Abstract
Background
Pioglitazone may have a protective effect against cardiovascular disease risk among type 2 diabetes (T2D) patients, but evidence from China is lacking. This study investigated the association using electronic health records (EHR) data from a Chinese cohort of T2D patients.
Methods
All T2D patients from the First Affiliated Hospital of Nanjing Medical University who were prescribed at least one oral antidiabetic drug and were aged ≥18 years between 1 July 2005 and 30 June 2017 were eligible for inclusion (n = 71 783). Pioglitazone use was determined in 6‐month study intervals, with outcome events of myocardial infarction (MI), ischemic stroke, and heart failure. Poisson regression was used to estimate adjusted rate ratios (RRs) with 95% confidence intervals (CIs).
Results
In multivariable analysis adjusted for potential confounders, pioglitazone use, compared with no use, was associated with a significant 39% decreased risk of MI (RR = 0.61; 95% CI = 0.42‐0.90; P = 0.012). Pioglitazone use was also associated with a non‐significant reduction in risk of heart failure or stroke. When MI, heart failure, and stroke were combined as a composite outcome, pioglitazone use was associated with a 30% decrease in risk (RR = 0.70; 95% CI = 0.56‐0.88; P = 0.002).
Conclusions
This study demonstrates that applying informatics tools to a large EHR database could be a good way to efficiently conduct clinical observational research. In addition, the findings validated the favorable effect of pioglitazone on the risk of MI among T2D patients in China, with the use of pioglitazone decreasing the risk of MI among those with T2D.
Keywords: cardiovascular disease, Chinese population, pioglitazone, Poisson regression, type 2 diabetes
Highlights
This study demonstrated that applying informatics tools to large electronic health record data could serve as an essential way to robustly and efficiently conduct clinical observational research.
The findings also validated, for the first time, the favorable effect of pioglitazone on the risk of myocardial infarction among type 2 diabetes (T2D) patients in China in a cohort of 71 783 T2D patients.
摘要
背景
吡格列酮可能对2型糖尿病患者的心血管疾病风险具有保护作用,但一直缺乏来自中国的研究证据。本研究利用中国的2型糖尿病患者的电子病历记录数据中来分析这种关联。
方法
将南京医科大学第一附属医院在2005年7月1日至2017年6月30日期间,开出的至少一种口服抗糖尿病药物且年龄≧18岁的所有2型糖尿病患者纳入研究队列(n = 71783)。以6个月为研究间隔,确定患者在该研究周期是否使用吡格列酮,观察的终点事件包括心肌梗死、缺血性中风和心力衰竭。泊松回归用于估计校正后的比率(RR)和95%置信区间(CI)。
结果
在校正混杂因素的多变量分析中,与未使用吡格列酮相比,使用吡格列酮组心肌梗死风险显著降低39%(RR = 0.61;95% CI = 0.42‐0.90;P = 0.012)。吡格列酮的使用与心力衰竭或中风风险降低没有显著相关性。当心肌梗死、心力衰竭和卒中综合作为复合结局时,吡格列酮的使用使风险降低30%(RR = 0.70;95% CI = 0.56‐0.88;P = 0.002)。
结论
本研究表明,将信息学工具应用于大型EHR数据库可能是有效开展临床观察研究的好方法。此外,该研究结果验证了吡格列酮对中国2型糖尿病患者心肌梗死风险的有利影响,使用吡格列酮降低了2型糖尿病患者心肌梗死的风险。
Keywords: 心血管疾病, 中国人群, 吡格列酮, 泊松回归, 2型糖尿病
1. INTRODUCTION
Due to the rapidly increasing prevalence and subsequent fast‐growing economic burden, diabetes has become one of the greatest public health challenges in China.1 It is estimated that the direct medical costs of type 2 diabetes (T2D) in China will reach USD 45 billion in 2030 and, in the next 10 years, 29% of global growth in the costs of diabetes care may occur in China.2, 3 Approximately 95% of diabetes patients have T2D, with major cardiovascular complications (eg, myocardial infarction [MI], stroke, and heart failure),4 which are the leading causes of death among T2D patients.5
Studies have shown that the risk of cardiovascular complications is closely linked to insulin resistance among patients with T2D, in addition to other accompanying conditions, such as hyperglycemia, dyslipidemia, hypertension, hyperinsulinemia, hypercoagulability, inflammation, endothelial dysfunction, and platelet hyperreactivity.6, 7, 8 Thiazolidinediones, a peroxisome proliferator‐activated receptor (PPAR) γ agonists, are a family of oral antidiabetic medications that have been widely used to reduce insulin resistance.9 Given the link between insulin resistance and cardiovascular complications, as well as the effects of thiazolidinediones in reducing insulin resistance, thiazolidinediones may decrease cardiovascular risk among T2D patients.
