Abstract
Purpose
Posterior lamellar corneal surgery is considered the standard of care for irreversible endothelial cell dysfunction. Pre‐cut grafts can be prepared either manually (Descemet stripping endothelial keratoplasty; DSEK) or mechanically (Descemet stripping automated endothelial keratoplasty; DSAEK). We performed a head‐to‐head clinical comparison between DSEK and DSAEK grafts.
Methods
All DSEK and DSAEK procedures performed by two corneal specialists at the University Medical Center Utrecht from 1 January 2016 through 31 October 2016 were prospectively included. Pre‐cut grafts were delivered by two eye banks, which either exclusively prepared the DSEK or DSAEK grafts. Preoperative and postoperative measurements were obtained, and all surgical events and adverse events were recorded.
Results
A total of 21 DSEK and 53 DSAEK procedures were included for analysis; the two groups were similar at baseline, with the exception of graft endothelial cell density, which was 2531 ± 67 versus 2748 ± 148 cells/mm2, respectively (p < 0.001). At the one‐year follow‐up visit, corrected distance visual acuity and endothelial cell loss were similar between the groups. Mean pachymetry was significantly lower in the DSEK group (521 ± 39 versus 588 ± 59 μm; p < 0.001), whereas the rebubbling rate was significantly higher in the DSEK group (47.6% versus 18.9%; p = 0.001). Finally, three grafts in the DSEK group experienced failure compared to one graft in the DSAEK group (14% versus 1.9%, respectively).
Conclusion
Manually dissected and microkeratome‐dissected grafts performed similarly with respect to vision and endothelial cell loss assessed one year after surgery. The higher incidence of graft failure among manually dissected (i.e. DSEK) grafts may be attributable to reduced relative thickness compared to DSAEK grafts and/or the resulting differences in tissue handling and the surgeon's learning curve.
Keywords: clinical evaluation, descemet stripping automated endothelial keratoplasty, descemet stripping endothelial keratoplasty, endothelial keratoplasty, pre‐cut tissue
Introduction
Fuchs endothelial corneal dystrophy (FECD) is the principal indication for corneal transplantation surgery, and posterior lamellar keratoplasty is currently the preferred method for treating FECD (Terry et al. 2011; Gain et al. 2016). During this procedure, the posterior layers of the cornea are replaced without compromising the cornea's structural integrity. Descemet stripping endothelial keratoplasty (DSEK) is an established endothelial keratoplasty technique involving the replacement of the diseased endothelium with the endothelium, Descemet membrane, and posterior stroma obtained from the donor tissue (Price & Price 2006a,2006b).
The graft tissue can be prepared either manually or automatically using a microkeratome (Terry & Ousley 2001; Terry et al. 2011; Romano et al. 2017). In addition, the graft tissue can be either prepared in the operating room or prepared at a tissue bank (i.e. pre‐cut). The use of a pre‐cut graft prepared at a tissue bank has gained widespread acceptance among corneal surgeons, as it provides similar results compared to grafts prepared during surgery (Terry 2009). In addition, the use of a pre‐cut graft reduces surgical time, reduces the risk of adverse events during preparation, avoids the investment of specialized equipment, and helps ensure consistent graft quality (Price et al. 2008; Palioura et al. 2017).
The most commonly used method for cutting the graft tissue is to use a microkeratome. This approach, referred to as Descemet stripping automated endothelial keratoplasty (DSAEK), is considered easy to perform (Choulakian et al. 2016). The DSEK preparation is performed manually, and it can result in thinner grafts when performed by a trained dissector (Rice et al. 2011; Anderson et al. 2014). However, the effects of using a pre‐cut DSEK or DSAEK graft with respect to clinical outcome have not been investigated.
In the Netherlands, corneal transplant surgeons obtain their donor tissue almost exclusively from two eye banks, Amnitrans EyeBank in Rotterdam, the Netherlands and the Cornea Bank in Beverwijk, the Netherlands, which use DSEK and DSAEK, respectively, to prepare pre‐cut corneal grafts. In this study, the Ophthalmology Department at the University Medical Center (UMC) Utrecht received pre‐cut tissue from both eye banks.
Here, we compared DSEK and DSAEK pre‐cut grafts with respect to visual outcome, postoperative pachymetry, endothelial cell density (ECD), and the rate of adverse events with a follow‐up period of one year.
