Abstract
Marine protected areas (MPAs) are important conservation tools that can support the resilience of marine ecosystems. Many countries, including Canada, have committed to protecting at least 10% of their marine areas under the Convention on Biological Diversity's Aichi Target 11, which includes connectivity as a key aspect. Connectivity, the movement of individuals among habitats, can enhance population stability and resilience within and among MPAs. However, little is known about regional spatial patterns of marine ecological connectivity, particularly adult movement. We developed a method to assess and design MPA networks that maximize inferred connectivity within habitat types for adult movement when ecological data are limited. We used the Northern Shelf Bioregion in British Columbia, Canada, to explore two different approaches: (1) evaluating sites important for inferred regional connectivity (termed hotspots) and (2) assessing MPA network configurations based on their overlap with connectivity hotspots and interconnectedness between MPAs. To assess inferred connectivity via adult movement, we used two different threshold distances (15 and 50 km) to capture moderate home ranges, which are most appropriate to consider in MPA design. We applied graph theory to assess inferred connectivity within 16 habitat and depth categories (proxies for distinct ecological communities), and used novel multiplex network methodologies to perform an aggregated assessment of inferred connectivity. We evaluated inferred regional connectivity hotspots based on betweenness and eigenvector centrality metrics, finding that the existing MPA network overlapped a moderate proportion of these regional hotspots and identified key areas to be considered as candidate MPAs. Network density among existing MPAs was low within the individual habitat networks, as well as the multiplex. This work informs an ongoing MPA planning process, and approaches for incorporating connectivity into MPA design when data are limited, with lessons for other contexts.
Keywords: British Columbia, ecological connectivity, graph theory, habitat proxy, marine conservation, marine reserve, marine spatial planning, multiplex network, network analysis, Northern Shelf Bioregion, population connectivity
Introduction
Countries are working toward protecting 10% of their coastal and marine areas as part of the Convention on Biological Diversity's Aichi Target 11, of which connectivity is a key aspect (Convention on Biological Diversity 2010). Connectivity is a fundamental attribute of MPA design and network development because of its importance for population persistence (Carr et al. 2017, Magris et al. 2018). Marine populations must have sufficient inputs of new individuals, whether through self‐recruitment or immigration from other areas, to avoid or recover from local extinctions (Botsford et al. 2001, Carr et al. 2017). Greater connectivity increases the stability and resilience of populations, thereby enabling MPAs to meet their ecological objectives (Botsford et al. 2001, Green et al. 2014). However, too much connectivity can also be detrimental to system stability, favoring the diffusion of diseases and other negative perturbations, as well as reducing the ability of prey to find refugia (Dakos et al. 2015, Hermoso et al. 2015).
Despite its ecological importance, connectivity is difficult to characterize and measure in marine ecosystems because data are limited and it encompasses many ecological processes, including dispersal (by larvae, juveniles, and adults), oceanographic conditions, ontogenetic shifts, migration, physical and ecological nutrient flow, invasive species, and risk due to anthropogenic impacts or disease (Gillanders et al. 2003, Robinson et al. 2005, Blowes and Connolly 2012). There are four commonly defined types of ecological connectivity: population, genetic, community, and ecosystem connectivity (Carr et al. 2017). Population and genetic connectivity pertain to a single species, resulting from the movement of individuals (e.g., adults, juveniles, larvae) between distinct areas and the movement of genes between populations, respectively (Carr et al. 2017). On a broader scale, community connectivity relates to the movement of multiple species within the same ecological community among spatially segregated patches (such as different patches of kelp forest beds), while ecosystem connectivity results from the movement of species, chemicals, energy, and materials between distinct ecosystems (Carr et al. 2017). Data necessary for measuring or modeling these types of connectivity are often limited, particularly at the regional scale, so methods that require large amounts of data may not be possible (e.g., Bode et al. 2016, Castorani et al. 2017). Studies incorporating connectivity into MPA planning have primarily focused on identifying locations that are important for these various types of connectivity as potential MPAs (e.g., Treml et al. 2008, Engelhard et al. 2017), but do not determine if these potential MPAs are connected to each other.
Population connectivity, the type of connectivity most commonly considered in MPA planning (e.g., Treml et al. 2008, Magris et al. 2016, Weeks 2017), is complex, with patterns varying by species and life history stage (Magris et al. 2014). The dispersal of individuals is an important ecological process, as it determines source–sink dynamics between areas (Botsford et al. 2001, Cowen and Sponaugle 2009). To be effective, MPAs must have sufficient self‐recruitment and/or immigration from other areas, protected or not, to offset mortality and emigration (Botsford et al. 2001). For many marine species, dispersal occurs through the movement of larvae, juveniles, and adults while seeking out potential reproductive partners, avoiding intra‐ or interspecies competition, or searching for suitable habitat and available resources. Species may also exhibit ontogenetic shifts, where different life stages display different habitat preferences, which will influence overall population connectivity (Gillanders et al. 2003). However, when juveniles and adults are moving between MPAs, they may be at risk of fishing or other human activities. Because dispersal abilities vary between species and life history stages, two MPAs may be functionally connected for one species or life history stage, but not another (Botsford et al. 2001).
Within the marine environment, connectivity due to adult movement has not been as well studied as for other life stages despite its importance for population persistence (Frisk et al. 2014, Bryan‐Brown et al. 2017). Adult movement distances are often reported as home ranges or maximum distance traveled, determined through methods such as tagging or tracking individuals (Gillanders et al. 2003, Grober‐Dunsmore et al. 2009). Connectivity via adult movement is most relevant in MPA planning for species with moderate adult movement distances, as individual MPA size rather than network connectivity should enable self‐recruitment for species with short distance adult dispersal (e.g., <1 km) (Kaplan et al. 2009, Carr et al. 2017), while the movement of wide‐ranging or pelagic species (e.g., turtles and tuna) may limit the effectiveness of MPAs as a conservation tool for these species (Moffitt et al. 2009, Green et al. 2014). Ideally, when species‐specific data are available, connectivity analysis incorporates details such as adult habitat preferences, resistance to movement within and between habitat types, influence of oceanic currents on adult movement, larval dispersal, density dependence, and species interactions (e.g., Rocha et al. 2002, Gillanders et al. 2003, Treml et al. 2008, Baggio et al. 2011, Caldwell and Gergel 2013, Pittman et al. 2014, Magris et al. 2016). However, such data rarely exist for multiple species.
In MPA planning processes where data are limited, benthic habitat data may be the only information available (Brooks et al. 2004). Indeed, conservation planners are often faced with limited data with which to make decisions, and may not have the time or resources to collect additional data (Ban 2009, Hansen et al. 2011). Benthic habitat data may be useful as a proxy for benthic‐associated species or communities, where species distributions are inferred based on the presence of suitable habitats (Ban 2009, Grober‐Dunsmore et al. 2009, Weeks 2017). It is uncertain how connectivity patterns identified using habitat proxies and modeling aligns with actual population connectivity for individual species. We coin the phrase “inferred connectivity” to emphasize this uncertainty and use it rather than “potential connectivity,” which combines landscape attributes with some information about species’ dispersal ability (Calabrese and Fagan 2004) or probability of dispersal between patches (Watson et al. 2010), because the latter implies a more detailed species‐specific understanding of connectivity patterns than a habitat proxy approach can provide. Delaying conservation action to collect more data may result in less effective protection, particularly in the presence of ongoing threats (Grantham et al. 2008, 2009). Therefore, it is critical to incorporate the best available information about ecological connectivity into MPA design (Carr et al. 2017), alongside other design objectives such as feature representation and replication (Margules and Pressey 2000, Roberts et al. 2003).
Graph theory is a common method to explore ecological connectivity (Urban and Keitt 2001, Grober‐Dunsmore et al. 2009, Galpern et al. 2011) and incorporate it into marine conservation (Treml et al. 2008, Anadón et al. 2013, Andrello et al. 2015). The graph's main components are “nodes,” typically the center of habitat patches or protected areas, and “edges,” which each indicate an ecological connection between two nodes (Urban and Keitt 2001, Grober‐Dunsmore et al. 2009, Treml and Kool 2018). Metrics to assess network connectivity include eigenvector centrality, betweenness, and network density among others (Treml et al. 2008, D'Aloia et al. 2017, Sayles and Baggio 2017). Multiplex networks are networks in which nodes are connected via multiple types of linkages (Battiston et al. 2014, De Domenico et al. 2014). Hence, in a multiplex network, a node (such as a location) is connected to another node via different ecological processes or different ecological attributes (Pilosof et al. 2017). Each node and type of link ensemble is called a layer (Battiston et al. 2014). Multiplex networks have recently been used to analyze and understand how structure may affect function in different complex systems (e.g., Battiston et al. 2014, Kivelä et al. 2014, Baggio et al. 2016), but their application is only just emerging in ecology (Pilosof et al. 2017).
