Summary
Objective
Patients with Lennox‐Gastaut syndrome (LGS) who completed 1 of 2 randomized, double‐blind, placebo‐controlled trials of add‐on cannabidiol (CBD) (GWPCARE3, NCT02224560 or GWPCARE4, NCT02224690) were invited to enroll in an open‐label extension (OLE) study evaluating the long‐term safety and efficacy of CBD (GWPCARE5, NCT02224573). Herein we present an interim analysis of the safety, efficacy, and patient‐reported outcomes from this trial.
Methods
Patients received a pharmaceutical formulation of highly purified CBD oral solution (Epidiolex; 100 mg/mL), titrated from 2.5 to 20 mg/kg/d over a 2‐week titration period, in addition to their existing medications. Doses could be reduced if not tolerated or increased up to 30 mg/kg/d if thought to be of benefit.
Results
This interim analysis was based on a November 2016 data cut. Of 368 patients who completed treatment in GWPCARE3 and GWPCARE4, 366 (99.5%) enrolled in the OLE study (GWPCARE5). Median treatment duration was 38 weeks at a mean modal dose of 23 mg/kg/d. Most patients (92.1%) experienced adverse events (AEs), primarily of mild (32.5%) or moderate (43.4%) severity. The most common AEs were diarrhea (26.8%), somnolence (23.5%), and convulsion (21.3%). Thirty‐five patients (9.6%) discontinued treatment due to AEs. Liver transaminase elevations were reported in 37 patients (10.1%), of whom 29 were receiving concomitant valproic acid; 34 cases resolved spontaneously or with dose modification of CBD or concomitant medication. Median reduction from baseline in drop seizure frequency (quantified monthly over 12‐week periods) ranged from 48% to 60% through week 48. Median reduction in monthly total seizure frequency ranged from 48% to 57% across all 12‐week periods through week 48. Eighty‐eight percent of patients/caregivers reported an improvement in the patient's overall condition per the Subject/Caregiver Global Impression of Change scale.
Significance
In this study, long‐term add‐on CBD treatment had an acceptable safety profile in patients with LGS and led to sustained reductions in seizures.
Keywords: antiepileptic drug, cannabinoid, childhood‐onset epilepsy, drop seizures
Key Points.
Three hundred sixty‐six patients with LGS were treated with long‐term CBD (mean modal dose 23 mg/kg/d; median treatment 263 days)
Most common AEs were diarrhea, somnolence, and convulsion, and most were mild to moderate in severity
Sustained reductions in drop and total seizures were observed through 48 weeks
Eighty‐eight percent of patients/caregivers reported improvement in overall condition after 48 weeks of treatment
1. INTRODUCTION
Lennox‐Gastaut syndrome (LGS) is a severe epileptic and developmental encephalopathy that typically arises by age 7 years, with peak onset between age 3 and 5 years.1, 2 The triad of LGS diagnostic criteria are the following: (a) multiple seizure types, mainly generalized, including tonic, atonic, and atypical seizures, with seizure types evolving over time; (b) abnormal electroencephalography (EEG) studies consisting mainly of interictal diffuse slow spike‐and‐wave complexes <3 Hz occurring during wakefulness and bouts of generalized paroxysmal fast activity; (c) cognitive impairment/intellectual disability.1, 2, 3 The classic seizure types associated with LGS, but not required for diagnosis, are tonic and atonic generalized seizures that may result in drop attacks and have the most potential to harm.
US Food and Drug Administration (FDA)–approved medications for LGS are felbamate, lamotrigine, topiramate, rufinamide, clobazam, clonazepam, and most recently GW Pharmaceuticals' formulation of cannabidiol (CBD), whereas rufinamide, topiramate, lamotrigine, and felbamate are approved in most countries of the European Union. Valproic acid is also a frequently used first‐line therapy, although it is not specifically approved for LGS.2 No monotherapy has high efficacy in the treatment of LGS, with polytherapy being the norm,4 and long‐term seizure control and cognitive outcomes are poor even with polypharmacologic treatment.2, 5, 6
Cannabidiol is a phytocannabinoid derived from Cannabis sativa that demonstrates antiseizure activity in vitro and in animal seizure models.7, 8, 9 Highly purified CBD is approved as Epidiolex (Greenwich Biosciences, Inc.) in the United States for seizures associated with LGS or Dravet syndrome in patients ≥2 years of age.10 Compared with other approved antiepileptic drugs (AEDs), CBD has a unique structure and potentially novel multimodal mechanism of action and does not activate or bind directly to cannabinoid receptors CB1 or CB2 at physiologically achievable concentrations.11, 12
Clinical experience has indicated that CBD has antiseizure efficacy in LGS. In a study of 214 patients with treatment‐resistant epilepsy enrolled in an early access program, which included 31 (19%) with LGS, CBD reduced seizure frequency and had an acceptable safety profile.13 In two, 14‐week randomized, double‐blind, placebo‐controlled studies that enrolled patients with LGS (GWPCARE3 and GWPCARE4), add‐on CBD resulted in a reduced frequency of drop seizures vs placebo, improved patient‐reported outcomes, and was well tolerated.14, 15
GWPCARE5 is an ongoing open‐label extension study of add‐on CBD in patients with LGS who completed treatment in GWPCARE3 or GWPCARE4 and patients with Dravet syndrome who completed treatment in one of two, phase 3 studies (GWPCARE1 and GWPCARE2). Herein we present an interim analysis of safety, efficacy, and patient‐reported outcomes for patients with LGS who received CBD in GWPCARE5.