Patients and Methods
Study group and design
The study was designed as an prospective cohort study to compare the 22 DSEK grafts that our centre received in 2016 alongside our regular used DSAEK grafts. All patients at the UMC Utrecht who underwent posterior lamellar surgery from 1 January 2016 through 31 October 2016 were included, regardless of the indication for surgery. The patients were pseudo‐randomized to receive either a DSEK or DSAEK graft, as the donor grafts were allocated by the Dutch Transplant Foundation (Nederlandse Transplantatie Stichting) in Leiden, the Netherlands, and the surgeons had no specific acceptance criteria for each recipient to receive either a DSEK or DSAEK graft. The study was approved by the Ethics Review Board of UMC Utrecht (Medical Ethics Committee file no. 18‐107C) and was performed in accordance with the Declaration of Helsinki and Dutch law regarding research involving human subjects.
Donor preparation
The DSEK and DSAEK grafts were prepared by two different eye banks, respectively, Amnitrans Eye Bank for DSEK grafts and the Cornea Bank for DSAEK donors. The same selection criteria for the donor tissues were used for both DSEK and DSAEK grafts and included a minimum ECD of 2300 cells/mm2. Between the time of enucleation and the time of arrival at the donor bank, the bulbi were stored under hypothermic conditions for a short period and subsequently decontaminated. The corneal‐scleral rim was dissected and stored in organ culture medium at 31°C until further processing (van Luijk et al. 2012). Different culture medium was used for DSEK and DSAEK grafts. Amnitrans EyeBank Rotterdam used CorneaMax (EuroBio, Courtaboeuf, France); the Cornea Bank Beverwijk used culture medium compromised minimum essential medium (Biowest, Nuaillé, France) supplemented with 20 mM HEPES, 26 mM sodium bicarbonate, 2% (vol/vol) newborn calf serum, 10 IU/ml penicillin, 0.1 mg/ml streptomycin, and 0.25 μg/ml amphotericin.
The DSEK donor tissues were stored in the organ culture medium until dissection and were therefore dissected and subsequently measured in an oedematous state. The donor corneas were mounted on an air‐filled artificial anterior chamber (Coronet; Network Medical, Ripon, UK) and manually dissected using a lamellar dissection set (DORC International, Zuidland, the Netherlands, Melles et al. 1998). The goal of this procedure was to achieve a lamellar graft thickness of 60–140 μm. The absolute dissection depth was measured using anterior segment SL‐optical coherence tomography (OCT; Heidelberg Engineering GmbH, Heidelberg, Germany). Predissected corneas were stored in CorneaMax at 31°C until surgery.
Prior to dissection, the DSAEK tissues were reduced to physiological thickness by incubating the tissue in organ culture medium supplemented with 6% dextran (Sigma‐Aldrich, St. Louis, MO, USA) for 24–48 hr to provoke deswelling. The DSAEK donor tissue was then dissected using a Gebauer SLc microkeratome (Gebauer Medizintechniek GmbH, Neuhausen, Germany) using the single‐pass technique, with the goal of achieving a central residual stromal bed thickness of 100 ± 40 μm for ultrathin DSAEK (Dickman et al. 2014, 2015). The donor (pre‐dissection) and lamellar (post‐dissection) central corneal thickness values were measured using a Casia SS‐1000 anterior segment OCT (Tomey, Nagoya, Japan). The prepared donor tissue was then stored with the anterior cap for transportation in culture medium supplemented with 6% dextran.
At several points during processing, the quality of the endothelium quality was evaluated using light microscopy. DSEK donor tissue was examined using computer‐assisted manual counting with an Axiovert 40C inverted light microscope (Carl Zeiss Meditec GmbH, Oberkochen, Germany); DSAEK donor tissue was counted manually using a grid in an Axioscope A1 microscope (Carl Zeiss Meditec GmbH). Extensive microbiological testing was performed on all grafts.
Surgical procedure
Surgeries were performed by two corneal surgeons (authors RW and CvL), and the surgical procedure was similar for both DSEK and DSAEK grafts. After inserting a Lewicky anterior chamber maintainer (DORC International, Zuidland, the Netherlands), the surgeon performed a descemetorhexis using a Price hook and a peripheral iridectomy. A 4‐mm sclerocorneal incision was made, and nylon 10‐0 non‐absorbable stitches (Ethicon, Sommersville, NJ, USA) were prepared.
All grafts were provided pre‐cut and were sufficiently rinsed and put in balanced salt solution (Alcon BV, the Netherlands) at the beginning of the procedure, to ensure any residual medium was washed. Grafts were trephined during the surgery at 8.5 mm. The donor graft was inserted using either a reusable Macaluso inserter (Janach Instruments, Como, Italy; n = 28) or a Tan Endoglide inserter (Angiotech Pharmaceuticals, Reading, PA, USA; n = 48). After the graft was inserted, air pressure was used in order to unfold and adhere the graft to the recipient stroma. Complete air fill with overpressure (approximately 65 mmHg) was then maintained for 12 min. The pressure was then normalized, and the air was partially replaced with balanced salt solution (Alcon Ltd.), leaving an air bubble approximately 8.5 mm in diameter (the same size as the transplant diameter). No venting incisions were made. After surgery, the patient remained in the supine position for 4 hr. If needed, a rebubbling procedure was performed using the same procedure used to adhere the graft during the initial surgery. Each patient received a peribulbar injection of dexamethasone (0.4%).
