Abstract
One of the more recently investigated adverse long‐term side effects of gonadotropin‐releasing hormone (GnRH) agonists for prostate cancer (PCa) is cardiovascular disease (CVD). Studies suggest lower risk of CVD following GnRH antagonists (degarelix) than GnRH agonists. This protocol describes precise codes used to extract variables from five European databases for a study that compares risk of CVD following GnRH agonists and antagonists for PCa.
PCa men on primary GnRH agonists or antagonists were identified from the UK THIN (The Health Improvement Network) database, National Health Service (NHS) Scotland, Belgian Cancer Registry (BCR), Dutch PHARMO Database Network and French National Database (SNIIRAM). Cohort entry was defined as date of treatment initiation. CVD event was defined as any first incident or fatal CVD after cohort entry. Readcodes in THIN and ICD codes in NHS Scotland, BCR, PHARMO and SNIIRAM were used to extract variables. Risk of Bias in Non‐randomised studies of Interventions (ROBINS‐I) tool was used to assess the potential risk of biases in this study. 51 572 men with a median follow‐up time of 2 years started on GnRH agonists and 2 417 men with a median follow‐up time of 1 year started on GnRH antagonists between 2010 and 2017 in the UK, Scotland, Belgium, the Netherlands and France. Data from five countries improved the study power and internal validity required to compare risk of CVD between GnRH agonists and antagonists, the latter being a fairly new drug with limited data in individual countries.
Keywords: cardiovascular disease, GnRH agonists, GnRH antagonists, prostate cancer, real‐world evidence
Introduction
Prostate cancer (PCa) is the most common cancer among men in Europe, with a further increase in projected incidence rates 1, 2. By decreasing male hormone levels, androgen deprivation therapy (ADT) serves as the mainstream treatment for symptomatic PCa. More specifically, ADT is commonly used in men with biochemical relapse after radical prostatectomy (RP), locally advanced PCa and metastasis 3, 4.
Several metabolic side effects have been reported for ADT, including increased body weight, insulin resistance, dyslipidaemia and hyperglycaemia 5, 6, 7, 8. One of the more recently investigated side effects of ADT is an increased risk of cardiovascular diseases (CVD), which is believed to be due to a reduced cardio‐protective effect of testosterone 6, 9, 10, 11, 12. In 2010, the findings from several observational studies 6, 9, 10, 11, 12, 13, 14 prompted the Food and Drug Administration (FDA) to issue a new requirement for manufacturers of certain types of ADT (gonadotropin‐releasing hormone (GnRH) receptor agonists) to add safety information to drug labels in order to warn users of the potential CVD risks involved.
It is therefore of interest to note that degarelix, a newly introduced GnRH receptor antagonist (2010), was suggested to be associated with a lower risk of CVD in PCa men 15, 16. These observations were also supported by preclinical mouse models showing less atherosclerosis and characteristics of metabolic syndrome in mice treated with degarelix as compared to those with orchiectomy or GnRH agonists 17. Even though a recent systematic review 18 suggested that GnRH antagonists may be appropriate for those men with significant CVD risk, existing osteopenia, lower urinary tract symptoms and significant metastatic disease, no results from randomized clinical trials (RCTs) are available to compare the risk of CVD between GnRH agonists and antagonists. The PRONOUNCE trial (ClinicalTrials.gov identifier: NCT02663908), a phase III RCT comparing CVD safety of leuprolide (GnRH agonist) and degarelix (GnRH antagonist), is currently recruiting patients with an anticipated completion date in December 2020 19. An observational study, which directly compared the risk of CVD between GnRH antagonists and GnRH agonists, detected no difference in risk of developing stroke and myocardial infarction (MI). However, overall CVD was not investigated as a specific outcome 20.
Even though the results of the PRONOUNCE trial will inform the long‐term side effects of GnRH analogues, it is equally important that any results obtained are applicable to the general PCa population. Observational studies, when well conducted, provide similar estimates of side effects to RCTs – which is the rationale behind phase IV studies 21. Elderly participants and those with comorbidities, two common characteristics of PCa patients receiving ADT, are often excluded from RCTs 22.
Therefore, we designed a study using real‐world evidence from five countries to provide results that are more applicable to the general PCa population. Moreover, as degarelix was only licensed in 2010, there was a need to combine data from different countries (the United Kingdom (UK), Scotland, Belgium, the Netherlands and France) to obtain a sufficient sample size. Preliminary results of this study were presented at the Annual Meeting of the European Association of Urology, 2018 23 and the Global Cardio‐Oncology Summit, 2018 24.
This study describes a methodological protocol which accounts for heterogeneity in the five databases by making study variables and analyses as homogenous as possible. In this protocol, we describe the codes used to extract study variables from the databases and the processes and challenges encountered in collecting and analysing real‐world data. When designing the protocol for this observational study, we followed the design of a target trial to assess all potential biases by using the Risk Of Bias In Non‐randomised Studies ‐ of Interventions (ROBINS‐I) tool 25.
Materials and methods
Study design
To investigate the association between GnRH agonists or GnRH antagonists and risk of CVD, we designed a prospective cohort study using databases (described in the ‘databases’ section below) from the UK, Scotland, Belgium, the Netherlands and France. The protocol was designed to obtain country‐specific hazard ratios (stage 1), which were pooled in a meta‐analysis (stage 2).
Target trial
A target trial is a pragmatic trial that emulates a hypothetical RCT in non‐randomized studies of interventions (NRSIs) and can thus be considered useful when designing an observational study to assess effects of different types of drugs. The results of NRSIs can be evaluated for any risk of bias (RoB) by using the ROBINS‐I tool 25. The latter is based on seven specific bias domains that address biases at pre‐intervention, during intervention and after intervention 25. To ensure a clinically applicable study design for our real‐world study, we used a modified version of the ROBINS‐I tool to emulate a target trial for risk of CVD following GnRH agonists or GnRH antagonists in men with PCa.
Study population
Men with PCa entered the cohort on the date of treatment (GnRH agonists or GnRH antagonists) initiation. In addition to exposure variable, cohort entry was also determined by the presence of advanced or metastatic PCa where stage of PCa was available (Belgium and the Netherlands). Once an individual entered the cohort, they stayed on that treatment regime until time of censoring.
Databases
The health improvement network
The Health Improvement Network (THIN) database is an electronic database that covers more than 11 million patients in the UK and is representative of 6.2% of the UK population 26, 27. The database comprises of longitudinal, anonymized data processed and validated by Cegedim Strategic Data (CSD) Medical Research UK. THIN is organized into seven different files (Figure 1 ), which are extracted from general practices (GP) in the UK using the VISION 28 system. The data are coded using standardized codes called the ‘readcodes’ 29 or ‘medcodes’ and ‘drugcodes’. As some individuals may be present in both THIN and National Health Service (NHS) Scotland databases, PCa men from Scotland were excluded from THIN. The study period used for this project extended from 2010 to 2016.
Figure 1.
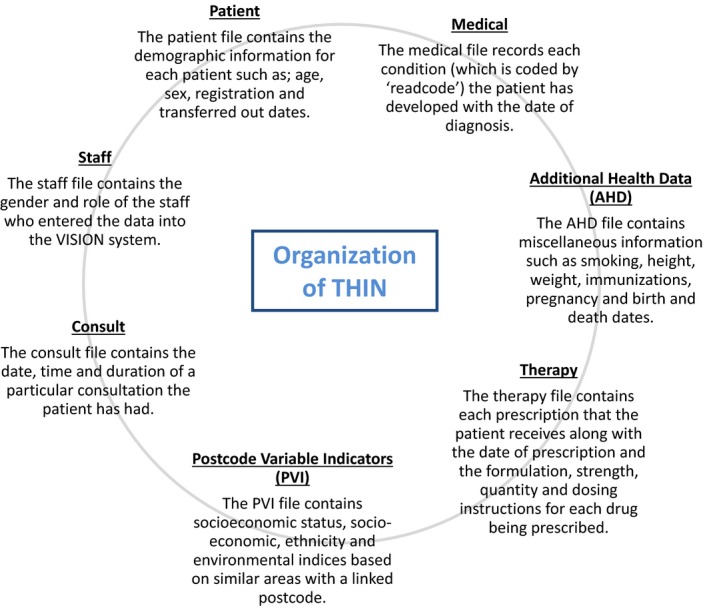
Organization of data in the THIN database.
National health service Scotland
Data were linked from five databases in Scotland 30: the Scottish Cancer Registry, the Scottish National Prescribing Information System (PIS), the General or Acute Inpatient and Day Case dataset (SMR01), the Outpatient Attendance dataset (SMR00) and the National Records of Scotland Death Records (NRSDR) using the unique identifier number, Community Health Index Number. The resulting dataset captures information on PCa diagnosis and treatment (from the Scottish Cancer Registry), community prescriptions in Scotland (PIS), hospital diagnoses and operations (SMR01), diagnoses and procedures from outpatient clinics (SMR00) and the date and cause of death (NRSDR) 30. Men diagnosed with PCa from 2010 to 2015 with follow‐up until 2017 were part of this study.