The objective of this research was to develop a method for incorporating connectivity into MPA planning through the mechanism of adult movement when data are limited, using a case study along the Pacific coast of Canada. We used the Northern Shelf Bioregion in British Columbia (BC), Canada, as our case study because MPA network planning is actively being pursued jointly by the Government of Canada, Province of British Columbia, and 16 First Nations who are working together as the Marine Protected Area Technical Team (MPATT; Department of Fisheries and Oceans Canada 2017), and our research is informing the planning process. Although tagging studies and genetic analyses can deepen our understanding of connectivity, such data are limited to only a few species and areas in the study region (e.g., Withler et al. 2001, Fong and Dunham 2007, Rechisky et al. 2017). We thus explore using existing habitat data, which are available, to ensure that a component of ecological connectivity can be incorporated into the MPA network planning process. We developed two ways to include inferred connectivity in MPA planning: (1) identifying sites important for inferred connectivity as proposed candidate MPAs and (2) assessing the inferred connectivity of existing and potential MPAs.
Methods
Case study description
Canada is committed to developing MPA networks in all 13 of its bioregions (Government of Canada 2011), with planning underway in five priority bioregions across the country. In BC, the Northern Shelf (~102,000 km2 in size) was selected as the planning area for the first MPA network in the Pacific Region. The planning team has identified connectivity as a key knowledge gap and as a highly important ecological attribute. MPATT has identified multiple marine conservation priorities in this planning process, including 55 fish, 15 crustaceans, and 19 molluscs (for complete list, see Department of Fisheries and Oceans Canada 2017). Of these, 26 are benthic conservation priority species with known adult movement ranges as reported by Burt et al. (2014) (Appendix S1: Table S1), and eight of these have moderate adult movement ranges. Fisheries and Oceans Canada does fishery‐independent surveys for many of these species, but these surveys do not cover the full extent of the bioregion as they do not venture into fjords, inlets, or parts of Hecate Strait (Chandler et al. 2017). Thus, they were not appropriate for use in this analysis.
Prior to the MPA Network planning process, there were 124 existing MPAs in the Northern Shelf Bioregion (Fig. 1) established in an ad hoc manner and with varying levels of protection from industry, fishing, and other anthropogenic impacts. We evaluated inferred connectivity to assess connections among existing MPAs and to inform the selection of new MPAs to ensure the resulting network was well connected. We also considered 311 “potential MPAs”: areas with boundaries but not yet designated as MPAs, which are likely to be considered in the MPA network planning process (i.e., Protection Management Zones (PMZs) proposed by the Marine Plan Partnership for the North Coast (MaPP 2016a, b); and Rockfish Conservation Areas (RCAs), partial fishing closures established to protect rockfish; Fig. 1). At the time of this study, the MPA planning process in BC was considering upgrading areas that had limited protection. Our analysis assumes that the MPAs, including existing ones, would need enhanced protection to provide ecological connectivity benefits.
Figure 1.
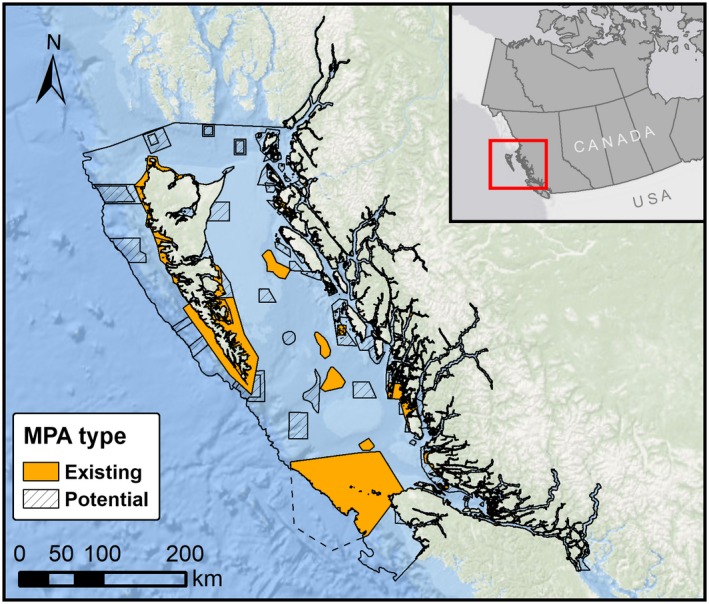
Existing and potential marine protected areas (MPAs) in the Northern Shelf Bioregion, British Columbia, Canada. Many small MPAs are not visible at the regional scale.
Data
We assessed inferred connectivity within benthic habitat types because species‐specific distribution and movement data were not available. For this study, habitat types were defined by their physical characteristics (e.g., rocky areas) or presence of canopy‐forming species (i.e., kelp beds and eelgrass meadows), resulting in six major benthic habitat types that comprise the Northern Shelf Bioregion. The region's entirety has been broadly classified into rocky, sandy, muddy, and “unknown” habitat types (Province of BC 2002), and biogenic habitat types: kelp beds, eelgrass meadows, and estuaries (Appendix S1: Table S2). Each habitat type was split into five biologically relevant depth ranges (Province of BC 2002) to generate distinct habitat patches in ArcGIS version 10.4.1 (ESRI 2016): 0–20 m (shallow), 20–50 m (photic), 50–200 m (mid‐depth), 200–1,000 m (deep), and 1,000 + m (abyssal). Although the best available data were used, there is some uncertainty associated with each of the spatial habitat data sets (e.g., positional accuracy and variation in data resolution; see Appendix S1: Table S2 for details). We defined “habitat category” as a specific combination of benthic habitat type and depth range. If a particular habitat category contained only one habitat patch, it was excluded as connectivity between habitat patches would not exist. Only habitat patches that were >1 km2 in area were included in the analysis due to computational feasibility.
Analyses
Given the lack of population level or individual movement data, we assumed that Euclidean distance between habitat patches is correlated to adult movement. Not only has this assumption has been used in other studies on connectivity (Minor and Urban 2008, Engelhard et al. 2017), previous studies have suggested that using distance as a proxy for connection probability involves the fewest additional assumptions, and therefore potential introduction of error, compared to more parameter‐rich methods such as dispersal modeling or least cost path analysis in the absence of movement information (Minor and Urban 2007, Galpern et al. 2011). We measured the distance from the centroid of each habitat patch to the centroids of all other patches within the same habitat category using visibility graphing (Tandy 1967, O'Sullivan and Turner 2001) and network analysis (Curtin 2007). Geographic Resources Analysis Support System software version 7.2.2 (GRASS Development Team 2017) was used to generate the visibility graphs, which were then processed in ArcGIS to find the shortest path from one habitat patch to another, while accounting for deviation around BC's complex coastline. The connectivity analyses were performed in R version 3.3.3 (R Core Team 2017), with the igraph package (Csardi and Nepusz 2006) using two approaches: (1) regional analyses, whereby we divided the Northern Shelf Bioregion into 1,597 100‐km2 planning units (smaller adjacent to land due to the complexity of the region's coastline), and (2) MPA analyses, where we assessed the distance between habitat patches within existing or potential MPAs. Although some biological processes occur on finer scales, planning unit resolution was restricted to 100 km2 due to data availability and computational feasibility. For planning units or MPAs with more than one habitat patch of the same category, the shortest distance to a habitat patch in another planning unit or MPA was used.
To assess inferred connectivity via adult movement, we used two different threshold distances to capture moderate adult movement ranges. Burt et al. (2014) conducted a literature review of BC marine species movement and dispersal in order to inform MPA design processes, assigning species to broad adult movement categories (0, <0.05, <1, 1–10, 10–50, 50–1,000, or >1,000 km). These natural groupings indicated that (a) 10 km and (b) 50 km were generalized moderate distance thresholds across BC species. Because the 10‐km distance threshold was the same size as the planning unit width, we adjusted that distance threshold to 15 km to capture diagonal connections and therefore to minimize distortion. Based on these inferred connections, adjacency matrices for each habitat category were generated at 15 and 50 km distance thresholds.
We used graph theory to evaluate inferred connectivity between planning units or MPAs, constructing networks with planning units or MPAs as nodes, and the inferred connections between them as edges (Fig. 2). The edges were undirected, indicating that movement could occur in both directions between the two nodes. A separate network was generated for each habitat category at each distance threshold, resulting in 32 networks.
Figure 2.