Standard postoperative medication included 0.3% ofloxacin EDO eye drops (Bausch & Lomb, Schiphol‐Rijk, the Netherlands) QID for 10 days, 0.5% prednisolone ointment (Ursapharm, Luik, Belgium) before bedtime for 1 month, and 0.1% monofree dexamethasone eye drops (Thea Pharma Benelux, Wetteren, Belgium) drops QID for 3 months. After this period, the use of topical steroids was tapered off; 12 months after surgery, topical steroids were switched to 0.1% fluorometholone eye drops (Allergan, Eindhoven, the Netherlands) once daily.
Baseline and follow‐up measurements
Each patient underwent an ophthalmic examination prior to surgery and 1 day, 1 week, 3 weeks, 3 months, 6 months, and 12 months after surgery. The preoperative (i.e. baseline) measurement and the 6‐ and 12‐month follow‐up measurements are reported in detail. The clinical assessment included a full slit‐lamp examination, fundus examination, intraocular pressure and ECD measurements (Tomey EM‐4000, Nürnberg, Germany), Scheimpflug tomography (Pentacam HR type 70900, Oculus GmbH, Wetzlar, Germany), anterior segment OCT (Visante OCT, Carl Zeiss Meditec GmbH, Oberkochen, Germany), automated refraction (KR8800, Topcon, Tokyo, Japan), and manifest refraction (CV3000, Topcon). Uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA) were measured using a visual acuity chart (CC100P, Topcon) at a distance of 6 metres.
Statistics
Data were analysed using spss 21.0 (IBM Corp., Armonk, NY, USA). Preoperative and postoperative measurements and complications were analysed using the Student's t‐test, anova with post hoc analysis, or Fischer's exact test. Graphs were generated using GraphPad Prism (GraphPad Software Inc., La Jolla, CA). Patients who experienced graft failure were considered to be lost to follow‐up from there onwards. The potential of inducing a type I error was considered minor due to the relatively small study size (Lachin 2000).
Results
Study group
A total of 76 surgeries were performed at our medical centre from 1 January 2016 through 31 October 2016. Two of these surgeries were excluded from the study because the graft was inadvertently not delivered as a pre‐cut graft. Thus, 21 DSEK and 53 DSAEK grafts were obtained from Amnitrans EyeBank Rotterdam and the Cornea Bank Beverwijk, respectively, and included for analysis. The study cohort included 29 males (39%) and 45 females (61%), and the mean age of the patients was 68 years. The male/female ratio and patient age were similar between the DSEK and DSAEK groups (p = 0.133, Student's t‐test).
The indications for surgery included Fuchs endothelial dystrophy (DSEK: n = 16; DSAEK: n = 32), pseudophakic bullous keratopathy (DSAEK: n = 5), graft failure after a previous endothelial keratoplasty (DSEK: n = 3; DSAEK: n = 8), and graft failure after a previous penetrating keratoplasty (DSEK: n = 2; DSAEK n = 8). The distribution of indications for surgery was similar between the DSEK and DSAEK groups (p = 0.551).
Graft properties and preoperative measurements
No difference was found between the DSEK and DSAEK groups with respect to their baseline measurements or donor characteristics, with the exception of endothelial cell density in the grafts (Table 1). It should be noted, however, that preoperative graft thickness in the DSEK group was measured when the grafts were swollen (i.e. in an oedematous state); graft thickness in this group was later determined to be 84 ± 28 μm based on postoperative anterior segment OCT readings performed in order to facilitate the comparison between the two groups. This recalculated graft thickness in the DSEK group differed significantly from the grafts in the DSAEK group (p < 0.005).
Table 1.
Preoperative graft properties and baseline measurements
| Graft properties | DSEK (n = 21)* | DSAEK (n = 53)* | p‐Value |
|---|---|---|---|
| Graft thickness, μm | 105 ± 21† | 106 ± 21 | 0.884 |
| ECD, cells/mm2 | 2531 ± 67 | 2748 ± 149 | <0.001 |
| Donor age, years | 63.5 ± 9.8 | 64.7 ± 9.4 | 0.630 |
| Baseline measurements | |||
| CDVA, logMAR | 0.43 ± 0.30 | 0.41 ± 0.35 | 0.980 |
| CDVA, logMAR (median, IQR) | 0.35 (IQR: 0.26–0.70) | 0.37 (IQR: 0.22–0.80) | |
| CDVA, decimal | 0.39 ± 0.27 | 0.36 ± 0.21 | 0.760 |
| CDVA, decimal (median, IQR) | 0.45 (IQR: 0.20–0.55) | 0.43 (IQR: 0.16–0.60) | |
| Manifest refraction | |||
| Spherical refraction, D | 0.82 ± 3.52 | 0.63 ± 4.40 | 0.870 |
| Cylindrical refraction, D | −1.87 ± 1.57 | −2.07 ± 1.76 | 0.654 |
| Pachymetry, μm | 657 ± 105 | 701 ± 160 | 0.280 |
| IOP, mmHg | 14 ± 2 | 14 ± 4 | 0.749 |
CDVA = corrected distance visual acuity, D = dioptre, ECD = endothelial cell density, IOP = intraocular pressure, IQR = interquartile range, logMAR = log of the minimum angle of resolution.
