Abstract
Surgical release of the lingual frenulum (frenotomy) has become an increasingly common procedure, performed from birth through to adulthood. Surprisingly, detailed anatomy of the in‐situ lingual frenulum has never been described, and no anatomical basis has been proposed for the individual variability in frenulum morphology. The lingual frenulum is frequently referred to as a “cord” or “submucosal band” of connective tissue, yet there is no evidence to support this anatomical construct. This paper aims to describe the anatomy of the in‐situ lingual frenulum and its relationship to floor of mouth structures. Fresh tissue microdissection of the lingual frenulum and floor of mouth was performed on nine adult cadavers with photo‐documentation and description of findings. The lingual frenulum is a dynamic structure, formed by a midline fold in a layer of fascia that inserts around the inner arc of the mandible, forming a diaphragm‐like structure across the floor of mouth. This fascia is located immediately beneath the oral mucosa, fusing centrally with the connective tissue on the tongue's ventral surface. The sublingual glands and submandibular ducts are enveloped by the fascial layer and anterior genioglossus fibers are suspended beneath it. Lingual nerve branches are located superficially on the ventral surface of the tongue, immediately deep to the fascia. The lingual frenulum is not a discrete midline structure. It is formed by dynamic elevation of a midline fold in the floor of mouth fascia. With this study, the clinical concept of ankyloglossia and its surgical management warrant revision. Clin. Anat. 32:749–761, 2019. © 2019 The Authors. Clinical Anatomy published by Wiley Periodicals, Inc. on behalf of American Association of Clinical Anatomists.
Keywords: ankyloglossia, tongue tie, lingual frenulum, frenotomy, lingual nerve, floor of mouth, fascia, congenital, oral cavity
INTRODUCTION
Lingual frenotomy is a surgical procedure that divides the lingual frenulum (Baker, 2015). Given the importance of sound anatomical knowledge prior to performing any surgical procedure, it is surprising that there are no publications documenting the anatomical structure of the in‐situ lingual frenulum. In anatomy textbooks it is often described in only one or two sentences using vague terminology (Sinnatamby and Last, 2011; Standring, 2016). No publications provide a structural explanation of the variability in frenulum morphology between individuals, which would enable an understanding of what encompasses normal anatomy and which variables have the potential to create functional limitation in tongue movement.
Only two publications exist on the histology of the human lingual frenulum further highlighting the absence of adequate evidence to describe this structure (Fuchs, 1966; Martinelli et al., 2014). Neither of these papers reviewed the attachments of the lingual frenulum nor its relationship to neighboring floor of mouth structures.
The terms “tongue tie” (TT) and “ankyloglossia” are used synonymously to represent a condition where movement of the tongue is assessed as being limited. This limitation is usually attributed to the lingual frenulum “tethering” the tongue, with the frenulum itself often being called a “tongue tie.” However, it is generally agreed ankyloglossia is not a purely anatomical or appearance‐based diagnosis, and that limitation of tongue movement is crucial to the diagnosis and in the decision to proceed to frenotomy (Suter and Bornstein, 2009; Puapornpong et al., 2014; Chinnadurai et al., 2015; Francis et al., 2015; Walsh and Tunkel, 2017). As yet no clear anatomical variables have been identified that have direct correlation with limitation of specific tongue movements, or improvement in any objective outcome measures following frenotomy. Consequently, major controversy still exists around when and how the frenulum is determined to be limiting movement, and when that limitation is sufficient to warrant surgical intervention.
The lingual frenulum must be considered a normal anatomical structure, with 99.5% of healthy infants reported as having an observable and/or palpable lingual frenulum (Haham et al., 2014). The lingual frenulum has been described in some texts as a midline mucosal fold passing between the under‐surface of the tongue and the floor of the mouth (Sinnatamby and Last, 2011). However, practitioners performing frenotomy commonly use descriptive terms such as; submucosal band, string, cord, or mast, with complete division of this discrete midline connective tissue structure being recommended to improve tongue mobility (Hong et al., 2010; Ghaheri, 2014; Watson‐Genna, 2017).
Individual variability in the location or height of attachment of the lingual frenulum on the ventral surface of the tongue is observed, with this feature forming the basis of grading systems for tongue ties developed by Kotlow (1999) and adapted later by Coryllos et al. (2004). The premise of these grading systems is based on using this single feature of the visual appearance of the lingual frenulum to categorize a frenulum into a grade of ‘“tongue tie.” An attachment of the lingual frenulum closer to the tip of the tongue, the more classically recognized appearance, is now commonly referred to as an “anterior tongue tie.” The term “posterior tongue tie” has been coined more recently to describe a frenulum with a lower ventral tongue attachment, or a frenulum that is “submucosal” and not at all visible, with “tension” or “restriction” in the floor of mouth needing to be palpated for diagnosis (Chu and Bloom, 2009; Hong et al., 2010; O'Callahan et al., 2013; Pransky et al., 2015; Ghaheri et al., 2017). As the categories of these grading systems encompass the full range of possible variation in frenulum appearance, they allow any frenulum to be categorized as a “tongue tie” and to therefore be labeled as “abnormal.” This creates a dilemma regarding when a lingual frenulum's appearance can be considered normal, and potentially drives an international trend for an increasing rate of diagnosis of ankyloglossia, reported in Canada, Unites States of America and Australia (Joseph et al., 2016; Walsh et al., 2017; Kapoor et al., 2018). These authors all voice concerns regarding the potential for overdiagnosis, and a need for improved diagnostic criteria to avoid unnecessary surgery. The absence of documentation of a relationship between the current “tongue tie” grading systems and the presence and/or severity of functional restriction (Messner et al., 2000; Hong et al., 2010) strongly suggests that other variables must also impact on tongue function other than this feature alone.
Traditionally frenotomy has been performed using scissors or a scalpel (also referred to as “cold steel” techniques, as no thermal energy is applied), with recent popularity in of the use of laser (Fiorotti et al., 2004; Kotlow, 2011; Barot et al., 2014; Baker, 2015). Some practitioners believe that there are deep attachments of the frenulum, warranting a deeper incision (Fabbie et al., 2016). Heated debates take place in social media bringing into question the “completeness” of a frenotomy procedure, particularly when improvement does not occur after a procedure (Ghaheri, 2018) and in these circumstances it is not uncommon for babies to be considered for a second or multiple frenotomies (Ghaheri et al., 2018).
We believe, given the clinical uncertainty around what is normal and abnormal frenulum anatomy, it is critical to obtain a detailed and accurate knowledge of the anatomy of the frenulum and to gain an anatomical understanding for the variability in morphology that occurs between individuals. This study aims to describe the surgical anatomy of the in‐situ lingual frenulum and floor of mouth, including a descriptive analysis of the variability of morphology between individuals.
METHODS
The nine human adult cadavers used in this research were donated to the Anatomy and Medical Imaging Department. Ethical consent was obtained under the Human Tissues Act 2008. Basic demographic data: Six male and three female, age at death ranged from 50 to 87 years, with an average of 72 years.
The specimens were harvested from fresh tissue cadavers to include; the body of the mandible, the whole tongue (including the posterior tongue down to the vallecula), mylohyoid and all the tissues of the floor of mouth. They were harvested from the cadavers using a bone saw to divide the mandible with a single cut on each side, adjacent to the ramus. The soft tissues were then dissected sharply from the inner surface of the mandibular ramus and released from the soft tissues below the hyoid. A single specimen was also divided (bone and soft tissue) in the mid‐sagittal plane. The specimens were frozen and later defrosted for dissection. No form of embalming had been used, allowing the tissues to remain soft and pliable, with normal passive mobility and tissue planes preserved. Prior to commencing dissection, all specimens were photographed. The tongue was moved passively by the researcher to photo‐document the change in morphology of the frenulum with tongue movement.