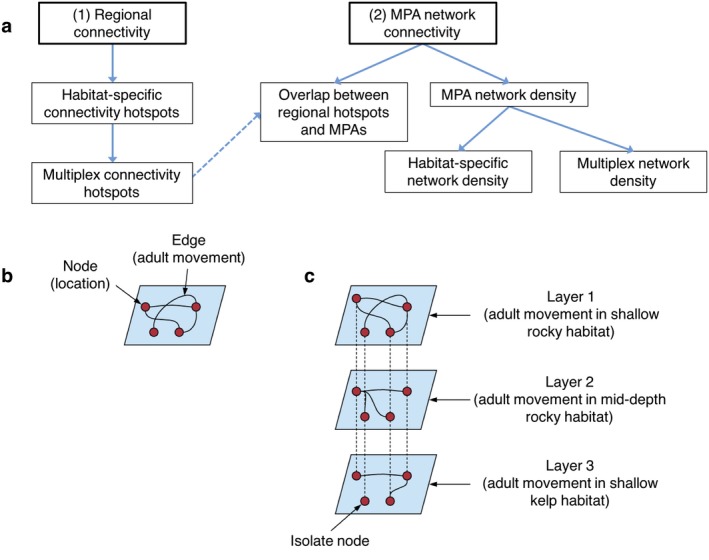
Conceptual diagram of the (a) analysis approach overview; (b) graph or network, showing key components; (c) multiplex network structure. Node or location may refer to a planning unit or MPA. “Isolate node” refers to a node that exists, but does not contain a particular habitat category or is not connected to any other nodes within that habitat category.
Approaches
Regional connectivity
We carried out regional analyses for the whole planning region to identify key areas for connectivity that could be considered in the MPA network planning process (Fig. 2). Dividing the study region into planning units enabled evaluation of the relative contribution of individual planning units to regional connectivity. We used two centrality indices to address different aspects of network connectivity: betweenness and eigenvector centrality. Nodes with the highest betweenness scores link otherwise disconnected parts of the network, acting as key stepping stone patches for animal movement (Freeman 1977, Urban and Keitt 2001, Gilarranz et al. 2015, Treml and Kool 2018). Eigenvector centrality evaluates the relative contribution of nodes to overall network connectivity, such that planning units with well‐connected neighbors may facilitate animal movement through a greater portion of the network (Battiston et al. 2014, De Domenico et al. 2014, White et al. 2014, Shanafelt et al. 2017).
For each habitat category, we calculated the centrality metrics (betweenness and eigenvector centrality) for all nodes that contained the habitat category, in the networks at the 15 and 50 km distance thresholds. This ensured that scores of 0 accurately reflected nodes that were not connected but had the potential to be. For every habitat category and distance threshold, we calculated the z score (number of standard deviations from the mean) associated with each planning unit's betweenness and eigenvector centrality metrics. Z scores ≥ 2 indicated a network hub (as in Battiston et al. 2014) for that habitat category, which was identified as a connectivity hotspot. These planning units may be important (with respect to connectivity only) to consider as possible areas to include in the MPA network to ensure the resulting network is well connected.
We then evaluated the planning units as a multiplex network, in which each layer represents a different habitat network type (Fig. 2). Analyzing the planning units as a multiplex allows consideration of the different types of links between areas (Baggio et al. 2016), where each link type represents a connection for a specific habitat category. We thus incorporated all of the habitat category matrices at the 15‐km distance threshold into a supra‐adjacency matrix, representing the layers in a multiplex, repeating this to create a separate multiplex for the 50‐km distance threshold. At each distance threshold, the same metrics for each planning unit in the multiplex were calculated (betweenness and eigenvector centrality, associated z scores), as well as the multiplex participation coefficient for each planning unit following the method described by Battiston et al. (2014). The participation coefficient (P i) of node i indicates how evenly spread the node's connections are across the habitat categories, where values close to 0 reflect participation in only a few habitat categories and values close to 1 reflect participation in most habitat categories. Participation is calculated for each multiplex, so there is only one set of highly participatory hotspots at each distance threshold (i.e., no difference between the centrality metrics). Planning units that were network hubs (z ≥ 2) or had a high participation coefficient (P i > 0.66) were identified as connectivity hotspots (the same thresholds as in Battiston et al. 2014). To assess how representative the multiplex connectivity hotspots were, we determined the proportion of hotspots identified in each habitat category that were also identified as multiplex hotspots, for each centrality metric at both distance thresholds.
MPA network connectivity
To assess the current state of inferred connectivity within existing and potential MPAs, we evaluated MPA network configurations rather than planning units (Fig. 2). We carried out two analyses: evaluating inferred connectivity for (1) existing MPAs, and (2) existing and potential MPAs. In order to evaluate the MPA network's performance with respect to regional connectivity, existing and potential MPAs were overlaid with the hotspots identified in the habitat networks and multiplexes, for all centrality metrics and distance thresholds (Fig. 2). We determined the proportion of hotspots that intersected (1) existing MPAs and (2) existing and potential MPAs.
In addition to assessing the MPAs’ performance for inferred regional connectivity, we evaluated inferred connectivity between MPAs to see how they functioned as a network (Fig. 2). For each habitat category and distance threshold, the network density, or the proportion of inferred connections to possible connections in the network (if every node was connected to every other node), was determined. The (1) existing MPA network densities and (2) existing and potential MPA network densities were compared at each distance threshold. MPA multiplex networks were created for each distance threshold the same way as in the regional planning unit analyses, where each habitat category formed a unique layer and MPAs corresponded to nodes that were present across layers. We summed the total number of inferred connections across the habitat categories for each distance threshold, and determined the total number of possible connections, then calculated the network densities of both network configurations (Battiston et al. 2014). The multiplex participation coefficients (P i) for all nodes were calculated, then averaged to determine the participation coefficient for the entire multiplex (P) at each distance threshold, as in Battiston et al. (2014). The multiplex network densities were used to compare the MPA network configurations for each distance threshold.
Results
Regional analysis
Out of 1,597 planning units, 1,261 were included in the analysis, comprised of 3,727 habitat patches (Appendix S1: Fig. S1). Some planning units were excluded because they contained only habitat patches that were of “unknown” type or <1 km2 in area; many naturally small discrete patches (like kelp, eelgrass, and some estuaries) were excluded because of the area minimum. The 16 habitat categories had variable patch numbers, from 6 patches in eelgrass meadows to 527 patches in mid‐depth rocky habitat (Appendix S1: Table S3). Eelgrass, kelp, estuaries, and abyssal rocky areas were more spatially restricted and had fewer habitat patches than the other categories. More links were present in habitat categories with more habitat patches because of the higher potential. The nearshore planning units tended to have the highest habitat diversity.
There was low similarity in the multiplex network hubs identified for each distance threshold and centrality metric (Fig. 3). This indicates that the planning units identified as important for connectivity, and perhaps as candidate MPAs, are highly dependent on the centrality metric and distance threshold (or model species) used in the planning process. No network hubs were identified with the eigenvector centrality metric at the 15‐km distance threshold, indicating that planning unit contributions to inferred regional connectivity were fairly similar across the bioregion. All 87 network hubs based on eigenvector centrality at the 50‐km distance threshold intersected the largest habitat patch in the bioregion; eigenvector centrality helps to confirm the importance of contiguous habitat for animal movement. In contrast, the network hubs identified using betweenness were often at the edge of habitat patches, where they would act as critical links to other patches in the same habitat category.
Figure 3.