Except where indicated otherwise, data are presented as the mean ± SD.
These grafts were measured in an oedematous state.
Thirty patients had visual impairing co‐morbidity, including glaucoma (n = 16), age‐related macular degeneration (n = 4), amblyopia (n = 2), and other pre‐existing conditions (n = 8). These co‐morbidities were distributed similarly between the DSEK and DSAEK groups. All treated eyes, including those with a co‐morbidity, were included in the analysis.
Surgical data
Ten of the 21 DSEK and 21 of the 53 DSAEK procedures (45% and 40%, respectively; p = 0.815, Fischer's exact test) were combined with phacoemulsification cataract extraction. Each surgeon performed half (37) of the 74 procedures, with similar numbers of DSEK or DSAEK cases performed by each surgeon, respectively, 12 versus 25 (RW) and 9 versus 28 (CvL) (p = 0.607, Fisher's exact test). The mean surgery time was 68 minutes (range: 40–111 min) and did not differ significantly between the DSEK and DSAEK groups, which had mean ± SD times of 71 ± 16 and 66 ± 16 min, respectively (p = 0.222, Student's t‐test).
Clinical outcomes
At the 6‐ and 12‐month follow‐up visits, clinical outcome in terms of restored visual acuity and endothelial cell density was similar between the DSEK and DSAEK groups (Fig. 1, Table 2). The sole exception was postoperative pachymetry, which was significantly lower in the DSEK group at both time‐points (Table 2).
Figure 1.
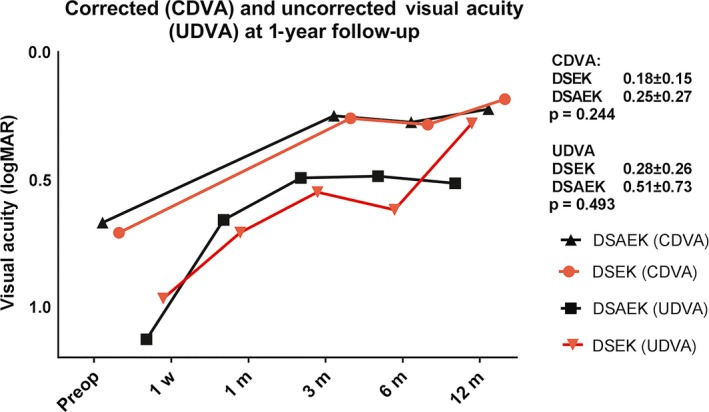
Plot of visual acuity at baseline (preoperative) and at the indicated follow‐up times after posterior lamellar surgery. Four patients had graft failure and are excluded from the follow‐up time‐points. CDVA = corrected distance visual acuity, DSAEK = descemet stripping automated endothelial keratoplasty, DSEK = descemet stripping endothelial keratoplasty, logMAR, log of the minimal angle of resolution, UDVA = uncorrected distance visual acuity.
Table 2.
Visual acuity, pachymetry and ECD measured 6 and 12 months after surgery
| DSEK | N | DSAEK | N | p‐Value | |
|---|---|---|---|---|---|
| CDVA at 6 months (logMAR) | 0.29 ± 0.24 | 18 | 0.28 ± 0.22 | 41 | 0.885 |
| CDVA at 12 months (logMAR) | 0.18 ± 0.15 | 18 | 0.25 ± 0.27 | 41 | 0.244 |
| Pachymetry at 6 months (μm) | 527 ± 39 | 17 | 584 ± 49 | 37 | <0.001 |
| Pachymetry at 12 months (μm) | 521 ± 39 | 15 | 588 ± 59 | 35 | <0.001 |
| ECD at 6 months (cells/mm2) | 1179 ± 128 (53% decrease*) | 12 | 1322 ± 502 (52% decrease*) | 37 | 0.382 |
| ECD at 12 months (cells/mm2) | 1156 ± 321 (54% decrease*) | 13 | 1320 ± 470 (52% decrease*) | 30 | 0.401 |
CDVA = corrected distance visual acuity, ECD = endothelial cell density, logMAR = log of the minimum angle of resolution.