All dissections were performed by the lead author who has 3 years' experience as a prosector and 20 years of clinical experience in otolaryngologic surgery. All dissections were assisted by magnification (Ziess EyeMag Smart Medical Loupes 2.5 magnification and Designs for Vision Loupes 3.5 extended field magnification) and LED illumination (Zeiss EyeMag Light II, 50,000 Lux illumination), using fine iris scissors and a scalpel (15 and 11 blades). Images were recorded with an S7 Samsung Phone Camera and a Canon EOS500D with a Macro EF 100 mm lens with an Amaran HC100 Halo ring flash.
Dissections were performed using a superficial‐to‐deep approach with photo‐documentation at each stage of the dissection. In each specimen, removal of the oral mucosa was carefully performed to display the underlying structures. Further dissection was then performed to define and determine the relationship of sublingual structures including connective tissues, salivary gland tissue and neurovascular structures. The tissues forming the lingual frenulum were identified and the morphological variability described, including the anterior and posterior attachment points of these tissues and the relationship to surrounding structures. A description of these findings was transcribed for each cadaver, then collated to summarize common findings and variations in anatomy between individuals. The described findings have been independently verified by the co‐authors.
RESULTS
Introducing the Floor of Mouth Fascia: Morphology and Attachments
The lingual frenulum is not a discrete midline connective tissue structure. In all specimens it is formed by a central fold in a layer of fascia that extends across the floor of mouth. This fascial layer attaches circumferentially around the inner surface of the mandible, with the fascia “flaring” into horizontal layers to give a broader vertical area of attachment where it fused with the mandibular periosteum (Fig. 1). The height of the superior‐most aspect of this attachment (around the inner surface of the mandible) determined the level of the “roof” of the floor of mouth, with the closely applied oral mucosa separating from the gingiva at this level. The fibers within the layers of the floor of mouth fascia passed centrally (in a radial fashion), closely following the contours of the oral mucosa, to merge with the dense submucosal connective tissue on the ventral surface of the tongue (epimysium). In the midline of the floor of mouth, the connective tissue fibers within the fascial layer pass obliquely across the midline, in a basket‐weave pattern. There was no discrete midline cord or band, and no organized connective tissue with an apparent antero‐posterior orientation. Anterior tongue movements create tension in the central region of the floor of mouth fascia, which then dynamically elevates into a midline fold, forming the lingual frenulum.
Figure 1.
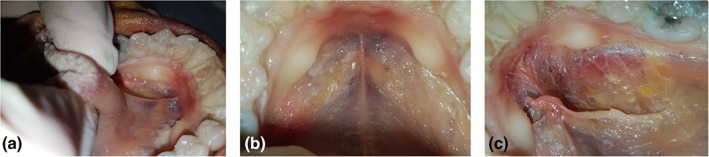
Floor of mouth fascia—mandibular attachment. (a, b) Mucosa intact, tongue elevated, and retracted to create tension along frenulum. (c) Mucosa removed, exposing floor of mouth fascia, and attachment of the fascia around the inner surface of the mandible. [Color figure can be viewed at http://wileyonlinelibrary.com]
Floor of Mouth Fascia: Variability in Thickness
Following careful removal of the overlying oral mucosa, the thickness of the anterior floor of mouth fascial layer was observed to vary between individuals, on a spectrum from dense and opaque, to thin and transparent (Fig. 2a–h). In all individuals, the fascia was thickest in the anterior floor of mouth, tapering to become gradually thinner as it passed postero‐laterally under the sides of the tongue (Fig. 3). From there, this very thin, more distensible fascia continued posteriorly in the submucosal plane into the piriform fossa of the pharynx (Fig. 3b). There was no relationship between fascial thickness and age or gender.
Figure 2.
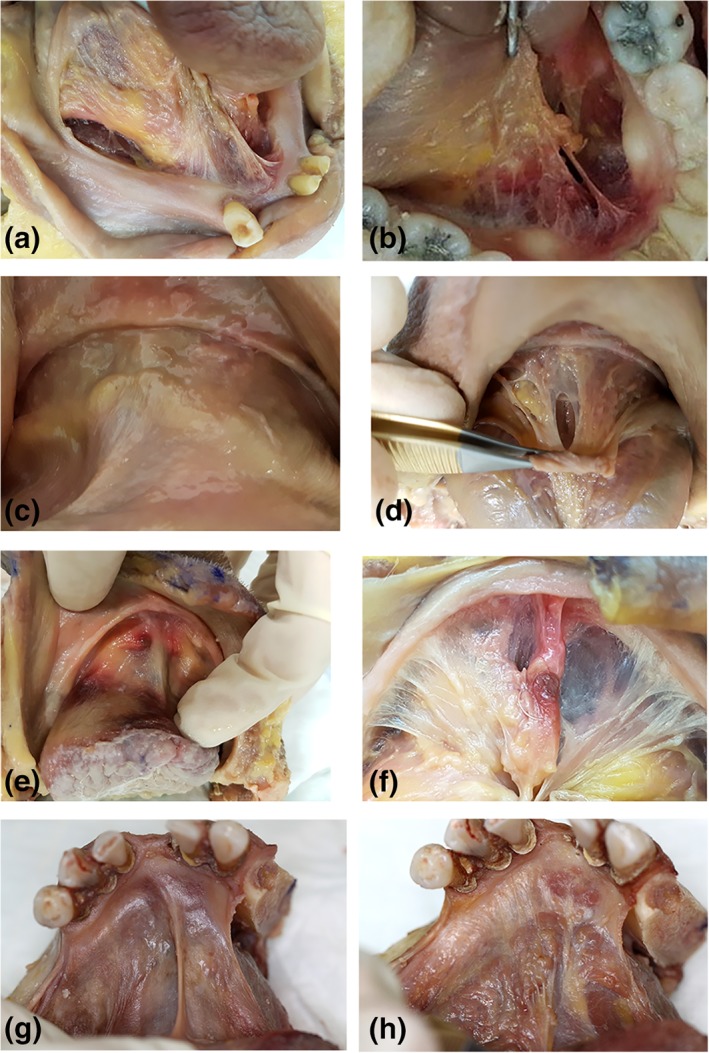
Variations in thickness and height of midline mandibular attachment of floor of mouth fascia. All images: tongue elevation and retraction to place fascia under tension. Specimen 1: (a) mucosa removed, thin/transparent fascia, elevated midline mandibular attachment. Specimen 2: (b) mucosa removed, thin/transparent fascia, elevated midline mandibular attachment. Specimen 3: (c) mucosa intact (d) mucosa removed: thick/opaque fascia, no elevation of midline mandibular attachment. Specimen 4: (e) mucosa intact (f) mucosa removed: thin/transparent fascia, slight elevation of midline mandibular attachment. Specimen 5: (g) mucosa intact, (h) mucosa removed: very thick/opaque fascia, slight elevation of midline mandibular attachment. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3.
