Abstract
Background:
Rising prostate-specific antigen (PSA) levels are associated with both increased risk of prostate cancer and prostatic inflammation. The confounding effects of inflammation on the utility of PSA kinetics to predict prostate cancer may be partially mitigated by anti-inflammatory drug use. We investigated the influence of anti-inflammatory drug use on the association of PSA kinetics with prostate cancer risk.
Methods:
We studied 488 prostate cancer case-control pairs (290 white, 198 African American (AA)) nested in a retrospective cohort of men with a benign prostate biopsy. A series of multivariable models estimated prostate cancer risk associated with PSA velocity (PSAV) at different levels of anti-inflammatory drug use while adjusting for the presence of both clinical and histologic prostatitis.
Results:
In men with one, two, or three or more courses of anti-inflammatory drug use, for each ng/mL/year increase in PSAV, prostate cancer risk increased 1.21-fold, 1.83-fold, and 1.97-fold, respectively (P < 0.0001). In controls with histologic prostatitis, anti-inflammatory drug use was associated with a significantly lower PSAV (P < 0.0001). This association was not observed in men with histologic prostatitis who were subsequently diagnosed with prostate cancer. A positive interaction between anti-inflammatory drug use and PSAV-associated prostate cancer risk was only observed in AA men, as well as a strong positive association between any anti-inflammatory drug use and clinical prostatitis (P = 0.004).
Conclusions:
In men with benign prostate biopsy, accounting for the presence of histologic prostatitis and anti-inflammatory drug use, particularly in AA men, may help distinguish between men with rising PSA because of prostatitis vs undiagnosed cancer.
Keywords: anti-inflammatory, benign, biopsy, effect modifier, inflammation, prostate cancer, prostate-specific antigen
1 |. INTRODUCTION
It has been suggested that the rate of prostate-specific antigen (PSA) change over time, or PSA velocity (PSAV), may help in differentiating between men with prostate cancer from those with PSA elevation because of benign causes (eg benign prostatic hyperplasia (BPH) or prostatitis).1 However, the utility of PSAV as a predictor of subsequent prostate cancer has been questioned, and in several studies PSAV was not a better predictor of prostate cancer than the absolute value of PSA.2–4 A complex relationship exists among PSAV, cancer detection, BPH, and histologic prostatitis. Generally, in the absence of prostate cancer, PSA levels remain relatively constant. If any measurable PSAV exists, it can generally be attributable to an enlarging prostate gland because of BPH. With prostate cancer, PSA rises gradually and modestly, causing a sustained PSAV. In contrast, when the prostate is inflamed, particularly in the settings of acute prostatitis, PSA levels often increase dramatically and subsequently decrease after treatment with anti-inflammatory medications,5 which will not have an effect on PSA rise because of prostate cancer.
The positive correlation between prostatic inflammation and circulating PSA levels 6 confounds studies aimed at determining an inflammation-cancer causative association. In patients with a benign biopsy performed because of elevated PSA, either undetected cancer or prostatitis, often subclinical, may serve as plausible explanations for the elevated PSA. Anti-inflammatory medications, commonly used in older men, can reduce PSA levels7 and possibly reduce prostate cancer risk.8 In the United States, where PSA screening is the primary tool of prostate cancer detection and the use of anti-inflammatory drugs is common, it is difficult to determine whether the observed reduced prostate cancer risk among anti-inflammatory medication users reflects a true causal disease link or is primarily a result of less prostate cancer detection.9,10
The potential modifying effects of race have not been considered in studies of PSAV, prostatic inflammation, and prostate cancer risk. African American men are at greater risk for prostate cancer11 and associations between prostatic inflammation and prostate cancer may vary by race. Histologic prostatitis and higher PSA levels may be more common in African American men with prostate cancer,12 but we found no racial differences in the prevalence of prostatic inflammation in benign prostate biopsies or transurethral resections.13 Whether clinical prostatitis is a prostate cancer risk factor is unclear.14–16 In our recent study, we found clinical prostatitis was associated with a lower prostate cancer risk in African American men.17 Evidence also exists of unequal PSA amounts between white and African American men with prostate cancer.18–20 while some have postulated that benign prostate tissue in African Americans may contribute more PSA to the circulation than that of Whites,21 when prostate weight and PSA hemodilution because of different body weights are accounted for,20 benign prostate tissue produces equal amounts of PSA in African American and white men.22
To increase the utility of PSA kinetics as a measure of prostate cancer risk, a better understanding of the effect of prostatic inflammation and concomitant anti-inflammatory drug use on longitudinal PSA changes in men with a subsequent cancer diagnosis and those who remained disease-free is needed. We address this question using a nested case-control design in a cohort of high-risk men with an initial benign prostate biopsy by testing how anti-inflammatory drug use modifies the association between prostatic inflammation, PSAV, and prostate cancer risk. We also investigate whether any joint associations of these factors with prostate cancer risk differs by race.