2. METHODS
Patients who completed the treatment period in trials GWPCARE3 (NCT02224560) and GWPCARE4 (NCT02224690) were eligible for enrollment in GWPCARE5 (NCT02224573), an open‐label extension (OLE) trial. All patients were aged 2‐55 years with a clinical diagnosis of LGS inadequately controlled by ≥1 current AED, had a history of slow (<3 Hz) spike‐and‐wave pattern EEG recordings, and had experienced ≥2 drop seizures per week during the 4‐week baseline period of the parent study. Drop seizures in these trials were defined as atonic, tonic, or tonic–clonic seizures involving the entire body, trunk, or head that led (or could have led) to a fall, injury, slumping in a chair, or hitting the patient's head on a surface.
No trial procedures were carried out on patients until written consent had been obtained from the patient or the patient's parent, caregiver, or legal representative, and, when possible, written assent had been obtained from the patient. The informed consent form, protocol, and amendments for this study were submitted to and approved by the institutional review board or independent ethics committee at each participating study site.
Patients received a pharmaceutical formulation of highly purified CBD derived from C. sativa L. plant in oral solution (100 mg/mL; Epidiolex in the United States; GW Research Ltd, Cambridge, UK), titrated from 2.5 to 20 mg/kg/d over a 2‐week titration period, and continued to receive this dose during the maintenance period. Patients received CBD in addition to their existing AEDs. Investigators could decrease the dose of CBD if a patient experienced intolerance or could increase the dose to a maximum of 30 mg/kg/d if thought to be of benefit by the physician. Patients could receive treatment for up to 1 year (United Kingdom, Spain, The Netherlands) or up to 3 years (United States, France, Poland). Findings reported in this study are specific to GW Pharmaceuticals' formulation of CBD and cannot be extrapolated to other CBD products. The data cut for this interim analysis was November 3, 2016. A more recent data cut is not yet available for publication due to ongoing clinical and regulatory activities.
The primary objective of this OLE study was to evaluate the long‐term safety and tolerability of adjunctive CBD treatment, based on treatment‐emergent adverse events (AEs), vital signs, 12‐lead electrocardiography, and clinical laboratory parameters, including serum levels of hepatic enzymes; drug‐induced liver injury was assessed as per Hy's law.16 Secondary objectives were to evaluate the efficacy of CBD as determined by changes in the frequency of drop and total seizures, seizure‐reduction responder rates, episodes of status epilepticus, and patient‐reported outcomes based on changes in the Subject/Caregiver Global Impression of Change (S/CGIC) scale.
Patients (or their caregivers) completed a daily paper diary to record AEs and daily usage of CBD, concomitant AEDs, and rescue medications. Information on seizure number and type was collected through an interactive voice recording system telephone diary completed weekly. The 7‐point S/CGIC scale (Appendix S1) was assessed at clinic visits at week 24, 38, and 48; if both caregiver and patient completed the S/CGIC, the caregiver score was used (Appendix S1). The percentage of patients reporting improvement on the S/CGIC scale was assessed using the number of patients who completed the questionnaire as the denominator at all time points; a conservative estimate was also performed to account for withdrawals using the total number of patients enrolled in the study as the denominator for the 24‐week visit window, since all enrolled patients could have reached this timepoint.
No formal sample size calculations were performed; all patients who wished to continue from the original placebo‐controlled studies were eligible for inclusion. Seizure frequencies (per 28 days) were determined for each 12‐week period of treatment. Percentage change in seizure frequency was calculated relative to the prerandomization baseline period from the parent placebo‐controlled trials. Analyses were repeated using inclusion of a last observation carried forward (LOCF) step, which is described in detail in the Appendix S1. Analyses were descriptive, and no formal hypothesis testing was conducted.