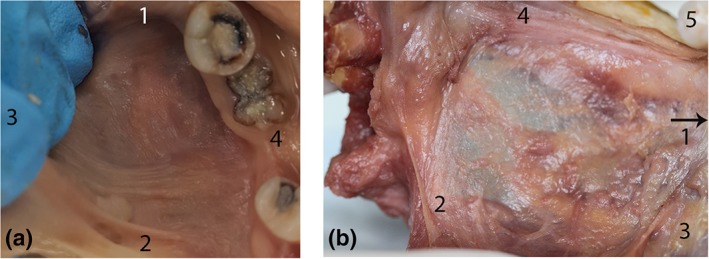
Continuity of floor of mouth fascia around lateral sides of the tongue. (a) Mucosa intact: lateral floor of mouth. Tongue retracted medially to create tension in fascia. (b) Posterior‐most aspect of floor of mouth fascia (left side, posterior tongue medialized to display space between tongue and mandible, mucosa removed). Here, the fascia is a thin, transparent layer, with high distensibility, continuous posteriorly as a submucosal layer extending into the piriform fossa. (1) Anterior (2) Posterior (3) Medial (tongue) (4) Lateral (mandible) (5) Molar tooth. [Color figure can be viewed at http://wileyonlinelibrary.com]
How Tongue Movements Mobilize the Floor of Mouth Fascia to Form the Lingual Frenulum
The floor of mouth fascia creates a diaphragm‐like “skirt” that suspends the tongue within the arc of the mandible. Its peripheral attachment to the mandible is fixed and stable. With the anterior tongue in a “resting” position and the tongue's ventral surface in contact with the anterior floor of mouth mucosa, the contour of the floor of mouth fascia is horizontal and it is not under tension (Fig. 4a). With all anterior tongue movements, the central attachment of the fascia to the ventral tongue surface creates passive movement of the floor of mouth fascia, together with the overlying oral mucosa. Anterior and mid tongue elevation and/or retraction create tension in the fascial layer, drawing the fascia and the overlying mucosa up into a midline sagittal fold that forms the lingual frenulum (Fig. 4b). The fold can become angulated, being low at its fixed mandibular attachment to the inner surface of the mandible and sloping upwards toward its ventral tongue attachment centrally (Fig. 4b).
Figure 4.
Tongue elevation creating tension in floor of mouth fascia. Both images of same specimen. (a) Tongue in “neutral” position: no tension in floor of mouth fascia. (b) Tongue elevated: tension drawing up the floor of mouth fascia (with overlying mucosa) to form a midline fold (recognizable as the lingual frenulum). [Color figure can be viewed at http://wileyonlinelibrary.com]
Variations in Lingual Frenulum Morphology
With tongue elevation, the prominence and visual appearance of the lingual frenulum fold varied significantly between individuals. The variables that contributed to differences in frenulum morphology have been summarized in Table 1; with the surface anatomy of the frenulum and important landmarks shown in Figure 5.
-
Height of midline floor of mouth fascial attachment to mandible (Fig. 5: point 4).
In the midline, the fascial attachment to the mandible could be higher than the level of attachment on either side of the mid‐line (Figs. 2a,b and 5). When placed under tension, this creates a visually more prominent frenulum, with the appearance of the attachment to the mandible being likened to the base of the Eiffel Tower (Fig. 6a–c). When the fascial insertion to the inner surface of the mandible was not elevated in the midline, the anterior aspect of the frenulum (under tension) visually merged with the floor of mouth (Figs. 2c,d and 7a–c).
-
Height of midline floor of mouth fascial attachment to ventral tongue (Fig. 5: point 2):
In a similar manner, but with more exaggerated variance, the fascia can attach in the midline anywhere along the full length of the ventral surface of the tongue (anywhere between the points 3 and 4 in Fig. 8), with this variable in morphology described and used in popular TT grading systems. If the distance or “height” of fascial attachment extends toward the tip of the tongue (Fig. 8: point 3) the frenulum creates a well‐defined, higher fold when under tension (Fig. 9a). If it is attached low on the ventral tongue surface, it creates a fold with minimal visual prominence when under tension (Fig. 9c). When the fascia attached at the lowest point on the ventral tongue, without elevation from the level of fascial attachment on either side of the mid‐line, tension created in the fascia by tongue retraction did not create a visible fold, but the tension created in the fascia could be palpated.
-
Height of midline mucosal attachment to ventral tongue (Fig. 5: point 1):
In some individuals, the floor of mouth mucosa merged with the tongue mucosa “higher” or further toward the tip of the tongue than the attachment of the fascial layer in the mid line. Fig. 5 shows the mucosal attachment to the ventral tongue at Point 1, with the fascial attachment visible slightly lower at Point 2. With tongue elevation, the floor of mouth mucosa could then glide into a transparent fold a variable distance above the fascial fold (as illustrated in Figs. 5 and 9a). An opaque frenulum formed in individuals where the mucosa and fascia both attached at the same level on the ventral tongue surface and these layers were drawn up together to the full height of the fold of the frenulum (Fig. 9b). In the individuals where genioglossus was suspended close to the inferior surface of the floor of mouth fascia, with tongue elevation genioglossus would be drawn up to the surface of the floor of mouth, usually creating a broad, ill‐defined frenulum fold (Fig. 9c). The morphology of the frenulum therefore varied on a spectrum, with the appearance closely correlated to how the layers (mucosa, fascia and genioglossus) were being drawn up into the fold of the frenulum in each specimen.
-
Length of the fold of the frenulum (Fig. 5: Length between points 1 and 4):
There was variability in the length of the fold of the frenulum between the anterior (mandibular) and posterior (ventral tongue) attachments in individual specimens. In the specimens where this dimension was shorter, the excursion of tongue movement required to create tension in the fascia and raise the fold of the frenulum was less when compared to specimens with more length between mandible and tongue attachments. This individual variability was not measured or quantified in this study.
-
Suspension of genioglossus from the floor of mouth fascia (Fig. 5: point 3):
In the midline, a sagittally orientated “curtain” of connective tissue (with vertically orientated fibers) suspends genioglossus as it passes from its anterior attachment to the mandible to merge posteriorly with the body of the tongue (Fig. 10A–G). This connective tissue “curtain” is continuous with the epimysium surrounding genioglossus. Tongue elevation creates tension in the floor of mouth fascia, raising the midline fold of the lingual frenulum, which then elevates the underlying genioglossus fibers via this connection. The height that genioglossus was suspended below the floor of mouth fascia varied. In some individuals the superior‐most genioglossus muscle fibers lay in close proximity to the floor of mouth fascia and were easily mobilized with the floor of mouth fascia up into the fold of the frenulum with tongue movement. Genioglossus fibers had a high degree of elasticity when passively stretched.
Table 1.
ANATOMICAL VARIABLES IN FRENULUM MORPHOLOGY (as observed with tongue elevated and frenulum placed under gentle tension)
| ANATOMICAL VARIABLES IN FRENULUM MORPHOLOGY | LOCATION IN FIGURE 5 |
|---|---|
| Height of midline mucosal attachment to ventral tongue (relative to height of fascial attachment) | 1 |
| Height of midline floor of mouth fascial attachment to ventral tongue | 2 |
| Height of midline floor of mouth fascial attachment to mandible | 4 |
| Length of frenulum | Length between points 1 & 4 |
| How far genioglossus is drawn up into fold of frenulum | 3 |
Figure 5.