2 |. MATERIALS AND METHODS
2.1 |. Patient sample and medical data
After institutional IRB approval, we assembled a historical cohort of 6692 men with a benign prostate specimen collected between January 1990 and December 2002.13,17 Eligibility criteria for the cohort included a recorded PSA level within a year of cohort entry and no history of a previous prostate cancer diagnosis. In this observational cohort, the date of cohort entry was defined as the date of the first benign prostate specimen in the Henry Ford Hospital pathology database that was collected after January 1990. For the present study, we further restricted the cohort to men with a benign prostate biopsy at cohort entry (n = 5432) excluding those with transurethral resection specimens. Using the “date of case diagnosis” as the date of first cancer-positive tissue biopsy or the date a clinician first reported a clinical diagnosis of prostate cancer, we identified 539 incident prostate cancer cases (Figure 1). Patients diagnosed with prostate cancer less than 1 year from the date of initial biopsy were ineligible for the study. Incidence density sampling with replacement was used to randomly select controls from all cohort members at risk at the time of case occurrence and one-to-one match them to cases on age at cohort entry (±2 years), date of cohort entry (±2 years), and race (African, American, or white). Controls were assigned a “reference date”, which corresponded to the date equal to the control’s cohort entry date plus the duration of time between the matched case’s cohort entry and diagnosis dates. Further restricting cases and controls to men with at least two PSA tests during follow-up resulted in a final analytic sample of 488 case-control pairs. Table 1 summarizes the analytic study population.
FIGURE 1.
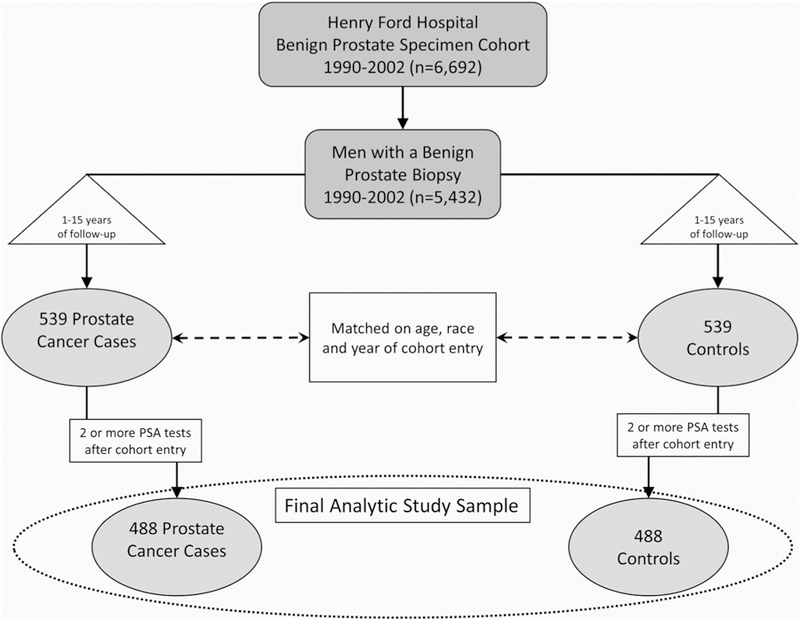
Diagram depicting formation of the analytic nested case-control study sample. PSA, prostate-specific antigen
TABLE 1.
Characteristics of analytic sample at baseline (488 Matched Pairs)
| Variable | Response | Cases | Controls | P value |
|---|---|---|---|---|
| Race* | white | 290 | 290 | – |
| African American | 198 | 198 | ||
| Mean Age at cohort entry, y* | 65.3 ± 7.4 | 65.4 ± 7.4 | – | |
| Date of 1st benign specimen* | ||||
| Median | 5/4/1995 | 4/12/1995 | – | |
| Range | 1/19/90–11/25/02 | 1/18/90–9/30/02 | ||
| Years to case diagnosis or follow-up time for controls* | ||||
| Median | 4.15 | 4.15 | – | |
| Range | 1.01–15.09 | 1.01–15.09 | ||
| Mean serum PSA at cohort entry, ng/mL | 7.82 ± 7.40 | 5.91 ± 5.49 | < 0.0001 | |
| Mean PSA velocity, ng/mL/year | 7.97 ± 97.12 | −0.06 ± 2.49 | < 0.0001 | |
| Mean number of PSA tests from cohort entry to diagnosis (cases) or reference (controls) date | 6.61 ± 3.56 | 5.55 ± 3.18 | < 0.0001 |
Abbreviation: PSA, prostate-specific antigen.
Paired Wilcoxon Sign Rank Test
Matching variable.
Clinical, demographic, and medication use data were abstracted from patients’ medical records 5 years before the date of cohort entry through the date of diagnosis (cases) or reference date (controls). Medical records were reviewed for any clinical note of “prostatitis” and information necessary to assign an NIH prostatitis category 23 was obtained. A “course” of anti-inflammatory drug use was defined as a notation of a patient using an anti-inflammatory drug and was stratified into steroidal and nonsteroidal categories (A). The number of anti-inflammatory drug courses between cohort entry and diagnosis/reference date was recorded and used as a semi-quantitative measure of anti-inflammatory drug exposure. The PSA level immediately before the initial benign biopsy was used as the baseline. Routine hematoxylin and eosin stained slides for all prostate biopsies at cohort entry were made and reviewed for the presence of inflammation by a single urological pathologist (ONK) blinded to outcomes.13
2.2 |. Statistical analyses
Chi-squared tests were used to investigate associations between inflammation, clinical prostatitis, and anti-inflammatory drug use. A mixed effect model was used to analyze PSA change over time because the PSA level observation dates varied from subject to subject. A polynomial fit was used for PSAV curves to estimate the trajectories of PSA level changes from cohort entry to diagnosis/reference date. The SAS procedure PROC MIXED (SAS version 9.4; SAS Institute, Inc, Cary, NC) with the full maximum likelihood estimation method and quadratic order was used for model fitting. The difference of PSAV among groups was estimated for 10 years before diagnosis/reference date. A Cochran-Armitage trend test was used to assess for a linear relationship between prostate cancer risk associated with histological inflammation and exposure to anti-inflammatory drugs.
