Abstract
Introduction
BAY 81‐8973 (Kovaltry®) is a full‐length, unmodified recombinant human factor VIII approved in China for prophylaxis and on‐demand treatment in patients with haemophilia A. Limited access to FVIII prophylaxis in China has historically led to this population being undertreated. This subanalysis of LEOPOLD II investigated whether the efficacy and safety of BAY 81‐8973 varied between Chinese and non‐Chinese patients.
Aim
To evaluate BAY 81‐8973 efficacy and safety in Chinese patients.
Methods
LEOPOLD II enrolled males aged 12‒65 years with severe haemophilia A who were receiving on‐demand treatment. Patients were randomly assigned to receive BAY 81‐8973 as low‐dose prophylaxis (20‒30 IU/kg twice‐weekly), high‐dose prophylaxis (30‒40 IU/kg 3 times weekly) or on‐demand for 1 year.
Results
Data were available from 23 Chinese and 57 non‐Chinese patients; Chinese patients had a higher prestudy bleeding rate and were more likely to have target joints than non‐Chinese patients. 74% of patients were assigned to prophylaxis. Annualized bleeding rates (ABRs) in Chinese and non‐Chinese patients receiving prophylaxis were significantly lower compared to patients treated on‐demand. Median ABRs for all bleeds in the last 6 months of the study were 2.0 and 1.0 for Chinese and non‐Chinese patients, respectively, in the combined prophylaxis groups, and 61.3 and 58.5 in the on‐demand group. A treatment‐related adverse event occurred in 1 Chinese patient; no patients developed FVIII inhibitors.
Conclusion
BAY 81‐8973 prophylaxis was efficacious and well tolerated in Chinese patients with severe haemophilia A, with ABRs comparable to those in non‐Chinese patients receiving prophylaxis.
Keywords: BAY 81‐8973, Chinese, clinical trial, factor VIII, haemophilia, prophylaxis
1. INTRODUCTION
Patients with severe haemophilia A experience spontaneous bleeding episodes into joints, muscles and soft tissue 1; over time, joint bleeding episodes can lead to haemophilic arthropathy.1 The standard of care for severe haemophilia A is prophylaxis with a factor VIII (FVIII) product, which can prevent bleeding episodes and joint damage.1, 2 Prophylaxis, however, is a costly and burdensome treatment, with infusions typically required several times per week.1 Consequently, in countries such as China, in which access to haemophilia care is limited and patient incomes are low, most patients with haemophilia are treated on‐demand, with FVIII administered at the time of a bleeding episode, and some patients may receive no treatment at all.3, 4 Lack of access to FVIII prophylaxis in some Chinese regions has historically led to this population being undertreated, which subsequently increases the likelihood of joint damage and compromises health‐related quality of life in patients with haemophilia A.6 It is possible that for this reason the joint status and treatment history of the Chinese patients differ from other populations. In 1 study of 213 Chinese patients with haemophilia A or haemophilia B, >90% had haemophilic arthropathy by age 6‐9 years.7
Ideally, patients with haemophilia in China should receive individualized prophylaxis as recommended by the World Federation of Hemophilia (WFH).1, 8 Low‐dose prophylaxis has been explored as a cost‐saving approach to treatment of Chinese patients with haemophilia 5, 9 and results have been encouraging. Patients have experienced fewer joint bleeding episodes and some improvement in daily activities compared with on‐demand treatment.10 For these reasons, it should be considered an initial step in haemophilia care in patients where high‐dose prophylaxis is not required or not possible due to the economic burden.8
BAY 81‐8973 (Kovaltry®; Bayer, Berkeley, CA) is a full‐length, unmodified recombinant human FVIII approved for prophylaxis and treatment of bleeding episodes in patients with haemophilia A11, 12 and according to Bayer's data on file has accumulated 8031 patient‐years of exposure as of 31st August 2018. The efficacy and safety of BAY 81‐8973 were demonstrated in the Long‐Term Efficacy Open‐Label Program in Severe Hemophilia A Disease (LEOPOLD) clinical trial program, which enrolled children, adolescents, and adults with severe haemophilia A at sites worldwide.13, 14 The efficacy of prophylaxis9, 16 and superiority of prophylaxis over on‐demand treatment17 have been demonstrated in Chinese patients treated with sucrose‐formulated recombinant FVIII (rFVIII‐FS; Kogenate® FS; Bayer), which has the same amino acid sequence as BAY 81‐8973.18 To evaluate the efficacy and safety of BAY 81‐8973 specifically in Chinese patients with haemophilia A, we examined data from the LEOPOLD II trial, which enrolled 23 patients from China. The subanalysis also explored whether treatment history in Chinese patients affected efficacy and safety.