3. RESULTS
3.1. Patients
Of the 368 patients who completed the GWPCARE3 and GWPCARE4 randomized controlled studies, 366 (99.5%) enrolled in this open‐label extension (Figure 1) at 53 centers in the United States and Europe. Slightly more than half of enrolled patients were male and most received ≥3 concurrent AEDs during the open‐label period, the most common being clobazam and valproic acid (Table 1). At the data cut, 67 patients (18.3%) had withdrawn from treatment, most commonly due to AEs or patient/parent/guardian decision (Figure 1). More than half of discontinuing patients withdrew during the first 24 weeks of the study period (Table S1). Treatment was ongoing in 299 patients. Median treatment duration was 263 days (38 weeks; range 3‐430 days), and 208 patients had completed 48 weeks of treatment. Two patients had treatment for <14 days; neither of these patients reported seizure data and therefore were not included in seizure frequency reduction analyses.
Figure 1.
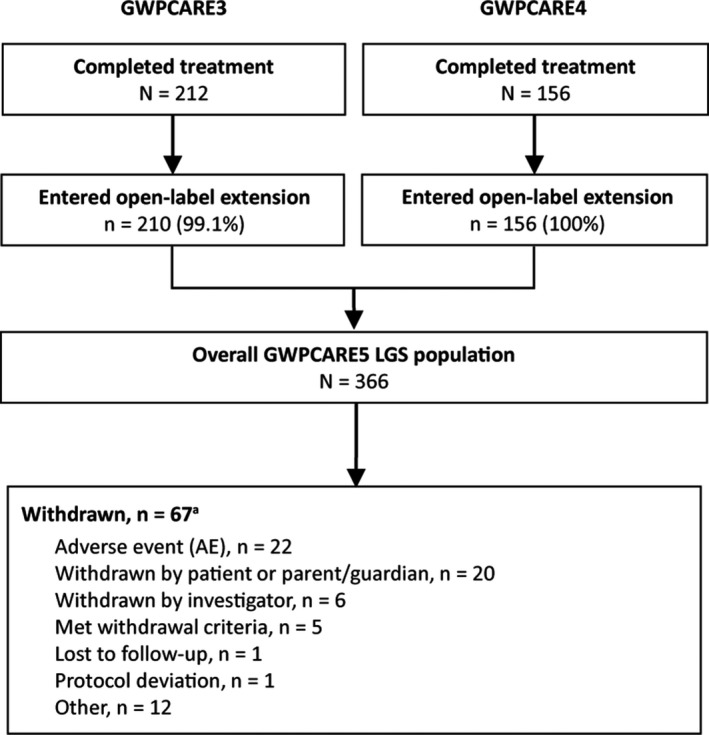
Patient disposition. LGS, Lennox‐Gastaut syndrome. aWithdrawals are shown by the primary reason for each patient
Table 1.
Patient demographics and baseline characteristics
| Parameter | CBD (N = 366) |
|---|---|
| Age at entry to OLE, (y) | |
| Mean (SD) | 15.9 (9.5) |
| Median (range) | 13.7 (3.0‐48.3) |
| Age group (y), n (%) | |
| 2‐5 | 36 (10) |
| 6‐11 | 121 (33) |
| 12‐17 | 89 (24) |
| 18‐55 | 120 (33) |
| Gender | |
| Male, n (%) | 198 (54) |
| Geographic region, n (%) | |
| United States | 284 (78) |
| Rest of world | 82 (22) |
| Race, n (%) | |
| White/Caucasian | 322 (88) |
| Black/African American | 14 (4) |
| American Indian/Alaska Native | 0 |
| Asian | 10 (3) |
| Other | 19 (5) |
| Unknowna | 1 (<1) |
| Body mass index at entry to OLE, mean (SD) | 20.2 (6.3) |
| Number of concomitant AEDs, median (range) | 3.0 (0, 9.0) |
| Concomitant AEDs (>20%), n (%) | |
| Clobazam | 188 (51) |
| Valproic acid | 136 (37) |
| Lamotrigine | 126 (34) |
| Levetiracetam | 122 (33) |
| Rufinamide | 104 (28) |
| Time on CBD treatment, median (range), d | 263 (3‐430) |
| Modal CBD dose, mean (SD), mg/kg/d | 22.82 (5.11) |
AEDs, antiepileptic drugs; CBD, cannabidiol; OLE, open‐label extension; SD, standard deviation.
Unknown due to country‐specific data protection law.
The mean modal dose was 22.8 mg/kg/d over the treatment period for all patients and remained stable for each 12‐week reporting interval, ranging from 21.2‐24.3 mg/kg/d over the first 48 weeks of treatment. During patients' last 12 weeks of treatment before the data cut, the mean modal dose was 23.0 mg/kg/d (n = 364).