Conditional logistic regression analyses were used to estimate both unadjusted and adjusted odds ratios and confidence intervals for prostate cancer risk and to account for the one-to-one matched design that controlled for age and race. Comparisons between the stratified models were assessed using a conditional logistic regression model that included interaction terms with the stratified variable that also allowed for estimates of stratified odds ratios.
3 |. RESULTS
Ever use of steroids or nonsteroidal anti-inflammatory drugs (NSAIDs) was not associated with the presence of histologic prostatitis but both showed a strong association with clinical prostatitis (Table 2). These associations were observed to a greater degree in African Americans. For instance, 81.9% of AA men with a history of clinical prostatitis used NSAIDs, but NSAID use was observed in only 64.8% of AA men without clinical prostatitis (P = 0.003). Similar associations between clinical prostatitis and anti-inflammatory drug use were observed in white men.
TABLE 2.
Prostate inflammation and use of anti-inflammatory medications
| Histologic inflammation |
Clinical prostatitis |
|||||
|---|---|---|---|---|---|---|
| Variable | Yes | No | P value | Yes | No | P value |
| Full sample (n = 902) | n = 517 | n = 385 | n = 159 | n = 743 | ||
| Any steroid use | 29.8% | 33.5% | 0.23 | 39.6% | 29.6% | 0.01 |
| Any NSAID use | 68.5% | 67.5% | 0.76 | 81.8% | 65.1% | < 0.0001 |
| Any NSAID or steroid use | 73.7% | 73.5% | 0.95 | 84.9% | 71.2% | 0.0004 |
| Whites (n = 549) | n = 310 | n = 239 | n = 76 | n = 473 | ||
| Any steroid use | 31.0% | 34.7% | 0.35 | 36.8% | 31.9% | 0.40 |
| Any NSAID use | 68.4% | 66.5% | 0.64 | 81.6% | 65.3% | 0.005 |
| Any NSAID or steroid use | 74.2% | 73.2% | 0.80 | 84.2% | 72.1% | 0.03 |
| African Americans (n = 353) | n = 207 | n = 146 | n = 83 | n = 270 | ||
| Any steroid use | 28.0% | 31.5% | 0.48 | 42.2% | 25.6% | 0.004 |
| Any NSAID use | 68.6% | 69.2% | 0.91 | 81.9% | 64.8% | 0.003 |
| Any NSAID or steroid use | 73.0% | 74.0% | 0.83 | 85.5% | 69.6% | 0.004 |
Abbreviation: NSAID, nonsteroidal anti-inflammatory drug.
The use of anti-inflammatory drugs did not show an association with prostate cancer risk either in the full or race-stratified samples (data not shown). We next investigated the joint association of anti-inflammatory drug use and PSAV with prostate cancer risk using a PSA velocity × anti-inflammatory use interaction term in a conditional logistic model that allowed for estimation of stratified odds ratios (Table 3). With greater exposure to anti-inflammatory drugs, sustained PSAV was a stronger predictor of a subsequent prostate cancer diagnosis. The effect modification of anti-inflammatory drug use on PSAV as a prostate cancer risk factor was more profound and consistent in African Americans. For instance, for each ng/mL/year increase in PSAV, prostate cancer risk increased 1.32-fold in African American men with no anti-inflammatory drug use, but this risk increased to 4.67 in African American men with three or more courses of anti-inflammatory drug use. In whites, only a marginally significant increase in the change of the PSAV odds ratio with increasing steroid use was observed (P = 0.06), but the PSAV odds ratio did not change appreciably between the lowest and highest level of NSAID exposure.
TABLE 3.