Belgian cancer registry
All new cancer cases are legally required to be registered in Belgium in the Belgian Cancer Registry (BCR) 31. The database constitutes of population‐based clinical–pathological information on new cancer diagnoses with almost complete coverage of the Belgian population since 2004. Administrative data on reimbursed medical acts and dispensed in‐ and outpatient medications are provided to the BCR by the health insurance companies (HIC), covering a period from 1 year before until 5 years after the date of cancer diagnosis 32. The HIC data contain information regarding the date and type of charged diagnostic and therapeutic procedures, and regarding the date, amount and dosages of dispensed medications. Following specific authorizations, hospital discharge data (HDD) covering hospitalizations of the patients registered by BCR from the year prior to the incidence date onwards are made available using specific codes 33. These records contain information on hospital admission and discharge dates, diagnoses and procedures for each hospitalization. Both HIC and HDD data are deterministically coupled to the BCR database, using the national social security number as a unique patient identifier. Cause of death information for all Belgian inhabitants is provided by the three different Belgian regions and probabilistically coupled to the BCR data (coupling percentage 98%). The current project used data from 2010 to 2013.
PHARMO Database Network
The PHARMO Database Network is a population‐based network of healthcare databases combining data from both primary and secondary healthcare settings in the Netherlands 34. These different data sources, including data from GPs, in‐ and outpatient pharmacies, clinical laboratories, hospitals, the cancer registry, pathology registry and perinatal registry, are linked on a patient level through validated algorithms. Detailed information on the methodology and the validation of the used record linkage method can be found elsewhere 35. For this study, data from the Out‐patient Pharmacy Database, Hospitalisation Database and Cancer Registry were used. The Out‐patient Pharmacy Database includes detailed information on GP or specialist prescribed healthcare products dispensed by outpatient pharmacies. The dispensing records include information on type of product, date, strength and dosage regimen, quantity, route of administration, prescriber specialty and costs. The Hospitalisation Database comprises of hospital admissions for more than 24 hours and admissions for less than 24 hours, for which a bed was required (i.e. inpatient records) from the Dutch Hospital Data Foundation. The records include information on hospital admission and discharge dates, discharge diagnoses and procedures. The Cancer Registry comprises information on newly diagnosed cancer patients in the Netherlands 34. For the current project, we used data from 2010 to 2015.
French Health National Database (SNIIRAM)
The French Health National Database based on claims data called the Système National d'Informations Inter‐Régimes de l'Assurance Maladie (SNIIRAM) was used for this study 36. SNIIRAM combines reimbursed claims from insurance plans with the National Hospital discharge Summaries database system (PMSI). As of 2016, the SNIIRAM includes 98.8% of the French population with follow‐up from birth to death 37. The database includes information on patient demographics, hospital and clinical visits, diagnoses of hospitalized patients (extracted using ICD‐10 codes from hospital visits) and chronic medical conditions. Data between 2010 and 2013 were used for this study.
Study variables
Exposure variable
The exposure variable was defined as prescription or dispensation of GnRH agonists or GnRH antagonists. PCa men who were hormone‐treatment naïve were followed from date of first prescription or dispensation until censoring (defined below).
Outcome variables
The outcome variable was defined as first (incident or fatal) CVD event (ICD‐10: I20‐I99, G45 or ICD‐9 equivalent) following GnRH agonists or antagonists initiation. In addition to overall CVD, the following five types of CVD were considered: ischaemic heart disease (IHD) (ICD‐10: I20‐I25), acute myocardial infarction (AMI) (ICD‐10: I21), arrhythmia (ICD‐10: I44‐I49), heart failure (HF) (ICD‐10: I50, I97.710, I97.790, I11.0) and stroke (ICD‐10: I60‐64, G45). The THIN database made use of already published readcodes 29 similar to the ICD codes.
Censoring
The censoring point was defined as any of the first occurring among the following: outcome, switch between GnRH agonists and antagonists and vice versa, orchiectomy, end of study period or death from other causes than CVD death during the study period, whichever came first. Since the six CVD outcomes were studied separately, only the first event of the interested outcome at the time of analysis was considered. For example, when IHD was studied as an outcome, men were censored at first incident or fatal IHD. Any CVD, AMI, arrhythmia, heart failure and stroke after treatment initiation were overlooked, even if these had occurred before the IHD event.
Other study variables
Age
Age was considered as a timescale in all analytical models and was defined at date of GnRH agonists or antagonists' initiation. 5 562 men in THIN had missing date of births which were imputed using multiple imputation. Age for all men in PHARMO was calculated using the same random day and month (12th June) as it only contained the year of birth.
Follow‐up time
The median follow‐up time and upper and lower quartiles were calculated for all countries. Follow‐up time began on the date of treatment initiation and ended when they reached any of the censoring criteria discussed above.
Year of PCa diagnosis
Year of PCa diagnosis was extracted for all countries except for France, where data for the year of PCa diagnosis was not available.
Stage of PCa
PCa stage was available for Scotland, Belgium and the Netherlands, recorded at the time of PCa diagnosis. It was defined as locally advanced (T3a/bT4 N0M0) and metastatic (TxNxM1), as most men with PCa on long‐term GnRH analogues are categorized into these stages. Further PCa stage subgroups were distinguished as: TxNxM1, TxN1M0, T3aNxMx, T3bNxMx and T4NxMx in Belgium.
Total Gleason Score
Total Gleason Score (GS) was available for Scotland and the Netherlands and was divided into Gleason 5–6, 7, 8, 9–10 and missing. In the Netherlands, men with invalid GS (nine patients) were included in the missing category.
Prostate‐specific antigen
Prostate‐specific antigen (PSA), only available for the Netherlands, was categorized into ≤ 10, 11–20, 21–50 and > 50 ng/mL.
Any prior PCa treatment
Some information on PCa treatment before GnRH initiation was available for all five countries. This included men who had undergone any form of PCa treatment prior to GnRH initiation such as radical prostatectomy, radical prostatectomy and adjuvant or salvage radiotherapy (Belgium only), radiotherapy, chemotherapy (the Netherlands only) and anti‐androgens. In Belgium, radiotherapy was further split into palliative radiotherapy (1–10 fractions) and long course external beam radiotherapy (+/‐ brachytherapy).
Type of ADT
This variable indicated whether ADT (only in the form of GnRH agonists or antagonists) was given as primary, adjuvant, neo‐adjuvant treatment or other (Belgium only). No distinction between primary, neo‐adjuvant and adjuvant ADT was made in the UK due to a lack of accurate data availability on radiotherapy given to men on ADT. An ADT prescription in Belgium and Scotland was considered neo‐adjuvant if it appeared in the database within 1 month before PCa incidence and the date of surgery or radiotherapy. An adjuvant ADT prescription was defined as a prescription of GnRH agonists or antagonists within a 6 months' period following surgery or radiotherapy. PCa men for whom a treatment (ADT) was found but had not fulfilled the definitions of primary, adjuvant or neo‐adjuvant ADT treatment (e.g. ADT treatment started more than 6 months following surgery) were classed into the ‘other’ category. In the Netherlands, the cancer registry only had treatment information given at PCa diagnosis and 6 months after diagnosis and combination treatment modalities were not derived for the study. In France, information for radiotherapy (especially dosages) was not available, and therefore, a distinction between primary, adjuvant and neo‐adjuvant was not made.
ADT specifics
This variable showed whether ADT was prescribed in combination with anti‐androgens as flare protection or combined androgen blockade (CAB). Flare protection was defined as receiving anti‐androgens for ≤ 30 days, whereas CAB was defined as receiving anti‐androgens for more than 30 days.
History of CVD indicator
History of CVD indicator (HCVDi) was defined as any of the following 12 months prior to entering the cohort: any CVD event (ICD‐10 codes: I20‐I99, G45), hypertension (ICD‐10 and ATC codes – Figure 2), dyslipidaemia (ATC codes or drugcodes – Table III) or diabetes (ATC codes or drugcodes – Table III). HCVDi was further subcategorized to specifically indicate history of hypertension, dyslipidaemia or diabetes 12 months prior to ADT initiation.
Figure 2.

Algorithm to define hypertension (HTA) used by France and the Netherlands (Panel 1) and modified algorithm used by Belgium (Panel 2).
Number of previous CVD events
The number of CVD events prior to entering the cohort was coded as 0, 1, 2 or ≥ 3 CVD events. As data in Belgium were only available 1 year before first ADT prescription, previous CVD events and time of last previous CVD were limited to the 12 months prior to entering the cohort. The previous history of CVD was stratified as time of last previous CVD, defined as no CVD, 0–3 months, 4–6 months, 7–12 months prior to treatment initiation.