Of the different types of thiazolidinediones, pioglitazone is the most commonly prescribed. The initial Prospective Pioglitazone Clinical Study in Macrovascular Events (PROactive) Trial, which assessed the effect of pioglitazone on cardiovascular complications among T2D patients, found that pioglitazone could reduce the risk of all‐cause mortality, non‐fatal MI, or stroke.10, 11 In a subsequent meta‐analysis of 19 available clinical trials, Lincoff et al.12 concluded that pioglitazone could reduce the risk of MI and stroke, but that it increased the risk of serious heart failure. In addition, in a recent trial among non‐diabetic patients who had insulin resistance and historical ischemic stroke or transient ischemic attack (TIA), pioglitazone was found to reduce the risk of MI or stroke.13 In addition, multiple studies have shown that pioglitazone may have a protective effect against the progression of atherosclerosis, which is directly related to common cardiovascular outcomes in T2D patients.14, 15
However, none of these studies was conducted in China, so it is unclear whether the potential protective effects of pioglitazone on cardiovascular outcomes among T2D patients can be generalized to the Chinese population. Hence, we conducted a retrospective cohort study to investigate the association between pioglitazone use and the risk of cardiovascular events in a Chinese population with T2D using a large database of electronic health records (EHR) from the First Affiliated Hospital of Nanjing Medical University, China. In addition, we developed an informatics tool that integrates natural language processing and other information retrieval methods to automatically and efficiently clean and organize data from this large EHR database for clinical epidemiological research.
2. METHODS
2.1. Data sources and study design
The data for the present study were obtained from the Clinical Data Repository (CDR) system of the First Affiliated Hospital of Nanjing Medical University in Jiangsu, China. The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (2013‐SRFA‐139, Jiangsu Province Hospital). Details that may have revealed the identity of the subjects in the study were omitted. The CDR system, initiated in 2005, contains deidentified real‐world patient‐level EHRs, including both inpatient and outpatient demographics, diagnoses, procedures, medications, images, and laboratory results. As of June 2017, the CDR system included 6.23 million patients (Figure 1). Of these, 104 312 had been diagnosed with T2D based on the International Classifications of Diseases (ICD)‐10 code E11.902 between July 2005 and June 2017. All T2D patients who were prescribed at least one oral antidiabetic drug and were aged ≥18 years were included in the present study (n = 71 783 patients for the final analysis).
Figure 1.