3.2. Safety
Treatment‐emergent AEs were reported in 337/366 patients (92.1%), 172/192 (89.6%) in those patients with modal dose ≤20 mg/kg/d and 165/174 (94.8%) in patients with modal dose >20 mg/kg/d. Most patients had AEs of mild (32.5%) or moderate (43.4%) severity. The most commonly reported AEs were diarrhea, somnolence, and convulsion; somnolence was reported in 57 of 188 patients (30.3%) who received concomitant clobazam, and 29 of 178 (16.3%) who did not receive clobazam. Decreased body weight was reported as an AE in 33 patients (9.0%) and was of mild or moderate severity in all cases. Serious AEs were reported in 94 patients (25.7%), and the most commonly reported were status epilepticus and convulsion (Table 2). Thirty‐five patients (9.6%) discontinued treatment due to AEs, with the most common (>1%) AEs leading to discontinuation being convulsion (n = 6 [1.6%]), vomiting (n = 5 [1.4%]), diarrhea (n = 5 [1.4%]), alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) increased (n = 5 [1.4%]), hepatic enzyme level increase (n = 4 [1.1%]), and somnolence (n = 4 [1.1%]). Four deaths were reported during the interim analysis period, due to respiratory failure as a complication of aspiration pneumonia (n = 1), bowel obstruction with necrotic bowel and severe septic shock (n = 1), seizure disorder as primary cause with severe cerebral and pulmonary edema as secondary causes (n = 1), and complications of seizure disorder due to perinatal hypoxic ischemic encephalopathy (n = 1). None were deemed treatment related by the investigator.
Table 2.
Adverse events
| CBD modal dose | CBD (N = 366) | ||
|---|---|---|---|
| ≤20 mg/kg/d (n = 192) | >20 mg/kg/d (n = 174) | ||
| All‐causality AEs, n (%) | 172 (89.6) | 165 (94.8) | 337 (92.1) |
| AEs leading to withdrawal,a n (%) | 28 (14.6) | 7 (4.0) | 35 (9.6) |
| Serious AEs, n (%) | 46 (24.0) | 48 (27.6) | 94 (25.7) |
| AEs reported in >10% of patients, n (%) | |||
| Diarrhea | 43 (22.4) | 55 (31.6) | 98 (26.8) |
| Somnolence | 43 (22.4) | 43 (24.7) | 86 (23.5) |
| Convulsion | 41 (21.4) | 37 (21.3) | 78 (21.3) |
| Pyrexia | 26 (13.5) | 43 (24.7) | 69 (18.9) |
| Decreased appetite | 40 (20.8) | 25 (14.4) | 65 (17.8) |
| Vomiting | 30 (15.6) | 35 (20.1) | 65 (17.8) |
| Upper respiratory tract infection | 25 (13.0) | 28 (16.1) | 53 (14.5) |
| Serious AEs reported in >1% of patients | |||
| Status epilepticus | 11 (5.7) | 15 (8.6) | 26 (7.1) |
| Convulsion | 9 (4.7) | 11 (6.3) | 20 (5.5) |
| Pneumonia | 3 (1.6) | 6 (3.4) | 9 (2.5) |
| AST increased | 4 (2.1) | 2 (1.1) | 6 (1.6) |
| ALT increased | 5 (2.6) | 1 (0.6) | 6 (1.6) |
| Pneumonia aspiration | 4 (2.1) | 2 (1.1) | 6 (1.6) |
| Hepatic enzymes increased | 4 (2.1) | 0 | 4 (1.1) |
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBD cannabidiol.
Includes all patients with AE listed as one of the reasons for withdrawal.
Increased ALT and/or AST >3 times the upper limit of normal (ULN) were observed in 29 of the 136 (21.3%) patients who were receiving concomitant valproic acid, and 8 of the 230 patients (3.5%) who did not receive valproic acid, for a total of 37 (10.1%) overall. No patient met the criteria for drug‐induced liver injury (AST or ALT >3 times ULN with concomitant total bilirubin >2 ULN).16 Overall, 13 patients withdrew due to elevated transaminases. At the time of this interim analysis, increased ALT/AST had resolved in 34 patients, either spontaneously (n = 11), following discontinuation of treatment (n = 12), or after dose reduction of CBD or concomitant AED (n = 11, of whom n = 6 reduced valproic acid).
3.3. Efficacy
Because this is an interim analysis of an ongoing trial, not all patients had reached the later visit windows; withdrawals during each 12‐week visit window are shown in Table S1.
Median drop seizure frequency reduction from baseline was 48.2% at weeks 1‐12 (a decrease from a median of 80.0 seizures per month at baseline to 37.7 per month) and was sustained through 48 weeks (Figure 2A). In the LOCF analysis, median percentage reductions ranged from 48.2% to 55.0% during each 12‐week visit window (Figure S1A). Twenty‐three patients (6.3%) were drop seizure‐free during their last 12 weeks of treatment prior to the data cut, and 8 (2.2%) patients were drop seizure‐free throughout the full treatment period at the data cut, with treatment durations ranging from 157 to 367 days. Almost half of patients showed seizure frequency reductions of ≥50% in the first 12‐week visit window, and this increased to over half of patients in the subsequent visit windows; responder rates at ≥25%, ≥50%, ≥75%, and 100% drop seizure frequency reduction thresholds are shown in Figure 3A. In the LOCF analysis, responder rates at the ≥50% threshold ranged from 49.2% to 54.4% (Figure S2A).