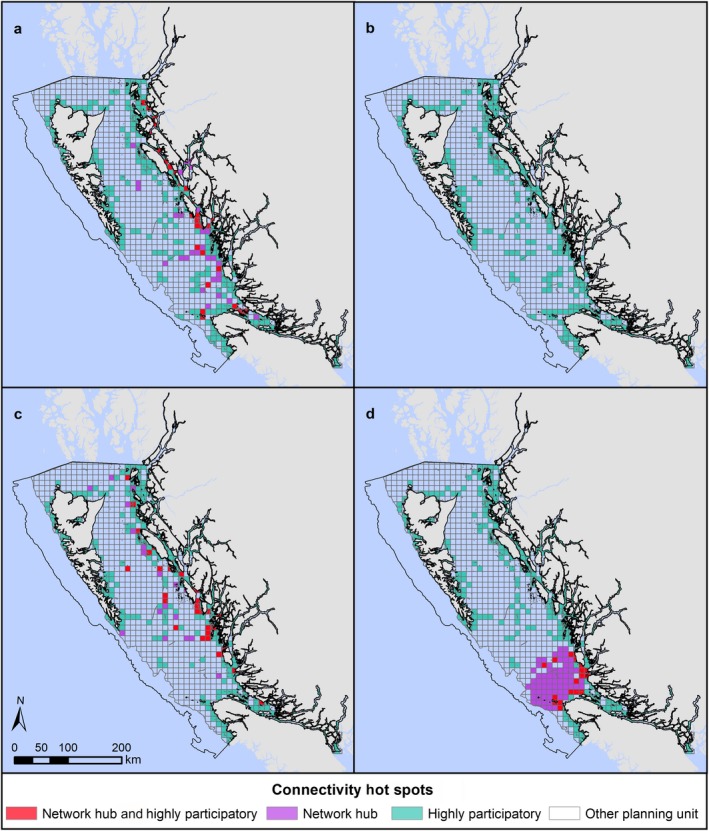
Inferred regional connectivity hotspots within the Northern Shelf Bioregion, as identified by planning units in the multiplex network with z score ≥ 2 for (a) betweenness hotspots at 15‐km distance threshold; (b) eigenvector centrality hotspots at 15‐km distance threshold; (c) betweenness hotspots at 50‐km distance threshold; (d) eigenvector centrality hotspots at 50‐km distance threshold. Highly participatory planning units are identical in panels a and b and in panels c and d.
The multiplex network had little overlap between the identified network hubs (z ≥ 2) and highly participatory (P i > 0.66) planning units. The majority of highly participatory planning units were in nearshore waters along the coast, where there is higher habitat diversity. The multiplex connectivity hotspots had variable representation of the hotspots identified for each habitat category (Table 1), with moderate representation overall.
Table 1.
Proportion of inferred connectivity hotspots (z ≥ 2) identified in each habitat category, for each distance threshold and centrality metric, which are represented by the inferred connectivity hotspots identified in the multiplex network analysis
| Habitat category | 15 km | 50 km | ||
|---|---|---|---|---|
| Betweenness | Eigenvector | Betweenness | Eigenvector | |
| Rocky | ||||
| 0–20 m | 0.86 | 0.79 | 1.00 | 0.86 |
| 20–50 m | 0.95 | 0.17 | 0.87 | 0.33 |
| 50–200 m | 0.58 | 0.25 | 0.78 | 0.34 |
| 200–1,000 m | 0.11 | 0 | 0.50 | 0.00 |
| 1,000+ m | 0 | a | a | a |
| Sandy | ||||
| 0–20 m | 0.14 | 0 | 0.50 | a |
| 20–50 m | 0.13 | 0 | 0.25 | a |
| 50–200 m | 1.00 | 0.04 | 0.80 | 1.00 |
| 200–1,000 m | 0.23 | 0 | 0.45 | 0.23 |
| Muddy | ||||
| 0–20 m | 0.77 | 0.80 | 0.86 | 0.82 |
| 20–50 m | 1.00 | 0.80 | 1.00 | a |
| 50–200 m | 1.00 | 0.45 | 0.79 | 0.48 |
| 200–1,000 m | 0.80 | 0.43 | 0.60 | 0.52 |
| Kelp | ||||
| 0–20 m | 1.00 | 0.75 | 1.00 | 1.00 |
| Estuary | ||||
| 0–20 m | 0 | 0.25 | 0.50 | 0.33 |
| Eelgrass | ||||
| 0–20 m | a | a | a | a |
The centrality metrics used were betweenness and eigenvector centrality; the distance thresholds used were 15 km and 50 km.
No inferred connectivity hotspots identified.
MPA analysis
There were 305 out of 435 existing and potential MPAs included in this analysis (Appendix S1: Fig. S2), made up of 65 existing MPAs (52% of existing MPAs considered) and 240 potential MPAs (77%). The other MPAs were excluded because there were no bathymetry data (3) or benthic habitat data (2) available at their location, or because they did not contain any habitat patches of a known type that were >1 km2 (125). A total of 1,642 habitat patches were located within MPA boundaries (Appendix S1: Table S4). Existing MPAs comprised 21% of the MPAs included in this analysis and made up 30% of the spatial area covered by existing and potential MPAs. About 4–5% of the links were from one existing MPA to another, closely matching the proportion expected from the potential connections within each network (i.e., 2,080 potential connections for a network of 65 MPAs vs. 46,360 for 305 MPAs).
We determined the number of regional connectivity hotspots that intersected with (1) existing MPAs, and (2) existing and potential MPAs (Table 2). On average, existing MPAs intersected 47% of all hotspots across the centrality metrics and distance thresholds considered. Within the existing MPAs, network hubs for betweenness were the least represented; the MPAs overlapped 30% and 35% of these network hubs at the 15‐ and 50‐km distance thresholds, respectively. Protecting a higher proportion of these hotspots may be prioritized in the planning process.
Table 2.
Proportion of regional connectivity hotspots identified by the multiplex network analysis for all centrality metrics and distance thresholds that intersect (1) existing MPAs, and (2) existing and potential MPAs in the Northern Shelf Bioregion
| Type of hotspot | 15 km distance threshold | 50 km distance threshold | ||
|---|---|---|---|---|
| Betweenness | Eigenvector | Betweenness | Eigenvector | |
| Existing MPAs | ||||
| Network hub | 0.30 | a | 0.35 | 0.61 |
| Highly participatoryb | 0.49 | 0.49 | 0.45 | 0.45 |
| Any hotspot | 0.46 | 0.49 | 0.44 | 0.48 |
| Existing and potential MPAs | ||||
| Network hub | 0.68 | a | 0.86 | 0.69 |
| Highly participatoryb | 0.81 | 0.81 | 0.80 | 0.80 |
| Any hotspot | 0.79 | 0.81 | 0.79 | 0.78 |
The centrality metrics used were betweenness and eigenvector centrality; the distance thresholds used were 15 and 50 km.
No inferred connectivity hotspots were identified.
There is one set of highly participatory planning units for each distance threshold.
For both distance thresholds and both MPA scenarios, the multiplex network densities were quite low and fairly similar to the network densities of individual habitat categories (Fig. 4). The exception to this was the eelgrass habitat network, made up of only eight MPAs (two existing MPAs), with much higher network densities of 0.39 for existing and potential MPAs and 1.00 for existing MPAs, resulting from a link between the only two MPAs with eelgrass habitat patches at both distance thresholds. As expected, network density at the 50‐km threshold was greater than at the 15‐km distance threshold, other than in the eelgrass networks.
Figure 4.
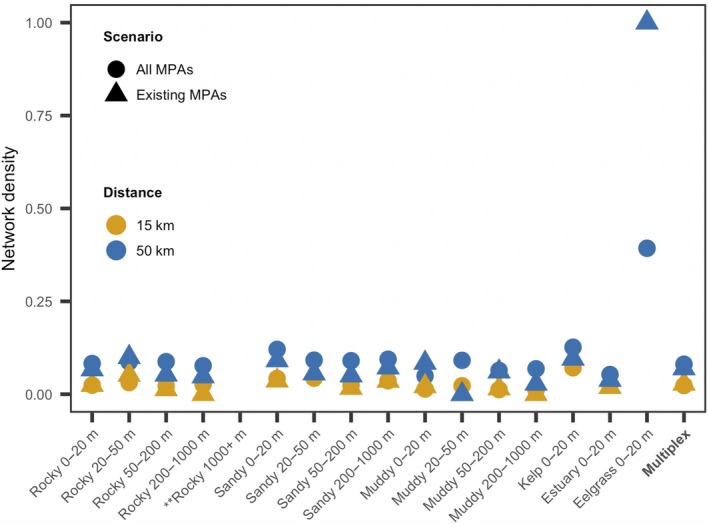
MPA network density for existing MPAs, as well as existing and potential MPAs, at two distance thresholds. Network density, the proportion of inferred connections to possible connections if every MPA was connected to every other MPA, was computed for 16 habitat categories and for a multiplex network. No MPAs contained rocky habitat with >1,000 m depth. Two habitat patches were eelgrass; eelgrass network densities at the 15‐km distance threshold are identical to, and therefore obscured by, the network densities at the 50‐km threshold. This figure generated using the ggplot2 package in R (Wickham 2016).
All multiplex networks had moderate participation coefficients for each entire network, but the networks containing existing and potential MPAs (P = 0.34 at 15 km; P = 0.44 at 50 km) had more even participation across habitat categories than those with existing MPAs only (P = 0.15 at 15 km; P = 0.25 at 50 km). This also indicates that connections at the 50‐km distance threshold were more evenly spread across habitat categories than at the 15‐km threshold for both MPA configurations, despite an unequal number of MPAs containing each habitat category.