Socio‐demographic variables
Body mass index (BMI), socio‐economic status (SES), civil status, smoking status and ethnicity were extracted in the UK using the readcodes (Table II for specific codes). BMI was defined as: underweight at ≤ 18.5 kg/m2, normal at 18.6–24 kg/m2, overweight at 25–30 kg/m2 and obese at ≥ 30 kg/m2. Townsend scores 38 were used to extract the SES of the study population. Townsend scores incorporated four different variables: unemployment, non‐car ownership, non‐home ownership and household overcrowding. The Townsend scores were given as quintiles (i.e. five groups of equal size ranging from 1 (least deprived) to 5 (most deprived) 38). In THIN, civil status was coded as 12 different codes that were combined to form three categories: single, married and unknown (Table II). Smoking status was defined as: current smokers, non‐smokers and past smokers. Ethnicity was defined as men with an origin of: Caucasian, Black, Asian and other (readcodes other than these three categories).
Analysis
The analysis was conducted in two stages: stage 1 analysis was used to assess heterogeneity and prescription patterns in different countries and stage 2 was a pooled analysis of PCa cohorts from five countries using meta‐analytical techniques to pool the results. Results of the meta‐analysis will be reported in the main study article.
Stage 1 analysis
Country‐specific estimates of hazard ratios were calculated using Cox proportional hazard models with age as a timescale. When using age as a timescale, men entered the cohort at baseline age (left‐truncation) and exited at CVD event age or censoring age. Stage 1 analysis was conducted in four separate steps: (i) age‐adjusted analysis with CVD as outcome, (ii) stratified analysis based on HCVDi, (iii) multivariable analysis including HCVDi and (iv) multivariable analysis including HCVDi and number of previous CVD events.
Stage 2 analysis
In the second stage, a random‐effects meta‐analytic model was performed to compare the pooled log‐transformed country‐specific hazard ratios for CVD following GnRH agonists and GnRH antagonists. The percentage of variation between the databases was assessed using the I 2 statistic. Each country in the meta‐analysis was weighted by the inverse of its variance (i.e. hazard ratios), and adjustment to the weight was made based upon the degree of heterogeneity between the five countries. Heterogeneity in the assessment of exposure and outcome data was further evaluated by performing sensitivity analyses. This included only those countries that had collected data in a similar way – incident CVD (ICD‐9‐CM codes) sourced from hospital discharge date and fatal CVD (ICD‐10 codes) sourced from death certificates in Belgium, ICD‐10 codes in Scotland, the Netherlands and France versus readcodes in the UK. Additional stratifications by HCVDi as well as age (< 75 and ≥ 75 years) were conducted to assess effect modification in all countries.
Results
Table 1 shows the modified ROBINS‐I tool used to compare a target trial with this study. This informed the inclusion/exclusion criteria for the real‐world study population as well as the definitions of all relevant exposures, outcomes and study variables. The aim of using the ROBINS‐I tool was to understand the types of biases and challenges involved when dealing with real‐world, heterogeneous data sources. The ROBINS‐I tool highlighted unmeasured confounding, channelling and misclassification biases.
Table 1.
Target trial using ROBINS‐I 25 tool
| Types of bias addressed | Trial characteristics | Challenges encountered | |
|---|---|---|---|
| Target trial | This study | ||
| Randomization distribution | 50/50 split | Uneven number of patients in GnRH agonists and GnRH antagonists | Observational data do not guarantee even distribution between trial arms |
| Information bias | Information on compliance to treatment | An individual is assumed to be in the same cohort at end of study as they are in at start of the study | There is no information on compliance in most observational databases |
| Unmeasured confounding | Lifestyle and socio‐demographic factors | Information used for lifestyle and socio‐demographic variables | Lifestyle factors are often not well recorded in healthcare databases leading to an unmeasured confounding. The UK was the only country with data on some lifestyle (BMI, smoking status) and socio‐economic (Townsend scores) factors recorded. However, due to high missing data, these variables were not added to the analytical models |
| Unmeasured confounding | Concomitant medications, history of specific diseases | History of CVD indicator | Although CVD risk factors such as HTN, DM and DYS were adjusted for in HCVDi, there may be other unmeasured concomitant medications that we may not have taken into account, leading to unmeasured confounding. This will be addressed in the main study article by calculating E‐values to assess the strength of unmeasured confounding in our study |
| Channeling bias | GnRH antagonists to patients with no history of CVD | Men with a history of CVD may be prescribed GnRH antagonists | GnRH antagonists may have been preferentially ‘channeled’ to patients who may have been at risk of a CVD leading to a channeling bias. This has to be considered when interpreting the results of this study |
| Classification bias | Uniform coding system to define exposure and outcome variables | Readcodes & drugcodes for UK and ATC codes & ICD codes for Scotland, Belgium, the Netherlands and France | It was difficult to homogenize the coding system fully across the five countries in this study, due to heterogeneity in the data collection methods |
| Immortal time bias | Information on GnRH agonists and GnRH antagonists dispensation | Prescription database in the UK. Dispensing database in Scotland, Belgium, the Netherlands and France | Prescription databases usually do not hold information on whether the patient has adhered to their prescribed treatment. For example, a man with PCa may be prescribed GnRH antagonists on 1st November but may not visit their health care professional on the same day for their injection. This introduces a lag time between the prescription date and dispensation/injection date resulting in an immortal time bias. A sensitivity analysis excluding the UK accounted for immortal time bias |
| Immeasurable time bias | Medications given at hospital visits during the follow‐up time | Hospital data were not available for the UK and medications from the inpatient pharmacy was not available for France and the Netherlands | Immeasurable time bias arises from the presence of an unidentified hospitalization within a database 49. Records of medications administered during a hospital visit may not have been available during the study period. Data for unidentified hospitalization were not available in the five countries |
HTN, Hypertension; DYS, dyslipidaemia; DM, diabetes mellitus.
Table 2 shows the study period, number of men with PCa on GnRH agonists and antagonists and follow‐up time (median and quartiles) for the UK, Scotland, Belgium, the Netherlands and France. Total median follow‐up time for the UK, Scotland, Belgium, the Netherlands and France was 2 (1.1–2.8) years for GnRH agonists and 1 (0.7–1.8) year for GnRH antagonists. High missing numbers for socio‐demographic confounders (BMI, SES, smoking status and civil status) resulted in an exclusion of these variables from the analytical models. Table 3 shows detailed codes used to extract study variables from four databases.
Table 2.
Study period, number of men with prostate cancer on GnRH agonists and antagonists, and median follow‐up time five European databases: the United Kingdom, Scotland, Belgium, the Netherlands and France
| United Kingdom (excluding Scotland) | Scotland | Belgium | Netherlands | France | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men on GnRH agonists | Men on GnRH antagonists | Men on GnRH agonists | Men on GnRH antagonists | Men on GnRH agonists | Men on GnRH antagonists | Men on GnRH agonists | Men on GnRH antagonists | Men on GnRH agonists | Men on GnRH antagonist | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Study period | 2010‐2016 | 2010‐2017 | 2010‐2015 | 2010‐2015 | 2010‐2013 | |||||
| Number of PCa men | 16 955 (99.3) | 118 (0.7) | 11 929 (94.0) | 768 (6.0) | 1 860 (78.1) | 522 (21.9) | 1 187 (92.5) | 97 (7.6) | 19 641 (83.9) | 912 (3.9) |
| Follow‐up time, years | ||||||||||
| Median | 0.6 | 0.5 | 2.1 | 0.8 | 1.7 | 1.1 | 2.1 | 1.3 | 2.4 | 2.3 |
| Lower quartile | 0.2 | 0.1 | 1.1 | 0.6 | 0.8 | 0.5 | 1.1 | 0.6 | 2.1 | 1.9 |
| Upper quartile | 1.8 | 1.2 | 2.9 | 1.1 | 3.0 | 1.8 | 3.4 | 2.2 | 2.7 | 2.6 |
Table 3.