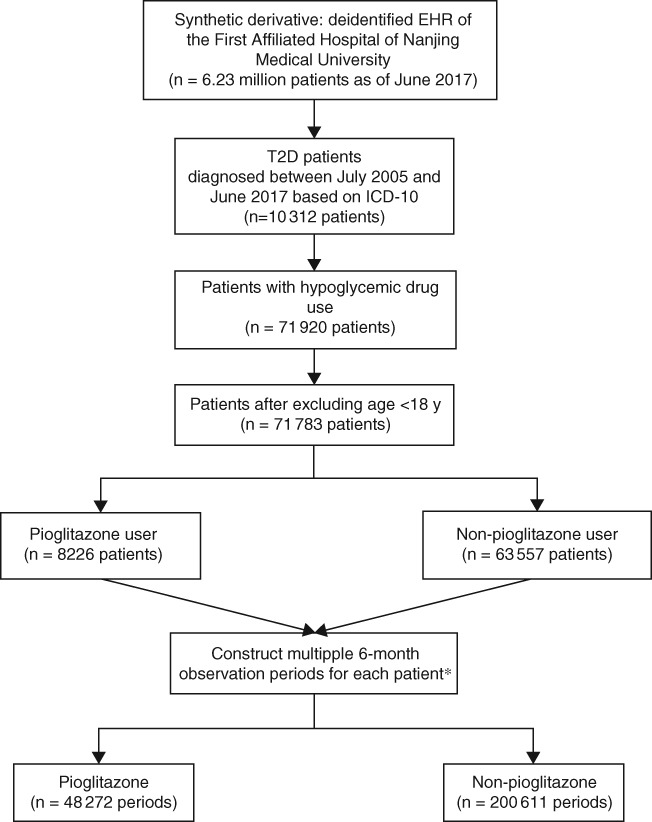
Study work flow. *Note, a patient could have up to 24 observation periods. T2D, type 2 diabetes
Because the study population could have received medical care outside the First Affiliated Hospital of Nanjing Medical University CDR system, some patients may have had a period that was not covered in the CDR system, resulting in incomplete records. To address this problem, we adopted an approach that was successfully implemented in a previous study conducted by Brownstein et al.16 In this approach, it was assumed that medical care encounters (inpatient or outpatient) could indicate receiving care in the CDR system over a certain study interval (eg, 6 months). In this study, we created a total of 24 6‐month study intervals (a total duration of 12 years) between 1 July 2005 (the earliest date in the CDR system at the time of study) and 30 June 2017 (the latest date in the CDR system). A study interval was included in the analysis if one or more inpatient or outpatient encounters occurred in this study interval, and a patient could have multiple (up to 24) study intervals. A patient entered the study when his or her first study interval met the criteria. If a medical encounter did not occur in one 6‐month study interval, yet it occurred in the subsequent study interval, the interval with no encounter would then be deleted and the subsequent interval would still be included in the analysis.
In terms of study interval classification, a study interval was classified as “pioglitazone use” if a pioglitazone prescription or dispensation occurred in this interval or lasted to this interval; otherwise, the interval was classified “no pioglitazone use”. For every patient, the duration of pioglitazone use and the duration of no pioglitazone use were assessed and accumulated in 6‐month increments. In all, 200 611 patient intervals were classified as no pioglitazone use and 48 272 patient intervals were classified as pioglitazone use (Figure 1). The outcome events were MI (ICD‐10 code I21.xx), ischemic stroke (ICD‐10 code I64.04), and heart failure (ICD‐10 code I50.xx) between 1 July 2005 and 30 June 2017. Events were associated with pioglitazone only when the events occurred after the pioglitazone prescription or dispensation.
2.2. Data extraction
Observational Health Data Sciences and Informatics (OHDSI) is “an international collaborative whose goal is to create and apply open‐source data analytic solutions to a large network of health databases to improve human health and wellbeing”.17, 18 It provides advanced, open‐source clinical research tools, including a common data model (CDM), standard vocabulary of clinical terminologies, and various software programs to assist with clinical research. The OHDSI China group is committed to leveraging the healthcare databases in China for clinical observational research, and the First Affiliated Hospital of Nanjing Medical University is part of the OHDSI China group.
First, the CDR data in the First Affiliated Hospital of Nanjing Medical University were transformed into OHDSI CDM version 5 (https://www.ohdsi.org/data-standardization/the-common-data-model/. Accessed December, 2018). Each element of the patient in the database must be mapped to the approved CDM vocabulary and placed in the data schema. Then, we developed an informatics tool in the C# programming language using natural language processing to automatically and efficiently clean and organize data that were transformed to the OHDSI CDM. The tool was developed by C Sharp and deployed on the hospital intranet server with a Windows operating system. The tool can output statistical data, such as diagnostics and drugs used by patients, depending on statistical requirements. The ICD‐10 diagnostic codes, which were validated for acute MI (I21.xx),19 stroke (I64.04), and heart failure (I50.xx), used by the CDR system20 were converted into a concept ID of the CDM model if the diagnosis name and diagnosis code could be directly matched in the CDM diagnosis vocabulary. Otherwise, the natural language processing approach and the cosine similarity of machine learning classification algorithm were combined to improve the accuracy of data extraction. Drug codes (eg, oral hypoglycemic agents, lipid‐lowering agents, antihypertensive agents etc.) were also processed with information technology rather than traditional data extraction and cleaning methods.