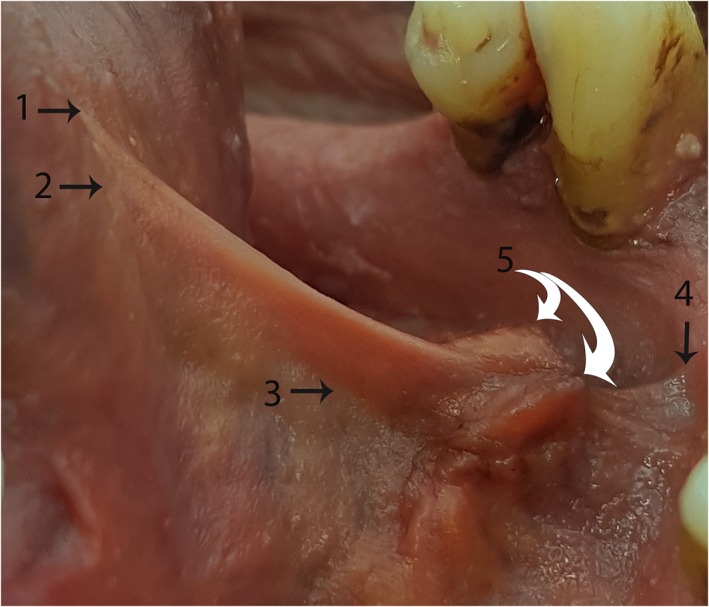
“Surface anatomy” of the lingual frenulum—Example 1 Tongue elevated to create tension in the floor of mouth fascia, raising the fold of the frenulum. (1) Highest point of midline mucosal attachment to ventral tongue. (2) Highest point of midline floor of mouth fascia attachment to ventral tongue. (3) Genioglossus—drawn into base of lingual frenulum (suspended from floor of mouth fascia). (4) Highest point of midline fascial attachment on the inner surface of mandible. (5) (White arrows): Submandibular duct openings—suspended from floor of mouth fascia. [Color figure can be viewed at http://wileyonlinelibrary.com]
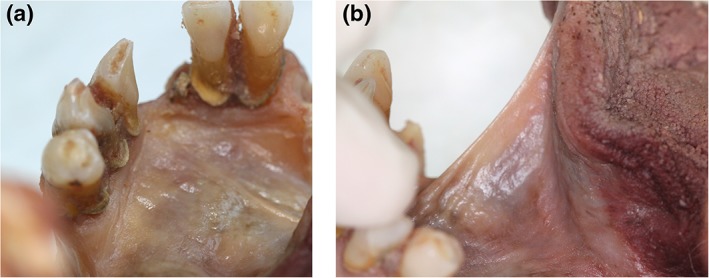
Figure 6.
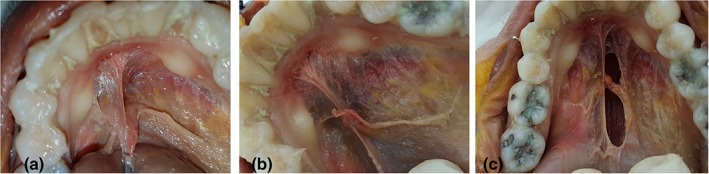
Height of midline fascial attachment to mandible—elevated. (a, b) Floor of mouth with mucosa removed. Fascial attachment to mandible elevated in midline, creating “eiffel tower” appearance. (c) Window created in fascia (to right of midline) to show underlying sublingual space and genioglossus. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 7.
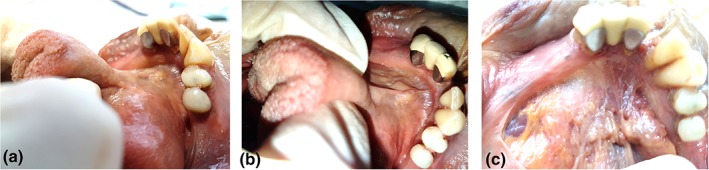
Height of midline fascial attachment to mandible—not elevated. (a and b): Mucosa in situ. (c) Mucosa removed: height of midline fascial attachment to mandible not elevated relative to height of fascial attachment on either side of midline. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 8.
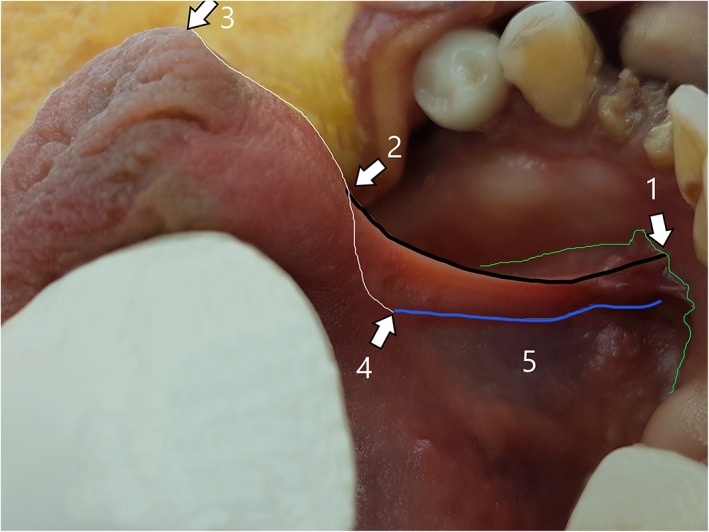
“Surface anatomy” of the lingual frenulum—Example 2. (1) midline mandibular attachment of floor of mouth fascia (higher than its attachment either side of midline). (2) Midline ventral tongue attachment of floor of mouth fascia (same height as mucosal attachment). (3) Tip of tongue (junction of ventral and dorsal tongue surfaces). (4) Location of genioglossus merging into body of tongue. (5) Superior edge of genioglossus fibers, drawn up into base of lingual frenulum with tongue elevation. Green line: height of attachment of floor of mouth fascia to mandible (with overlying mucosa closely applied) nb: the attachment is higher in midline, creating an “eiffel tower” appearance. Black line: (between points 1 and 2): fold of the lingual frenulum—opaque, with the fascial layer elevated up to the top of the fold. White line: (between points 3 and 4): midline ventral tongue surface. Distance between points 2 and 3: the “free length” of the tongue. Blue line: superior‐most aspect of genioglossus fibers, being drawn up into frenulum. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 9.
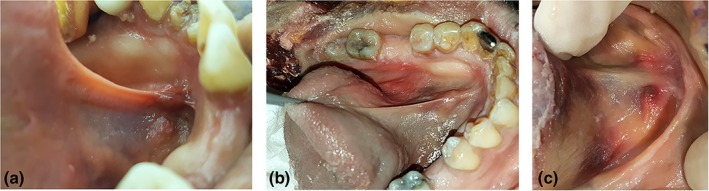
Variable morphology of lingual frenulum under tension. (a) Transparent: mucosal fold elevating just above fascial fold, genioglossus at base of frenulum. (b) Opaque: mucosa and fascia drawn up to top of fold, genioglossus drawn up into mid‐frenulum. (c) Thick/bulky: genioglossus drawn up into frenulum together with mucosa and fascia, less defined fold. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 10.