2. METHODS
2.1. Study design and patients
LEOPOLD II was a multinational, open‐label, randomized, crossover phase 2/3 study designed to demonstrate the superiority of BAY 81‐8973 prophylaxis over on‐demand treatment in patients with severe haemophilia A.13 The design and results of this study have been previously described.13 In brief, patients were eligible for LEOPOLD II if they were males aged 12‐65 years, had severe haemophilia A, were receiving on‐demand treatment at screening and had not received regular prophylaxis for >6 consecutive months in the previous 5 years, had ≥150 exposure days (EDs) to any FVIII product and had no current or history of FVIII inhibitors. Patients were enrolled at 30 centres in 11 countries in Europe, South Africa, North America, South America and Asia.
Patients were randomly assigned to receive BAY 81‐8973 as low‐dose prophylaxis (20‐30 IU/kg twice‐weekly), as high‐dose prophylaxis (30‐40 IU/kg 3 times weekly), or on‐demand for 1 year. In the prophylaxis groups, the dose per infusion was selected by the investigator within the designated dosing range (low‐dose group: 20, 25 or 30 IU/kg; high‐dose group: 30, 35 or 40 IU/kg); dosing for on‐demand treatment and treatment of breakthrough bleeds in the prophylaxis groups depended on the location and severity of the bleeding episode.
2.2. Efficacy, safety and pharmacokinetic assessments
The primary efficacy endpoint was the annualized bleeding rate (ABR) for all bleeding episodes, defined as spontaneous bleeds, trauma‐related bleeds, untreated bleeds and unspecified events for which treatment was administered. Additional predefined efficacy endpoints included ABR for joint, spontaneous and trauma‐related bleeds; percentage of bleeding episodes treated with ≤2 infusions; and FVIII recovery. All patients were monitored for adverse events (AEs), including the development of FVIII inhibitors. Incremental FVIII recovery was assessed at the start and after 6 months of BAY 81‐8973 treatment in patients receiving prophylaxis; FVIII levels were determined using the chromogenic assay and the one‐stage assay. Pharmacokinetic (PK) assessments in LEOPOLD II were performed only in a subset of patients recruited at centres in Japan. PK data for Chinese patients, including three paediatric patients (age <18 years), were available from the LEOPOLD I study; methods and results for both of these studies have previously been published.14, 15
2.3. Statistical analysis
A subanalysis was performed to assess the efficacy of prophylaxis versus on‐demand treatment in all Chinese patients receiving BAY 81‐8973 in the LEOPOLD II study. Evaluated bleed outcomes included ABR for all bleeds, joint bleeds, spontaneous bleeds and trauma‐related bleeds for both Chinese and non‐Chinese patients and were calculated arithmetically in addition to calculations of mean, standard deviation, median and quartiles. Direct comparisons between Chinese and non‐Chinese patients were made for all bleed and joint bleed ABRs. Data were analysed for the 12 months prestudy, the first and last 6 months on‐study, and the total 12‐month study period. Wilcoxon rank sum tests were used for all comparisons.