Prostate cancer risk associated with each ng/mL/year increase in PSA velocity stratified by level of anti-inflammatory drug use in nested case-control sample
| 95% Confidence |
||||
|---|---|---|---|---|
| Variable | Odds ratio* | Interval | P value | Interaction P value |
| Full sample (n = 488 pairs) | ||||
| No steroid use | 1.28 | (1.17–1.40) | < 0.0001 | < 0.0001 |
| One course | 2.45 | (1.70–3.53) | < 0.0001 | |
| Two or more courses | 4.56 | (2.16–9.61) | < 0.0001 | |
| No NSAID use | 1.39 | (1.17–1.65) | 0.0002 | 0.17 |
| One course | 1.21 | (1.03–1.43) | 0.02 | |
| Two courses | 1.29 | (1.05–1.57) | 0.01 | |
| Three or more courses | 1.90 | (1.29–2.79) | 0.001 | |
| No anti-inflammatory use | 1.25 | (1.08–1.45) | 0.003 | 0.001 |
| One course | 1.21 | (1.08–1.35) | 0.0007 | |
| Two courses | 1.83 | (1.29–2.60) | 0.0008 | |
| Three or more courses | 1.97 | (1.43–2.71) | < 0.0001 | |
| Whites (n = 290 pairs) | ||||
| No steroid use | 1.28 | (1.11–1.46) | 0.0004 | 0.06 |
| One course | 1.62 | (1.12–2.35) | 0.01 | |
| Two or more courses | 4.03 | (1.41–11.51) | 0.009 | |
| No NSAID use | 1.29 | (1.07–1.55) | 0.007 | 0.1 |
| One course | 1.90 | (1.32–2.73) | 0.0005 | |
| Two courses | 1.15 | (0.94–1.40) | 0.17 | |
| Three or more courses | 1.41 | (0.99–2.00) | 0.055 | |
| No anti-inflammatory use | 1.22 | (1.02–1.46) | 0.03 | 0.15 |
| One course | 1.83 | (1.32–2.55) | 0.0003 | |
| Two courses | 1.23 | (0.96–1.58) | 0.1 | |
| Three or more Courses | 1.47 | (1.06–2.02) | 0.02 | |
| African Americans (n = 198 pairs) | ||||
| No steroid use | 1.56 | (1.28–1.91) | < 0.0001 | < 0.0001 |
| One course | 6.66 | (2.85–15.56) | < 0.0001 | |
| Two or more courses | 5.39 | (1.77–16.38) | 0.004 | |
| No NSAID use | 2.03 | (1.31–3.14) | 0.001 | 0.0002 |
| One course | 1.08 | (0.98–1.18) | 0.11 | |
| Two courses | 2.87 | (1.04–7.93) | 0.04 | |
| Three or more courses | 5.77 | (2.17–15.33) | 0.0005 | |
| No anti-inflammatory use | 1.32 | (0.99–1.76) | 0.06 | 0.0001 |
| One course | 1.34 | (1.10–1.64) | 0.004 | |
| Two courses | 3.78 | (1.81–7.90) | 0.0004 | |
| Three or more courses | 4.67 | (2.19–9.96) | < 0.0001 | |
Abbreviation: NSAID, nonsteroidal anti-inflammatory drug.
adjusted for a number of PSA tests, histologic inflammation, and presence of high grade prostatic intraepithelial neoplasia.