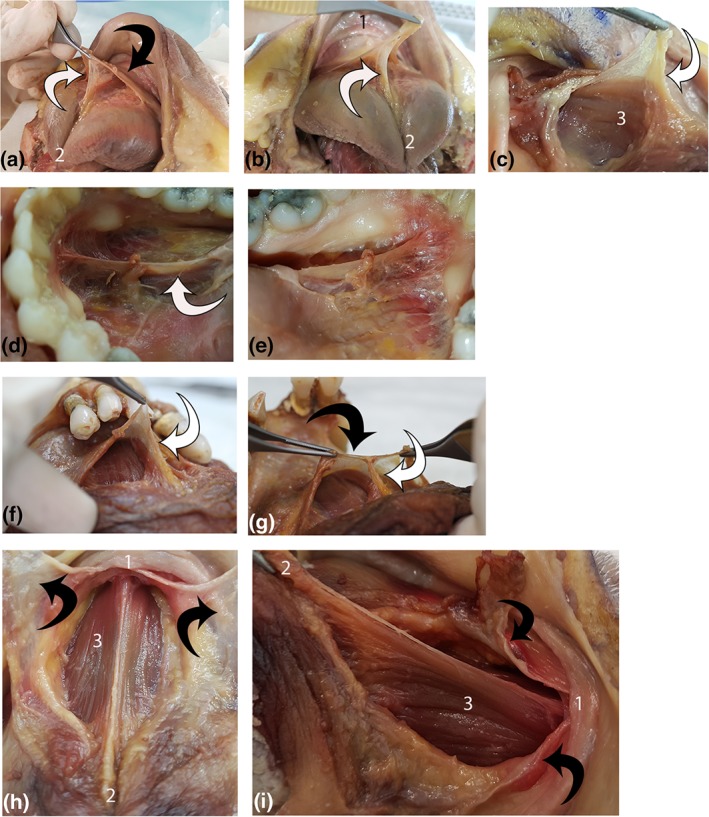
Suspension of genioglossus from floor of mouth fascia. Each line shows multiple views of a single specimen (four specimens total). (1) mandible, (2) ventral tongue tip, and (3) genioglossus. White arrow: connective tissue suspending genioglossus. Black arrow: floor of mouth fascia. (a, b): Suspension of genioglossus from floor of mouth fascia. (c) Connective tissue suspending genioglossus continuous with genioglossus epimysium. (d, e) Left and right lateral view of connective tissue suspending genioglossus. (f, g) Midline suspension of genioglossus from floor of mouth fascia. (h, i) Floor of mouth fascia divided (midline sagittal incision) and retracted, exposing genioglossus (under tension with tongue elevated and retracted). Suspending connective tissue and epimysium removed. [Color figure can be viewed at http://wileyonlinelibrary.com]
The appearance of the lingual frenulum (with the tongue elevated and/or retracted) could therefore vary on a spectrum between individuals: from being a thin and transparent fold, to an opaque fold, to thick and poorly defined fold, or having no clearly visible frenulum fold at all. We have shown this to correlate with the variability of how the mucosa and fascial layers and genioglossus fibers are mobilized into the fold of the lingual frenulum when the fascia is placed under tension. Figure 11 uses line drawings to illustrate an anatomically based understanding of this variability in frenulum morphology, to show how this varies from the popular “presumed” understanding of frenulum structure.
Figure 11.
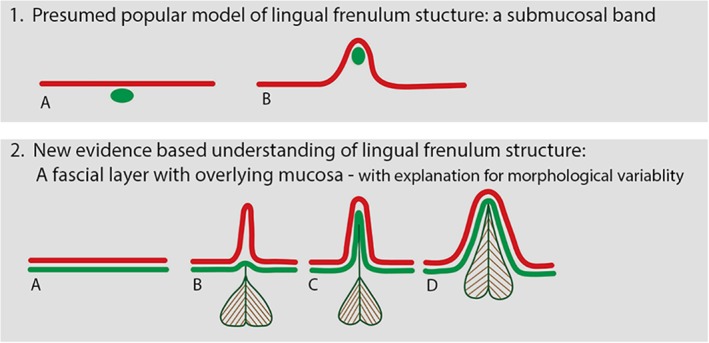
Anatomically based understanding of lingual frenulum structure. Diagram illustrating coronal section of floor of mouth: (1) Current “presumed” understanding of lingual frenulum structure: a submucosal band: (a): tongue relaxed, (b): tongue elevated, raising lingual frenulum. Red line: oral mucosa green oval: coronal section of connective tissue “band.” (2) Our newly proposed anatomically based understanding of lingual frenulum structure: red line: oral mucosa green line: floor of mouth fascia, with genioglossus suspended from fascia. (a): Tongue relaxed, floor of mouth fascia immediately beneath mucosa. (b–d) Variations in frenulum morphology with tongue elevated to raise frenulum. (b) “Transparent” frenulum—mucosal fold elevates above fascia to form fold, with fascia remaining low/at base of fold. (c) “Opaque” frenulum—mucosal and fascia elevate together to form fold. (d) “Thick” frenulum”—mucosa and fascia elevate together, with genioglossus also drawn into fold. [Color figure can be viewed at http://wileyonlinelibrary.com]
Suspension of Sublingual Glands
On either side of the midline, the deep layers of the floor of mouth fascia separate to envelope and suspend the sublingual glands, submandibular duct, and sublingual venous plexus from its inferior surface (Figures 12, 13, 14). With anterior tongue movements, the suspended floor of mouth structures glide smoothly over the underlying genioglossus, with the plane of movement being in the loose connective tissue and adipose tissue below the sublingual glands. The openings of the submandibular ducts pass through the fascial layer, immediately adjacent to the midline fold of the frenulum. The fascial layer surrounding the submandibular duct openings is thicker, and the overlying mucosa is tightly adherent. Therefore, the submandibular duct openings are mobilized passively together with the fascia with any tongue movements and become “draped” onto the lateral sides of the fold as tension in the fascia raises the frenulum.
Figure 12.
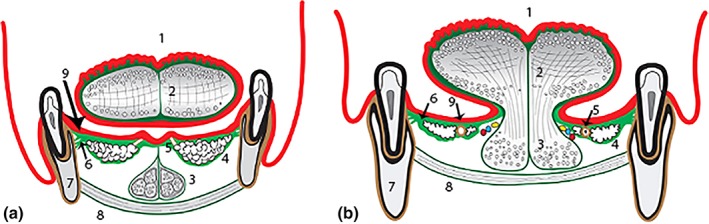
Diagram of floor of mouth fascia (coronal section). (a) anterior floor of mouth (under blade of tongue). (b) postero‐lateral floor of mouth (under lateral sides of tongue). (1) Tongue—dorsal surface, (2) Tongue—intrinsic muscles (median septum and superficial connective tissue—dark green), (3) Anterior fibers of genioglossus (in diagram a: suspended from floor of mouth fascia, in diagram; b: merging into body of tongue), (4) Sublingual glands (enveloped by and suspended from floor of mouth fascia), (5) Submandibular duct (in diagram a: entering papilla at mucosal surface, in diagram b: embedded in fascia with sublingual glands), (6) Floor of mouth fascia—spans floor of mouth (bright green)—insertion into mandible immediately beneath oral mucosa), (7) Mandible, (8) Mylohyoid, and (9) Oral mucosa (red layer). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 13.