Discussion
Despite its importance for MPA effectiveness, connectivity has rarely been incorporated directly into MPA network planning (Magris et al. 2014). We developed an approach for incorporating connectivity via adult movement into MPA network planning for consideration alongside other MPA design objectives (e.g., representation), when data are limited. Connectivity via adult movement has not been as well studied as larval dispersal (Sala et al. 2002, Cowen and Sponaugle 2009, Anadón et al. 2013; but see Freiwald 2012, Pittman et al. 2014). Rather, previous studies have considered how adult movement may decrease efficacy of an MPA or affect MPA sizing (Walters et al. 2007, Moffitt et al. 2009, Grüss et al. 2011a). Our approach best aligns with species that have strong benthic associations as adults, and has the potential to represent animals for which species‐specific information may not be available. It is widely applicable because it can inform planning where resources and data are limited, but also contains many assumptions.
Our results are being integrated into the interactive platform that MPATT is using to create and evaluate alternative MPA network configurations, enabling inferred connectivity as an additional design objective in the Northern Shelf Bioregion planning process. We received iterative guidance from MPATT in creating this method to ensure that this work remained applicable as the planning process and our research developed. Many of the planning units that emerged as inferred regional connectivity hotspots have conservation importance for other reasons. For example, many multiplex hotspots have some spatial overlap with areas previously identified as Ecologically and Biologically Significant Areas in the planning area (Department of Fisheries and Oceans Canada 2012). Protection of these overlapped hotspots would result in progress toward multiple conservation targets. There was low interconnectedness among existing MPAs (network density), particularly at the 15‐km distance threshold. To increase connectivity between existing areas in the region, planners should prioritize areas that may act as stepping stones among existing MPAs. However, planners may also want to keep MPAs disconnected intentionally, as isolation contributes to uniqueness and resistance to disease transmission between MPAs (Dakos et al. 2015, Hermoso et al. 2015), and it is unclear what the threshold for adequate MPA interconnectedness would be. In addition, network density is likely to be affected by the number of nodes and the spatial extent of the planning region, so it may be more useful to compare the network density of multiple potential MPA network configurations to determine that which is most interconnected, rather than evaluating a single network configuration against a target density.
Our approach can also be used to prioritize MPAs for upgraded protection, if it is not possible to increase protection in all MPAs. Potential MPAs could be assessed individually to determine their overlap with inferred regional connectivity hotspots and contribution to network density, identifying those that would best complement the existing MPA network (with respect to inferred connectivity only). An important result from our study was that the centrality metrics and distance thresholds we explored highlighted different planning units as regional connectivity hotspots and therefore which MPAs were identified as contributing to inferred regional connectivity. This finding aligns with other studies, such as those that have investigated larval dispersal with multiple planktonic larval duration times or connectivity metrics (Treml et al. 2008, Magris et al. 2016, D'Aloia et al. 2017). Thus it is important to consider multiple approaches when evaluating connectivity and ensure they align with priorities identified for the particular MPA planning process.
Our analysis incorporated multiple ways in which connectivity can be used in MPA planning: (1) identifying areas that were important for inferred connectivity within the study region and (2) evaluating existing and potential MPA networks with respect to regional inferred connectivity and interconnectedness. Other studies have identified regional connectivity hotspots through modeling (e.g., larval dispersal by oceanic currents), then used Marxan to produce an optimal or near‐optimal configuration of planning units based on connectivity (Magris et al. 2016, D'Aloia et al. 2017, Krueck et al. 2017). This is valuable for determining priority sites to consider as MPA candidates in planning processes, as well as evaluating the performance of existing or proposed networks in protecting these hotspots. However, these methods do not assess the extent to which MPAs within the resulting network are connected to each other. Evaluating inferred connectivity between existing and potential MPAs can be used to explore and enhance network interconnectedness, but the “best” network configuration may or may not include regional connectivity hotspots. Objectives for connectivity in MPA design are often unclear and qualitative (Magris et al. 2014); it is important that both regional connectivity and MPA interconnectedness are incorporated into planning processes. Our two approaches complement and may provide feedback to each other as a network configuration is developed. Both can also be used to compare possible network configurations alongside other MPA design objectives.
We aggregated inferred connectivity across multiple habitat types using multiplex networks, useful for integrating multiple habitat types, as well as species, in an MPA planning process. To our knowledge, this was the first application of multiplex networks to incorporating connectivity into MPA network planning. Multiplex network hubs take into account that some areas can be important only for one habitat category, whereas others can be important for multiple habitat categories (Battiston et al. 2014, De Domenico et al. 2014). For our case study, highly participatory planning units contribute to inferred regional connectivity in multiple habitat categories, so planners may prioritize these planning units as highly as the network hubs with high contributions to inferred connectivity in one or a few habitat categories. Multiplex network density and participation may be useful in comparing proposed MPA network configurations, as these metrics provide an indication of how well connected the proposed MPAs are, and how evenly these connections are distributed across conservation priorities. In our results, the multiplex inferred regional connectivity hotspots and network densities were moderately representative of those identified for each habitat category, although individual habitat categories varied in level of representation. Inferred multiplex hotspots could be biased toward habitat categories with more links, resulting from greater habitat distribution throughout the region, but not necessarily with higher network density. Planners may prioritize (or deprioritize) habitats with less areal extent or fewer connections, as well as habitats that are important for threatened species or vulnerable life stages (e.g., Department of Fisheries and Oceans Canada 2017; Roberts et al. 2003); weighting of layers within the multiplex can be altered to reflect this. Multiplex networks may also be used for evaluating connectivity for multiple species or communities (i.e., assigning each layer to one species or community), and between different habitat or ecosystem types (Gilarranz et al. 2015, Pilosof et al. 2017). If data are available, multiplex networks can integrate connection weight and directionality for both intra‐ and inter‐layer edges (Battiston et al. 2014, De Domenico et al. 2014; see Baggio et al. 2016 for an example of a directed, weighted, multiplex network analysis in a socioecological context).
Limitations
Our study had several limitations and assumptions that should guide the use and interpretation of both the methods and results. First, our study was constrained by limited data about connectivity (but see, e.g., Robinson et al. 2005, Fong and Dunham 2007), leading us to use habitat data as the best available spatial data at the scale of the region. However, these data also have limitations, as they came from multiple sources with different survey methodologies and may not have not been ground truthed.
Second, there was limited information about adult movement to inform our modeling efforts. Ideally, we could incorporate information about species‐specific movement barriers (e.g., unsuitable substrate or depth range, freshwater influence, thermal or pH tolerance threshold, habitat degradation), habitat preferences, and behaviors (e.g., foraging) (Caldwell and Gergel 2013, Engelhard et al. 2017), but such data were too limited or incomplete in our study region for our analysis. Species‐specific movement patterns may also be influenced by many other factors including oceanic currents, density dependence, species interactions, site fidelity, seasonality, and fishing mortality between MPAs (Moffitt et al. 2009, Grüss et al. 2011a, Botsford et al. 2014, Daigle et al. 2015, Green et al. 2015), but again such data were not available for the study region.
Finally, the data and information limitations lead us to use simplifying assumptions. In particular, we assumed that all nodes were effective sources and sinks, although we excluded habitat patches <1 km2 (because of computational feasibility); however, the minimum patch size necessary to support a viable population is species dependent. Population viability is an important consideration as some of the connections identified may not exist if there are no or small source populations (Cowen and Sponaugle 2009, Pe'er et al. 2014, D'Aloia et al. 2017). We also assumed that all connections were bidirectional and had the same strength and that animals traveled to the nearest MPA using the shortest path. The shortest path may cross multiple habitat categories and is thus relevant for habitat generalist species like Sebastolobus alascanus or Metacarcinus magister. Habitat specialist species may not be able to travel through unsuitable habitat. In addition, individual habitat patches vary in their suitability, attractiveness, quality, and carrying capacity so their contributions as population sources and sinks would also vary (i.e., actual links could be stronger or weaker than assumed by analysis), but we lacked this information. Because of this, some of the nodes that we identified as inferred connectivity hotspots may not contribute very much to network connectivity, while other nodes may have more important contributions than assumed. Overall, actual connectivity patterns may differ from what we have inferred through this analysis, as is expected with many modeling approaches (Fulton et al. 2015). These limitations (and resulting uncertainty) should be considered when interpreting this study, particularly in a management context (Fulton et al. 2015). As more data become available, they can be easily integrated into our approaches to gain a better understanding of connectivity. Ongoing monitoring of the MPA network, once established, is important for assessing actual connectivity, as is adaptive management if different connectivity patterns are detected (Lubchenco and Grorud‐Colvert 2015, Carr et al. 2017).