3. RESULTS
3.1. Patients
A total of 80 patients were treated with BAY 81‐8973 in LEOPOLD II, including 23 patients enrolled at five sites in China and 57 patients enrolled at sites outside China. Median age was similar for the Chinese and non‐Chinese patients, but differences were seen between groups in clinical characteristics, including bleeding history and the percentage of patients with target joints (Table 1). Specifically, 100% of Chinese patients had target joints at baseline, compared with 86% of non‐Chinese patients. Compared with non‐Chinese patients, Chinese patients also had a higher median age at diagnosis (1.3 vs 2.3 year) and a higher median age at first treatment (1.6 vs 2.9 year). BAY 81‐8973 treatment assignments were identical for Chinese and non‐Chinese patients, with 74% of patients in each group receiving prophylaxis and 26% treated on‐demand (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics and BAY 81‐8973 Treatment Assignment for Chinese and Non‐Chinese Patients in LEOPOLD II
| Chinese (n = 23) | Non‐Chinese (n = 57) | |
|---|---|---|
| Median (range) age, y | 27.0 (15‐48) | 30.0 (14‐59) |
| Race, n (%) | ||
| Asian | 23 (100) | 9 (15.8) |
| White | n/a | 36 (63.2) |
| Black | n/a | 4 (7.0) |
| Hispanic | n/a | 8 (14.0) |
| Target joint present, n (%) | 23 (100) | 49 (86.0) |
| Median (range) number of target joints | 2.0 (1‒7) | 3.0 (0‒9)a |
| Median (range) number of bleeds in past 12 mo | 60.0 (14‒106) | 33.0 (3‒106)b |
| Median (range) number of joint bleeds in past 12 mo | 43.0 (1‒90) | 23.5 (3‒104)b |
| Median (range) age at diagnosis, y | 2.3 (0‒26) | 1.3 (0‒22) |
| Median (range) age at first treatment, y | 2.9 (0‒18) | 1.6 (0‒22) |
| BAY 81‐8973 treatment, n (%) | ||
| Combined prophylaxis | 17 (73.9) | 42 (73.7) |
| 2×/wk prophylaxis | 7 (30.4) | 21 (36.8) |
| 3×/wk prophylaxis | 10 (43.5) | 21 (36.8) |
| On‐demand | 6 (26.0) | 15 (26.3) |
Data available for 56 patients.
Data available for 54 patients.
3.2. Treatment exposure
Patients were only considered eligible for the study if they had accumulated at least 150 ED. Median number of days in the study for all 80 patients treated in LEOPOLD II was 365.5 (range, 140‐375), and median EDs for all patients were 117.5 (range, 8‐187). Among Chinese patients, median days in the study were 369.6 (range, 361‐375) for the combined prophylaxis group and 364.6 (range, 358‐371) for the on‐demand group; median EDs were 153.0 (range, 106‐182) and 76.5 (range, 54‐118), respectively.
For Chinese patients, median (range) number of infusions was 154.0 (106‒182) for the combined prophylaxis group and 77.5 (56‒164) for the on‐demand group. Median (range) nominal dose per infusion was 31.4 (21.6‒41.9) IU/kg and 21.6 (15.0‒23.5) IU/kg for prophylaxis and on‐demand treatment, respectively; median (range) nominal dose per year was 4916.1 (2305‒6541) IU/kg and 1642.3 (1225‒2599) IU/kg, respectively.