Figure 2.
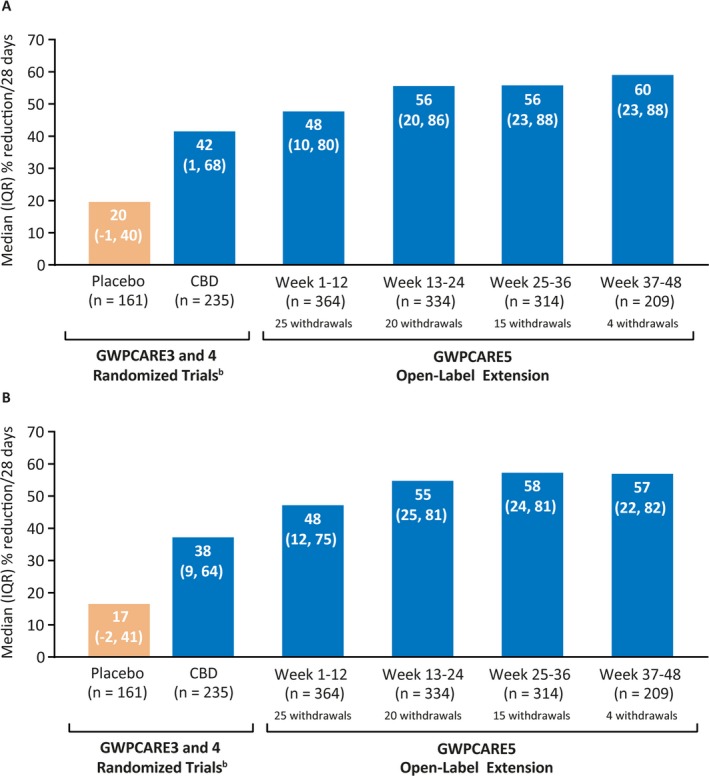
Reduction from baselinea in (A) drop seizure frequency and (B) total seizure frequency. CBD, cannabidiol; IQR, interquartile range. aReduction from baseline of parent randomized trial. bPooled data from randomized controlled trials during 14‐wk treatment period (2‐wk titration followed by 12‐wk maintenance); n = 235 patients in CBD group includes patients receiving 10 and 20 mg/kg/d CBD
Figure 3.
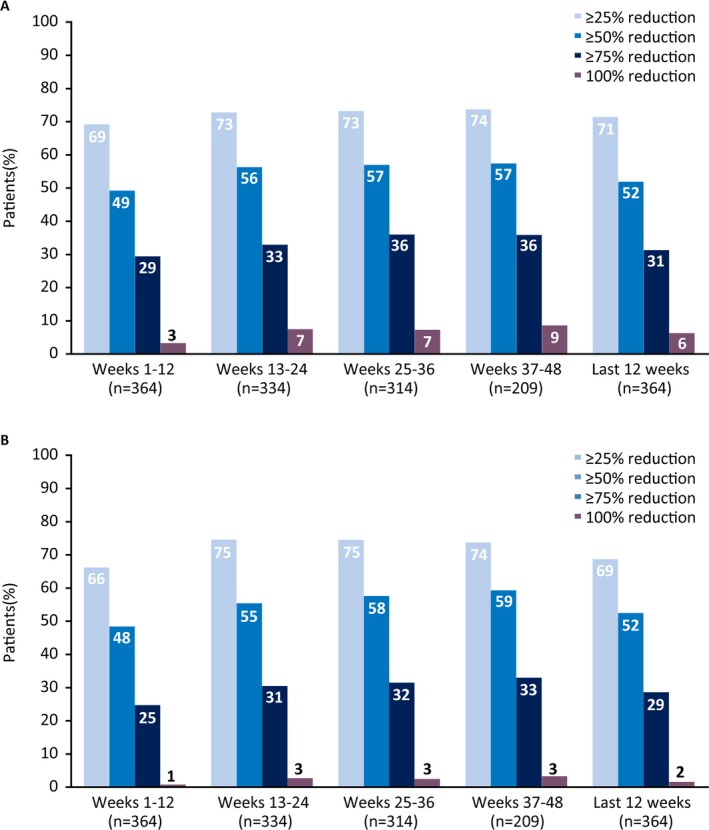
Responder rates at (A) drop and (B) total seizure reduction thresholds
Median total seizure frequency reduction from baseline was 47.7% at weeks 1‐12 (a decrease from a median of 167.6 seizures per month at baseline to 79.3 per month); in the subsequent three, 12‐week visit windows, reductions ranged from 55.3% to 57.4% (Figure 2B). In the LOCF analysis, median reductions ranged from 47.7% to 54.3% during each 12‐week visit window (Figure S1B). Six patients (1.6%) were seizure‐free during their last 12 weeks of treatment, and none were seizure‐free through the full treatment period. More than half of patients showed total seizure frequency reductions after the first 12‐week period; responder rates at ≥25%, ≥50%, ≥75%, and 100% total seizure‐reduction thresholds are shown in Figure 3B. Responder rates were generally similar in the LOCF analysis (Figure S2B), with ≥50% responder rates ranging from 48.4% to 54.4%.