2.3. Statistical analysis
Selected characteristics of study patients stratified according to pioglitazone use were compared using Student's t test for continuous variables and the Chi‐squared test for categorical variables. The risks of MI, ischemic stroke, and heart failure among pioglitazone intervals were compared with those among non‐pioglitazone intervals. Poisson regression was used to estimate crude and adjusted rate ratios (RR) with 95% confidence intervals (CIs) for the outcome events, with duration of exposure or non‐exposure (in 6‐month increments) as the offset. Adjustments were made for potential risk factors, including age, sex, calendar year, concurrent medications (metformin, sulfonylurea, acarbose, insulin, lipid‐lowering drugs, and antihypertensive drugs), comorbidities (hypertension, prior coronary heart disease, hyperlipidemia, and chronic kidney disease), and Charlson score. All statistical analyses were performed in Stata 13.0 (StataCorp LP, College Station, Texas). Statistical significance was set at two‐sided P < 0.05.
3. RESULTS
Selected subject characteristics according to pioglitazone use (63 557 non‐pioglitazone users and 8226 pioglitazone users) are presented in Table 1. Compared with non‐pioglitazone users, pioglitazone users were younger (P < 0.001), less likely to be male (P < 0.001), less likely to be in a more recent calendar year (P < 0.001), less likely to be using concurrent metformin (P < 0.001), acarbose (P < 0.001), insulin (P < 0.001), lipid‐lowering drugs (P < 0.001), or antihypertensive drugs (P < 0.001), more likely to be on a concurrent sulfonylurea (P < 0.001), less likely to have concurrent hypertension (P < 0.001), prior coronary heart disease (P < 0.001), hyperlipidemia (P < 0.001), or chronic kidney disease (P < 0.001), and more likely to have a lower Charlson score (P < 0.001).
Table 1.
Patient characteristics according to pioglitazone use
| Pioglitazone use | P‐value | ||
|---|---|---|---|
| No (n = 63 557 patients) | Yes (n = 8226 patients) | ||
| Age (y) | 57.9 ± 14.4 | 55.1 ± 13.4 | <0.001 |
| Sex | |||
| Male | 35 511(55.87) | 4248 (51.64) | |
| Female | 28 038 (44.11) | 3977 (48.35) | |
| Missing | 8 (0.01) | 1 (0.01) | <0.001 |
| Calendar year | |||
| 2007 and prior | 9568 (15.05) | 2797 (34.00) | |
| 2008 | 5134 (8.08) | 975 (11.85) | |
| 2009 | 5289 (8.32) | 1049 (12.75) | |
| 2010 | 5217 (8.21) | 786 (9.56) | |
| 2011 | 5497 (8.65) | 479 (5.82) | |
| 2012 | 6106 (9.61) | 443 (5.39) | |
| 2013 | 6227 (9.80) | 429 (5.22) | |
| 2014 | 5815 (9.15) | 352 (4.28) | |
| 2015 | 5665 (8.91) | 377 (4.58) | |
| 2016 and after | 9039 (14.22) | 539 (6.55) | <0.001 |
| Concurrent medications | |||
| Metformin | 30 274 (47.63) | 3465 (42.12) | <0.001 |
| Sulfonylurea | 34 234 (53.86) | 7161 (87.05) | <0.001 |
| Acarbose | 24 538 (38.61) | 2078 (25.26) | <0.001 |
| Insulin | 17 079 (26.87) | 1907 (23.18) | <0.001 |
| Lipid‐lowering drugs | 16 644 (26.19) | 1457 (17.71) | <0.001 |
| Antihypertensive drugs | 26 989 (42.46) | 2834 (34.45) | <0.001 |
| Comorbidities | |||
| Hypertension | 21 729 (34.19) | 1515 (18.42) | <0.001 |
| Prior coronary heart disease | 5605 (8.82) | 339 (4.12) | <0.001 |
| Hyperlipidemia | 4720 (7.43) | 429 (5.22) | <0.001 |
| Chronic kidney disease | 473 (0.74) | 18 (0.22) | <0.001 |
| Charlson score | |||
| 0 | 58 901 (92.67) | 7916 (96.23) | |
| 1 | 2395 (3.77) | 150 (1.82) | |
| ≥2 | 2261 (3.56) | 160 (1.95) | <0.001 |
Data are given as the mean ± SD or as n (%).