3.3. Efficacy
BAY 81‐8973 prophylaxis significantly decreased ABR in Chinese and non‐Chinese patients who had previously been treated on‐demand, compared with those who continued on‐demand treatment during LEOPOLD II (Table 2 and Figure 1). In Chinese patients, median ABR for all bleeds was 2.0 (quartile 1; quartile 3 [Q1; Q3], 1.0; 5.0) for patients receiving prophylaxis and 63.5 (52.1; 100.2) for those treated on‐demand (P < 0.0001; Figure 1A), indicating a significant reduction in bleeding episodes with prophylaxis treatment. Median (Q1; Q3) ABRs for Chinese patients receiving low‐dose or high‐dose prophylaxis were 5.0 (2.0; 11.0) and 2.0 (0; 2.0), respectively, and this difference was not statistically significant. In non‐Chinese patients, median ABR for all bleeds was 2.0 (0; 7.0) for patients receiving prophylaxis and 52.0 (32.4; 76.3) for those treated on‐demand (Figure 1B). ABRs for Chinese and non‐Chinese patients receiving prophylaxis were significantly lower than those for patients treated on‐demand during both the first and last 6 months of the study (Table 2). Thus, although Chinese patients receiving prophylaxis during LEOPOLD II generally had higher prestudy ABRs compared with non‐Chinese patients, ABRs during the study were comparable for both groups (Table 2).
Table 2.
Annualized bleeding rate before and during BAY 81‐8973 treatment in Chinese and non‐Chinese patients treated on#x2010;demand or with prophylaxis
| On‐demand | Prophylaxis (Combined)a | P valueb | |
|---|---|---|---|
| Median (Q1; Q3) | Median (Q1; Q3) | ||
| Chinese patients | n = 6 | n = 17 | |
| All bleedsc | |||
| Prestudy | 69.5 (60.0; 76.0) | 50.0 (24.0; 76.0) | 0.289 |
| First 6 mo | 65.9 (59.6; 100.0) | 2.0 (1.9; 4.2) | 0.002* |
| Last 6 mo | 61.3 (44.5; 89.1) | 2.0 (0; 3.8) | 0.002* |
| Joint bleeds | |||
| Prestudy | 50.0 (28.0; 50.0) | 40.0 (11.0; 50.0) | 0.534 |
| First 6 mo | 42.1 (37.7; 58.6) | 1.9 (0; 4.0) | 0.002* |
| Last 6 mo | 46.5 (32.4; 61.4) | 0 (0; 2.1) | 0.001* |
| Non‐Chinese patients | n = 15 | n = 42 | |
| All bleedsc | |||
| Prestudy | 27.0 (23.0; 58.0) | 36.0 (18.0; 51.0) | 0.992 |
| First 6 mo | 51.6 (36.1; 72.7) | 3.8 (0.0; 7.9) | <0.0001* |
| Last 6 mo | 58.5 (37.5; 77.0) | 1.0 (0; 8.0) | <0.0001* |
| Joint bleeds | |||
| Prestudy | 22.0 (15.0; 39.0) | 24.0 (13.0; 45.0) | 0.985 |
| First 6 mo | 36.9 (20.4; 58.5) | 2.0 (0; 6.1) | <0.0001* |
| Last 6 mo | 40.2 (22.5; 67.8) | 1.0 (0; 6.2) | <0.0001* |
Q1 = quartile 1; Q3 = quartile 3.
Prestudy refers to the 12‐mo period before LEOPOLD II.
Combined prophylaxis includes all patients who received BAY 81‐8973 prophylaxis in LEOPOLD II.
Combined prophylaxis vs on‐demand.
All bleeds included spontaneous, trauma‐related and untreated bleeds as well as bleeds with missing reason.
P values < 0.05 were considered significant.
Figure 1.