Codes used for the databases from the UK, France, Belgium and the Netherlands to extract study variables
| Variables | Definitions | Codes used | |||
|---|---|---|---|---|---|
| United Kingdom | France | Belgiuma | Netherlands | ||
| Exposures defined by drugcodes in UK and ATC codes in France, Belgium and the Netherlands | |||||
| GnRH agonists | Prescription or dispensation of: Leuprorelin Acetate (Eligard), Leuprorelin Acetate (Lucrin), Leuprorelin Acetate (Generics) | Drugcodes: 39797978, 42604978, 61916979, 61917979, 61918979, 61919979, 88842998, 88845998, 92404979, 92405979, 92412979, 96945998, 97368998, 81332998, 81333998 | ATC: L02AE02 | ATC: L02AE02 | ATC: L02AE02 |
| Prescription or dispensation of: Goserelin (Zoladex) | Drugcodes: 82646998, 91057998, 91058998, 92434979, 92436979, 92439979, 92444979, 94894998, 94895998 | ATC: L02AE03 | ATC: L02AE03 | ATC: L02AE03 | |
| Prescription or dispensation of: Buserelin | Drugcodes: 94133997, 94133998, 97430997, 97430998 | ATC: L02AE02 | ATC: L02AE02 | ATC: L02AE02 | |
| Triptorelin (Decapeptyl or Gonapeptyl Depot) | Drugcodes: 81648998, 81649998, 81699998, 81700998, 87670998, 87671998, 87744998, 87745998, 91336998, 91337998 | ATC: L02AE04 | ATC: L02AE04 | ATC: L02AE04 | |
| GnRH antagonist | Prescription or dispensation of: Degarelix | Drugcodes: 82881998, 82882998, 82886998, 82887998 | ATC: L02BX02 | ATC: L02BX02 | ATC: L02BX02 |
| Outcomes defined by readcodes in UK and ICD codes in France, Belgium and the Netherlands | |||||
| Any CVD | First incident or fatal any CVD | G3…00, G3…11, G3…12, G3…13, G30..00, G30..12, G30..13, G30..15, G30..16, G30..17, G300.00, G301.00, G301000, G301100, G301z00, G302.00, G303.00, G304.00, G305.00, G306.00, G307.00, G307000, G307100, G308.00, G309.00, G30B.00, G30X.00, G30X000, G30y.00, G30y100, G30y200, G30yz00, G30z.00, G31..00, G310.00, G310.11, G311.00, G311.11, G311.12, G311.13, G311.14, G311000, G311011, G311100, G311200, G311300, G311500, G311z00, G31y.00, G31y000, G31y200, G31y300, G31yz00, G32..00, G32..11, G32..12, G33..00, G330.00, G330000, G330z00, G331.00, G331.11, G332.00, G33z.00, G33z000, G33z100, G33z200, G33z300, G33z400, G33z500, G33z600, G33z700, G33zz00, G34..00, G340.00, G340.11, G340.12, G340000, G340100, G342.00, G343.00, G344.00, G34y.00, G34y000, G34y100, G34yz00, G34z.00, G34z000, G35..00, G350.00, G351.00, G353.00, G35X.00, G360.00, G362.00, G363.00, G364.00, G365.00, G38..00, G380.00, G381.00, G384.00, G38z.00, G3y..00, G3z..00, G574011, G575.00, G575.11, G575.12, G575000, G575100, G575z00, G61..00, G61..11, G61..12, G610.00, G611.00, G612.00, G613.00, G614.00, G615.00, G616.00, G617.00, G618.00, G61X.00, G61X000, G61X100, G61z.00, G63..00, G63..11, G632.00, G63y000, G63y100, G64..12, G64..13, G640.00, G640000, G641.00, G641.11, G641000, G64z.00, G64z.11, G64z.12, G64z000, G64z111, G64z200, G64z300, G64z400, G66..00, G66..11, G66..12, G66..13, G661.00, G662.00, G663.00, G664.00, G665.00, G666.00, G667.00, G668.00, G671.00, G671000, G671z00, G676000, G6W..00, G6X..00, G70..00, G700.00, G73..00, G73..12, G73y.00, G73yz00, G73z.00, G73z000, G73z011, G73zz00, G742z00, G74y300, G76z000, Gyu3.00, Gyu3200, Gyu3300, Gyu3400, Gyu3600, Gyu6200, Gyu6300, Gyu6400, Gyu6F00, Gyu6G00, Gyu7400 | ICD 10: I20‐I99, G45 | ICD 10: I20‐I99; G45ICD 9 : 4111 ‐ 4131 ‐ 4130 ‐ 4139 – 41000 ‐ 41001 ‐ 41002 ‐ 41010 ‐ 41011 ‐ 41012 ‐ 41030 ‐ 41031 –41032 ‐ 41020 ‐ 41021 ‐ 41022 ‐ 41040 ‐ 41041 ‐ 41042 ‐ 41050 – 41051 ‐ 41052 ‐ 41060 ‐ 41061 ‐41062 ‐ 41080 ‐ 41081 ‐ 41082 – 41090 ‐ 41091 ‐ 41092 ‐ 41070 ‐ 41071 ‐ 41072 ‐ 42979 ‐ 41181 –4110 ‐ 41189 ‐ 41400 ‐ 41401 ‐ 4292 ‐ 412 ‐ 41410 ‐ 41419 – 41411 ‐ 41412 ‐ 4148 ‐ 41402 – 41403 ‐ 41404 ‐ 41405 ‐ 41406 – 41407 ‐ 4142 ‐ 4143 ‐ 4144 ‐4149 ‐ 42611 ‐ 42612 ‐ 42613 – 4260 ‐ 42610 ‐ 42650 ‐ 4262 – 4263 ‐ 4264 ‐ 42651 ‐ 42652 ‐ 42653 ‐ 42654 ‐ 4266 – 4267 ‐ 42681 ‐ 42682 ‐ 42689 –4269 ‐ 4275 ‐ 4270 ‐ 4271 ‐ 4272 – 42731 ‐ 42732 ‐ 42741 ‐ 42742 – 42761 ‐ 42769 ‐ 42760 ‐ 42781 –42789 ‐ 4279 ‐ 4281 ‐ 42820 – 42821 ‐ 42822 ‐ 42823 ‐ 42830 – 42831 ‐ 42832 ‐ 42833 ‐ 42840 –42841 ‐ 42842 ‐ 42843 ‐ 4280 – 4289 ‐ 9971 ‐ 40201 ‐ 40211 – 40291 ‐ 430 ‐ 431 ‐ 4321 ‐ 4320 –4329 ‐ 43321 ‐ 43311 ‐ 43391 – 43301 ‐ 43401 ‐ 43411 ‐ 43491 – 43331 ‐ 43381 ‐ 4350 ‐ 4351 –4353 ‐ 4358 ‐ 4377 ‐ 4352 – 4359 | ICD 10: I20‐I99, G45 |
| Ischaemic Heart Disease | First incident or fatal IHD | G3…00, G3…11, G3…12, G3…13, G30..00, G30..11, G30..12, G30..13, G30..14, G30..15, G30..16, G30..17, G300.00, G301.00, G301000, G301100, G301z00, G302.00, G303.00, G304.00, G305.00, G306.00, G307.00, G307000, G307100, G308.00, G309.00, G30A.00, G30B.00, G30X.00, G30X000, G30y.00, G30y000, G30y100, G30y200, G30yz00, G30z.00, G31..00, G310.00, G310.11, G311.00, G311.11, G311.12, G311.13, G311.14, G311000,, G311011, G311100, G311200, G311300, G311400, G311500, G311z00, G312.00, G31y.00, G31y000, G31y100, G31y200, G31y300, G31yz00, G32..00, G32..11, G32..12, G33..00, G330.00, G330000, G330z00, G331.00, G331.11, G332.00, G33z.00, G33z000, G33z100, G33z200, G33z300, G33z400,G33z500, G33z600, G33z700, G33zz00, G34..00, G340.00, G340.11, G340.12, G340000, G340100, G342.00, G343.00, G344.00, G34y.00, G34y000, G34y100, G34yz00, G34z.00, G34z000, G35..00, G350.00, G351.00, G353.00, G35X.00, G36..00, G360.00, G361.00, G362.00, G363.00, G364.00, G365.00, G366.00, G38..00, G380.00, G381.00, G384.00, G38z.00, G3y..00, G3z..00 | ICD 10: I20‐I25 | ICD 10: I20‐I25ICD 9 : 4111 ‐ 4131 ‐ 4130 – 4139 ‐ 41000 ‐ 41001 ‐ 41002 – 41010 ‐ 41011 ‐ 41012 ‐ 41030 –41031 ‐ 41032 ‐ 41020 ‐ 41021 – 41022 ‐ 41040 ‐ 41041 ‐ 41042 – 41050 ‐ 41051 ‐ 41052 ‐ 41060 –41061 ‐ 41062 ‐ 41080 ‐ 41081 – 41082 ‐ 41090 ‐ 41091 ‐ 41092 – 41070 ‐ 41071 ‐ 41072 ‐ 42979 –41181 ‐ 4110 ‐ 41189 ‐ 41400 – 41401 ‐ 4292 ‐ 412 ‐ 41410 – 41419 ‐ 41411 ‐ 41412 ‐ 4148 – 41402 ‐ 41403 ‐ 41404 ‐ 41405 – 41406 ‐ 41407 ‐ 4142 ‐ 4143 – 4144 ‐ 4149 | ICD 10: I20‐I25 |
