according to the presence/absence of sulfonamide group.
Table S10d. Reporting odds ratios of hypersensitivity reactions using broad scope definition for individual NSAIDs within 5 years after market approval.
Acknowledgement
The authors thank the World Health Organization (WHO) Collaborating Centre in Uppsala, Sweden, as the data provider and the national pharmacovigilance centers as contributors in the WHO‐ADR database.
References
- 1. Blanca‐Lopez N., J Torres M., Doña I. et al. Value of the clinical history in the diagnosis of urticaria/angioedema induced by NSAIDs with cross‐intolerance. Clin. Exp. Allergy (2013) 43 85–91. [DOI] [PubMed] [Google Scholar]
- 2. Petrisor C., Gherman N., Bologa R. et al. Epidemiology of self‐reported drug‐induced immediate‐type hypersensitivity reactions in the surgical population: a 5‐year single‐center survey in a Romanian allergo‐anaesthesia center. Clujul. Med. (2013) 86 321–326. [PMC free article] [PubMed] [Google Scholar]
- 3. Brockow K. Time for more clinical research on non‐steroidal anti‐inflammatory drug‐induced urticaria/angioedema and anaphylaxis. Clin. Exp. Allergy (2013) 43 5–7. [DOI] [PubMed] [Google Scholar]
- 4. Giavina‐Bianchi P., Aun M.V., Jares E.J., Kalil J. Angioedema associated with nonsteroidal anti‐inflammatory drugs. Curr. Opin. Allergy Clin. Immunol. (2016) 16 323–332. [DOI] [PubMed] [Google Scholar]
- 5. Downing A., Jacobsen J., Sorensen H.T., McLaughlin J.K., Johnsen S.P. Risk of hospitalization for angioedema among users of newer COX‐2 selective inhibitors and other nonsteroidal anti‐inflammatory drugs. Br. J. Clin. Pharmacol. (2006) 62 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borges M.S., Capriles‐Hulett A., Caballero‐Fonseca F., Pérez C.R. Tolerability to new COX‐2 inhibitors in NSAID‐sensitive patients with cutaneous reactions. Ann. Allergy Asthma Immunol. (2001) 87 201–204. [DOI] [PubMed] [Google Scholar]
- 7. Goksel Ö., Aydin O., Misirligil Z., Demirel Y.S., Bavbek S. Safety of meloxicam in patients with aspirin/non‐steroidal anti‐inflammatory drug‐induced urticaria and angioedema. J. Dermatol. (2010) 37 973–979. [DOI] [PubMed] [Google Scholar]
- 8. Valero A., Baltasar M., Enrique E. et al. NSAID‐sensitive patients tolerate rofecoxib. Allergy (2002) 57 1214–1215. [DOI] [PubMed] [Google Scholar]
- 9. Colanardi M.C., Nettis E., Traetta P. et al. Safety of parecoxib in patients with nonsteroidal anti‐inflammatory drug‐induced urticaria or angioedema. Ann. Allergy Asthma Immunol. (2008) 100 82–85. [DOI] [PubMed] [Google Scholar]
- 10. Garcia‐Rodriguez R.M., Hinojosa M., Camacho‐Garrido E., Berges Gimeno P., Martín García C. Celecoxib, safe in NSAID intolerance. Allergy (2002) 57 1085–1086. [DOI] [PubMed] [Google Scholar]
- 11. van Puijenbroek E.P., Egberts A.C., Meyboom R.H., Leufkens H.G. Different risks for NSAID‐induced anaphylaxis. Ann. Pharmacother. (2002) 36 24–29. [DOI] [PubMed] [Google Scholar]
- 12. Wiholm B.E. Identification of sulfonamide‐like adverse drug reactions to celecoxib in the World Health Organization database. Curr. Med. Res. Opin. (2001) 17 210–216. [DOI] [PubMed] [Google Scholar]
- 13. Lindquist M. VigiBase, the WHO global ICSR database system: basic facts. Drug Inf. J. (2008) 42 409–419. [Google Scholar]
- 14. Uppsala Monitoring Centre . WHO Programme Members. 2016. 12 April [cited 2016 May 9, 2016]; Available from: http://www.who-umc.org/DynPage.aspx?id=100653&mn1=7347&mn2=7252&mn3=7322&mn4=7442.