Through 48 weeks of treatment, the number of patients with episodes of either convulsive or nonconvulsive status epilepticus was <4%, with no increase in incidence with continuing treatment; during the baseline period, 3.8% and 4.6% of patients had convulsive and nonconvulsive status epilepticus, respectively (Table S2).
Of the 299 patients/caregivers who completed the S/CGIC at week 24, 88% considered the patient's overall condition improved with CBD treatment, and this percentage was similar at weeks 38 and 48 (Figure 4). Of the 366 patients who enrolled in the study (all of whom enrolled early enough to complete 24 weeks of treatment), 72% reported improvement in overall condition after 24 weeks of treatment.
Figure 4.
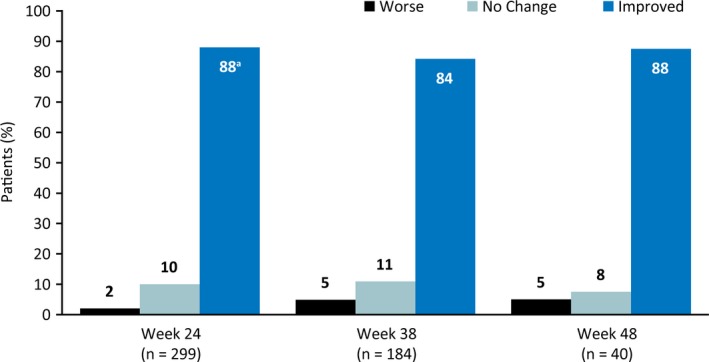
Patient/caregiver ratings of overall condition on the S/CGIC scale. S/CGIC, Subject/Caregiver Global Impression of Change. aOf the 366 patients who enrolled in study (all of whom enrolled early enough to complete 24 wks of treatment), 72% reported improvement in overall condition after 24 wks of treatment
4. DISCUSSION
This long‐term open‐label trial demonstrated that treatment with add‐on CBD was well tolerated and resulted in sustained reductions in the frequency of drop seizures and total seizures.
The safety profile of this open‐label study was similar to that observed in the 2 original 14‐week studies. Median duration of treatment in this extension study was 38 weeks, with some patients treated for up to 61 weeks. Incidence of AEs was similar to GWPCARE3/4, with most patients experiencing ≥1 AE. The most frequently reported AEs in this trial were also the most frequent in GWPCARE3/4 (diarrhea, somnolence, pyrexia, decreased appetite, vomiting, and upper respiratory tract infection), with the exception of convulsion, which was more frequent in the present trial. The rate of AEs leading to withdrawal was generally similar to that observed in the original randomized controlled trials (10%) despite a longer duration of exposure to CBD (7% in GWPCARE3 20 mg/kg/d, 14% in GWPCARE4).
Findings from previous OLE studies of AEDs suggest that long‐term retention rate can be a surrogate outcome for assessing treatment benefit.17 When taking into consideration the duration of follow‐up in the present study at the time of this analysis (median 38 weeks), there was a relatively low withdrawal rate of 18%. Although study burden can increase discontinuations in OLE studies, the lack of a commercially available pharmaceutical formulation of CBD may have encouraged patients to remain in the study. Real‐world analysis of long‐term retention rates on CBD is warranted after CBD becomes commercially available.
The rate of somnolence as an AE was higher in patients receiving concomitant clobazam vs those who were not (30.3% vs 16.3%, respectively). Previous clinical trial experience has shown that somnolence is a frequent AE in patients receiving clobazam, in that 16%‐22% of patients in 2 randomized controlled studies of clobazam and 17% in the subsequent OLE study had somnolence as an AE,18, 19, 20, 21 whereas the majority of patients who experienced somnolence as an AE in GWPCARE3 and GWPCARE4 were receiving concomitant clobazam.14, 15
Liver enzyme abnormalities were observed mainly in patients receiving concomitant valproic acid, which is consistent with previous clinical study experience with CBD.14, 15, 22 Although elevated transaminases led to treatment withdrawal in some patients, most elevations resolved while the patient continued on treatment either with the same treatment regimen or with reduced CBD or concomitant AED doses.