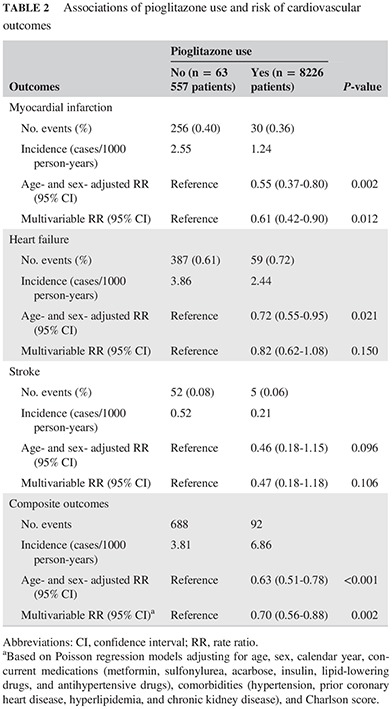
Table 2 details the associations of pioglitazone use with the risk of cardiovascular events (MI, heart failure, and stroke). Compared with the non‐pioglitazone group, lower incidence rates were found in the pioglitazone group for MI (2.55 vs 1.24 cases/1000 person‐years, respectively), heart failure (3.86 vs 2.44 cases/1000 person‐years, respectively), and stroke (0.52 vs 0.21 cases/1000 person‐years, respectively; Table 2). For composite outcomes (MI, heart failure, and stroke), the incidence rate was higher in pioglitazone than non‐pioglitazone group (6.86 vs 3.81 cases/1000 person‐years, respectively; Table 2). In multivariable analysis adjusted for potential confounders, pioglitazone use compared with no use was associated with a significant 39% decreased risk of MI (RR = 0.61; 95% CI = 0.42‐0.90; P = 0.012), a non‐significant 18% decreased risk of heart failure (RR = 0.82; 95% CI = 0.62‐1.08; P = 0.150), and a non‐significant 53% decreased risk of stroke (RR = 0.47; 95% CI = 0.18‐1.18; P = 0.106). When MI, heart failure, and stroke were combined as a composite outcome, pioglitazone use was associated with a 30% decreased risk (RR = 0.70; 95% CI = 0.56‐0.88; P = 0.002). The results were similar in the sensitivity analysis after excluding those patients who had prior cardiovascular diseases before entering the study (data not shown).
Table 2.
Associations of pioglitazone use and risk of cardiovascular outcomes
| Outcomes | Pioglitazone use | P‐value | |
|---|---|---|---|
| No (n = 63 557 patients) | Yes (n = 8226 patients) | ||
| Myocardial infarction | |||
| No. events (%) | 256 (0.40) | 30 (0.36) | |
| Incidence (cases/1000 person‐years) | 2.55 | 1.24 | |
| Age‐ and sex‐ adjusted RR (95% CI) | Reference | 0.55 (0.37‐0.80) | 0.002 |
| Multivariable RR (95% CI) | Reference | 0.61 (0.42‐0.90) | 0.012 |
| Heart failure | |||
| No. events (%) | 387 (0.61) | 59 (0.72) | |
| Incidence (cases/1000 person‐years) | 3.86 | 2.44 | |
| Age‐ and sex‐ adjusted RR (95% CI) | Reference | 0.72 (0.55‐0.95) | 0.021 |
| Multivariable RR (95% CI) | Reference | 0.82 (0.62‐1.08) | 0.150 |
| Stroke | |||
| No. events (%) | 52 (0.08) | 5 (0.06) | |
| Incidence (cases/1000 person‐years) | 0.52 | 0.21 | |
| Age‐ and sex‐ adjusted RR (95% CI) | Reference | 0.46 (0.18‐1.15) | 0.096 |
| Multivariable RR (95% CI) | Reference | 0.47 (0.18‐1.18) | 0.106 |
| Composite outcomes | |||
| No. events | 688 | 92 | |
| Incidence (cases/1000 person‐years) | 3.81 | 6.86 | |
| Age‐ and sex‐ adjusted RR (95% CI) | Reference | 0.63 (0.51‐0.78) | <0.001 |
| Multivariable RR (95% CI)a | Reference | 0.70 (0.56‐0.88) | 0.002 |
Abbreviations: CI, confidence interval; RR, rate ratio.