- 15. Warner T.D., Giuliano F., Vojnovic I., Bukasa A., Mitchell J.A., Vane J.R. Nonsteroid drug selectivities for cyclooxygenase‐1 rather than cyclooxygenase‐2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc. Natl Acad. Sci. (1999) 96 7563–7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cryer B., Feldman M. Cyclooxygenase‐1 and cyclooxygenase‐2 selectivity of widely used nonsteroidal anti‐inflammatory drugs. Am. J. Med. (1998) 104 413–421. [DOI] [PubMed] [Google Scholar]
- 17. Grossman C., Wiseman J., Lucas F.S., Trevethick M.A., Birch P.J. Inhibition of constitutive and inducible cyclooxygenase activity in human platelets and mononuclear cells by NSAIDs and Cox 2 inhibitors. Inflamm. Res. (1995) 44 253–257. [DOI] [PubMed] [Google Scholar]
- 18. Kawai S. Cyclooxygenase selectivity and the risk of gastrointestinal complications of various non‐steroidal anti‐inflammatory drugs: a clinical consideration. Inflamm. Res. (1998) 47 102–106. [DOI] [PubMed] [Google Scholar]
- 19. Carbone L.D., Tylavsky F.A., Cauley J.A. et al. Association between bone mineral density and the use of nonsteroidal anti‐inflammatory drugs and aspirin: impact of cyclooxygenase selectivity. J. Bone Miner. Res. (2003) 18 1795–1802. [DOI] [PubMed] [Google Scholar]
- 20. Abraham N.S., El‐Serag H.B., Hartman C., Richardson P., Deswal A. Cyclooxygenase‐2 selectivity of non‐steroidal anti‐inflammatory drugs and the risk of myocardial infarction and cerebrovascular accident. Aliment. Pharmacol. Ther. (2007) 25 913–924. [DOI] [PubMed] [Google Scholar]
- 21. Danelich I.M., Wright S.S., Lose J.M., Tefft B.J., Cicci J.D., Reed B.N. Safety of nonsteroidal anti‐inflammatory drugs in patients with cardiovascular disease. Pharmacotherapy (2015) 35 520–535. [DOI] [PubMed] [Google Scholar]
- 22. De Bruin M., Pettersson M., Meyboom R.H., Hoes A.W., Leufkens H.G. Anti‐HERG activity and the risk of drug‐induced arrhythmias and sudden death. Eur. Heart J. (2005) 26 590–597. [DOI] [PubMed] [Google Scholar]
- 23. Hoffman K.B., Dimbil M., Erdman C.B., Tatonetti N.P., Overstreet B.M. The Weber effect and the United States Food and Drug Administration's Adverse Event Reporting System (FAERS): analysis of sixty‐two drugs Approved from 2006 to 2010. Drug Saf. (2014) 37 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thiessard F., Roux E., Miremont‐Salamé G. et al. Trends in spontaneous adverse drug reaction reports to the French Pharmacovigilance System (1986—2001). Drug Saf. (2005) 28 731–740. [DOI] [PubMed] [Google Scholar]
- 25. Bavbek S., Celik G., Pasaoglu G., Misirligil Z. Rofecoxib, as a safe alternative for acetylsalicylic acid/nonsteroidal anti‐inflammatory drug‐intolerant patients. J. Investig. Allergol. Clin. Immunol. (2006) 16 57–62. [PubMed] [Google Scholar]
- 26. Bavbek S., Celik G., Ozer F., Mungan D., Misirligil Z. Safety of selective COX‐2 inhibitors in aspirin/nonsteroidal anti‐inflammatory drug‐intolerant patients: comparison of nimesulide, meloxicam, and rofecoxib. J. Asthma (2004) 41 67–75. [DOI] [PubMed] [Google Scholar]
- 27. Strom B.L., Carson J.L., Morse M.L., West S.L., Soper K.A. The effect of indication on hypersensitivity reactions associated with zomepirac sodium and other nonsteroidal anti‐inflammatory drugs. Arthritis Rheum. (1987) 30 1142–1148. [DOI] [PubMed] [Google Scholar]
- 28. Onakpoya I.J., Heneghan C.J., Aronson J.K. Worldwide withdrawal of medicinal products because of adverse drug reactions: a systematic review and analysis. Crit. Rev. Toxicol. (2016) 46 477–489. [DOI] [PubMed] [Google Scholar]