Figure 2A graphically shows the mixed model adjusted difference between PSAV in cases and controls stratified by anti-inflammatory drug use. Among cases, men with exposure to anti-inflammatory drugs had a higher PSAV compared with unexposed cases (P < 0.0001). The opposite was observed for controls, but the difference did not reach statistical significance (P = 0.09). Overall, PSAV in controls was much lower than in cases. A similar pattern for PSAV was observed when stratifying cases and controls by evidence of histologic prostatitis. Cases with histologic inflammation had a slightly lower PSAV than those without inflammation (Figure 2B; P = 0.14). When considering both histologic prostatitis and anti-inflammatory use, we found that in men subsequently diagnosed with prostate cancer, PSAV was lowest among cases with histologic inflammation and no exposure to anti-inflammatory drugs (Figure 3A). The PSAV trajectory in these cases was significantly lower than cases without inflammation who used anti-inflammatory drugs (P = 0.05). Among controls (Figure 3B), stratifying by prostatic inflammation and anti-inflammatory drug use resulted in different PSAV trajectories with the greatest PSAV difference between men with prostatic inflammation who were and were not exposed to anti-inflammatory drugs (P < 0.0001).
FIGURE 2.
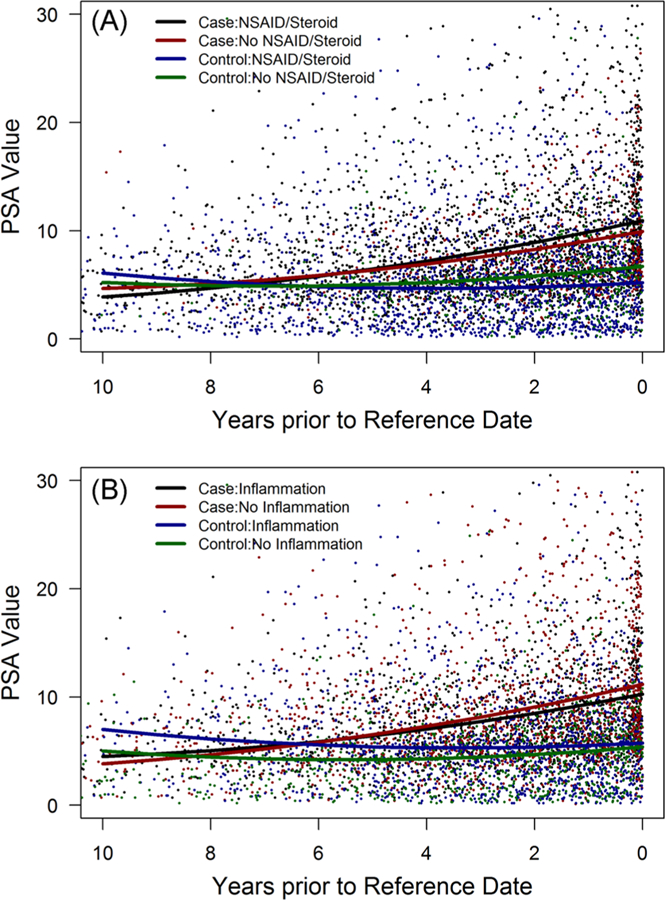
PSA velocity from cohort entry until diagnosis (cases) or reference (controls) date stratified by case/control status and anti-inflammatory drug use (A) or presence of prostatic inflammation (B). NSAID, nonsteroidal anti-inflammatory drug; PSA, prostate-specific antigen [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
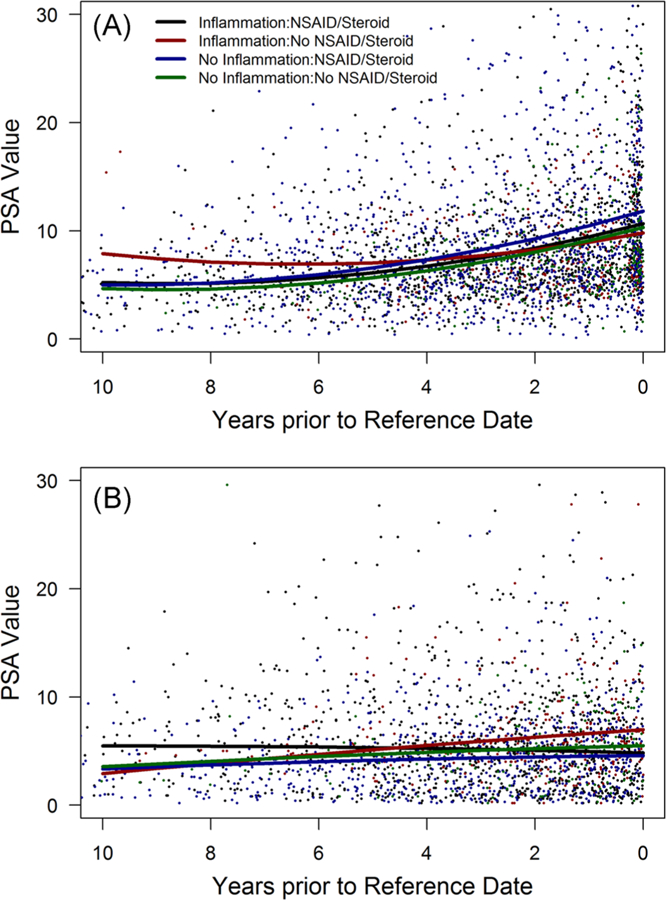
PSA velocity between cohort entry and diagnosis (reference date) stratified by prostatic inflammation and anti-inflammatory drug use among prostate cancer cases (A) and controls (B). NSAID, nonsteroidal anti-inflammatory drug; PSA, prostate-specific antigen [Color figure can be viewed at wileyonlinelibrary.com]
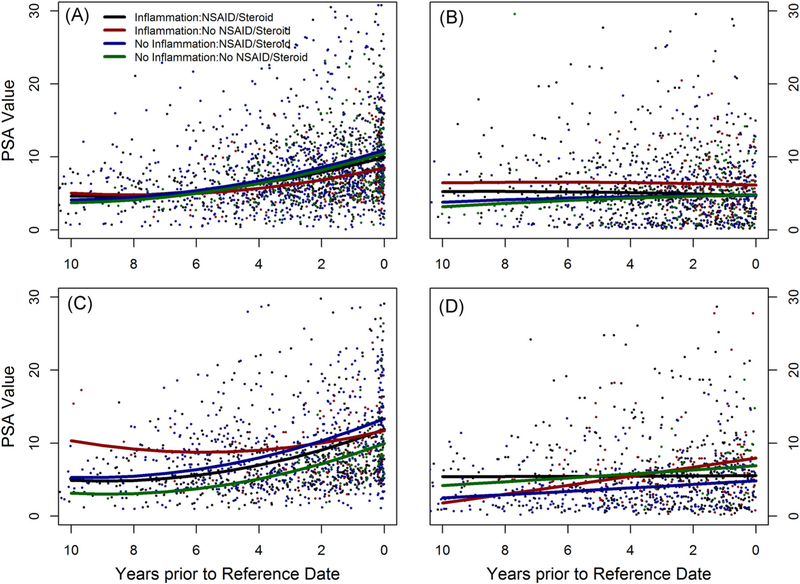
Stratifying PSAV trajectories by race and case/control status, we found the presence of prostatic inflammation and anti-inflammatory drug use did not alter PSAV trajectories in white cases (Figure 4A), but in African American cases prostatic inflammation shifted PSAV upwards whereas anti-inflammatory drug use shifted it downwards (Figure 4C). Similar to the full sample, PSAV was lowest among African American cases with histologic prostatitis and no exposure to anti-inflammatory drugs and most different from African American cases without prostatic inflammation who used anti-inflammatory drugs (P = 0.08). Among white controls, histologic prostatitis and anti-inflammatory drug use did not significantly differentiate PSAV (Figure 4B), but in African American controls the opposite was true (Figure 4D). The largest difference was between men with histologic prosta-titis who were and were not exposed to anti-inflammatory drugs (P < 0.0001).
FIGURE 4.