Based on Poisson regression models adjusting for age, sex, calendar year, concurrent medications (metformin, sulfonylurea, acarbose, insulin, lipid‐lowering drugs, and antihypertensive drugs), comorbidities (hypertension, prior coronary heart disease, hyperlipidemia, and chronic kidney disease), and Charlson score.
4. DISCUSSION
In this large retrospective cohort study that made efficient use of EHR data by using novel informatics tools, we found that the previously reported protective effect of pioglitazone on the risk of MI among Western T2D patients was also observed among T2D patients in China. Pioglitazone use was also associated with a non‐significant reduction in the risk of heart failure and ischemic stroke.
Previous studies have shown a significant reduction in MI risk associated with the use of pioglitazone in Western countries,12, 21 and our results provide the first evidence supporting this association among patients in a large provincial medical institution in China. Although it the mechanism underlying the lower rates of MI among pioglitazone users remains unclear, a recent study in an adult spontaneously hypertensive rat (SHR) model22 provided evidence to partially answer this question. In that study, Hernanz et al.22 found that pioglitazone increased the cyclo‐oxygenase (COX)‐2 levels and improved COX‐2‐derived prostaglandin I2 secretion, in addition to increasing serum nitric oxide levels and decreasing reactive oxygen species, which all contribute to the favorable effects of pioglitazone on the cardiovascular system. In addition, Zhao et al.23 observed that the miR‐711‐Sp1 transcription factor‐collagen‐I pathway may be involved in the cardiovascular protective effects of pioglitazone. Moreover, the use of pioglitazone was associated with a reduction in the progression of carotid intima‐media thickness, which plays an essential role in the development of cardiovascular disease development.24, 25 There is also evidence showing that pioglitazone is associated with increased endothelial function in T2D patients, which has its unique metabolic changes.26
In this study we found a non‐significant reduction in the risk of heart failure associated with the use of pioglitazone, which is in contrast with the findings of some previous studies that showed that pioglitazone use could increase the risk of serious heart failure.12 However, in a clinical trial among non‐diabetic patients who had insulin resistance and historical ischemic stroke or TIA, pioglitazone use was not found to be associated with the risk of heart failure.13 In addition, another short‐term study from the Kaiser Permanente Medical Care Program (Kaiser Permanente Northern California Diabetes Registry) did not find an association between pioglitazone use and an elevated risk of congestive heart failure hospitalization.27, 28 The possible reason for the conflicting findings could be that we were not able to separate serious heart failure from mild heart failure because it was difficult to determine the severity of heart failure based on structured EHR data only.
There are few efficient ways to used increasingly rich EHR data for clinical research, and the OHDSI is one proposed approach that is gaining a lot of interest in the research community. In the OHDSI approach, clinical data are converted into a structured, universal, standard format for research purposes. This standard format can greatly enhance the efficiency of clinical observational research and promote collaboration across institutions. This study is the first in China to use the OHDSI approach to investigate the effect of pioglitazone use on cardiovascular outcomes among T2D patients. We demonstrated the great significance of using clinical medical data for observational research, as well as the potential of the OHDSI approach in mining the value of clinical medical data.