| Acute Myocardial Infarction | First incident or fatal AMI | 323..00, 3233.00, 3234.00, 3235.00, 3236.00, 323Z.00, 889A.00, G30..00, G30..11, G30..12, G30..13, G30..14, G30..15, G30..16, G30..17, G300.00, G301.00, G301000, G301100, G301z00, G302.00, G303.00, G304.00, G305.00, G306.00, G307.00, G307000, G307100, G308.00, G309.00, G30A.00, G30B.00, G30X.00, G30X000, G30y.00, G30y000, G30y100, G30y200, G30yz00, G30z.00, G310.11, G31y100, G35..00, G350.00, G351.00, G353.00, G35X.00, G36..00, G360.00, G361.00, G362.00, G363.00, G364.00, G365.00, G366.00, G38..00, G380.00, G381.00, G384.00, G38z.00, G501.00, Gyu3400 | ICD 10: I21 | ICD 10: I21ICD 9 : 41000 ‐ 41001 ‐ 41002 – 41010 ‐ 41011 ‐ 41012 ‐ 41030 – 41031 ‐ 41032 ‐ 41020 ‐ 41021 –41022 ‐ 41040 ‐ 41041 ‐ 41042 – 41050 ‐ 41051 ‐ 41052 ‐ 41060 – 41061 ‐ 41062 ‐ 41080 ‐ 41081 ‐41082 ‐ 41090 ‐ 41091 ‐ 41092 – 41070 ‐ 41071 – 41072 | ICD 10: I21 |
| Arrhythmia | First incident or fatal arrhythmia | 14AN.00, 14AR.00, 212R.00, 327..00, 3272, 3273, 328..00, 3282, 328Z.00, 662S.00, 6A9..00, 7936A00, 8CMW200, 8HTy.00, 9Os..00, 9Os0.00, 9Os1.00, 9Os2.00, 9Os3.00, 9Os4.00, 9hF..00, 9hF1.00, G559.00, G55A.11, G56..00, G56..11, G567400, G56y.00, G56y000, G56zz00, G57..00, G57..11, G570.00, G570000, G570100, G570200, G570300, G570z00, G571.00, G571.11, G572.00, G572000, G572z00, G573.00, G573000, G573100, G573200, G573300, G573400, G573500, G573600, G573z00, G574.00, G574100, G574z00, G576300, G576400, G576500, G57y.00, G57y600, G57y900, G57yA00, G57yz00, G57z.00, Gyu5a00, I45.6, I47, I47.0, I47.1, I47.2, I47.9, I48, I49, I49.0, I49.1, I49.2, I49.3, I49.4, I49.5, R00.0 | ICD 10: I44‐ I49 | ICD 10: I44‐ I49ICD 9 : 42611 ‐ 42612 ‐ 42613 – 4260 ‐ 42610 ‐ 42650 ‐ 4262 – 4263 ‐ 4264 ‐ 42651 ‐ 42652 – 42653 ‐ 42654 ‐ 42650 ‐ 4266 – 4267 ‐ 42681 ‐ 42682 ‐ 42689 –4269 ‐ 4275 ‐ 4270 ‐ 4271 ‐ 4272 – 42731 ‐ 42732 ‐ 42741 ‐ 42742 – 42761 ‐ 42769 ‐ 42760 ‐ 42781 –42789 – 4279 | ICD 10: I44‐ I49 |
| Heart Failure | First incident or fatal HF | 1O1..00, 402 C, 4270, 4270C, 4270CC, 4270D, 4270DR, 4270LW, 4270R, 4271, 4271A, 4271H, 428 A, 7824A, 7824AC, 7824FC, 7824FH, G1yz100, G232.00, G234.00, G58..00, G58..11, G580.00, G580.11, G580.12, G580.13, G580.14, G580000, G580100, G580200, G580300, G581.00, G581.11, G581.12, G581.13, G581000, G582.00, G58z.00, G58z.12 | ICD 10: I50, I97.710, I97.790, I11.0 | ICD 10: I50, I97.710, I97.790, I11.0ICD 9 :4281 ‐ 42820 ‐ 42821 – 42822 ‐ 42823 ‐ 42830 ‐ 42831 – 42832 ‐ 42833 ‐ 42840 ‐ 42841 – 42842 ‐ 42843 ‐ 4280 ‐ 4289 – 9971 ‐ 40201 ‐ 40211 – 40291 | ICD 10: I50, I97.710, I97.790, I11.0 |
| Stroke | First incident or fatal stroke | 4350AT, 4359AT, 4360A, 4360B, 4369A, 4369AL, 4369AR, 4369B, 4369BN, G61..00, G61..11, G61..12, G610.00, G611.00, G612.00, G613.00, G614.00, G615.00, G616.00G618.00, G61X.00, G61X000, G61X100, G61z.00, G63y000, G63y100, G64..00, G64..11, G64..12, G64..13, G640.00, G640000, G641.00, G641.11, G641000, G64z.00, G64z.11, G64z.12, G64z000, G64z100, G64z111, G64z200, G64z300, G64z400, G65..00, G65..11, G65..12, G65..13, G650.00, G650.11, G651.00, G651000, G652.00, G653.00, G654.00, G656.00, G65y.00, G65z.00, G65z000, G65z100, G65zz00, G66..00, G66..11, G66..12, G66..13, G660.00, G661.00, G662.00, G663.00, G664.00, G665.00, G666.00, G667.00, G668.00, G6W..00, G6X..00, Gyu6200, Gyu6300, Gyu6400, Gyu6500, Gyu6600, Gyu6G00, L440.11, L440.12 | ICD 10: I60‐64, G45 | ICD 10: I60‐64, G45ICD 9 : 430 ‐ 431 ‐ 4321 ‐ 4320 – 4329 ‐ 43321 ‐ 43311 ‐ 43391 – 43301 ‐ 43401 ‐ 43411 ‐ 43491 –43331 ‐ 43381 ‐ 4350 ‐ 4351 – 4353 ‐ 4358 ‐ 4377 ‐ 4352 – 4359 | ICD 10: I60‐64, G45 |
| Other variables defined by readcodes or drugcodes in UK and ICD or ATC codes in France, Belgium and the Netherlands | |||||
| Hypertension | At baseline | Algorithm using readcodes, ATC codes, BNF codes and drugcodes | Panel 1 (Figure 2) | Panel 2 (Figure 2) + ATC codes :C09AA01 ‐ C09AA02 ‐ C09AA03 – C09AA04 ‐ C09AA05 ‐ C09AA06 – C09AA07 ‐ C09AA08 ‐ C09AA09 –C09AA10 ‐ C09AA11 ‐ C09AA12 – C09AA13 ‐ C09AA14 ‐ C09AA15 – C09AA16 ‐ C07AA01 ‐ C07AA02 –C07AA03 ‐ C07AA05 ‐ C07AA06 – C07AA07 ‐ C07AA12 ‐ C07AA14 ‐ C07AA15 ‐ C07AA16 ‐ C07AA17 –C07AA19 ‐ C07AA23 ‐ C07AA27 – C07AB01 ‐ C07AB02 ‐ C07AB03 – C07AB04 ‐ C07AB05 ‐ C07AB06 –C07AB07 ‐ C07AB08 ‐ C07AB09 – C07AB10 ‐ C07AB11 ‐ C07AB12 – C07AB13 ‐ C07AB14 ‐ C08CA01 –C08DB01 ‐ C08CA02 ‐ C08CA03 – C08CA04 ‐ C08CA05 ‐ C08CA55 – C08DA01 ‐ C08DA51 ‐ C03AA01 –C03AA02 ‐ C03AA03 ‐ C03AA04 – C03AA05 ‐ C03AA06 ‐ C03AA07 – C03AA08 ‐ C03AA09 ‐ C03AA13 –C03AB01 ‐ C03AB02 ‐ C03AB03 – C03AB04 ‐ C03AB05 ‐ C03AB06 – C03AB07 ‐ C03AB08 ‐ C03AB09 ‐C07BA02 ‐ C07BA05 ‐ C07BA06 – C07BA07 ‐ C07BA12 ‐ C07BA68 – C07BB02 ‐ C07BB03 ‐ C07BB04 –C07BB06 ‐ C07BB07 ‐ C07BB12 – C07BB52 ‐ C09CA09 ‐ C09CA06 – C09DB07 ‐ C09DA06 ‐ C09CA02 –C09DA02 ‐ C09CA04 ‐ C09DB05 – C09DA04 ‐ C09CA01 ‐ C09DB06 – C09DA01 ‐ C09CA08 ‐ C09DB02 –C09DA08 ‐ C09DX03 ‐ C09CA07 – C09DB04 ‐ C09DA07 ‐ C09CA03 – C09DX02 ‐ C09DB01 ‐ C09DA03 –C09DB08 ‐ C09DX04 ‐ C09DX01 – C02AA01 ‐ C02AA02 ‐ C02AA03 – C02AA04 ‐ C02AA05 ‐ C02AA06 –C02AA07 ‐ C02AA52 ‐ C02AA53 ‐ C02AA57 ‐ C02AB01 ‐ C02AB02 – C02AC01 ‐ C02AC02 ‐ C02AC04 –C02AC05 ‐ C02AC06 ‐ C02BA01 – C02BB01 ‐ C02CA01 ‐ C02CA02 – C02CA03 ‐ C02CA04 ‐ C02CA06 –C02CC01 ‐ C02CC02 ‐ C02CC03 – C02CC04 ‐ C02CC05 ‐ C02CC06 – C02CC07 ‐ C02DG01 ‐ C02DA01 –C02DB01 ‐ C02DB02 ‐ C02DB03 – C02DB04 ‐ C02DC01 ‐ C02DD01 – C02KA01 ‐ C02KB01 ‐ C02KC01 –C02KD01 ‐ C02KX01 ‐ C02KX02 – C02KX03 ‐ C02KX04 ‐ C02KX05 – C02LA01 ‐ C02LA02 ‐ C02LA03 –C02LA04 ‐ C02LA07 ‐ C02LA08 – C02LA09 ‐ C02LA50 ‐ C02LA51 ‐ C02LA52 ‐ C02LA71 ‐ C02LB01 –C02LC01 ‐ C02LC05 ‐ C02LC51 – C02LE01 ‐ C02LF01 ‐ C02LG01 – C02LG02 ‐ C02LG03 ‐ C02LG51 –C02LG73 ‐ C02LK01 ‐ C02LL01 – C02LX01 ‐ C02BC ‐ C02LN ‐ C02N | Panel 1 (Figure 2) |