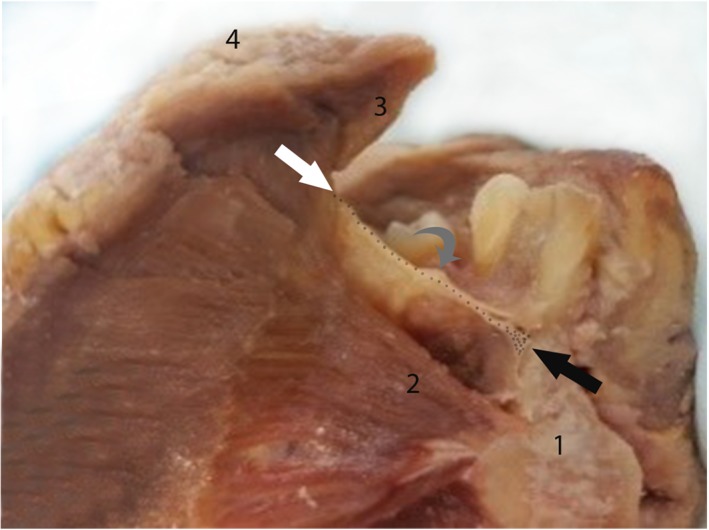
Hemi‐mandible: mid‐sagittal section through tongue and floor of mouth. Floor of mouth fascia (dotted line) with insertion anteriorly onto mandible (black arrow) and posteriorly onto ventral tongue (white arrow). “Window” through to sublingual space beneath fascia, showing submandibular duct (gray arrow) and sublingual glands suspended from fascia (visible as irregular tan‐colored tissue on under surface of fascia, between submandibular duct and mandibular insertion). (1) Mandible (2) Genioglossus (3) Ventral tongue surface (4) Dorsal tongue surface. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 14.
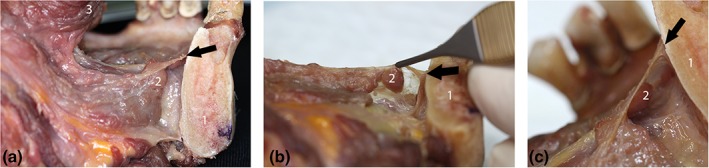
Section through mandible in parasagittal plane: showing floor of mouth fascia and sublingual space. All images are of same specimen: demonstrating suspension of sublingual glands from inferior surface of floor of mouth fascia. (a) Lateral view of specimen: showing ventral tongue surface and mandible, with floor of mouth fascia spanning between and sublingual space visible beneath fascia. (b) Specimen tilted to show inferior surface of fascia, with suspended sublingual glands. (c) Close‐up lateral view, showing mandibular attachment of fascia and suspended sublingual glands. Black arrow: indicating location of floor of mouth fascia attachment to mandible. (1) Mandible, (2) Sublingual glands (3) Ventral tongue surface. [Color figure can be viewed at http://wileyonlinelibrary.com]
Lingual Nerve Branches
As the lingual nerve crosses the submandibular duct lateral to the body of the tongue, anterior branches of the nerve pass on the ventral surface of the tongue from lateral toward the midline (Fig. 15). With the mucosa and fascia removed, branches of the lingual nerve are visible on the surface of genioglossus, with some branches penetrating the muscle and others continuing to pass superficially immediately beneath the fascia, toward the tip of the tongue or onto the connective tissue suspending genioglossus in the midline.
Figure 15.
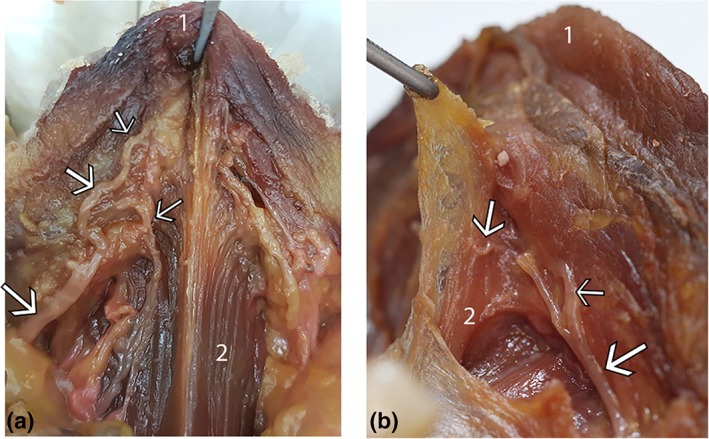
Lingual nerve branches: location on ventral tongue. Two specimens (a and b): floor of mouth fascia removed, nerve branches shown to be located immediately beneath the fascia on the surface of the muscle, with branches passing toward the tongue tip and onto the connective tissue suspending genioglossus. (1) Ventral tongue tip (2) Genioglossus. White arrows: lingual nerve branches. [Color figure can be viewed at http://wileyonlinelibrary.com]
SUMMARY AND DISCUSSION
This study has provided new, comprehensive detail of the in‐situ anatomy of the lingual frenulum and floor of mouth using fresh cadaveric dissection. The lingual frenulum is a dynamic structure formed by a central fold of fascia that spans the floor of mouth and together with the overlying oral mucosa it forms the “roof” of the sublingual space. From its broad connection around the inner arc of the mandible, the fascia connects around the anterior and lateral ventral surfaces of the tongue, to stabilize tongue position while allowing freedom of movement. The anterior fibers of genioglossus are suspended from the fascia's inferior surface as it passes from its mandibular insertion toward the body of the tongue. The sublingual glands and submandibular ducts are enveloped and suspended by the fascia and the lingual nerve branches are located superficially on the ventral surface of the tongue, immediately deep to the fascial layer.
The Lingual Frenulum Is a Fold of Fascia, Not a Band
The lingual frenulum is not composed of connective tissue fibers that have an anteroposterior orientation nor is it a discrete cord or band as often described in literature (Fig. 11‐(1)) (Hong et al., 2010; Ghaheri, 2014; Watson‐Genna, 2017). In contrast, the fascial fibers that form the frenulum have a basket‐weave orientation as they cross the midline. This finding is supported by the histological study of adult cadavers from 1966 that reported a diagonal orientation of the collagenous fibers of the frenulum that crossed each other to form a scaffold like framework (Fuchs, 1966). Consequently, the use of the terms such as cord, string, mast or band to describe the lingual frenulum is misleading and should be discontinued. We suggest the structure of the lingual frenulum is described as a “midline fold.” This term is inclusive of the morphological variations of mucosa, floor of mouth fascia and genioglossus fibers that may elevate into the fold that forms the frenulum with tongue elevation (Fig. 11‐(2)). Furthermore, the diagnosis and classification of a “posterior tongue tie” is based on the construct of a midline “submucosal band” and is not supported by these dissections. In those individuals with low attachment of the floor of mouth fascia to the ventral tongue, a defined midline fold may be visible with tongue elevation, but if sufficient tension is placed on the fascia, it can be palpated as non‐distensible tissue. The role of surgery in dividing the floor of mouth fascia in this subgroup and the impact of surgical intervention on tongue biomechanics in these individuals are not known and warrant further research.
Variations in Frenulum Morphology—A Structural Explanation
Our research supports the concept that frenulum morphology varies across a spectrum (summarized in Figs. 9 and 11), prohibiting identification of a discrete finding which would lead a definitive visual diagnosis of aberrant anatomy. At one extreme of the spectrum of anatomic variation, where the tongue tip is tethered directly to the mandible, most practitioners would agree the findings would limit tongue range of movement and would be considered clinically significant. However, at which point on the spectrum the visual appearance of a frenulum is considered abnormal is subjective and currently remains a point of controversy.
Our findings suggest that the location of the attachment of the frenulum fold on the ventral surface of the tongue, currently the basis for both the Kotlow (1999) and Coryllos et al. (2004) grading systems for “tongue ties” is insufficient in isolation to diagnose or define the severity of ankyloglossia. The in‐depth knowledge of anatomy of frenulum morphology provided by this study gives the foundation for future research to determine the impact of a broader range of anatomical variables of the frenulum on tongue function. This information is critical for a more objective, evidenced based approach to determine which individuals are most likely to benefit from frenotomy and reducing the current trend for over‐diagnosis and treatment.