PSA velocity between cohort entry and diagnosis (reference date) stratified by prostatic inflammation and anti-inflammatory drug use among white cases (A) and controls (B) and African American cases (C) and controls (D). NSAID, nonsteroidal anti-inflammatory drug; PSA, prostate-specific antigen [Color figure can be viewed at wileyonlinelibrary.com]
4 |. DISCUSSION
We assembled a large matched case-control sample of men who were followed for a significant period of time to study the potential effect of anti-inflammatory drug use on PSA kinetics in men with a benign prostate biopsy who subsequently were either diagnosed with prostate cancer or remained disease-free. In both men diagnosed with cancer and those that remained disease-free, the use of anti-inflammatory drugs in the absence of prostatic inflammation did not significantly alter PSAV. Although 57.3% of men had histologic prostatitis, only 17.6% had documented clinical prostatitis with African Americans having a significantly higher prevalence than whites. As expected, men with symptomatic prostatitis used anti-inflammatory drugs significantly more often, but the presence of histologic prostatitis, often asymptomatic, was not associated with anti-inflammatory drug use. Frequency of anti-inflammatory drug use did not differ between cases and controls. Stratifying by the presence of prostatic inflammation and anti-inflammatory drug use resulted in different PSAV trajectories among cases and controls. Cases without prostatic inflammation exposed to anti-inflammatory drugs showed the highest PSAV whereas the opposite group, men with prostatic inflammation unexposed to anti-inflammatory drugs had the highest PSAV in controls. These differences between cases and controls can be explained in the context of how inflammation and cancer jointly increase PSA levels. In cases, continuous PSA elevation was likely driven primarily by increasing cancer volume with less contribution by inflammation or anti-inflammatory drug use. In controls, where PSA elevation is often related to prostatic inflammation, both anti-inflammatory drug use and prostatic inflammation were associated with PSAV. Hence, accounting for both of these factors among men with a benign biopsy may provide the information needed to identify men at higher risk for subsequent cancer on the basis of their follow-up PSAV.
In our study, a sustained PSAV for men using anti-inflammatory drugs was a harbinger for a subsequent cancer diagnosis. In the full and race-stratified samples, the risk of prostate cancer progressively increased in patients whose PSAV was not diminished with increasing courses of anti-inflammatory drug use. NSAIDs had a slightly different modifying effect on the association of PSAV with prostate cancer by race. In whites, we could not demonstrate the alteration of cancer risk associated with PSAV regardless of the number of NSAID courses. African Americans with three or more NSAID courses had a nearly six-fold increase of prostate cancer risk for each ng/mL/year of PSAV. It is not clear if this dichotomy could be explained by an underlying physiological mechanism. Approximately 85% of symptomatic white and African American men were treated with NSAIDs. Since African Americans with prostatic inflammation were symptomatic nearly twice as often as whites, NSAID treatment of the former could have effectively controlled PSA rise in men with prostatitis-related PSA elevation, whereas in whites NSAID administration occurred less often in the presence of clinical prostatitis.
Limited data exist regarding the effect of anti-inflammatory drug use on PSA kinetics and subsequent cancer risk in men with benign prostate biopsy with inflammation. Gallo et al5 studied 70 pairs of Italian men with benign prostate biopsy with and without inflamma-tion. All men were given an intense three-month course of combined anti-inflammatory drugs with subsequent PSA measurement and repeat biopsy. Average PSA level declined from 7.2 to 4.6 ng/mL in men with inflammation and only 7.2 to 7 ng/mL in those without – an observation similar to ours that in the absence of inflammation anti-inflammatory drugs do not significantly alter PSAV or PSA level. Although the design of Gallo et al5 study is different from ours, it highlights the utility of using profound decreases in PSA levels after anti-inflammatory drug therapy as a way to stratify men at high and low risk for prostate cancer. In our cohort, controls more often had histologic prostatitis. This group of men, who may be serially biopsied because of asymptomatic prostatitis-driven PSA elevation, was more likely to have an inverse association between PSAV and anti-inflammatory drug use.
Potts et al24 studied 122 patients with elevated PSA in whom evidence of asymptomatic prostatitis (NIH Category IV) was assessed by white blood cell count in expressed prostatic secretion. Seventy-one men without laboratory evidence of prostatitis were biopsied with 36 (51%) showing cancer. Fifty-one had inflammation and after treatment, PSA decreased below a biopsy threshold in 22 (43%). The cancer was detected in 9 of 29 (31%) biopsied men or 18% (9/51) of the entire cohort with inflammation. This observation was confirmed by a recent study where in nearly half of the patients with prostatitis, PSA decreased to below the biopsy threshold after a 4-week course of antibiotics and NSAIDs.25 These PSA alterations after a short-term therapy support our observations in men with up to 15 years of follow-up. The American Urological Association recommends early PSA screening in men with a family history of prostate cancer and in African American men. PSAV is often a decisive factor for prostate biopsy in these groups who often present with PSA below 4 ng/mL. Given our and others’ observations, accounting for prostatitis (either clinical or subclinical in biological specimens, such as expressed prostatic secretion or tissue) and anti-inflammatory drug therapy may help make a decision when original or repeat prostate biopsy is considered because of elevated PSA or sustained PSAV, particularly in African American men.
Our study had significant strengths including its nested case-control matched design, which accounted for temporal effects in clinical practice. We had a large, racially diverse study sample with long follow-up, and blinded focused pathology re-review. Most men underwent a sextant biopsy in contrast to at least 12-core biopsy currently used.26 Repeat biopsies were on the basis of clinical indications and no patients underwent MRI-targeted biopsy. We did not incorporate prostate volume or body mass index into analyses, which would have controlled for increasing gland volume over time or potential hemodilution effects, respectively.20 We did not analyze specific anti-inflammatory drugs but rather used broad drug groups as a variable. The medical record data used to determine anti-inflammatory drug use precluded having analyzable data on dose or duration, however, we used the number of medical chart notations as a surrogate for greater drug exposure. Our use of medical records likely resulted in an underestimate of anti-inflammatory drug use, with less complete data capture of non-prescription anti-inflammatory drug use.27 Because we matched on age, the main factor that appears to influence the accuracy of NSAID data in medical records,28,29 anti-inflammatory drug use misclassification in our study is likely non-differential and would bias our effect estimates towards the null. The severity of prostatic inflammation was not factored in either, although most patients had mild inflammation.13 Finally, because our study was observational, PSA data were not evenly distributed across study participants and anti-inflammatory medication use was not randomly assigned. However, given the robust nature of our findings and the large numbers in the risk strata we studied, our results should be generalizable to men with an indication for prostate biopsy.