| Dyslipidaemia | At baseline | Algorithm using readcodes, ATC codes, BNF codes and drugcodes | ICD 10: E78 | ATC codes : C10AA01 ‐ C10AA02 – C10AA03 ‐ C10AA04 ‐ C10AA05 – C10AA06 ‐ C10AA07 ‐ C10AA08 – C10AB01 ‐ C10AB02 ‐ C10AB03 – C10AB04 ‐ C10AB05 ‐ C10AB06 – C10AB07 ‐ C10AB08 ‐ C10AB09 – C10AB10 ‐ C10AB11 ‐ C10AC01 – C10AC02 ‐ C10AC03 ‐ C10AC04 – C10AD01 ‐ C10AD02 ‐ C10AD03 – C10AD04 ‐ C10AD05 ‐ C10AD06 – C10AD52 ‐ C10AX03 ‐ C10AX05 – C10AX06 ‐ C10AX07 ‐ C10AX08 – C10AX09 ‐ C10AX10 ‐ C10AX11 – C10AX12 ‐ C10AX13 ‐ C10AX14 | ICD 10: E78 |
| Diabetes | At baseline | Algorithm using readcodes, ATC codes, BNF codes and drugcodes | ICD 10: E10‐E14 | ATC codes : A10BA02 ‐ A10BD02 – A10BD03 ‐ A10BD05 ‐ A10BD07 – A10BD08 ‐ A10BD10 ‐ A10BD11 – A10BD13 ‐ A10BD14 ‐ A10BD15 –A10BD16 ‐ A10BD17 ‐ A10BD18 – A10BD20 ‐ A10BB01 ‐ A10BB02 – A10BB03 ‐ A10BB04 ‐ A10BB05 –A10BB06 ‐ A10BB07 ‐ A10BB08 – A10BB09 ‐ A10BB10 ‐ A10BB11 – A10BB12 ‐ A10BB31 ‐ A10AB | ICD 10: E10‐E14 |
| Prior PCa Treatment | Radical prostatectomy before GnRH initiation | Readcodes: 7B20000, 7B20200, 7B36.00, 7B36.11, 7B36000, 7B36100, 7B36111, 7B36200, 7B36300, 7B36400, 7B36411, 7B36500, 7B36600, 7B36700, 7B36y00, 7B36z00, 7B36z11, 7B37.00, 7B37000, 7B37200, 7B37y00, 7B37z00, 7B3E.00, 7B3Ez00 | ICD 10: M6180, M6182, M34.1, M61.2, M61.3, M61.4 | Nomenclature codes (from health insurance companies : http://ondpanon.riziv.fgov.be/nomen/fr/search) :261796 – 261800694610 ‐ 694621154851 ‐ 154862777114 ‐ 777125 | ICD 10: M6180, M6182, M34.1, M61.2, M61.3, M61.4 |
| Prior PCa Treatment | Radiotherapy | 5149, 5151., 59…00, 597..00, 5971.00, 597Z.00, 59Z..00, 5A…11, 5A1..00, 5A16.00, 5A16.11, 5A16.12, 5A17.00, 5A1Z.00, 5A2..00, 5A27.00, 5A28.00, 5A2Z.00, 5A3..00, 5A33.00, 5A3Z.00, 5A4..00, 5A59.00, 5A65.00, 5A8Z.00, 7M37100, XalpH | ICD 10: M70.6, M71.2, Z42.2, Z42.2 | Nomenclature codes (from health insurance companies : http://ondpanon.riziv.fgov.be/nomen/fr/search):444113 ‐ 444124444135 ‐ 444146444150 ‐ 444161444172 ‐ 444183444216 ‐ 444220444253 ‐ 444264444290 ‐ 444301444312 ‐ 444323 | ICD 10: M70.6, M71.2, Z42.2, Z42.2 |
| Smoking Status | Current smokers | Readcodes: 137%, 8CAL%, 8H%, 8IAj.00, 9NS0200, 9ko% | N/A | N/A | N/A |
| Smoking Status | Non‐smokers | Readcodes: 1371%, 9kn% | N/A | N/A | N/A |
| Smoking Status | Smokers | Readcodes: 1377, 1378, 1379, 137(A,B,F,K,N,O,S,Tj), 1371.00, 9 km% | N/A | N/A | N/A |
| Ethnicity | Caucasian | Readcodes: 9S1%, 9SA9%, 9SI%, 9i1%, 9i2% | N/A | N/A | N/A |
| Ethnicity | Black | Readcodes: 9S2%, 9S3%, 9S4%, 9iB%, 9iC%, 9iC%, 9iD% | N/A | N/A | N/A |
| Ethnicity | Asian | Readcodes: 9S6%,9S7%,9S8%,9SA8%,9SH%,9i8%,9i9%,9iA% | N/A | N/A | N/A |
| Ethnicity | Other | Readcodes: 9S5%, 9S9%,9SA2%,9SA4%,9SAA%, 9SB%,9SJ%,9i3%‐9i6%,9iA3%,9iA7%,9iE%,9iF% | N/A | N/A | N/A |
| BMI | underweight at ≤ 18.5 | Readcodes: 22K3.00, 22K6.00, EMISNQBO29 | N/A | N/A | N/A |
| BMI | normal at 18.6–24 | Readcodes: 22K1.00, 22K8.00, JHCBO5 | N/A | N/A | N/A |
| BMI | overweight at 25–30 | Readcodes: 22K2.00, 22K4.00 | N/A | N/A | N/A |
| BMI | obese at ≥ 30 | Readcodes: 22KC.00, 22KD.00, 22KE.00, 22K7.00, 22K5.00 | N/A | N/A | N/A |
| Socio‐economic Status | Lowest | 1 ‐ least deprived | N/A | N/A | N/A |
| Socio‐economic Status | Low | 2 | N/A | N/A | N/A |
| Socio‐economic Status | Middle | 3 | N/A | N/A | N/A |
| Socio‐economic Status | High | 4 | N/A | N/A | N/A |
| Socio‐economic Status | Highest | 5 ‐ most deprived | N/A | N/A | N/A |
| Socio‐economic Status | French ‘poor income’ | N/A | N/A | N/A | |
| Civil Status | Single | Single (01), widowed (03), divorced (04), separated(05) | N/A | N/A | N/A |
| Civil Status | Married | Engaged (07), co‐habiting (08), remarried (09), stable relationship (10), civil partnership (11) | N/A | N/A | N/A |
Further information on nomenclature in Belgium can be found on the RIZIV/INAMI 50.
An algorithm of ICD and ATC codes (Figure 2) in Belgium, the Netherlands and France was used to identify men with hypertension as using ICD codes alone resulted in a very low number of hypertensive men in an aged population.
Discussion
This is the first study to combine real‐world data from five European countries to compare risk of CVD following GnRH agonists and GnRH antagonists in men with PCa. The ROBINS‐I tool allowed detailed investigation of our real‐world study design with an emulated RCT in an attempt to avoid misclassification and unmeasured confounding biases. Extraction of baseline and clinical characteristics defined variables that were to be included in country‐specific analytical models. Homogenous variables in the five countries were then used in the meta‐analytical models.
Real‐world data or population‐based observational studies have enabled large‐scale studies that allow linkages between databases, such as cancer registries, hospital records and epidemiological databases 39. According to Booth and Tannock, the way forward in research is to apply RCTs and real‐world data in a complementary manner. Whereas RCTs provide information on how to improve efficacy and quality of life of cancer patients (because they collect lifestyle factors along with other measurements), real‐world data provide evidence of improvement in outcome (including safety) at the level of the general population 39. Therefore, the ROBINS‐I tool helped generate a pragmatic approach to our study design to mimic a target trial. It specifically allowed a detailed investigation of trial characteristics, types of biases involved and challenges encountered when using different databases. The ROBINS‐I tool highlighted some evident and unavoidable biases associated with observational data such as uneven randomization distribution and unmeasured confounding. Although unmeasured confounding is often unavoidable in real‐world data, VanderWeele (2017) suggests a ‘straightforward’ E‐value calculation to quantify the minimum strength of association that an unmeasured confounder would need to have with treatment and outcome in order to explain the treatment‐outcome association 40. For example, higher E‐values suggest stronger unmeasured confounder associations to explain the estimated effect.