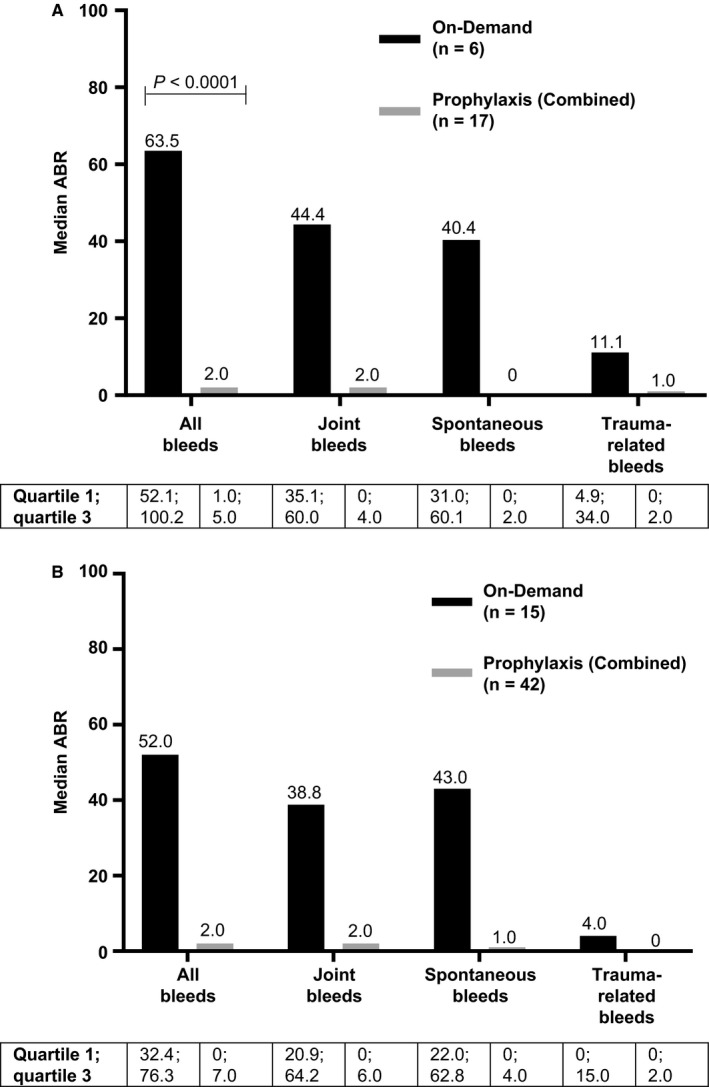
Median annualized bleeding rates during BAY 81‐8973 treatment in (A) Chinese patients and (B) non‐Chinese patients treated on‐demand or with prophylaxis. Combined prophylaxis includes all patients who received BAY 81‐8973 prophylaxis in LEOPOLD II. All bleeds included spontaneous, trauma‐related and untreated bleeds as well as bleeds with missing reason
During LEOPOLD II, a total of 499 bleeding episodes occurred in Chinese patients (432 in the on‐demand group; 67 in the combined prophylaxis group). Most bleeding episodes were spontaneous (69.5% for on‐demand; 74.2% for combined prophylaxis) and occurred in joints (72.7% for on‐demand; 83.6% for combined prophylaxis). In the on‐demand group, 314 bleeding episodes occurred in patients with target joints, of which 262 (83.4%) were into target joints; in the combined prophylaxis group, of 56 bleeding episodes occurring in patients with target joints, 31 (55.4%) were into target joints. Most bleeds were considered mild or moderate (97.2% in the on‐demand group; 92.5% in the combined prophylaxis group), as rated by the patient.
More than 90% of bleeding episodes in Chinese patients in both the on‐demand and combined prophylaxis groups were treated with ≤2 BAY 81‐8973 infusions; doses to treat acute bleeds varied between on‐demand and prophylaxis groups (Table 3). Response to treatment of bleeding episodes with BAY 81‐8973 was rated by patients as excellent or good for 54.9% of 432 bleeds in the on‐demand group and 62.9% of 62 bleeds in the combined prophylaxis group (treatment response was missing for 5 bleeds in the prophylaxis group).
Table 3.