In summary, the greater duration of anti-inflammatory drug(s) exposure, the more utility sustained PSAV provides as a predictor of prostate cancer. Our study results suggest that monitoring anti-inflammatory drug use and PSAV may help differentiate men with elevated PSA because of prostatic inflammation from those who have underlying cancer, particularly in AA men. In patients with cancer, PSA will continue to rise irrespective of anti-inflammatory drug use whereas men with elevated PSA because of prostatic inflammation may be prospectively identified by decreasing PSAV with anti-inflammatory drug use. Published evidence suggests that screening for asymptomatic prostatitis and its treatment may have merits at an original presentation of high-risk men.5,24,25 In men with benign prostate biopsy and rising PSA, accounting for the presence of prostatitis and use of anti-inflammatory drugs may help in the clinical use of the benign biopsy results or decision making when a repeat biopsy is considered.
ACKNOWLEDGMENT
This study was supported by NIH grant # 5R01-ES011126 (BAR).
Funding information
Center for Scientific Review, Grant/Award Number: NIH grant # 5R01-ES011126
APPENDIX A
| Non-Steroidal Anti-inflammatories used | Steroids used | ||
|---|---|---|---|
| Advil | Rezulin | Advair | Megace |
| Anaprox | Salicylate | Aerobid | Nasacort AQ |
| Ansaid | Salsalate | Androgel | Prednisone |
| Arthrotec | Sulindac | Avodart | Pulmicort |
| Aspirin | Tolectin | Azmacort | Rhinocort Aqua |
| Bextra | Toradol | Beclovent | Testosterone |
| Cataflam | Trilisate | Beconase | Triamcinolone Acetonide |
| Celebrex, ql | Vicoprofen | Celestone | |
| Clinoril | Vioxx | Cortisol | |
| Cox 2 | Voltaren | Cortisone | |
| Daypro | Zorprin | Cortisporin | |
| Diclofenac sodium | Deca-Durabolin | ||
| Difenac | Decadron | ||
| Disalcid | Depo-Medrol | ||
| Doan’s pills | Dermajet Kenalog | ||
| Dolobid | Derma-Smoothe | ||
| Easprin | Desonide Cream | ||
| Ecotrin | Dexacort Turbinaire | ||
| Edecrin | Dexamethasone | ||
| Feldene | Dhea | ||
| Ibuprofen | Dilacort | ||
| Indocin | Diprolene | ||
| Indomethacin | Diprosone cream | ||
| Ketoprofen | Dosepak | ||
| Lodine | Elocon | ||
| Meclomen | Flonase | ||
| Motrin | Florinef Acetate | ||
| Naprosyn | Flovent | ||
| Naproxen | Fludrocortisone Acetate | ||
| Norgesic | Fluticasone | ||
| Orudis | Halotestin | ||
| Oruvail | Hydrocortisone | ||
| Piroxicam | Medrol | ||
REFERENCES
- 1.Berger AP, Deibl M, Steiner H, et al. Longitudinal PSA changes in men with and without prostate cancer: assessment of prostate cancer risk. Prostate. 2005;64:240–245. [DOI] [PubMed] [Google Scholar]
- 2.Ulmert D, Serio AM, O’Brien MF, et al. Long-term prediction of prostate cancer: prostate-specific antigen (PSA) velocity is predictive but does not improve the predictive accuracy of a single PSA measurement 15 years or more before cancer diagnosis in a large, representative, unscreened population. J Clin Oncol. 2008;26:835–841. [DOI] [PubMed] [Google Scholar]
- 3.Vickers AJ, Till C, Tangen CM, Lilja H, Thompson IM. An empirical evaluation of guidelines on prostate-specific antigen velocity in prostate cancer detection. JNCI. 2011;103:462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loughlin KR. PSA velocity: a systematic review of clinical applications. Urol Oncol. 2014;32:1116–1125. [DOI] [PubMed] [Google Scholar]
- 5.Gallo L. The effect of a pure anti-inflammatory therapy on reducing prostate-specific antigen levels in patients diagnosed with a histologic prostatitis. Urology. 2016;94:198–203. [DOI] [PubMed] [Google Scholar]
- 6.Schatteman PH, Hoekx L, Wyndaele JJ, Jeuris W, Van Marck E. Inflammation in prostate biopsies of men without prostatic malignancy or clinical prostatitis: correlation with total serum PSA and PSA density. Eur Urol. 2000;37:404–412. [DOI] [PubMed] [Google Scholar]
- 7.Singer EA, Palapattu GS, van WE. Prostate-specific antigen levels in relation to consumption of nonsteroidal anti-inflammatory drugs and acetaminophen: results from the 2001-2002 National Health and Nutrition Examination Survey. Cancer. 2008;113:2053–2057. [DOI] [PubMed] [Google Scholar]
- 8.Vidal AC, Howard LE, Moreira DM, Castro-Santamaria R, Andriole GL, Freedland SJ. Aspirin, NSAIDs, and risk of prostate cancer: results from the REDUCE study. Clin Cancer Res. 2015;21:756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Platz EA, Rohrmann S, Pearson JD, et al. Nonsteroidal anti-inflammatory drugs and risk of prostate cancer in the Baltimore Longitudinal Study of Aging. Cancer Epidemiol Biomarkers Prev. 2005;14:390–396. [DOI] [PubMed] [Google Scholar]