ROBINS‐I tool further highlighted indication bias also known as channeling bias in pharmacoepidemiology. Qayyim Said in Yang and West‐Strum (2010) describes channeling bias as one of the most common type of bias found in pharmacoepidemiology studies. Channeling bias arises when the physician treating patients for a particular disease prescribes certain drugs based on patient characteristics such as severity of disease, age or gender 41. We accounted for channeling bias by conducting a stratified meta‐analysis by HCVDi. Stratification by HCVDi allowed for estimating hazard ratios across two strata: those who had a history of CVD and those who had no history of CVD.
The use of different codes in the five databases proved difficult to fully homogenize variable definitions. While readcodes and drugcodes were used to identify study variables in the UK, ICD and ATC codes were used in Scotland, Belgium, the Netherlands and France. Moreover, due to missing observations in socio‐demographic data in the UK, further analyses of lifestyle factors were not possible.
An algorithm combining ICD and ATC codes (Figure 2 ) in Belgium, the Netherlands and France was used to extract hypertensive men as using ICD codes alone resulted in a very low number of hypertensive men in an aged population. We attempted to avoid classification biases in the five databases by ensuring that data availability, study variable definitions and cohort definitions were as uniform as possible (Table 3). As information on compliance to treatment was not available in our databases, this information bias will have to be accounted for when interpreting the results of stage 1 and stage 2 analyses.
The representativeness of the European PCa population by incorporating five different databases across Europe adds strong value to this study. THIN is a primary healthcare database which represents approximately 6.2% of the UK population 39. Whereas THIN is a primary healthcare database, data in the other four databases were of other origins. NHS Scotland provides nationwide medical record linkages between cancer registry, hospital inpatient and outpatient admission, dispensed medications and death certificates 30. BCR derives information from standard cancer registration, health insurance companies, hospital discharge data and cause of death data 31. The PHARMO Database Network obtains data from both primary and secondary healthcare settings which meant that both cancer registration and follow‐up visits were reliably available for a patient 35. The SNIIRAM database combines a claims database (derived from insurance funds) with hospital‐derived data to form a large database representative of the French population 36. The use of primary healthcare, secondary healthcare and claims databases thus ensured the inclusion of rare, adverse events that may not have been identified in a RCT and adds additional strength to the study.
We used a two‐stage approach for the study by investigating heterogeneity in country‐specific analysis. The country‐specific analysis was used to describe prescription patterns and the PCa population in the five countries. Stage 2 meta‐analysis assessed the risk of outcome due to the two exposures investigated. As there was no possibility of combining data at the individual level (due to legal and ethical restrictions), using pooled log‐transformed hazard ratios of CVD outcomes were the only way to combine the data. In addition to creating a homogenous study protocol, we attempted to further account for heterogeneity using stratified and sensitivity analyses, as described in the methods section.
The effect of other treatment modalities in addition to GnRH agonists or antagonists needs to be considered when assessing the risk of CVD. This was not considered in detail for this study because full chemotherapy and radiotherapy profiles were not available for all countries. Chemotherapy and radiotherapy are treatment modalities given in a hospital setting, and our data source was limited in this aspect. As a result, we were not able to consider other combination treatment modalities that may have affected CVD outcome. Moreover, data on follow‐up treatment modalities affecting CVD outcome were missing and were therefore a limitation to the study.
A further limitation to the study was that CVD history was only considered 12 months prior to GnRH initiation. Although Belgium received information on CVD history for a maximum of 12 months prior to PCa diagnosis, the first GnRH prescription was given immediately or a maximum of four months after PCa diagnosis (90%). In France, access to CVD history was only available during the study period (2010–2013) and accurate information on PCa diagnosis date was not available. As CVD history was not consistently available for more than 12 months across the countries, we defined CVD history to be 12 months prior to first GnRH prescription. Therefore, all men included in the study had a minimum of 12 months of CVD history. In order to keep study definitions homogenous across the five countries, we assessed history of CVD using the variable HCVDi.
The variations in prescription patterns of GnRH antagonists in the five included countries may have influenced the delivery of GnRH antagonists to a specific class of PCa men who were predisposed by factors such as comorbidities and physician preferences. For instance, a physician may have prescribed GnRH antagonists to an individual with a history of CVD based on previous evidence 42. This means that GnRH antagonists may have been channeled to this class of PCa men (channeling bias discussed in Table 1). Channeling may also explain the different proportions of PCa men on GnRH antagonists across the five countries. A lower number of men on GnRH antagonists was observed in the UK compared to the other four countries, owing to specific guidelines (CG175) 43, 44 only allowing the use of GnRH antagonists in certain PCa men. Although these specific guidelines determined the prescription of GnRH antagonists to advanced hormone‐dependent PCa men during the study period, current UK National Institute of Clinical Excellence (NICE) guidelines suggest that GnRH antagonists should be prescribed to advanced staged PCa men with a spinal metastasis (NICE TA404) 45.
In Belgium, GnRH antagonists were specified for advanced stage hormone‐dependent PCa; however, no specifications were made concerning the exact definition of advanced stage, which left room for interpretation by the physician 46. In France, although set regulations defined classes of patients for whom GnRH antagonists were prescribed, the decisions were steered mostly by the physicians who may have included PCa men with all T‐stages with nodal involvement and metastatic disease 47. In the Netherlands, the need for rapid testosterone decline was achieved by using GnRH antagonists, with switch to a GnRH agonist after a few months 48. Potential differences in prescription and delivery of GnRH antagonists between UK, Scotland, Belgium, the Netherlands and France may thus explain the data heterogeneity between the countries.
Conclusion
When considering the potential heterogeneity introduced by the variation in the means of recording real‐world data, pooling data from five different databases were found to be a challenge. However, for the first time we were able to use databases from the UK, Scotland, Belgium, the Netherlands and France to include a heterogeneous PCa population (in contrast to the selected PCa population in RCTs) across Europe to provide results that are more applicable to the general PCa population. The results from this study will help us understand the variations in risk of long‐term CVD outcomes following GnRH agonists and GnRH antagonists in men with PCa.
Acknowledgements
This work was supported by an independent research grant from Ferring Pharmaceuticals. The funder had no influence on the study design, analyses or interpretation of the results. The authors would also like to thank all the healthcare providers and patients contributing information to the UK THIN database, NHS Scotland database, Belgian Cancer Registry, the PHARMO Database Network and the French SNIIRAM database.
Joint first authors: Gincy George, Lucie‐Marie Scailteux.
References
- 1. Ferlay J., Steliarova‐Foucher E., Lortet‐Tieulent J. et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur. J. Cancer (2013) 49 1374–1403. [DOI] [PubMed] [Google Scholar]
- 2. Kockelbergh R., Hounsome L. Mayer E. The epidemiology of urological cancer 2001–2013. J. Clin. Urol. (2017) 10(1S) 3–8. [Google Scholar]
- 3. Perlmutter M.A., Lepor H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev. Urol. (2007) 9(Suppl 1) S3–S8. [PMC free article] [PubMed] [Google Scholar]
- 4. Khan M.A., Partin A.W. Management of high‐risk populations with locally advanced prostate cancer. Oncologist (2003) 8 259–269. [DOI] [PubMed] [Google Scholar]
- 5. Braga‐Basaria M., Dobs A.S., Muller D.C. et al. Metabolic syndrome in men with prostate cancer undergoing long‐term androgen‐deprivation therapy. J. Clin. Oncol. (2006) 24 3979–3983. [DOI] [PubMed] [Google Scholar]
- 6. Keating N.L., O'Malley A.J., Smith M.R. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J. Clin. Oncol. (2006) 24 4448–4456. [DOI] [PubMed] [Google Scholar]
- 7. McLeod D.G., Iversen P., See W.A. et al. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int. (2006) 97 247–254. [DOI] [PubMed] [Google Scholar]
- 8. Petrylak P.J., Moul J.W. Androgen Ablation for Prostate Cancer: Mechanisms and Modalities, in: Kantoff P.W., Carroll P.R., D'Amico A.V. (Eds), Prostate cancer: principles and practice. Williams & Wilkins, Lippincott, Philadelphia, 2002, pp. 518–523. [Google Scholar]
- 9. Saigal C.S., Gore J.L., Krupski T.L. et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer (2007) 110 1493–1500. [DOI] [PubMed] [Google Scholar]
- 10. Jones R.D., Nettleship J.E., Kapoor D. et al. Testosterone and atherosclerosis in aging men: purported association and clinical implications. Am. J. Cardiovasc. Drugs (2005) 5 141–154. [DOI] [PubMed] [Google Scholar]
- 11. D'Amico A.V., Denham J.W., Crook J. et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J. Clin. Oncol. (2007) 25 2420–2425. [DOI] [PubMed] [Google Scholar]
- 12. Tsai H.K., D'Amico A.V., Sadetsky N. et al. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J. Natl Cancer Inst. (2007) 99 1516–1524. [DOI] [PubMed] [Google Scholar]
- 13. O'Farrell S., Garmo H., Holmberg L. et al. Risk and timing of cardiovascular disease after androgen‐deprivation therapy in men with prostate cancer. J. Clin. Oncol. (2015) 33 1243–1251. [DOI] [PubMed] [Google Scholar]
- 14. Van Hemelrijck M., Garmo H., Holmberg L. et al. Absolute and relative risk of cardiovascular disease in men with prostate cancer: results from the Population‐Based PCBaSe Sweden. J. Clin. Oncol. (2010) 28 3448–3456. [DOI] [PubMed] [Google Scholar]
- 15. Barkin J. Risks, benefits, and approaches to hormonal blockade in prostate cancer. Highlights from the European Association of Urology Meeting, March 20–24, 2015, Madrid, Spain. Can. J. Urol. (2015) 22 7847–7852. [PubMed] [Google Scholar]
- 16. Albertsen P.C., Klotz L., Tombal B. et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur. Urol. (2014) 65 565–573. [DOI] [PubMed] [Google Scholar]
- 17. Hopmans S.N., Duivenvoorden W.C., Werstuck G.H. et al. GnRH antagonist associates with less adiposity and reduced characteristics of metabolic syndrome and atherosclerosis compared with orchiectomy and GnRH agonist in a preclinical mouse model. Urol. Oncol. (2014) 32 1126–1134. [DOI] [PubMed] [Google Scholar]
- 18. Rosario D.J., Davey P., Green J. et al. The role of gonadotrophin‐releasing hormone antagonists in the treatment of patients with advanced hormone‐dependent prostate cancer in the UK. World J. Urol. (2016) 34 1601–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. ClinicalTrials.gov . A trial comparing cardiovascular safety of degarelix versus leuprolide in patients with advanced prostate cancer and cardiovascular disease (PRONOUNCE). (2018) Available from: https://clinicaltrials.gov/ct2/show/study/NCT02663908 (Accessed date: 05/04/2018).