The decrease in ECD compared to the graft baseline value.
We found a significant difference between the DSEK and DSAEK groups with respect to postoperative complications (Table 3). Overall, the DSEK group had a higher frequency of complications compared to the DSAEK group (p = 0.001, Fisher exact test). Specifically, a higher percentage of patients in the DSEK group required a rebubbling procedure, as shown in Table 3 (p < 0.01). Figure 2 shows the distribution of grafts that were dislocated, dislocated with rebubbling, or underwent rejection/failure plotted against graft thickness. We found that the majority of grafts that required rebubbling were thinner than the mean graft thickness (p < 0.001, Student's t‐test). In contrast, no other factors were identified, including the surgeon, insertion device, preoperative co‐morbidity, prior corneal grafting surgery, or age (data not shown).
Table 3.
Summary of graft dislocations, graft dislocations with rebubbling, and graft failures in the DSEK and DSAEK groups
| Complication | DSEK (n = 21) | DSAEK (n = 53) | p‐Value |
|---|---|---|---|
| Graft dislocation | 2 (10%) | 3 (6%) | 0.618 |
| Graft dislocation with rebubbling | 10 (47%) | 10 (19%) | 0.006 |
| Graft failure/rejection | 3 (14%) | 1 (2%) | 0.066 |
Figure 2.
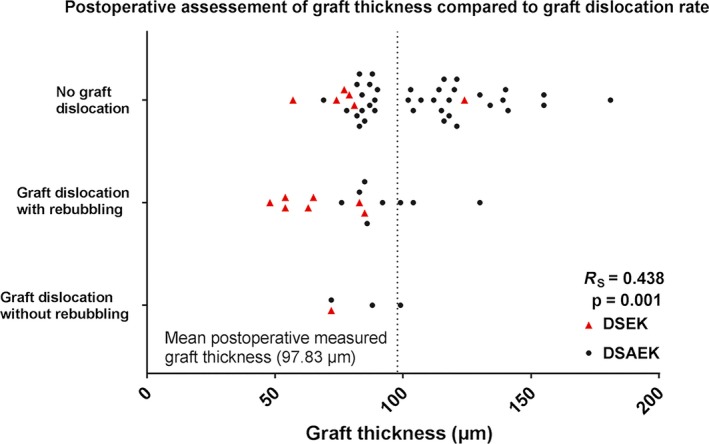
Overview of grafts that were not dislocated and grafts that dislocated with or without the need to rebubble, plotted against postoperative measured graft thickness. The mean postoperative measured graft thickness is indicated by the vertical dotted line at 105 μm. DSAEK = descemet stripping automated endothelial keratoplasty, DSEK = descemet stripping endothelial keratoplasty.
Both surgeons reported marked differences between the two graft types with respect to handling the tissues. Specifically, they reported that the manually dissected DSEK grafts were more sticky and had a tendency to curl outward—similar to a much thinner Descemet membrane endothelial keratoplasty (DMEK) scroll—rather than inwards (like a DSAEK lamella).(Dapena et al. 2011) Both surgeons reported that the thinner grafts were more difficult to manipulate, as they were more likely to fold rather than shift to the desired position. However, the more difficult handling and higher detachment rate among the DSEK grafts did not appear to have a negative effect on clinical outcome measured at the 1‐year follow‐up visit (although early graft failures were excluded from further analysis). Three graft failures occurred in the DSEK group (<1, 5, and 6 months after surgery), and one graft failure occurred in the DSAEK group (6 months after surgery). The first two graft failures in the DSEK group occurred in patients who experienced extensive endothelial damage during surgery.
Discussion
We found that visual acuity and endothelial cell loss measured up to 12 months after surgery was similar between patients who received a manually prepared graft (DSEK) and patients who received a mechanically prepared graft (DSAEK). The endothelial cell loss (>50%), although higher than average, is comparable with other studies (Dickman et al. 2016; Ishii et al. 2016) and normally high in the first year after which cell count stabilizes (Dickman et al. 2016; Price et al. 2016). The increased cell loss in our study could possibly be attributed to a high count of combined cataract extraction procedures and rebubbling procedures (Price & Price 2008). Strikingly, mean postoperative pachymetry was significantly thinner in the DSEK group (approximately 60 μm) compared to the DSAEK group. Postoperative anterior segment OCT showed that the mean thickness of the DSEK grafts was lower than initially measured (see Fig. 3); the difference between the DSEK and DSAEK groups may be attributed to differences in the dissection methods, as the grafts in the DSEK group were dissected in an oedematous state and were therefore transplanted while swollen, reaching physiological thickness after transplantation (Nieuwendaal et al. 2006). The DSAEK grafts used were stored in an dextran supplemented medium, which has deswelling properties, and were dissected in near physiological thickness (Dickman et al. 2015). Importantly, we also found that the prevalence of graft detachment was higher in the DSEK group. In addition, nearly half of the DSEK grafts required rebubbling due to dislocation, which is higher than previously reported postoperative dislocation rates (Price & Price 2006a,2006b). Moreover, we found that graft dislocations were more prevalent for thinner grafts, regardless of the group (see Fig. 2).
