Summary
Organ preservation and re‐conditioning using machine perfusion technologies continue to generate promising results in terms of viability assessment, organ utilization and improved initial graft function. Here, we summarize the latest findings and study the results of ex‐vivo/ex‐situ hypothermic (HMP) and normothermic machine perfusion (NMP) in the area of abdominal organ transplantation (kidney, liver, pancreas and intestine). We also consider the potential role of normothermic regional perfusion (NRP) to re‐condition donors after circulatory death organs before retrieval. The findings from clinical studies reported to date suggest that machine perfusion will offer real benefits when compared with conventional cold preservation. Several randomized trials are expected to report their findings within the next 2 years which may shed light on the relative merits of different perfusion methods and could indicate which perfusion parameters may be most useful to predict organ quality and viability. Further work is needed to identify composite endpoints that are relevant for transplanted organs that have undergone machine preservation. Multi‐centre trials to compare and analyse the combinations of NRP followed by HMP and/or NMP, either directly after organ retrieval using transportable devices or when back‐to‐base, are needed. The potential applications of machine preservation technology beyond the field of solid organ transplantation are also considered.
Keywords: hypothermic, normothermic, organ preservation, regional perfusion, transplantation
Introduction
The organ preservation process is a fundamental part of transplantation and has been the focus of research for over half a century. It is broadly defined as “the process by which organs are kept viable outside of the organism from which they were removed” in which the preservation method should ideally mimic the natural state within the organism 1. In the context of transplantation, preservation is required to maintain the quality of organs from the point at which they are removed from the donor, during storage and transportation, until transplantation into the recipient 2. These preservation methods include static cold storage (SCS) and more recently, a variety of dynamic perfusion techniques have come to the fore (Fig. 1).
Figure 1.
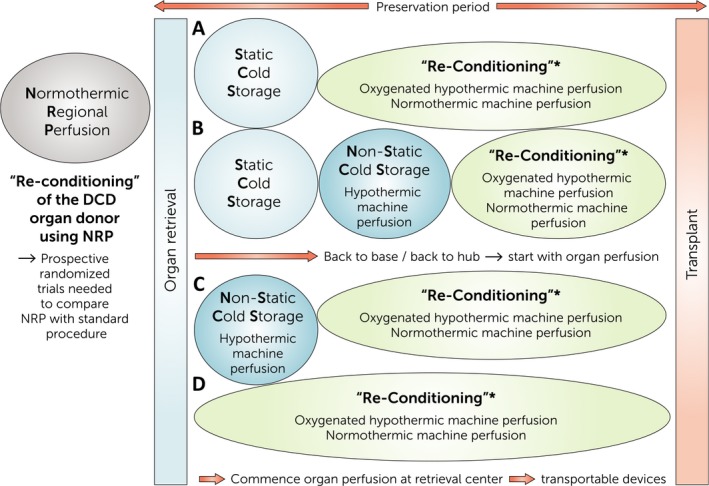
Future aspects of organ preservation and re‐conditioning with several possibilities to arrange preservation methods/technologies currently used clinically. Prospective randomized multicentre trials are needed to compare strategies (a–d) in regard to their effects and transplant outcomes. In addition, trials comparing (a–d) will deliver evidence if there are significant advantages/disadvantages of starting preservation using perfusion devices immediately after organ retrieval or in a back‐to‐base/hub approach following static cold storage. *Re‐Conditioning offers viability assessment of the organ, treatment of the organ with supplemental biologicals and stem cells as therapeutic agents. Pharmacological testing can be undertaken. The treatment to be applied or the drug/substance to be tested will determine if oxygenated hypothermic machine perfusion is enough or if a nearly physiological environment at 37 °C is needed.
The terms “organ perfusion” and “re‐conditioning” when applied to preservation, inherently emphasize the utilization of nonstatic preservation methods rather than counting merely on the current standard of care, SCS.