Implications of New Anatomy Findings for Frenotomy Procedure
The floor of mouth fascia is continuous with the superficial layer of connective tissue on the ventral tongue, with no deep extensions into genioglossus and no fibers extending directly into the median septum of the tongue (Figs. 10, 11, 12). Further, we found no evidence of tongue restriction caused by deep connective tissue or genioglossus, therefore there is no anatomical indication during frenotomy for deep incisions into muscle. Deeper incisions would lead to increased pain and local inflammation and increased risk of significant scarring.
Lingual nerve branches were located superficially on the ventral tongue, immediately beneath the fascia, with these branches providing sensory innervation to the frenulum and the anterior tongue (Fig. 14). This finding raises concern regarding potential injury to lingual nerve branches when performing any frenulum surgery, particularly if the incision creates a wide “diamond” (being more likely to injure the larger branches that have a more lateralized position) and/or when the surgical technique involves any thermal energy that will be absorbed into tissues immediately deep to the incision. Injury to these nerve branches risks temporary or permanently compromise to sensation of the anterior tongue, with this complication not able to be objectively measured in neonates and therefore being potentially overlooked or missed. Research suggests anterior tongue sensation is involved in reflexive tongue shaping and movement by intrinsic muscles (Mu and Sanders, 2010), so injury to these branches of the lingual nerve would be of particular concern in infants having difficulty with breastfeeding.
A New Biomechanical Model: The Role of the Floor of Mouth Fascia in Tongue Stability and Mobility
The floor of mouth fascia forms a diaphragm‐like structure within the arc of the mandible (Figs. 1–5) which we propose leads to it having a primary role in suspending the tongue. The tongue has unique anatomy and function in the human body being a muscular hydrostat in that it is able to change its shape and contours without changing its volume and without skeletal support (Gilbert et al., 2007; Smith and Kier, 1989; Stavness et al., 2012). The functional tasks of the tongue require complex contour shaping and involve coordinated sequential movement with precise timing and exact positioning of the tongue within the oral cavity. This requires fine neuromotor control, and the ability to move the tongue through a wide range of movements. The floor of mouth fascia appears to have two roles relating to tongue function: providing tongue stability while facilitating tongue mobility. These two roles are potentially conflicting; however, we have demonstrated that the location of fascial attachments and some laxity of the fascial layer enable a wide range of movements of the tongue before the layer is brought under tension, while the fascia's low distensibility once under tension stabilizes tongue position against the resulting diverse vectors of forces, critical for the manipulation and control of a liquid or solid bolus for example. In some individuals, anatomical variation in frenulum morphology may create limitation in tongue movement, such that there is an imbalance between these roles of stability and mobility. Research on task specific tongue biomechanics helps us understand how limitation of movement caused by the lingual frenulum may impact variably on different tongue activities (Jackson, 1988; Hiiemae et al., 2002; Green and Wang, 2003; Perrier et al., 2003; Geddes et al., 2008a, 2008b; Ono et al., 2009; Stavness et al., 2012; Elad et al., 2014; Xu, 2017). Further research is required to correlate the impact of specific morphological variables of the frenulum into a clinical context. We encourage researchers to include assessment of other anatomic variables including mandible size and position, hard palate height and contour and dimensions of the tongue, to help understand the impact of the lingual frenulum in the context of a broader range of anatomical factors that may also impact on sucking biomechanics.
The anatomical architecture of the floor of mouth fascia forming a diaphragm‐like structure does not appear to be replicated elsewhere in the body, with the diaphragms of the abdomen and pelvis being functionally and structurally very different. Suspension of floor of mouth has traditionally been a role attributed to mylohyoid, but our research suggests that the floor of mouth structures and the tongue itself are suspended from and stabilized by the floor of mouth fascial layer. With anterior tongue movements, the major plane of movement in the sublingual space is between the inferior surface of the salivary glands and the superior surface of mylohyoid as it passes from its mandibular insertion into the body of the tongue. Mylohyoid is located in a spatially separate, deeper layer and does not appear to have a primary role in support of the contents of the floor of mouth.
Strengths and Limitations of Study
We acknowledge that a potential limitation of the current study is that all the specimens dissected were from adult cadavers. The oral mucosa with its associated connective tissue develops by 23 weeks gestation, such that oral epithelium with adult characteristics is present by this stage of development (Winning and Townsend, 2000). We hypothesize that that the floor of mouth fascial layer demonstrated in this research is present in neonates and have embarked on further research to test this hypothesis.
CONCLUSION
This study provides a foundation for comprehensive research that will redefine the concept of ankyloglossia. Our dissections have shown that the lingual frenulum is a dynamic three‐dimensional structure that varies in morphology on a spectrum. We have clarified that the connective tissues that form the lingual frenulum are created by a sheet of fascia, rather than the presumed discrete midline band. This diaphragm‐like structure suspends the tongue and the floor of mouth structures within the arc of the mandible, creating a balance between mobility and stability.
This new understanding of frenulum anatomy provides crucial information to guide clinical examination of structure and function of the lingual frenulum, decision‐making regarding frenotomy, and an appreciation of potential risks or complications when recommending or proceeding with surgical intervention.
REFERENCES
- Baker A. 2015. Surgical treatment of ankyloglossia. Oper Tech Otolaryngol Head Neck Surg 26:28–32. [Google Scholar]
- Barot VJ, Vishnoi SL, Chandran S, Bakutra GV. 2014. Laser: The torch of freedom for ankyloglossia. Indian J Plastic Surg 47:418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnadurai S, Francis D, Epstein R, Morad A, Kohanim S, McPheeters M. 2015. Treatment of ankyloglossia for reasons other than breastfeeding: A systematic review. Pediatrics 135:1467–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu MW, Bloom DC. 2009. Posterior ankyloglossia: A case report. Int J Pediatr Otorhinolaryngol 73:881–883. [DOI] [PubMed] [Google Scholar]
- Coryllos E, Watson Genna, C , Salloum A. 2004. Congenital tongue tie and its impact on breastfeeding. American Academy of Pediatrics (Summer): Breastfeeding: Best for Mother and Baby:1–6.