Treatment of acute bleeds with BAY 81‐8973 in Chinese patients
| On‐demand (n = 6) | Prophylaxis (Combined)a (n = 17) | |
|---|---|---|
| Bleeds treated with: | (N = 432 bleeds) | (N = 67 bleeds) |
| ≤2 infusions, n (%) | 420 (97.2) | 61 (91.0) |
| Not treated, n (%) | 2 (0.5) | 4 (6.0) |
| Median (range) injections per bleed | 1.0 (0‒3) | 1.0 (0‒4) |
| Median (range) nominal dose per infusion, IU/kg/infusion | 21.4 (15.0‒23.5) | 27.3 (23.5‒41.6) |
| Median (range) nominal dose per year, IU/kg/y | 1642.3 (1225‒2599) | 120.6 (47‒608) |
Combined prophylaxis includes all patients who received BAY 81‐8973 prophylaxis in LEOPOLD II.
3.4. Pharmacokinetics and FVIII recovery
Pharmacokinetic data from the LEOPOLD studies were available for 10 Asian patients (6 from China and 4 from Japan) and 32 non‐Asian patients. No significant differences were seen in half‐life, dose‐normalized area under the curve, or dose‐normalized maximum concentration between Asian and non‐Asian patients (Table 4).
Table 4.
Range of pharmacokinetic variables in Asian and non‐Asian patients in the LEOPOLD clinical trials
| Asiana (n = 10) | Non‐Asian (n = 32) | |
|---|---|---|
| AUCnorm, kg/h/dL | 22.1‐50.7 | 13.5‐68.2 |
| Cmax, norm, kg/dL | 1.2‐3.6 | 0.9‐3.5 |
| Half‐life, h | 9.6‐23.7 | 7.7‐18.8 |
AUCnorm, dose‐normalized area under the curve; Cmax, norm, dose‐normalized maximum concentration.
Includes six Chinese patients.
Median FVIII recovery in Chinese patients measured using the chromogenic assay and based on a nominal dose of 50 IU/kg was 1.7‐2.1 kg/dL at the start of BAY 81‐8973 dosing and 1.7‐2.0 kg/dL after 6 months. FVIII recovery measured using the one‐stage assay did not differ relevantly from results obtained using the chromogenic assay.
3.5. Safety
Adverse events were reported in 9 of the 23 Chinese patients (39.1%) in LEOPOLD II. One AE (mild allergic dermatitis) was considered treatment related. No treatment‐related serious AEs occurred, and no patients discontinued treatment because of AEs. No patients tested positive for FVIII inhibitors.
4. DISCUSSION
In a subanalysis of LEOPOLD II data, the efficacy and safety of BAY 81‐8973 for prophylaxis and treatment of bleeding episodes in previously treated Chinese patients with severe haemophilia A were comparable to that of the full LEOPOLD II patient population. Chinese patients had higher bleeding rates for all bleeds and joint bleeds prestudy, as well as all having target joints, indicating more severe disease compared with non‐Chinese patients. However, on‐study ABR for all bleeds and joint bleeds was similar in Chinese and non‐Chinese patients, demonstrating that treatment with BAY 81‐8973 was successful. For both Chinese and non‐Chinese patients, ABRs for all bleeds and joint bleeds were significantly lower with prophylaxis versus on‐demand treatment during both the first and last 6 months on‐study. For the entire 12 months, both Chinese and non‐Chinese patients also demonstrated significantly lower ABRs for all bleeds, joint bleeds, spontaneous bleeds and trauma‐related bleeds with prophylaxis versus on‐demand treatment. These results confirm a lack of ethnic differences in the improved control of bleeding episodes seen with BAY 81‐8973 prophylaxis compared with on‐demand treatment. Response to treatment of bleeds was good or excellent for 54.9% and 62.9% of patients in the on‐demand and combined prophylaxis groups, respectively; these results could be a reflection of current clinical practice in China, where lower doses are used. The percentage of bleeds that required ≤2 infusions was 97% for on‐demand and 91% for prophylaxis patients. It should also be noted that the dosage in the prophylaxis groups was to some extent at the discretion of the investigators, but had to be within the prespecified range for the respective group (low‐dose group: 20, 25 or 30 IU/kg; high‐dose group: 30, 35 or 40 IU/kg). BAY 81‐8973 was well tolerated in Chinese patients, with only 1 treatment‐related AE (mild allergic dermatitis) and no FVIII inhibitor development in any patient.