Given data and information limitations, and the assumptions we used, our results likely represent the best possible case for inferred connectivity via adult movement. While there was some buffering capacity in our analysis because we calculated path distance between habitat patch centroids, rather than patch edges, these distances are still likely lower than what would occur in reality because of our assumption that individuals traveled along the shortest path from one MPA to the next. If movement barriers exist, we may have identified connections that do not exist in nature. This is particularly important for areas identified as betweenness hotspots; if crucial “bridging” links between otherwise disconnected regions do not exist, the associated nodes may have only marginal importance for network connectivity. It is also possible that there are links present (perhaps unidirectional) that were not identified by our modeling efforts (e.g., individuals moving downstream with currents may be able to traverse greater distances because of lower energetic cost), so some nodes may have higher importance for network connectivity than concluded by this analysis. However, reliable understanding of most species’ movement patterns is still needed (Pe'er et al. 2014). In regions where this information is available, our approach could be modified by calculating distance between habitat patches using least cost path analysis instead of visibility graphing (Galpern et al. 2011, Caldwell and Gergel 2013, Pittman et al. 2014).
The approaches that we developed can be expanded upon in future work. Adult movement is an important aspect of ecological connectivity to be considered in marine planning, whether through single‐ or multiple‐species assessments (Frisk et al. 2014). It would be valuable to incorporate fishing mortality and larval dispersal into future analyses, as each has significant impacts on population persistence and connectivity (Shanks et al. 2003, Grüss et al. 2011b). In addition, connectivity measurements through tagging, surveys, and genetic analyses would help ground truth our inferred connectivity work (e.g., Buonomo et al. 2017, Zemeckis et al. 2017). After determining connections between nodes through connectivity models or measurements, there may be more appropriate network analysis metrics to use than betweenness and eigenvector centrality, depending on the specific conservation planning objectives. Planning processes should include social‐political considerations, particularly where multiple jurisdictions exist, as these influence the MPA network's effectiveness and potential for monitoring and enforcement (Schill et al. 2015, Dehens and Fanning 2018). Future research should also assess shifting connectivity patterns with climate change (Magris et al. 2014, Andrello et al. 2015). Data on present and predicted future ocean conditions (e.g., temperature, pH, and current shifts) or species distributions can be integrated into our approaches to explore how regional connectivity hotspots and MPA network interconnectedness may change over time. Most importantly, these approaches can be applied to other MPA planning processes, even when data are limited.
Supporting information
Acknowledgments
We gratefully thank Matthew Poirier for assisting with geospatial analysis, all data providers, and the Marine Protected Area Technical Team for their guidance and feedback during this project. We thank Rafael Magris and the Marine Ethnoecology Research laboratory for providing comments on the manuscript, plus two anonymous reviewers for their insights. This research is sponsored by the NSERC Canadian Healthy Oceans Network and its Partners: Department of Fisheries and Oceans Canada and INREST (representing the Port of Sept‐Îles and City of Sept‐Îles). S. Friesen acknowledges the support of the University of Victoria, and the Natural Sciences and Engineering Research Council of Canada (NSERC) through a Canada Graduate Scholarship (Master's). S. Friesen conducted the analyses and drafted the initial manuscript; all authors contributed equally to designing the study, interpreting the results, and revising the manuscript.
Friesen, S. K. , Martone R., Rubidge E., Baggio J. A., Ban N. C.. 2019. An approach to incorporating inferred connectivity of adult movement into marine protected area design with limited data. Ecological Applications 29(4):e01890 10.1002/eap.1890
Corresponding Editor: Éva E. Plaganyi.
Data Availability
The British Columbia Marine Conservation Analysis (https://bcmca.ca) data repository has links to the data custodians for the substrate and depth data sets used in this analysis (Appendix S1: Table S2); these custodians may be contacted directly. Data from the connectivity analyses are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.ts0jb2s (Friesen et al. 2019).
Literature Cited
- Anadón, J. D. , del Mar Mancha‐Cisneros M., Best B. D., and Gerber L. R.. 2013. Habitat‐specific larval dispersal and marine connectivity: implications for spatial conservation planning. Ecosphere 4:1–15. [Google Scholar]
- Andrello, M. , Mouillot D., Somot S., Thuiller W., and Manel S.. 2015. Additive effects of climate change on connectivity between marine protected areas and larval supply to fished areas. Diversity and Distributions 21:139–150. [Google Scholar]
- Baggio, J. A. , Salau K., Janssen M. A., Schoon M. L., and Bodin Ö.. 2011. Landscape connectivity and predator‐prey population dynamics. Landscape Ecology 26:33–45. [Google Scholar]
- Baggio, J. A. , BurnSilver S. B., Arenas A., Magdanz J. S., Kofinas G. P., and De Domenico M.. 2016. Multiplex social ecological network analysis reveals how social changes affect community robustness more than resource depletion. Proceedings of the National Academy of Sciences USA 113:13708–13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban, N. C. 2009. Minimum data requirements for designing a set of marine protected areas, using commonly available abiotic and biotic datasets. Biodiversity and Conservation 18:1829–1845. [Google Scholar]
- Battiston, F. , Nicosia V., and Latora V.. 2014. Structural measures for multiplex networks. Physical Review E—Statistical, Nonlinear, and Soft Matter Physics 89:32804. [DOI] [PubMed] [Google Scholar]
- Blowes, S. A. , and Connolly S. R.. 2012. Risk spreading, connectivity, and optimal reserve spacing. Ecological Applications 22:311–321. [DOI] [PubMed] [Google Scholar]
- Bode, M. , Williamson D. H., Weeks R., Jones G. P., Almany G. R., Harrison H. B., Hopf J. K., and Pressey R. L.. 2016. Planning marine reserve networks for both feature representation and demographic persistence using connectivity patterns. PLoS ONE 11:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford, L. W. , Hastings A., and Gaines S. D.. 2001. Dependence of sustainability on the configuration of marine reserves and larval dispersal distance. Ecology Letters 4:144–150. [Google Scholar]
- Botsford, L. W. , White J. W., Carr M. H., and Caselle J. E.. 2014. Marine protected area networks in California, USA Pages 205–251 in Johnson M. L. and Sandell J., editors. Advances in marine biology. Volume 69, Marine Managed Areas and Fisheries Elsevier Ltd, Amsterdam, The Netherlands. [DOI] [PubMed] [Google Scholar]
- Brooks, T. M. , da Fonseca G. A. B., and Rodrigues A. S. L.. 2004. Protected areas and species. Conservation Biology 18:616–618. [Google Scholar]
- Bryan‐Brown, D. N. , Brown C. J., Hughes J. M., and Connolly R. M.. 2017. Patterns and trends in marine population connectivity research. Marine Ecology Progress Series 585:243–256. [Google Scholar]
- Buonomo, R. , Assis J., Fernandes F., Engelen A. H., Airoldi L., and Serrão E. A.. 2017. Habitat continuity and stepping‐stone oceanographic distances explain population genetic connectivity of the brown alga Cystoseira amentacea . Molecular Ecology 26:766–780. [DOI] [PubMed] [Google Scholar]
- Burt, J. M. , Akins P., Latham E., Beck M., Salomon A. K., and Ban N. C.. 2014. Marine protected area network design features that support resilient human‐ocean ecosystems—Applications for British Columbia, Canada. Simon Fraser University, Vancouver, British Columbia, Canada. [Google Scholar]
- Calabrese, J. M. , and Fagan W. F.. 2004. A comparison‐shopper's guide to connectivity metrics. Frontiers in Ecology and the Environment 2:529–536. [Google Scholar]
- Caldwell, I. R. , and Gergel S. E.. 2013. Thresholds in seascape connectivity: influence of mobility, habitat distribution, and current strength on fish movement. Landscape Ecology 28:1937–1948. [Google Scholar]