- 10.Fowke JH, Motley SS, Smith JA Jr., et al. Association of nonsteroidal anti-inflammatory drugs, prostate specific antigen and prostate volume. J Urol. 2009;181:2064–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin. 2013;63:151–166. [DOI] [PubMed] [Google Scholar]
- 12.Eastham JA, May RA, Whatley T, Crow A, Venable DD, Sartor O. Clinical characteristics and biopsy specimen features in African-American and white men without prostate cancer. J Natl Cancer Inst. 1998;90:756–760. [DOI] [PubMed] [Google Scholar]
- 13.Kryvenko ON, Jankowski M, Chitale DA, et al. Inflammation and preneoplastic lesions in benign prostate as risk factors for prostate cancer. Mod Pathol. 2012;25:1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarma AV, McLaughlin JC, Wallner LP, et al. Sexual behavior, sexually transmitted diseases and prostatitis: the risk of prostate cancer in black men. J Urol. 2006;176:1108–1113. [DOI] [PubMed] [Google Scholar]
- 15.Cheng I, Witte JS, Jacobsen SJ, et al. Prostatitis, sexually transmitted diseases, and prostate cancer: the California Men’s Health Study. PLoS One. 2010;5:e8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perletti G, Monti E, Magri V, Cai T, Cleves A, Trinchieri A, Montanari E, et al. The association between prostatitis and prostate cancer. Systematic review and meta-analysis. Arch Ital Urol Androl. 2017;89:259–265. [DOI] [PubMed] [Google Scholar]
- 17.Rybicki BA, Kryvenko ON, Wang Y, et al. Racial differences in the relationship between clinical prostatitis, presence of inflammation in benign prostate and subsequent risk of prostate cancer. Prostate Cancer Prostatic Dis. 2016;19:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chinea FM, Lyapichev K, Epstein JI, et al. Understanding PSA and its derivatives in prediction of tumor volume: addressing health disparities in prostate cancer risk stratification. Oncotarget. 2017;8:20802–20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tewari A, Horninger W, Badani KK, et al. Racial differences in serum prostate-specific antigen (PSA) doubling time, histopathological variables and long-term PSA recurrence between African-American and white American men undergoing radical prostatectomy for clinically localized prostate cancer. BJUInt. 2005;96:29–33. [DOI] [PubMed] [Google Scholar]
- 20.Kryvenko ON, Diaz M, Matoso A, et al. Prostate-specific antigen mass density--a measure predicting prostate cancer volume and accounting for overweight and obesity-related prostate-specific antigen hemodilution. Urology. 2016;90:141–147. [DOI] [PubMed] [Google Scholar]
- 21.Fowler JE Jr., Bigler SA, Kilambi NK, Land SA. Relationships between prostate-specific antigen and prostate volume in black and white men with benign prostate biopsies. Urology. 1999;53:1175–1178. [DOI] [PubMed] [Google Scholar]
- 22.Kryvenko ON, Epstein JI, Cote RJ. Do Black NonHispanic men produce less prostate specific antigen in benign prostate tissue or cancer compared to white NonHispanic men with gleason score 6 (Grade Group 1) prostate cancer? J Urol. 2016;196:1659–1663. [DOI] [PubMed] [Google Scholar]
- 23.Krieger JN, Nyberg L Jr., Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236–237. [DOI] [PubMed] [Google Scholar]
- 24.Potts JM. Prospective identification of National Institutes of Health category IV prostatitis in men with elevated prostate specific antigen. J Urol. 2000;164:1550–1553. [PubMed] [Google Scholar]
- 25.Bozeman CB, Carver BS, Eastham JA, Venable DD. Treatment of chronic prostatitis lowers serum prostate specific antigen. J Urol. 2002;167:1723–1726. [PubMed] [Google Scholar]
- 26.Kryvenko ON, Diaz M, Meier FA, Ramineni M, Menon M, Gupta NS. Findings in 12-core transrectal ultrasound-guided prostate needle biopsy that predict more advanced cancer at prostatectomy: analysis of 388 biopsy-prostatectomy pairs. Am J Clin Pathol. 2012;137:739–746. [DOI] [PubMed] [Google Scholar]
- 27.Abdolrasulnia M, Weichold N, Shewchuk R, Saag K, Cobaugh DJ, LaCivita C, Weissman N, Allison J, et al. Agreement between medical record documentation and patient-reported use of nonsteroidal antiinflammatory drugs. Am J Health Syst Pharm. 2006;63:744–747. [DOI] [PubMed] [Google Scholar]
- 28.West SL, Savitz DA, Koch G, Strom BL, Guess HA, Hartzema A. Recall accuracy for prescription medications: self-report compared with database information. Am J Epidemiol. 1995;142:1103–1112. [DOI] [PubMed] [Google Scholar]
- 29.West SL, Strom BL, Freundlich B, Normand E, Koch G, Savitz DA. Completeness of prescription recording in outpatient medical records from a health maintenance organization. J Clin Epidemiol. 1994;47: 165–171. [DOI] [PubMed] [Google Scholar]