Pharmacokinetic data were available from 10 Asian patients, including six patients from China who had PK parameters measured in the LEOPOLD I study. In general, PK data for Asian patients were within the range of values seen for non‐Asian patients. This finding is as expected because ethnic differences in the PK parameters of BAY 81‐8973 are unlikely, given that clearance of endogenous human FVIII is not mediated by drug‐metabolizing enzymes having a genetic polymorphism.19
Caring for patients with haemophilia in some regions of China is challenging owing to insufficient healthcare infrastructure and experience, lack of treatment affordability and accessible insurance, and low disease awareness in the community. As a result, in some areas of China, many patients receive little or no treatment.4 Even low‐dose, short‐term prophylaxis treatment provides some clinical benefit for these patients,9 indicating that standard‐dose prophylaxis as recommended by the WFH would have substantial effects on clinical outcomes and quality of life.1, 8 Indeed, results of the present study indicate that the benefits of standard‐dose prophylaxis for Chinese patients would likely be similar to those observed for the rest of the world.
5. CONCLUSIONS
BAY 81‐8973 was efficacious and well tolerated in previously treated Chinese patients with severe haemophilia A treated on‐demand or with prophylaxis in the LEOPOLD II clinical trial, with results in Chinese patients consistent with the full LEOPOLD II population. The significant decrease in bleeding rates for all bleeds and joint bleeds seen in Chinese patients receiving BAY 81‐8973 prophylaxis compared with those continuing on‐demand treatment support the use of prophylaxis in this patient population.
DISCLOSURES
Renchi Yang has received speaker/consultancy fees from Bayer, Shire, Novo Nordisk and Pfizer. Yongqiang Zhao has received fees for consulting and speaking from Bayer, Novo Nordisk and Shire. Jing Sun, Xuefeng Wang and Depei Wu have no conflicts of interest to report. Despina Tseneklidou‐Stoeter, Junde Wu and Nikki Church are employees of Bayer.
AUTHOR CONTRIBUTION
Renchi Yang, Jing Sun, Yongqiang Zhao, Xuefeng Wang, and Depei Wu performed the research. Junde Wu contributed to the data analysis. Despina Tseneklidou‐Stoeter and Nikki Church contributed to data analysis and interpretation.
ACKNOWLEDGEMENTS
The authors thank Horst Beckmann and Stephan Rauchensteiner of Bayer for their input and guidance on this paper. Medical writing assistance was provided by Karen L. Zimmermann from Complete Healthcare Communications, LLC (North Wales, PA, USA), and was fully funded by Bayer.
Yang R, Sun J, Zhao Y, et al. Efficacy and safety of prophylaxis with BAY 81‐8973 in Chinese patients with severe haemophilia A enrolled in the LEOPOLD II trial. Haemophilia. 2019;25:e153–e158. 10.1111/hae.13751
REFERENCES
- 1. Srivastava A, Brewer AK, Mauser‐Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1‐e47. [DOI] [PubMed] [Google Scholar]
- 2. Manco‐Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535‐544. [DOI] [PubMed] [Google Scholar]
- 3. Ozelo MC, Matta MA, Yang R. Meeting the challenges of haemophilia care and patient support in China and Brazil. Haemophilia. 2012;18(Suppl 5):33‐38. [DOI] [PubMed] [Google Scholar]
- 4. Poon MC, Luke KH. Haemophilia care in China: achievements of a decade of World Federation of Hemophilia treatment centre twinning activities. Haemophilia. 2008;14:879‐888. [DOI] [PubMed] [Google Scholar]