The present study has several strengths. First, we developed an informatics tool to automatically and efficiently clean and organize data from a massive EHR database (6.2 million patients with data size >5 Tb, not including image data for the present study), and using traditional methods to clean and organize the data would be very time and resource consuming. This tool can be used in future studies using large EHR databases for clinical epidemiological research. Second, we investigated, for the first time, the association between pioglitazone use and the risk of subsequent cardiovascular events in a large provincial medical institution patient population using real‐world patient clinical data. Third, the statistical methods we used could deal with the commonly encountered situation where the study population does not receive health care exclusively within one system, leading to incomplete records.
Despite its strengths, the present study does have limitations. First, because the study population could receive medical care outside the First Affiliated Hospital of Nanjing Medical University CDR system, some patients may have had a period that was not covered in the CDR system, resulting in incomplete records. However, we used a statistical method to overcome this limitation, and the estimated rates of cardiovascular events are comparable to the national rates in China. For example, the observed incidence rate of MI was 2.30 cases/1000 person‐years among our study cohort of T2D patients, and this rate is consistent with the reported rate of 1.51 cases/1000 person‐years among patients with diabetes or prediabetes in another study from China in which complete follow‐up was achieved.24 Second, like any other observational study, the present study could not exclude the possibility of residual confounding due to unknown or uncontrolled factors. Finally, even though we had a large cohort of T2D patients, the number of cardiovascular events in the pioglitazone group was relatively small, limiting the statistical power for stratified analyses of interest.
5. CONCLUSION
The present study demonstrates that with proper research design, EHR data can be used for clinical epidemiological studies. Applying informatics approaches to large EHR datasets serves as an essential way to robustly and efficiently conduct clinical observational research. The study findings also validated the favorable effects of pioglitazone on the risk of MI in a provincial medical institution patient population, which may be helpful for personalized decision making in T2D treatment.
CONFLICTS OF INTEREST
None declared.
ACKNOWLEDGEMENTS
The authors thank Observational Health Data Sciences and Informatics (OHDSI) Alliance for sharing the data model. In addition, the authors thank the funding bodies that supported this study.
Miao S, Dong X, Zhang X, et al. Detecting pioglitazone use and risk of cardiovascular events using electronic health record data in a large cohort of Chinese patients with type 2 diabetes. Journal of Diabetes. 2019;11:684–689. 10.1111/1753-0407.12894
Shumei Miao and Xiao Dong contributed equally to this work.
Funding information the 2016 Industry Prospecting and Common Key Technology Key Projects of Jiangsu Province Science and Technology Department, Grant/Award Number: BE2016002‐4; the 2016 Projects of Nanjing Science Bureau, Grant/Award Number: 201608003; the 2017 Projects of Jiangsu Provincial Department of Finance, Grant/Award Number: 2150510; UTHealth Innovation for Cancer Prevention Research Training Program Pre‐doctoral Fellowship (Cancer Prevention and Research Institute of Texas), Grant/Award Number: RP160015
REFERENCES
- 1. Wang J. Diabetes: a challenge for China in the 21st century. Lancet Diabetes Endocrinol. 2014;2:e6‐e7. [DOI] [PubMed] [Google Scholar]
- 2. Wang W, McGreevey WP, Fu C, et al. Type 2 diabetes mellitus in China: a preventable economic burden. Am J Manag Care. 2009;15:593‐601. [PubMed] [Google Scholar]
- 3. Alcorn T, Ouyang Y. Diabetes saps health and wealth from China's rise. Lancet. 2012;379:2227‐2228. [DOI] [PubMed] [Google Scholar]
- 4. Lagani V, Koumakis L, Chiarugi F, Lakasing E, Tsamardinos I. A systematic review of predictive risk models for diabetes complications based on large scale clinical studies. J Diabetes Complicat. 2013;27:407‐413. [DOI] [PubMed] [Google Scholar]