- 29. Dona I., Blanca‐Lopez N., Torres M.J. et al. NSAID‐induced urticaria/angioedema does not evolve into chronic urticaria: a 12‐year follow‐up study. Allergy Asthma Immunol. Res. (2014) 69 438–444. [DOI] [PubMed] [Google Scholar]
- 30. Hermans M.A.W., Otten R., Karim A.F., van Maaren M.S. Nonsteroidal anti‐inflammatory drug hypersensitivity: not always an allergy!. Neth. J. Med. (2018) 76 52‐59. [PubMed] [Google Scholar]
- 31. Brackett C.C. Sulfonamide allergy, and cross‐reactivity. Curr. Allergy Asthma Rep. (2007) 7 41–48. [DOI] [PubMed] [Google Scholar]
- 32. Wulf N.R., Matuszewski K.A. Sulfonamide cross‐reactivity: is there evidence to support broad cross‐allergenicity? Am. J. Health Syst. Pharm. (2013) 70 1483–1494. [DOI] [PubMed] [Google Scholar]
- 33. Strom B.L., Schinnar R., Apter A.J. et al. Absence of cross‐reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics. N. Engl. J. Med. (2003) 349 1628–1635. [DOI] [PubMed] [Google Scholar]
- 34. Weber J. Epidemiology of adverse reactions to nonsteroidal anti‐inflammatory drugs. Adv. Inflamm. Res. (1984) 6 1–7. [Google Scholar]
- 35. Dusetzina S.B., Higashi A.S., Dorsey E.R. et al. Impact of FDA drug risk communications on health care utilization and health behaviors: a systematic review. Med. Care (2012) 50 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sessler N.E., Walker E., Chickballapur H., Kacholakalayil J., Coplan P.M. Disproportionality analysis of buprenorphine transdermal system and cardiac arrhythmia using FDA and WHO postmarketing reporting system data. Postgrad. Med. (2017) 129 62–68. [DOI] [PubMed] [Google Scholar]
- 37. Piening S., Haaijer‐Ruskamp F.M., de Vries J.T. et al. Impact of safety‐related regulatory action on clinical practice. Drug Saf. (2012) 35 373–385. [DOI] [PubMed] [Google Scholar]
- 38. Fournier J.P., Sommet A., Durrieu G. et al. Drug interactions between antihypertensive drugs and non‐steroidal anti‐inflammatory agents: a descriptive study using the French Pharmacovigilance database. Fundam. Clin. Pharmacol. (2014) 28 230–235. [DOI] [PubMed] [Google Scholar]
- 39. Alharbi F.F., Kholod A.A.V., Souverein P.C. et al. The impact of age and sex on the reporting of cough and angioedema with renin‐angiotensin system inhibitors: a case/noncase study in VigiBase. Fundam. Clin. Pharmacol. (2017) 31 676–684. [DOI] [PubMed] [Google Scholar]
- 40. La Rochelle P., Lexchin J., Simonyan D. Analysis of the drugs withdrawn from the US market from 1976 to 2010 for safety reasons. Pharm. Med. (2016) 30 277–289. [Google Scholar]
- 41. U.S. Food & Drug Administration . Orange book: approved drug products with therapeutic equivalence evaluations. 2017. 25 April 2017 [cited 2017 April 26]; Available from: https://www.accessdata.fda.gov/scripts/cder/ob/default.cfm.
- 42. Willemen M.J., Mantel‐Teeuwisse A.K., Straus S.M., Meyboom R.H., Egberts T.C., Leufkens H.G. Use of dipeptidyl peptidase‐4 inhibitors and the reporting of infections: a disproportionality analysis in the World Health Organization VigiBase. Diabetes Care (2011) 34 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bate A., Lindquist M., Edwards I. The application of knowledge discovery in databases to post‐marketing drug safety: example of the WHO database. Fundam. Clin. Pharmacol. (2008) 22 127–140. [DOI] [PubMed] [Google Scholar]
- 44. Lumley L.E., Walker S.R., Hall C.G., Staunton N., Grob P.R. The under‐reporting of adverse drug reactions seen in general practice. Pharm. Med. (1986) 1 205–212. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Diagnoses of hypersensitivity reactions in MedDRA browser.