Atonic and tonic seizures resulting in falls (drop seizures) are characteristic of LGS.1, 2 The median reduction in drop seizure frequency with CBD in the GWPCARE3 and GWPCARE4 studies was 46% from baseline (vs 20% with placebo)14, 15; this level of reduction was sustained through 48 weeks of the OLE. The reduction in total seizure frequency observed in GWPCARE3/GWPCARE4 (median 44% reduction from baseline) was also sustained through 48 weeks of the OLE.
The S/CGIC scale is a patient/caregiver‐reported outcome measurement tool used in previous clinical studies to evaluate the clinical relevance of reductions in seizure frequency.22, 23, 24 The majority of patients/caregivers in this OLE study reported improvement in overall condition on the S/CGIC (>70% of patients who initially enrolled in the study; >80% of patients who completed the questionnaire). Although few patients experienced complete seizure freedom, results from these outcomes indicate that the majority of patients/caregivers perceived a clinically meaningful benefit with long‐term CBD treatment.
Median modal CBD dose was generally consistent from weeks 1‐12 to the last 12 weeks of data for each patient, suggesting that the study population did not develop tolerance to treatment and that increased dosing of CBD was not necessary to maintain seizure frequency reductions.
The findings of this study should be considered in the context of the following limitations. As an OLE, the study lacked a placebo comparator group. Because efficacy and patient/caregiver‐reported outcomes were determined as percentage changes from pretreatment baseline from original randomized trials, CBD exposure differed between patients originally randomized to placebo and those who received CBD throughout the original randomized trials. As this was an interim analysis, patients had different durations of exposure at the time of data cut; not all patients had completed later treatment windows. In addition, the majority of patients in this study were Caucasian. There is the potential for different results to be observed in other races due to differences in drug pharmacokinetics, such as differences in cytochrome P450 2C metabolism in Asian populations.25
This OLE demonstrated that long‐term add‐on CBD treatment had an acceptable safety profile and was well tolerated. The reductions in the frequency of drop and total seizures observed in the original placebo‐controlled trials were sustained with long‐term CBD treatment, and these reductions were considered meaningful by patients and their caregivers, as indicated by S/CGIC scores. The findings of this study support the long‐term safety and utility of add‐on CBD therapy in the control of seizures in patients with LGS.
CONFLICTS OF INTEREST
Elizabeth Thiele has served as a study investigator for GW Pharmaceuticals. Eric Marsh has served as a consultant for Eisai Pharma and Cydan, and as a study investigator for GW Pharmaceuticals. Maria Mazurkiewicz‐Beldzinska has served as a study investigator for GW Pharmaceuticals. Jonathan J. Halford has served as a consultant for Brain Sentinel and as a study investigator for GW Pharmaceuticals. Boudewijn Gunning has served as a study investigator for GW Pharmaceuticals. Orrin Devinsky has served as a consultant/advisor to GW Pharmaceuticals and Pairnomix, and as a study investigator for GW Pharmaceuticals, has equity interest in Tevard, Empatica, Privateer Holdings, and Receptor Life Sciences and has equity ownership of Papa & Barkley. Daniel Checketts is employed by GW Research Ltd. Claire Roberts was employed by GW Research Ltd at the time of this study and is now affiliated with Eisai Ltd. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
AUTHOR CONTRIBUTIONS
ET, EM, MMB, JJH, BG, and OD helped design the study in collaboration with the sponsor and/or collected the data as a study site investigator. DC and CR contributed to the design of study and analysis of results. All authors contributed to interpretation of the data. EM and ET developed the draft manuscript with medical writing support funded by the sponsor. All authors reviewed and revised the manuscript and approved the final submission.
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank the patients, their families, and the staff at sites that participated in this study. Medical writing support was provided to authors by Jeremy Kennard, PhD, and Dena McWain of Ashfield Healthcare Communications, Middletown, CT, and funded by Greenwich Biosciences, Inc.
Thiele E, Marsh E, Mazurkiewicz‐Beldzinska M, et al. Cannabidiol in patients with Lennox‐Gastaut syndrome: Interim analysis of an open‐label extension study. Epilepsia. 2019;60:419–428. 10.1111/epi.14670
Funding information
This study was sponsored by GW Research Ltd, Cambridge, UK.
Correction added on 19 February 2019, after first online publication: Conflict of interest statement for Orrin Devinsky updated.
Contributor Information
Elizabeth Thiele, Email: ethiele@mgh.harvard.edu.
Eric Marsh, marshe@email.chop.edu.