- Carr, M. H. , Robinson S. P., Wahle C., Davis G., Kroll S., Murray S., Schumacker E. J., and Williams M.. 2017. The central importance of ecological spatial connectivity to effective coastal marine protected areas and to meeting the challenges of climate change in the marine environment. Aquatic Conservation: Marine and Freshwater Ecosystems 27:6–29. [Google Scholar]
- Castorani, M. C. N. , Reed D. C., Raimondi P. T., Alberto F., Bell T. W., Cavanaugh K. C., Siegel D. A., and Simons R. D.. 2017. Fluctuations in population fecundity drive variation in demographic connectivity and metapopulation dynamics. Proceedings of the Royal Society B 284:20162086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler, P. C. , King S. A., and Boldt J.. 2017. State of the physical, biological and selected fishery resources of Pacific Canadian Marine Ecosystems in 2016. Canadian Technical Report of Fisheries and Aquatic Sciences 3225. [Google Scholar]
- Convention on Biological Diversity . 2010. The strategic plan for biodiversity 2011–2020 and the Aichi Biodiversity Targets. Conference of the Parties to the Convention on Biological Diversity, Nagoya., Japan. UNEP/CBD/COP/DEC/X/2. United Nations Environment Program, Nairobi, Kenya [Google Scholar]
- Cowen, R. K. , and Sponaugle S.. 2009. Larval dispersal and marine population connectivity. Annual Review of Marine Science 1:443–466. [DOI] [PubMed] [Google Scholar]
- Csardi, G. , and Nepusz T.. 2006. The igraph software package for complex network research. InterJournal 1695:1–9. [Google Scholar]
- Curtin, K. M. 2007. Network analysis in geographic information science: review, assessment, and projections. Cartography and Geographic Information Science 34:103–111. [Google Scholar]
- Daigle, R. M. , Monaco C. J., and Baldridge A. K.. 2015. An adaptable toolkit to assess commercial fishery costs and benefits related to marine protected area network design. F1000Research 4:1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakos, V. , Quinlan A., Baggio J. A., Bennett E., Bodin Ö., and BurnSilver S.. 2015. Principle 2—Manage connectivity Pages 80–104 in Biggs R., Schluter, M. and Schoon M. L., editors. Principles for building resilience: sustaining ecosystem services in social‐ecological systems. Cambridge University Press, Cambridge, UK. [Google Scholar]
- D'Aloia, C. C. , Daigle R. M., Côté I. M., Curtis J. M. R., Guichard F., and Fortin M.‐J.. 2017. A multiple‐species framework for integrating movement processes across life stages into the design of marine protected areas. Biological Conservation 216:93–100. [Google Scholar]
- De Domenico, M. , Solé‐Ribalta A., Cozzo E., Kivelä M., Moreno Y., Porter M. A., Gómez S., and Arenas A.. 2014. Mathematical formulation of multilayer networks. Physical Review X 3:41022. [Google Scholar]
- Dehens, L. A. , and Fanning L. M.. 2018. What counts in making marine protected areas (MPAs) count? The role of legitimacy in MPA success in Canada. Ecological Indicators 86:45–57. [Google Scholar]
- Department of Fisheries and Oceans Canada . 2012. Evaluation of proposed ecologically and biologically significant areas in marine waters of British Columbia. Canadian Science Advisory Secretariat Science Advice Report 2012/075. Centre for Science Advice, Pacific Region, Fisheries and Oceans Canada, Nanaimo, British Columbia, Canada. [Google Scholar]
- Department of Fisheries and Oceans Canada . 2017. A framework for identification of ecological conservation priorities for marine protected area (MPA) network design and its application in the Northern Shelf Bioregion. Canadian Science Advisory Secretariat Science Advice Report 2017/019. Centre for Science Advice, Pacific Region, Fisheries and Oceans Canada, Nanaimo, British Columbia, Canada. [Google Scholar]
- Engelhard, S. L. , Huijbers C. M., Stewart‐Koster B., Olds A. D., Schlacher T. A., and Connolly R. M.. 2017. Prioritising seascape connectivity in conservation using network analysis. Journal of Applied Ecology 54:1130–1141. [Google Scholar]
- ESRI Inc . 2016. ArcGIS Desktop 10.4.1. Redlands, California, USA.
- Fong, K. H. , and Dunham J. S.. 2007. Inshore Tanner Crab (Chionoecetes bairdi) biology in a central coast inlet, British Columbia, Canada. Journal of Shellfish Research 26:581–595. [Google Scholar]
- Freeman, L. C. 1977. A set of measures of centrality based on betweenness. Sociometry 40:35–41. [Google Scholar]
- Freiwald, J. 2012. Movement of adult temperate reef fishes off the west coast of North America. Canadian Journal of Fisheries and Aquatic Sciences 69:1362–1374. [Google Scholar]
- Friesen, S. K. , Martone R., Rubidge E., Baggio J. A., and Ban N. C.. 2019. Data from: an approach to incorporating inferred connectivity of adult movement into marine protected area design with limited data. Dryad Digital Repository. 10.5061/dryad.ts0jb2s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisk, M. G. , Jordaan A., and Miller T. J.. 2014. Moving beyond the current paradigm in marine population connectivity: Are adults the missing link? Fish and Fisheries 15:242–254. [Google Scholar]
- Fulton, E. A. , et al. 2015. Modelling marine protected areas: insights and hurdles. Philosophical Transactions of the Royal Society B 370:20140278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galpern, P. , Manseau M., and Fall A.. 2011. Patch‐based graphs of landscape connectivity: a guide to construction, analysis and application for conservation. Biological Conservation 144:44–55. [Google Scholar]
- Gilarranz, L. J. , Sabatino M., Aizen M. A., and Bascompte J.. 2015. Hot spots of mutualistic networks. Journal of Animal Ecology 84:407–413. [DOI] [PubMed] [Google Scholar]
- Gillanders, B. M. , Able K. W., Brown J. A., Eggleston D. B., and Sheridan P. F.. 2003. Evidence of connectivity between juvenile and adult habitats for mobile marine fauna: an important component of nurseries. Marine Ecology Progress Series 247:281–295. [Google Scholar]
- Government of Canada . 2011. National framework for Canada's network of marine protected areas. Department of Fisheries and Oceans Canada, Ottawa, Ontario, Canada. [Google Scholar]
- Grantham, H. S. , Moilanen A., Wilson K. A., Pressey R. L., Rebelo T. G., and Possingham H. P.. 2008. Diminishing return on investment for biodiversity data in conservation planning. Conservation Letters 1:190–198. [Google Scholar]
- Grantham, H. S. , Wilson K. A., Moilanen A., Rebelo T., and Possingham H. P.. 2009. Delaying conservation actions for improved knowledge: how long should we wait? Ecology Letters 12:293–301. [DOI] [PubMed] [Google Scholar]
- GRASS Development Team . 2017. Geographic Resources Analysis Support System (GRASS) Software. Open Source Geospatial Foundation; https://grass.osgeo.org [Google Scholar]
- Green, A. L. , Fernandes L., Almany G., Abesamis R., McLeod E., Aliño P. M., White A. T., Salm R., Tanzer J., and Pressey R. L.. 2014. Designing marine reserves for fisheries management, biodiversity conservation, and climate change adaptation. Coastal Management 42:143–159. [Google Scholar]
- Green, A. L. , Maypa A. P., Almany G. R., Rhodes K. L., Weeks R., Abesamis R. A., Gleason M. G., Mumby P. J., and White A. T.. 2015. Larval dispersal and movement patterns of coral reef fishes, and implications for marine reserve network design. Biological Reviews 90:1215–1247. [DOI] [PubMed] [Google Scholar]
- Grober‐Dunsmore, R. , Pittman S. J., Caldow C., Kendall M. S., and Frazer T. K.. 2009. A landscape ecology approach for the study of ecological connectivity across tropical marine seascapes Pages 493–530 in Nagelkerken I., editor. Ecological connectivity among tropical coastal ecosystems. Springer, Dordrecht, The Netherlands. [Google Scholar]
- Grüss, A. , Kaplan D. M., Guénette S., Roberts C. M., and Botsford L. W.. 2011a. Consequences of adult and juvenile movement for marine protected areas. Biological Conservation 144:692–702. [Google Scholar]
- Grüss, A. , Kaplan D. M., and Hart D. R.. 2011b. Relative impacts of adult movement, larval dispersal and harvester movement on the effectiveness of reserve networks. PLoS ONE 6:e19960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, G. J. A. , Ban N. C., Jones M. L., Kaufman L., Panes H. M., Yasué M., and Vincent A. C. J.. 2011. Hindsight in marine protected area selection: a comparison of ecological representation arising from opportunistic and systematic approaches. Biological Conservation 144:1866–1875. [Google Scholar]
- Hermoso, V. , Januchowski‐Hartley S. R., and Linke S.. 2015. Systematic planning of disconnection to enhance conservation success in a modified world. Science of the Total Environment 536:1038–1044. [DOI] [PubMed] [Google Scholar]