Figure 3.
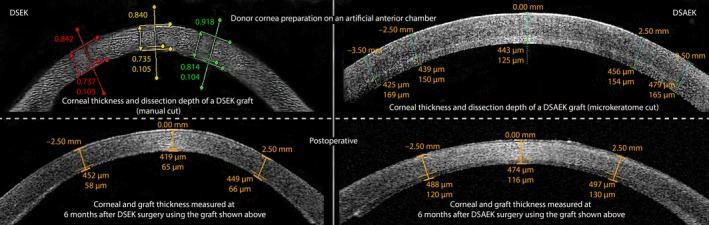
Example of postoperative deswelling observed using anterior segment OCT images of a DSEK graft (left) and a DSAEK graft (right) measured while mounted on an artificial anterior chamber (upper images) and measured at the 6‐month follow‐up visit (lower images). Using calipers, the CCT, preoperative dissection depth and postoperative graft thickness are measured (preoperative DSEK; CCT 840 μm, graft 105 μm; postoperative DSEK; CCT 484 μm, graft 65 μm, preoperative DSAEK; CCT: 568 μm, graft: 125 μm, postoperative DSAEK; CCT: 590 μm, graft: 116 μm). CCT = central corneal thickness, DSAEK = descemet stripping automated endothelial keratoplasty, DSEK = descemet stripping endothelial keratoplasty.
Several studies investigated the effects of graft thickness. For example, a small—albeit not significant—difference in detachment was reported between thicker automated and thinner manually dissected surgeon‐cut grafts, with fewer detachments when using automated cut grafts (Price & Price 2006a,2006b). However, no difference in detachment rate was found between microkeratome cut ultrathin DSAEK and conventional thickness DSAEK grafts (Dickman et al. 2016). In addition, in general higher graft detachment rate of the thinner DMEK, resembling the handling of the DSEK graft, was reported compared to DS(A)EK (Stuart et al. 2018). In general, thinner grafts have different properties than thicker grafts and are subsequently more difficult to handle, as is reported for DMEK grafts (Li et al. 2017). This raises the question of whether the thinner graft itself is more prone to detachment or whether the increased rate of detachment is due in part to the steeper learning curve for the surgeon.
The experiences reported by the corneal surgeons in our study indicate that the learning curve is likely a factor in the increased rate of detachment. The surgeons reported that thinner grafts were more prone to inward rolling (like a DMEK scroll). Difficulties in unfolding the graft may increase the amount of manipulation needed, leading to subsequent graft damage and potential increased risk of detachment (Maier et al. 2015). Given that the DSEK grafts were transplanted in an oedematous (i.e. swollen) state, it is unclear whether similarly thick DSEK and DSAEK grafts are similar with respect to ease of handling; regardless, the detachment rate was higher than we would expect for surgeons experienced with this technique. Another factor that may have affected the detachment rate in our cohort is the relatively high percentage of patients who had prior corneal grafting surgery (approximately 28%), as prior grafting surgery can increase the risk of graft detachment (Nahum et al. 2017). Nevertheless, we found no association between the rate of detachment and prior corneal grafting surgery.
In addition the composition of the donor medium could have affected graft dislocation rates; DSAEK grafts were stored in culture medium supplemented with 6% dextran, whereas the DSEK graft was not. Corneal swelling in culture medium is more pronounced in the posterior stroma (Meek et al. 2003; Dickman et al. 2015) which could have influenced the graft adherence compared to a deswelled graft. Although the results of this study provide valuable insight into clinical outcome achieved using two distinct preparation methods, the study has limitations that warrant discussion. One limitation is the relatively steep learning curve associated with posterior lamellar keratoplasty. DSEK grafts were not used prior in our centre and an Australian group recently reported that graft survival is significantly worse when a posterior lamellar procedure is performed by low‐volume surgeons (i.e. surgeons who have performed fewer than 57 posterior lamellar keratoplasties, Keane et al. 2017). Although both surgeons in our study were experienced (having performed >100 posterior lamellar keratoplasties each), both had limited prior experience with manually dissected grafts. However, the notable differences in tissue handling of manually prepared grafts could have invoked a learning curve which resulted in a higher rebubbling or graft failure rate despite the two preparation methods resulted in the same type of graft. The current trend in corneal grafting surgery is to use thinner grafts, and DSEK grafts are consistent with this preference. Moreover, DSEK is a validated method for preparing the graft. In experienced hands, this technique can produce a thinner graft at lower cost; however, with respect to handling, DSEK grafts are more similar to DMEK grafts than DSAEK grafts (Dapena et al. 2009). Nevertheless, the differences in tissue handling suggest that DSEK and DSAEK grafts are not equal and that clinical outcome depends to a large extent on the surgeon's experience with the specific preparation technique. From the surgeon's perspective, it is therefore unclear whether these methods of preparing the tissue are truly interchangeable.