- 20. Scailteux L.M., Vincendeau S., Balusson F. et al. Androgen deprivation therapy and cardiovascular risk: no meaningful difference between GnRH antagonist and agonists ‐ a nationwide population‐based cohort study based on 2010‐2013 French Health Insurance data. Eur. J. Cancer (2017) 77 99–108. [DOI] [PubMed] [Google Scholar]
- 21. Miettinen O.S. The need for randomization in the study of intended effects. Stat. Med. (1983) 2 267–271. [DOI] [PubMed] [Google Scholar]
- 22. Benson K., Hartz A.J. A comparison of observational studies and randomized, controlled trials. N. Engl. J. Med. (2000) 342 1878–1886. [DOI] [PubMed] [Google Scholar]
- 23. George G., Scailteux L.M., Garmo H. et al. The risk of cardiovascular disease following GnRH agonists versus antagonists: real‐world evidence from four European countries. Poster presented at: Expert‐guided Poster Presentation. 33rd Annual European Association of Urology Congress, Copenhagen, Denmark, (2018a), pp. 16–20.
- 24. George G., Scailteux L.M., Garmo H. et al. Real‐world insights into risk of cardiovascular disease following GnRH agonists and antagonists in men with prostate cancer. Poster presented at: Poster Reception. Global Cardio‐Oncology Summit 2018, Florida, United States of America, (2018b) 27th ‐ 28th September.
- 25. Sterne J.A.C., Hernán M.A., Reeves B.C. et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. Br. Med. J. (2016) 355 i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lodwick R.K. THIN database. (2015) Available from: https://www.ucl.ac.uk/pcph/research-groups-themes/thin-pub/database (Accessed date: 05/04/2018).
- 27. IMS Health Incorporated . Data content. (2015) Available from: http://csdmruk.cegedim.com/our-data/data-content.shtml (Accessed date: 10/10/2017).
- 28. Vision Health. Vision Health . (2017) Available from: https://www.visionhealth.co.uk/ (Accessed date: 05/04/2018).
- 29. Booth N. What are the read codes? Health Lib. Rev. (1994) 11 177–182. [DOI] [PubMed] [Google Scholar]
- 30. Alvarez‐Madrazo S., McTaggart S., Nangle C. et al. Data resource profile: the Scottish national prescribing information system (PIS). Int. J. Epidemiol. (2016) 45 714–715f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Belgian Cancer Registry . Belgian Cancer Registry. (2017) Available from: http://www.kankerregister.org/Belgian%20Cancer%20Registry (Accessed date: 05/04/2018).
- 32.Belgian Law: Deliberation No. 09/071 of 15 September 2009, last amended on 18 February 2014, relating to the communication of personal data by insurers to the Cancer Registry Foundation under Article 45 quinquies of the Royal Decree number 78 of 10 November 1967 concerning the practice of health care professions.
- 33.Belgian Law: Deliberation No. 16/021 of 15 March 2016 on the communication of coded personal health data by the technical unit to the Cancer Registry Foundation for the assessment of comorbidity in cancer patients in the context of scientific research projects.
- 34. Van Herk‐Sukel M.P., Van de Poll‐Franse L.V., Lemmens V.E. et al. New opportunities for drug outcomes research in cancer patients: the linkage of the Eindhoven Cancer Registry and the PHARMO Record Linkage System. Eur. J. Cancer (2010) 46 395–404. [DOI] [PubMed] [Google Scholar]
- 35. PHARMO Institute . PHARMO Database Network. (2017) Available from: http://pharmo.nl/what-we-have/pharmo-database-network/ (Accessed date: 05/04/2018).
- 36.Ameli. SNIIRAM (2016) Available from: https://www.ameli.fr/l-assurance-maladie/statistiques-et-publications/sniiram/finalites-du-sniiram.php (Accessed date: 05/04/2018).
- 37. Bezin J., Duong M., Lassalle R. et al. The national healthcare system claims databases in France, SNIIRAM and EGB: Powerful tools for pharmacoepidemiology. Pharmacoepidemiol. Drug Saf. (2017) 26 954–962. [DOI] [PubMed] [Google Scholar]
- 38. Mackenbach J.P. Health and deprivation in: Townsend P., Phillimore P., Beattie A. (Eds), Inequality and the North. Croom Helm Ltd: London, 1987, pp. 221, ISBN 0‐7099‐4352‐0. Health Policy. (1988) 10(2), 207. [Google Scholar]
- 39. Booth C.M., Tannock I.F. Randomised controlled trials and population‐based observational research: partners in the evolution of medical evidence. Br. J. Cancer (2014) 110 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. VanderWeele T.J., Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann. Intern. Med. (2017) 167 268–274. [DOI] [PubMed] [Google Scholar]
- 41. Said Q. Other methodological issues, in: Yang Y., West‐Strum D. (Eds), Understanding pharmacoepidemiology. The McGraw‐Hill Companies, United States of America, 2010, pp. 105–120. [Google Scholar]
- 42. Clyne M. Prostate cancer: tipping the balance in favour of degarelix for ADT. Nat. Rev. Urol. (2014) 11 67. [DOI] [PubMed] [Google Scholar]
- 43. National Institute for Health and Care Excellence . Degarelix for treating advanced hormone‐dependent prostate cancer. Final Scope (2013) Available from: https://www.nice.org.uk/guidance/ta404/documents/prostate-cancer-advanced-hormone-dependent-degarelix-depot-final-scope2 (Accessed date: 21/02/2018).
- 44. National Institute for Health and Care Excellence . Prostate Cancer: diagnosis and treatment. (2014) Available from: https://www.nice.org.uk/guidance/cg175/evidence/full-guideline-191710765 (Accessed date: 21/02/2018).
- 45. National Institute for Health and Care Excellence . Final Appraisal Determination: Degarelix for treating advanced hormone‐dependent prostate cancer. (2014) Available from: https://www.nice.org.uk/guidance/ta404/documents/prostate-cancer-advanced-hormone-dependent-degarelix-depot-fad-document2 (Accessed date: 06/04/2018).
- 46. Royal Decree of 31/12/2001 – IV – 2360000 – Law on various health provisions of December 13, 2006, article 39. Moniteur Belge (2006).
- 47. Haute Autorité De Santé . Transparency committee opinion Firmagon. (2009) Available from: https://www.has-sante.fr/portail/upload/docs/application/pdf/2011-02/firmagon_ct_6725.pdf (Accessed date: 06/04/2018).
- 48. De Boer J.E. CFH‐rapport 09/19: degarelix (Firmagon®). (2009) Available from: https://www.zorginstituutnederland.nl/binaries/zinl/documenten/rapport/2009/07/27/degarelix-firmagon-bij-hormoonafhan0kelijke-prostaatkanker-in-een-vergevorderd-stadium/Degarelix+(Firmagon)+bij+hormoonafhankelijke+prostaatkanker+in+een+vergevorderd+stadium.pdf (Accessed date: 06/04/2018).
- 49. Suissa S. Immeasurable time bias in observational studies of drug effects on mortality. Am. J. Epidemiol. (2008) 168 329–335. [DOI] [PubMed] [Google Scholar]
- 50. INAMI‐RIZIV . Nomenclature: research criteria. (2018) Available from: http://ondpanon.riziv.fgov.be/nomen/fr/search (Accessed date: 06/04/2018).