- 5. Yao W, Xiao J, Cheng X, et al. The efficacy of recombinant FVIII low‐dose prophylaxis in Chinese pediatric patients with severe hemophilia A: a retrospective analysis from the ReCARE study. Clin Appl Thromb Hemost. 2017;23:851‐858. [DOI] [PubMed] [Google Scholar]
- 6. Wang T, Zhang L, Li H, Zhao H, Yang R. Assessing health‐related quality‐of‐life in individuals with haemophilia in China. Haemophilia. 2004;10:370‐375. [DOI] [PubMed] [Google Scholar]
- 7. Wu R, Wu X, Zhang N, et al. Joint disease status of severe and moderate haemophilia patients at the Beijing Children's Hospital: early onset and rapid increasing severity of arthropathy in 90% of patients by 6 years of age. Haemophilia. 2014;20:e227‐e230. [DOI] [PubMed] [Google Scholar]
- 8. Dunkley S, Lam J, John MJ, et al. Principles of haemophilia care: the Asia‐Pacific perspective. Haemophilia. 2018;24:366‐375. [DOI] [PubMed] [Google Scholar]
- 9. Tang L, Wu R, Sun J, et al. Short‐term low‐dose secondary prophylaxis for severe/moderate haemophilia A children is beneficial to reduce bleed and improve daily activity, but there are obstacle in its execution: a multi‐centre pilot study in China. Haemophilia. 2013;19:27‐34. [DOI] [PubMed] [Google Scholar]
- 10. Wu R, Luke KH. The benefit of low dose prophylaxis in the treatment of hemophilia: a focus on China. Expert Rev Hematol. 2017;10:995‐1004. [DOI] [PubMed] [Google Scholar]
- 11. Kovaltry® (antihemophilic factor [recombinant]). Full prescribing information. Bayer HealthCare LLC, Whippany, NJ;2016.
- 12. Kovaltry (recombinant human coagulation factor VIII). Summary of product characteristics. Bayer AG,Leverkusen, Germany: 2017.
- 13. Kavakli K, Yang R, Rusen L, et al. Prophylaxis vs. on‐demand treatment with BAY 81–8973, a full‐length plasma protein‐free recombinant factor VIII product: results from a randomized trial (LEOPOLD II). J Thromb Haemost. 2015;13:360‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ljung R, Kenet G, Mancuso ME, et al. BAY 81–8973 safety and efficacy for prophylaxis and treatment of bleeds in previously treated children with severe haemophilia A: results of the LEOPOLD Kids Trial. Haemophilia. 2016;22:354‐360. [DOI] [PubMed] [Google Scholar]
- 15. Saxena K, Lalezari S, Oldenberg J, et al. Efficacy and safety of BAY 81–8973, a full‐length recombinant factor VIII: results from the LEOPOLD I trial. Haemophilia. 2016;22:706‐712. [DOI] [PubMed] [Google Scholar]
- 16. Shi J, Zhao Y, Wu J, Sun J, Wang L, Yang R. Safety and efficacy of a sucrose‐formulated recombinant factor VIII product for the treatment of previously treated patients with haemophilia A in China. Haemophilia. 2007;13:351‐356. [DOI] [PubMed] [Google Scholar]
- 17. Zhao Y, Xiao J, Yang R, et al. Efficacy of standard prophylaxis versus on‐demand treatment with Bayer's sucrose‐formulated recombinant FVIII (rFVIII‐FS) in Chinese children with severe hemophilia A. Pediatr Hematol Oncol. 2017;34:138‐148. [DOI] [PubMed] [Google Scholar]
- 18. Garger S, Severs J, Regan L, et al. BAY 81–8973, a full‐length recombinant factor VIII: manufacturing processes and product characteristics. Haemophilia. 2017;23:e67‐e78. [DOI] [PubMed] [Google Scholar]
- 19. Kepa S, Horvath B, Reitter‐Pfoertner S, et al. Parameters influencing FVIII pharmacokinetics in patients with severe and moderate haemophilia A. Haemophilia. 2015;21:343‐350. [DOI] [PubMed] [Google Scholar]