Conclusions
Here, we report that manually dissected and microkeratome‐dissected corneal lamellar transplants yield a similar clinical outcome both 6 and 12 months after grafting. Importantly, neither the change in visual acuity nor endothelial cell density differed between DSEK and DSAEK grafts. Mean pachymetry was significantly lower in the DSEK group. The higher incidence of graft detachment and graft failure in the DSEK group may be due to thinner lamellae and/or differences in tissue handling, suggesting that the learning curve can differ between DSEK and DSAEK grafts.
Conflict of interest: Marc Muijzer is supported by an unrestricted grant from the Dr. F.P. Fischer Stichting, facilitated by the Stichting Vrienden van het UMC Utrecht. Robert Wisse and Chantal van Luijk have no financial or proprietary interest in the materials presented herein. The Cornea Bank Beverwijk and Amnitrans EyeBank provided the donor corneas used in this study. All authors had full access to the data and agree with the contents of this manuscript.
References
- Anderson D, Tsatsos M, Konstantopoulos A, Hossain P & Anderson D (2014): Presoaking with BSS used for thin manually dissected DSEK (TMDSEK): a viable option for thin DSEK. Eye 28: 701–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choulakian MY, Li JY, Ramos S & Mannis MJ (2016): Single‐pass microkeratome system for eye bank DSAEK tissue preparation. Cornea 35: 95–99. [DOI] [PubMed] [Google Scholar]
- Dapena I, Ham L & Melles GRJ (2009): Endothelial keratoplasty: DSEK/DSAEK or DMEK – the thinner the better? Curr Opin Ophthalmol 20: 299–307. [DOI] [PubMed] [Google Scholar]
- Dapena I, Moutsouris K, Droutsas K, Ham L, van Dijk K & Melles G (2011): Standardized ‘No‐Touch' technique for descemet membrane endothelial keratoplasty. Arch Ophthalmol 129: 88. [DOI] [PubMed] [Google Scholar]
- Dickman MM, van Maris MPFHL, van Marion FW, Schuchard Y, Steijger‐Vermaat P, van den Biggelaar FJHM, Berendschot TTJM & Nuijts RMMA (2014): Surface metrology and 3‐Dimensional confocal profiling of femtosecond laser and mechanically dissected ultrathin endothelial lamellae. Investig Ophthalmol Vis Sci 55: 5183–5190. [DOI] [PubMed] [Google Scholar]
- Dickman MM, Kruit PJ, Van Den Biggelaar FJHM, Berendschot TTJM & Nuijts RMMA (2015): Single‐pass dissection of ultrathin organ‐cultured endothelial lamellae using an innovative microkeratome system. Cornea 35: 100–104. [DOI] [PubMed] [Google Scholar]
- Dickman MM, Kruit PJ, Remeijer L et al. (2016): A randomized multicenter clinical trial of ultrathin descemet stripping automated endothelial keratoplasty (DSAEK) versus DSAEK. Ophthalmology 123: 2276–2284. [DOI] [PubMed] [Google Scholar]
- Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F & Thuret G (2016): Global survey of corneal transplantation and eye banking. JAMA Ophthalmol 134: 167. [DOI] [PubMed] [Google Scholar]
- Ishii N, Yamaguchi T, Yazu H, Satake Y, Yoshida A & Shimazaki J (2016): Factors associated with graft survival and endothelial cell density after Descemet's stripping automated endothelial keratoplasty. Sci Rep 6: 25276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane MC, Mills RADA, Coster DJ & Williams KA (2017): Is there evidence for a surgeon learning curve for endothelial keratoplasty in Australia? Clin Exp Ophthalmol 45: 575–583. [DOI] [PubMed] [Google Scholar]
- Lachin J (2000): Statistical considerations in the intent‐to‐treat principle. Control Clin Trials 16: 7–189. [DOI] [PubMed] [Google Scholar]
- Li S, Liu L, Wang W, Huang T, Zhong X, Yuan J & Liang L (2017): Efficacy and safety of Descemet's membrane endothelial keratoplasty versus Descemet's stripping endothelial keratoplasty: a systematic review and meta‐analysis. PLoS ONE 12: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Luijk CM, Bruinsma M, van der Wees J, Lie JT & Ham LMG (2012): Combined chlorhexidine and PVP‐I decontamination of human donor eyes prior to corneal preservation. Cell Tissue Bank 13: 333–339. [DOI] [PubMed] [Google Scholar]