REFERENCES
- 1. Arzimanoglou A, Resnick T. All children who experience epileptic falls do not necessarily have Lennox‐Gastaut syndrome… but many do. Epileptic Disord 2011; 13(suppl 1): S3–13. [DOI] [PubMed] [Google Scholar]
- 2. Cross JH, Auvin S, Falip M, et al. Expert opinion on the management of Lennox‐Gastaut syndrome: treatment algorithms and practical considerations. Front Neurol. 2017;8:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gastaut H, Roger J, Soulayrol R, et al. Childhood epileptic encephalopathy with diffuse slow spike‐waves (otherwise known as “petit mal variant”) or Lennox syndrome. Epilepsia. 1966;7:139–79. [DOI] [PubMed] [Google Scholar]
- 4. Hancock EC, Cross JH. Treatment of Lennox‐Gastaut syndrome. Cochrane Database Syst Rev. 2013;(2):CD003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Camfield P, Camfield C. Long‐term prognosis for symptomatic (secondarily) generalized epilepsies: a population‐based study. Epilepsia. 2007;48:1128–32. [DOI] [PubMed] [Google Scholar]
- 6. Kim HJ, Kim HD, Lee JS, et al. Long‐term prognosis of patients with Lennox–Gastaut syndrome in recent decades. Epilepsy Res. 2015;110:10–9. [DOI] [PubMed] [Google Scholar]
- 7. Jones NA, Glyn SE, Akiyama S, et al. Cannabidiol exerts anti‐convulsant effects in animal models of temporal lobe and partial seizures. Seizure. 2012;21:344–52. [DOI] [PubMed] [Google Scholar]
- 8. Jones NA, Hill AJ, Smith I, et al. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J Pharmacol Exp Ther. 2010;332:569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mechoulam R, Shvo Y, Hashish I. The structure of cannabidiol. Tetrahedron. 1963;19:2073–8. [DOI] [PubMed] [Google Scholar]
- 10. EPIDIOLEX (cannabidiol) oral solution US prescribing information. Carlsbad, CA: Greenwich Biosciences Inc.; 2018. [Google Scholar]
- 11. Devinsky O, Cilio MR, Cross H, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaplan JS, Stella N, Catterall WA, et al. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A. 2017;114:11229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment‐resistant epilepsy: an open‐label interventional trial. Lancet Neurol. 2016;15:270–8. [DOI] [PubMed] [Google Scholar]
- 14. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N Engl J Med. 2018;378:1888–97. [DOI] [PubMed] [Google Scholar]
- 15. Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2018;391:1085–96. [DOI] [PubMed] [Google Scholar]
- 16. Robles–Diaz M, Lucena MI, Kaplowitz N, et al. Use of Hy's Law and a new composite algorithm to predict acute liver failure in patients with drug‐induced liver injury. Gastroenterology. 2014;147(109–118):e105. [DOI] [PubMed] [Google Scholar]
- 17. Toledo M, Beale R, Evans JS, et al. Long‐term retention rates for antiepileptic drugs: a review of long‐term extension studies and comparison with brivaracetam. Epilepsy Res. 2017;138:53–61. [DOI] [PubMed] [Google Scholar]
- 18. Conry JA, Ng YT, Kernitsky L, et al. Stable dosages of clobazam for Lennox‐Gastaut syndrome are associated with sustained drop‐seizure and total‐seizure improvements over 3 years. Epilepsia. 2014;55:558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Conry JA, Ng YT, Paolicchi JM, et al. Clobazam in the treatment of Lennox‐Gastaut syndrome. Epilepsia. 2009;50:1158–66. [DOI] [PubMed] [Google Scholar]
- 20. Ng YT, Conry JA, Drummond R, et al. Randomized, phase III study results of clobazam in Lennox‐Gastaut syndrome. Neurology. 2011;77:1473–81. [DOI] [PubMed] [Google Scholar]
- 21. OnfiTM (clobazam) tablets for oral use and oral suspension [prescribing information]. Deerfield, IL: Lundbeck; 2016. [Google Scholar]
- 22. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug‐resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011–20. [DOI] [PubMed] [Google Scholar]
- 23. Cramer JA, de la Loge C, Brabant Y, et al. Determining minimally important change thresholds for the Seizure Severity Questionnaire (SSQ). Epilepsy Behav. 2014;31:286–90. [DOI] [PubMed] [Google Scholar]
- 24. Zadeh WW, Escartin A, Byrnes W, et al. Efficacy and safety of lacosamide as first add‐on or later adjunctive treatment for uncontrolled partial‐onset seizures: a multicentre open‐label trial. Seizure. 2015;31:72–9. [DOI] [PubMed] [Google Scholar]
- 25. Kim K, Johnson JA, Derendorf H. Differences in drug pharmacokinetics between East Asians and Caucasians and the role of genetic polymorphisms. J Clin Pharmacol. 2004;44:1083–105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