- 5. Abajobir AA, Abbafati C, Abbas KM, et al. Global, regional, and national agesex specific mortality for 264 causes of death, 1980‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mather KJ, Steinberg HO, Baron AD. Insulin resistance in the vasculature. J Clin Invest. 2013;123:1003‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Semenkovich CF. Review series. Insulin resistance and atherosclerosis. J Clin Invest. 2006;116:1813‐1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kernan WN, Inzucchi SE, Viscoli CM, Brass LM, Bravata DM, Horwitz RI. Insulin resistance and risk for stroke. Neurology. 2002;59:809‐815. [DOI] [PubMed] [Google Scholar]
- 9. Inzucchi SE, Maggs DG, Spollett GR, et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338:867‐873. [DOI] [PubMed] [Google Scholar]
- 10. Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. J Vasc Surg. 2006;43:639‐640. [DOI] [PubMed] [Google Scholar]
- 11. Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke. 2007;38:865‐873. [DOI] [PubMed] [Google Scholar]
- 12. Lincoff M, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta‐analysis of randomized trials. JAMA. 2007;298:1180‐1188. [DOI] [PubMed] [Google Scholar]
- 13. Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321‐1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nissen SE, Nicholls SJ, Wolski K, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561‐1573. [DOI] [PubMed] [Google Scholar]
- 15. Mazzone T, Meyer PM, Feinstein SB, et al. Effect of pioglitazone compared with glimepiride on carotid intima‐media thickness in type 2 diabetes: a randomized trial. JAMA. 2006;296:2572‐2581. [DOI] [PubMed] [Google Scholar]
- 16. Brownstein JS, Murphy SN, Goldfine AB, et al. Rapid identification of myocardial infarction risk associated with diabetes medications using electronic medical records. Diabetes Care. 2010;33:526‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hripcsak G, Duke JD, Shah NH, et al. Observational health data sciences and informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform. 2015;216:574‐578. [PMC free article] [PubMed] [Google Scholar]
- 18. Hripcsak G, Ryan PB, Duke JD, et al. Characterizing treatment pathways at scale using the OHDSI network. Proc Natl Acad Sci USA. 2016;113(27):7329‐7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel AB, Quan H, Welsh RC, et al. Validity and utility of ICD‐10 administrative health data for identifying ST‐ and non‐ST‐elevation myocardial infarction based on physician chart review. CMAJ Open. 2015;3:e413‐e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCormick N, Bhole V, Lacaille D, Avina‐Zubieta JA. Validity of diagnostic codes for acute stroke in administrative databases: a systematic review. PLoS One. 2015;10:e0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jong M, Worp HB, Graaf Y, et al. Pioglitazone and the secondary prevention of cardiovascular disease: a meta‐analysis of randomized‐controlled trials. Cardiovasc Diabetol. 2017;16(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hernanz R, Martín Á, Pérez‐Girón JV, et al. Pioglitazone treatment increases COX‐2‐derived prostacyclin production and reduces oxidative stress in hypertensive rats: role in vascular function. Br J Pharmacol. 2012;166:1303‐1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao N, Yu HY, Yu HT, et al. miRNA‐711‐SP1‐collagen‐I pathway is involved in the anti‐fibrotic effect of pioglitazone in myocardial infarction. Sci China Life Sci. 2013;56:431‐439. [DOI] [PubMed] [Google Scholar]
- 24. DeFronzo R, Tripathy D, Schwenke DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364:1104‐1115. [DOI] [PubMed] [Google Scholar]
- 25. Saremi A, Schwenke DC, Buchanan TA, et al. Pioglitazone slows progression of atherosclerosis in prediabetes independent of changes in cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2013;33:393‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martens FM, Visseren FL, de Koning EJ, Rabelink TJ. Short‐term pioglitazone treatment improves vascular function irrespective of metabolic changes in patients with type 2 diabetes. J Cardiovasc Pharmacol. 2005;46:773‐778. [DOI] [PubMed] [Google Scholar]
- 27. Karter AJ, Ahmed AT, Liu J, Moffet HH, Parker MM. Pioglitazone initiation and subsequent hospitalization for congestive heart failure. Diabet Med. 2005;22:986‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang J, Li J, Wang A, et al. Dipstick proteinuria and risk of myocardial infarction and all‐cause mortality in diabetes or pre‐diabetes: a population based cohort study. Sci Rep. 2017;7(1):11986. [DOI] [PMC free article] [PubMed] [Google Scholar]