- Maier AKB, Gundlach E, Schroeter J et al. (2015): Influence of the difficulty of graft unfolding and attachment on the outcome in descemet membrane endothelial keratoplasty. Graefe's Arch Clin Exp Ophthalmol 253: 895–900. [DOI] [PubMed] [Google Scholar]
- Meek KM, Leonard DW, Connon CJ, Dennis S & Khan S (2003): Transparency, swelling and scarring in the corneal stroma. Eye 17: 927–936. [DOI] [PubMed] [Google Scholar]
- Melles G, ten Hoope G, Rietveld F, Beekhuis W & Binder S (1998): Depth predictability of stromal pockets in the posterior cornea. Cornea 17: 174–179. [DOI] [PubMed] [Google Scholar]
- Nahum Y, Leon P, Mimouni M & Busin M (2017): Factors associated with graft detachment after primary descemet stripping automated endothelial keratoplasty. Cornea 36: 265–268. [DOI] [PubMed] [Google Scholar]
- Nieuwendaal CP, Lapid‐Gortzak R, Van Der Meulen IJ & Melles GJR (2006): Posterior lamellar keratoplasty using descemetorhexis and organ‐cultured donor corneal tissue (Melles technique). Cornea 25: 933–936. [DOI] [PubMed] [Google Scholar]
- Palioura S, MD P, Colby K & MD P (2017): Outcomes of descemet stripping endothelial keratoplasty using eye bank‐prepared preloaded grafts. Cornea 36: 21–25. [DOI] [PubMed] [Google Scholar]
- Price FW & Price MO (2006a): Descemet's stripping with endothelial keratoplasty in 200 eyes. Early challenges and techniques to enhance donor adherence. J Cataract Refract Surg 32: 411–418. [DOI] [PubMed] [Google Scholar]
- Price MO & Price FW (2006b): Descemet's stripping with endothelial keratoplasty. comparative outcomes with microkeratome‐dissected and manually dissected donor tissue. Ophthalmology 113: 1936–1942. [DOI] [PubMed] [Google Scholar]
- Price MO & Price FW (2008): Endothelial cell loss after descemet stripping with endothelial keratoplasty. Influencing factors and 2‐year trend. Ophthalmology 115: 857–865. [DOI] [PubMed] [Google Scholar]
- Price MO, Baig KM, Brubaker JW & Price FW (2008): Randomized, prospective comparison of precut vs surgeon‐dissected grafts for descemet stripping automated endothelial keratoplasty. Am J Ophthalmol 14: 6. [DOI] [PubMed] [Google Scholar]
- Price MO, Calhoun P, Kollman C, Price FW & Lass JH (2016): Descemet stripping endothelial keratoplasty ten‐year endothelial cell loss compared with penetrating keratoplasty. Ophthalmology 123: 1421–1427. [DOI] [PubMed] [Google Scholar]
- Rice A, Spokes DM, Anand S & Ball JL (2011): Endothelial cell survival and graft profile analysis in descemet stripping endothelial keratoplasty. Cornea 30: 865–871. [DOI] [PubMed] [Google Scholar]
- Romano V, Steger B, Myneni J, Batterbury M, Willoughby CE & Kaye SB (2017): Preparation of ultrathin grafts for Descemet‐stripping endothelial keratoplasty with a single microkeratome pass. J Cataract Refract Surg 43: 12–15. [DOI] [PubMed] [Google Scholar]
- Stuart AJ, Romano V, Virgili G & Shortt AJ (2018): Descemet's membrane endothelial keratoplasty (DMEK) versus Descemet's stripping automated endothelial keratoplasty (DSAEK) for corneal endothelial failure. Cochrane Database Syst Rev 6: 1465–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MA (2009): Endothelial keratoplasty: a comparison of complication rates and endothelial survival between precut tissue and surgeon‐cut tissue by a single dsaek surgeon. Trans Am Ophthalmol Soc 107: 184–193. [PMC free article] [PubMed] [Google Scholar]
- Terry M & Ousley P (2001): Deep lamellar endothelial keratoplasty in the first United States patients: early clinical results. Cornea 20: 239–243. [DOI] [PubMed] [Google Scholar]
- Terry MA, Shamie N, Straiko MD, Friend DJ & Davis‐Boozer D (2011): Endothelial keratoplasty. Ophthalmology 118: 36–40. [DOI] [PubMed] [Google Scholar]