- Elad D, Kozlovsky P, Blum O, Laine A, Po MJ, Botzer E, Dollberg S, Zelicovich M, Sira LB. 2014. Biomechanics of milk extraction during breast‐feeding. Proc Natl Acad Sci USA 111:5230–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbie P, Kundel L, Vitruk P. 2016. Tongue‐tie functional release ‐ LightScalpel. Dental Sleep Practice:40–45. URL: http://www.lightscalpel.com/publications/tongue-tie-functional-release/
- Fiorotti R, Bertolini M, Nicola J, Nicola E. 2004. Early lingual frenectomy assisted by CO2 laser helps prevention and treatment of functional alterations caused by ankyloglossia. Int J Orofacial Myology 30:64–71. [PubMed] [Google Scholar]
- Francis D, Krishnaswami S, McPheeters M. 2015. Treatment of ankyloglossia and breastfeeding outcomes: A systematic review. Pediatrics 135:1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs G. 1966. Histological examinations of the frenum of the tongue. [Histologische Untersuchungen des Zungenbandchens]. Dtsch Stomatol 16:575–580. [PubMed] [Google Scholar]
- Geddes D, Kent J, Mitoulas L, Hartmann P. 2008a. Tongue movements and intra‐oral vacuum in breastfeeding infants. Early Hum Dev 84:471–477. [DOI] [PubMed] [Google Scholar]
- Geddes D, Langton D, Gollow I, Jacobs L, Hartmann P, Simmer K. 2008b. Frenulotomy for breastfeeding infants with ankyloglossia: Effect on milk removal and sucking mechanism as imaged by ultrasound. Pediatrics 122:188–194. [DOI] [PubMed] [Google Scholar]
- Ghaheri B, Cole M, Fausel S, Chuop M, Mace J. 2017. Breastfeeding improvement following tongue‐tie and lip‐tie release: A prospective cohort study. Laryngoscope 127:1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaheri B, Cole M, Mace J. 2018. Revision lingual frenotomy improves patient‐reported breastfeeding outcomes: A prospective cohort study. J Hum Lact 34:566–574. [DOI] [PubMed] [Google Scholar]
- Ghaheri B. 2014. Rethinking tongue tie anatomy: Anterior vs posterior is irrelevant. URL: https://www.drghaheri.com/blog/2014/3/22/rethinking-tongue-tie-anatomy-anterior-vs-posterior-is-irrelevant [accessed Sep. 2018]
- Ghaheri B. 2018. What constitutes a complete tongue tie release? URL:https://www.facebook.com/DrGhaheriMD/posts/1085195651641810 [accessed Sep. 2018]
- Gilbert R, Napadow V, Gaige T, Wedeen V. 2007. Anatomical basis of lingual hydrostatic deformation. J Exp Biol 210:4069–4082. [DOI] [PubMed] [Google Scholar]
- Green J, Wang Y. 2003. Tongue‐surface movement patterns during speech and swallowing. J Acoust Soc Am 113:2820–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haham A, Marom R, Mangel L, Botzer E, Dollberg S. 2014. Prevalence of breastfeeding difficulties in newborns with a lingual frenulum: A prospective cohort series. Breastfeed Med 9:438–441. [DOI] [PubMed] [Google Scholar]
- Hiiemae K, Palmer J, Medicis S, Hegener J, Jackson B, Lieberman D. 2002. Hyoid and tongue surface movements in speaking and eating. Arch Oral Biol 47:11–27. [DOI] [PubMed] [Google Scholar]
- Hong P, Lago D, Seargeant J, Pellman L, Magit A, Pransky S. 2010. Defining ankyloglossia: A case series of anterior and posterior tongue ties. Int J Pediatr Otorhinolaryngol 74:1003–1006. [DOI] [PubMed] [Google Scholar]
- Jackson M. 1988. Analysis of tongue positions: Language‐specific and cross‐linguistic models. J Acoust Soc Am 84:124–143. [DOI] [PubMed] [Google Scholar]
- Joseph K, Kinniburgh B, Metcalfe A, Razaz N, Sabr Y, Lisonkova S. 2016. Temporal trends in ankyloglossia and frenotomy in British Columbia, Canada, 2004–2013: A population‐based study. CMJA Open 4:E33–E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor V, Douglas P, Hill P, Walsh L, Tennant M. 2018. Frenotomy for tongue‐tie in Australian children, 2006‐2016: An increasing problem. Med J Austr 208:88–89. [DOI] [PubMed] [Google Scholar]
- Kotlow L. 1999. Ankyloglossia (tongue‐tie): A diagnostic and treatment quandary. Quintessence Int 30:259–262. [PubMed] [Google Scholar]
- Kotlow L. 2011. Diagnosis and treatment of ankyloglossia and tied maxillary fraenum in infants using er: YAG and 1064 diode lasers. Eur Arch of Paediat Dent 12:106–112. [DOI] [PubMed] [Google Scholar]
- Martinelli R, Marchesan I, Gusmão R, Rodrigues A, Berretin‐Felix G. 2014. Histological characteristics of altered human lingual frenulum. Int J Pediatr Child Health 2:5–9. [Google Scholar]
- Messner A, Lalakea M, Aby J, Macmahon J, Bair E. 2000. Ankyloglossia: Incidence and associated feeding difficulties. Arch Otolaryngol Head Neck Surg 126:36–39. [DOI] [PubMed] [Google Scholar]
- Mu L, Sanders I. 2010. Human tongue neuroanatomy: Nerve supply and motor endplates. Clin Anat 23:777–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callahan C, Macary S, Clemente S. 2013. The effects of office‐based frenotomy for anterior and posterior ankyloglossia on breastfeeding. Int J Pediatr Otorhinolaryngol 77:827–832. [DOI] [PubMed] [Google Scholar]
- Ono T, Hori K, Tamine K, Maeda Y. 2009. Evaluation of tongue motor biomechanics during swallowing. Jpn Dent Sci Rev 45:65–74. [Google Scholar]
- Perrier P, Payan Y, Zandipour M, Perkell J. 2003. Influences of tongue biomechanics on speech movements during the production of velar stop consonants: a modeling study. J Acoust Soc Am 114:1582–1599. [DOI] [PubMed] [Google Scholar]
- Pransky S, Lago D, Hong P. 2015. Breastfeeding difficulties and oral cavity anomalies: the influence of posterior ankyloglossia and upper‐lip ties. Int J Pediatr Otorhinolaryngol 79:1714–1717. [DOI] [PubMed] [Google Scholar]
- Puapornpong P, Raungrongmorakot K, Mahasitthiwat V, Ketsuwan S. 2014. Comparisons of the latching on between newborns with tongue‐tie and normal newborns. J Med Assoc Thai 97:255–259. [PubMed] [Google Scholar]
- Sinnatamby CS, Last RJ. 2011. Last's Anatomy: Regional and Applied Edinburgh. New York: Churchill Livingstone/Elsevier. [Google Scholar]
- Smith K, Kier W. 1989. Trunks, tongue and tentacles: moving with skeletons of muscle. Am Sci 77:28–35. [Google Scholar]
- Standring S. 2016. Gray's Anatomy: The Anatomical Basis of Clinical Practice. Philadelphia, PA: Elsevier Limited. [Google Scholar]
- Stavness I, Lloyd J, Fels S. 2012. Automatic prediction of tongue muscle activations using a finite element model. J Biomech 45:2841–2848. [DOI] [PubMed] [Google Scholar]
- Suter V, Bornstein M. 2009. Ankyloglossia: Facts and myths in diagnosis and treatment. J Periodontol 80:1204–1219. [DOI] [PubMed] [Google Scholar]
- Walsh J, Links A, Boss E, Tunkel D. 2017. Ankyloglossia and lingual frenotomy: National trends in inpatient diagnosis and management in the United States 1997–2012. Otolaryngol Head Neck Surg 156:735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J, Tunkel D. 2017. Diagnosis and treatment of ankyloglossia in newborns and infants: A review. JAMA Otolaryngol Head Neck Surg 143:1032–1039. [DOI] [PubMed] [Google Scholar]
- Watson‐Genna C. 2017. Supporting Sucking Skills in Breastfeeding Infants. Burlington, MA: Jones & Bartlett Learning. [Google Scholar]
- Winning T, Townsend G. 2000. Oral mucosal embryology and histology. Clin Dermatol 18:499–511. [DOI] [PubMed] [Google Scholar]
- Xu K. 2017. 3D tongue motion visualization based on the B‐mode ultrasound tongue images. Computer Aided Engineering. Université Pierre et Marie Curie—Paris VI, 2016. (English).