Table S2. Categorization of NSAIDs based on COX selectivity as the ratios of inhibitory concentration 80% (IC80) against COX‐2 and COX‐1 enzymes.
Table S3a. Reporting odds ratios of hypersensitivity reactions for any NSAIDs based on cyclooxygenase selectivity.
Table S3b. Reporting odds ratios of hypersensitivity reactions for any NSAIDs based on chemical group.
Table S3c. Reporting odds ratios of hypersensitivity reactions for any NSAIDs according to the presence/absence of sulfonamide group.
Table S3d. Reporting odds ratios of hypersensitivity reactions for individual NSAIDs within 5 years after market approval.
Table S4a. Reporting odds ratios of urticaria for any NSAIDs based on cyclooxygenase selectivity.
Table S4b. Reporting odds ratios of urticaria for any NSAIDs based on chemical group.
Table S4c. Reporting odds ratios of urticaria for any NSAIDs according to the presence/absence of sulfonamide group.
Table S4d. Reporting odds ratios of urticaria for individual NSAIDs within 5 years after market approval.
Table S5a. Reporting odds ratios of angiodema for any NSAIDs based on cyclooxygenase selectivity.
Table S5b. Reporting odds ratios of angioedema for any NSAIDs based on chemical group.
Table S5c. Reporting odds ratios of angioedema for any NSAIDs according to the presence/absence of sulfonamide group.
Table S5d. Reporting odds ratios of angioedema for individual NSAIDs within 5 years after market approval.
Table S6a. Reporting odds ratios of anaphylactic shock for any NSAIDs based on cyclooxygenase selectivity.
Table S6b. Reporting odds ratios of anaphylactic shock for any NSAIDs based on chemical group.
Table S6c. Reporting odds ratios of anaphylactic shock for any NSAIDs according to the presence/absence of sulfonamide group.
Table S6d. Reporting odds ratios of anaphylactic shock for individual NSAIDs within 5 years after market approval.
Table S7a. Reporting odds ratios of hypersensitivity reactions for NSAIDs according to cyclooxygenase selectivity stratified by reporting countries.
Table S7b. Reporting odds ratios of hypersensitivity reactions for any NSAIDs based on chemical group stratified by reporting countries.
Table S7c. Reporting odds ratios of hypersensitivity reactions for any NSAIDs according to the presence/absence of sulfonamide group stratified by reporting countries.
Table S7d. Reporting odds ratios of hypersensitivity reactions for individual NSAIDs within 5 years after market approval stratified by reporting countries.
Table S8a. Reporting odds ratios of hypersensitivity reactions for any NSAIDs based on cyclooxygenase selectivity stratified by age.
Table S8b. Reporting odds ratios of hypersensitivity reactions for any NSAIDs based on chemical group stratified by age.
Table S8c. Reporting odds ratios of hypersensitivity reactions for any NSAIDs according to the presence/absence of sulfonamide group stratified by age group.
Table S8d. Reporting odds ratios of hypersensitivity reactions for individual NSAIDs within 5 years after market approval stratified by age group.
Table S9a. Reporting odds ratios of hypersensitivity reactions for any NSAIDs based on cyclooxygenase selectivity stratified by sex.
Table S9b. Reporting odds ratios of hypersensitivity reactions for any NSAIDs based on chemical group stratified by sex.
Table S9c. Reporting odds ratios of hypersensitivity reactions for any NSAIDs according to the presence/absence of sulfonamide group stratified by sex.
Table S9d. Reporting odds ratios of hypersensitivity reactions for individual NSAIDs within 5 years after market approval stratified by sex.
Table S10a. Reporting odds ratios of hypersensitivity reactions using broad scope definition for any NSAIDs based on cyclooxygenase selectivity.
Table S10b. Reporting odds ratios of hypersensitivity reactions using broad scope definition for any NSAIDs based on chemical group.
Table S10c. Reporting odds ratios of hypersensitivity reactions using broad scope definition for any NSAIDs according to the presence/absence of sulfonamide group.
Table S10d. Reporting odds ratios of hypersensitivity reactions using broad scope definition for individual NSAIDs within 5 years after market approval.