- Kaplan, D. M. , Botsford L. W., O'Farrell M. R., and Gaines S. D.. 2009. Model‐based assessment of persistence in proposed marine protected area designs. Ecological Applications 19:433–448. [DOI] [PubMed] [Google Scholar]
- Kivelä, M. , Arenas A., Barthelemy M., Gleeson J. P., Moreno Y., and Porter M. A.. 2014. Multilayer networks. Journal of Complex Networks 2:203–271. [Google Scholar]
- Krueck, N. C. , Ahmadia G. N., Green A., Jones G. P., Possingham H. P., Riginos C., Treml E. A., and Mumby P. J.. 2017. Incorporating larval dispersal into MPA design for both conservation and fisheries. Ecological Applications 27:925–941. [DOI] [PubMed] [Google Scholar]
- Lubchenco, J. , and Grorud‐Colvert K.. 2015. Making waves: the science and politics of ocean protection. Science 350:382–383. [DOI] [PubMed] [Google Scholar]
- Magris, R. A. , Pressey R. L., Weeks R., and Ban N. C.. 2014. Integrating connectivity and climate change into marine conservation planning. Biological Conservation 170:207–221. [Google Scholar]
- Magris, R. A. , Treml E. A., Pressey R. L., and Weeks R.. 2016. Integrating multiple species connectivity and habitat quality into conservation planning for coral reefs. Ecography 39:649–664. [Google Scholar]
- Magris, R. A. , Andrello M., Pressey R. L., Mouillot D., Dalongeville A., Jacobi M. N., and Manel S.. 2018. Biologically representative and well‐connected marine reserves enhance biodiversity persistence in conservation planning. Conservation Letters 11:e12439. [Google Scholar]
- Margules, C. R. , and Pressey R. L.. 2000. Systematic conservation planning. Nature 405:243–253. [DOI] [PubMed] [Google Scholar]
- Marine Plan Partnership for the North Pacific Coast . 2016a. MaPP Implementation Strategy 2015–2020. Marine Plan Partnership for the North Pacific Coast, North Pacific Coast, British Columbia, Canada. [Google Scholar]
- Marine Plan Partnership for the North Pacific Coast . 2016b. Regional action framework, Marine Plan Partnership for the North Pacific Coast, North Pacific Coast, British Columbia, Canada. [Google Scholar]
- Minor, E. S. , and Urban D. L.. 2007. Graph theory as a proxy for spatially explicit population models in conservation planning. Ecological Applications 17:1771–1782. [DOI] [PubMed] [Google Scholar]
- Minor, E. S. , and Urban D. L.. 2008. A graph‐theory framework for evaluating landscape connectivity and conservation planning. Conservation Biology 22:297–307. [DOI] [PubMed] [Google Scholar]
- Moffitt, E. A. , Botsford L. W., Kaplan D. M., and O'Farrell M. R.. 2009. Marine reserve networks for species that move within a home range. Ecological Applications 19:1835–1847. [DOI] [PubMed] [Google Scholar]
- O'Sullivan, D. , and Turner A.. 2001. Visibility graphs and landscape visibility analysis. International Journal of Geographical Information Science 15:221–237. [Google Scholar]
- Pe'er, G. , et al. 2014. Toward better application of minimum area requirements in conservation planning. Biological Conservation 170:92–102. [Google Scholar]
- Pilosof, S. , Porter M. A., Pascual M., and Kéfi S.. 2017. The multilayer nature of ecological networks. Nature Ecology & Evolution 1:101. [DOI] [PubMed] [Google Scholar]
- Pittman, S. J. , Monaco M. E., Friedlander A. M., Legare B., Nemeth R. S., Kendall M. S., Poti M., Clark R. D., Wedding L. M., and Caldow C.. 2014. Fish with chips: tracking reef fish movements to evaluate size and connectivity of Caribbean marine protected areas. PLoS ONE 9:e96028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Province of British Columbia . 2002. British Columbia Marine Ecological Classification: Marine ecosections and ecounits. Resources Information Standards Committee, Victoria, British Columbia, Canada.
- R Core Team . 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org [Google Scholar]
- Rechisky, E. L. , Stevenson C., Porter A. D., Welch D. W., Furey N. B., and Hinch S. G.. 2017. Telemetry‐based estimates of early marine survival and residence time of juvenile salmon in the Strait of Georgia and Queen Charlotte Strait Pages 196–201 in Chandler P., King S., and Boldt J., editors. State of the physical, biological and selected fishery resources of Pacific Canadian Marine Ecosystems in 2016. Canadian Technical Report of Fisheries and Aquatic Sciences 3225. [Google Scholar]
- Roberts, C. M. , et al. 2003. Ecological criteria for evaluating candidate sites for marine reserves. Ecological Applications 13:S199–S214. [Google Scholar]
- Robinson, C. L. K. , Morrison J., and Foreman M. G. G.. 2005. Oceanographic connectivity among marine protected areas on the north coast of British Columbia, Canada. Canadian Journal of Fisheries and Aquatic Sciences 62:1350–1362. [Google Scholar]
- Rocha, L. A. , Bass A. L., Robertson D. R., and Bowen B. W.. 2002. Adult habitat preferences, larval dispersal, and the comparative phylogeography of three Atlantic surgeonfishes (Teleostei: Acanthuridae). Molecular Ecology 11:243–252. [DOI] [PubMed] [Google Scholar]
- Sala, E. , Aburto‐Oropeza O., Paredes G., Parra I., Barrera J. C., and Dayton P. K.. 2002. A general model for designing networks of marine reserves. Science 298:1991–1993. [DOI] [PubMed] [Google Scholar]
- Sayles, J. S. , and Baggio J. A.. 2017. Social–ecological network analysis of scale mismatches in estuary watershed restoration. Proceedings of the National Academy of Sciences 114:E1776–E1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schill, S. R. , Raber G. T., Roberts J. J., Treml E. A., Brenner J., and Halpin P. N.. 2015. No reef is an island: integrating coral reef connectivity data into the design of regional‐scale marine protected area networks. PLoS ONE 10:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanafelt, D. W. , Salau K. R., and Baggio J. A.. 2017. Do‐it‐yourself networks: a novel method of generating weighted networks. Royal Society Open Science 4:171227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks, A. L. , Grantham B. A., and Carr M. H.. 2003. Propagule dispersal distance and the size and spacing of marine reserves. Ecological Applications 13:S159–S169. [Google Scholar]
- Tandy, C. R. 1967. The isovist method of landscape survey Pages 9–10 in Murray H. C., editor. Methods of landscape analysis. Landscape Research Group, London, UK. [Google Scholar]
- Treml, E. A. , and Kool J.. 2018. Networks for quantifying and analysing seascape connectivity Pages 293–318 in Pittman S. J., editor. Seascape ecology. John Wiley & Sons, Oxford, UK. [Google Scholar]
- Treml, E. A. , Halpin P. N., Urban D. L., and Pratson L. F.. 2008. Modeling population connectivity by ocean currents, a graph‐theoretic approach for marine conservation. Landscape Ecology 23:19–36. [Google Scholar]
- Urban, D. , and Keitt T.. 2001. Landscape connectivity: a graph‐theoretic perspective. Ecology 82:1205–1218. [Google Scholar]
- Walters, C. J. , Hilborn R., and Parrish R.. 2007. An equilibrium model for predicting the efficacy of marine protected areas in coastal environments. Canadian Journal of Fisheries and Aquatic Sciences 64:1009–1018. [Google Scholar]
- Watson, J. R. , Mitarai S., Siegel D. A., Caselle J. E., Dong C., and McWilliams J. C.. 2010. Realized and potential larval connectivity in the southern California bight. Marine Ecology Progress Series 401:31–48. [Google Scholar]
- Weeks, R. 2017. Incorporating seascape connectivity in conservation prioritisation. PLoS ONE 12:e0182396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J. W. , Schroeger J., Drake P. T., and Edwards C. A.. 2014. The value of larval connectivity information in the static optimization of marine reserve design. Conservation Letters 7:533–544. [Google Scholar]
- Wickham, H. 2016. ggplot2: Elegant graphics for data analysis. Springer‐Verlag, New York, New York, USA. [Google Scholar]
- Withler, R. E. , Beacham T. D., Schulze A. D., Richards L. J., and Miller K. M.. 2001. Co‐existing populations of Pacific ocean perch, Sebastes alutus, in Queen Charlotte Sound, British Columbia. Marine Biology 139:1–12. [Google Scholar]
- Zemeckis, D. R. , Liu C., Cowles G. W., Dean M. J., Hoffman W. S., Martins D., and Cadrin S. X.. 2017. Seasonal movements and connectivity of an Atlantic cod (Gadus morhua) spawning component in the western Gulf of Maine. ICES Journal of Marine Science 74:1780–1796. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The British Columbia Marine Conservation Analysis (https://bcmca.ca) data repository has links to the data custodians for the substrate and depth data sets used in this analysis (Appendix S1: Table S2); these custodians may be contacted directly. Data from the connectivity analyses are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.ts0jb2s (Friesen et al. 2019).