The idea of perfusing organs with a perfusate composed with the intention of mimicking the physiological environment of the body is not novel itself. The recent resurgence of normothermic organ preservation techniques represents the revival of techniques used to keep organs alive in the early days of transplantation. Long before the first attempts of clinical organ transplantation and before even the development of SCS techniques, preservation was already the main focus of transplantation science. In the early 19th century, Le Gallois philosophized whether it would be feasible to replace the human heart with a form of injection using a substitute for arterial blood and thus effortlessly maintain active metabolism in the entire body for an indefinite period of time 3. Since these early endeavours, the 21st century transformation of preservation techniques has progressed in the reverse sequence to its 20th century trajectory. During the 20th century, whilst solid organ transplantation was becoming a clinical reality, efforts were made to evolve the more complex technology of ex vivo normothermic machine perfusion (NMP), first applied by Carrel and Lindbergh 4 to a technology offering the advantages of simplicity, permanent availability and cost‐effectiveness. Normothermic organ perfusion from the 1930s was replaced by Belzer's simpler hypothermic machine perfusion (HMP) technology in the early 1960s 5, before Collins et al. 6 published a method to transport kidneys on ice using a preservation solution later in the same decade. This heralded the era of SCS and the icebox emerged as the storage vessel, ubiquitous to organ transplantation. The implementation of SCS enabled satisfactory results after solid organ transplantation, with relative ease and low cost. However, although SCS has been acceptable and yielded good outcomes for high quality organs, its use has been limited in the context of higher‐risk or marginal organs. During the hypoxic conditions of SCS, anaerobic metabolism continues leading to accumulation of metabolites which contribute later to the occurrence of ischaemia‐reperfusion injury (IRI) after reperfusion of the organ in the recipient 7, 8. This is the key factor limiting the applicability of SCS in marginal organs. In the 21st century, an ageing donor population with increasing co‐morbidities, has resulted in an inevitable increase in marginal organs in the donor pool. This has necessitated a re‐consideration of past preservation technologies; to enhance the preservation of these marginal organs and reduce the IRI to which they are particularly susceptible. The need to transplant higher‐risk organs in larger numbers has revitalized research on alternative preservation methods and brought machine perfusion back to the fore. A landmark study for HMP in deceased‐donor kidney transplantation was published by Moers et al. 9 in 2009 and this heralded a new era in organ preservation and machine perfusion. Subsequently, normothermic preservation has been explored, with the added advantage of negating cold ischaemia time (CIT) which remains an important risk factor for adverse outcomes. Normothermic preservation entails several concepts: the organ is continuously perfused with oxygenated blood/oxygen carrier, medications and nutrients at body temperature during storage (+/− transport). This has the following advantages: (i) enabling normal cellular metabolism with recovery of cellular energy status; (ii) allowing repair of reversible injury; (iii) facilitating functional testing of the organ before transplantation through the measurement of perfusion and biochemical parameters during preservation. Within the field of NMP, a milestone was achieved when researchers tested the technique in liver transplantation head‐to‐head against SCS, in the first randomized clinical trial of its kind, with the results published in spring 2018 10.
This review provides an overview of the current state of research with regard to abdominal allotransplantation and provides a perspective on the potential for organ perfusion, preservation and re‐conditioning for the future.
Perfusion and re‐conditioning methods for abdominal organs
Prior to organ recovery: normothermic regional perfusion in the organ donor
To improve the outcomes from organs obtained from controlled and uncontrolled donors after circulatory death (DCD), abdominal normothermic regional perfusion (NRP) was introduced as a method of re‐conditioning organs in the donor prior to explantation. It has been proposed that restoring blood flow (using ECMO‐technology) before organ recovery in DCD donors is more beneficial than conventional rapid recovery techniques to counteract ischaemic damage and improve the recipients’ outcome. The NRP‐team in the United Kingdom published their first experience in 2014 11. A period of NRP in the donor with the aim of reversing the detrimental effects of warm ischaemia was established after asystole via aortic and caval cannulation and was maintained for 2 h. Forty‐nine patients were transplanted including 32 kidney transplants, 11 liver transplants, two combined pancreas‐kidney transplants, one islet transplant and three double lung transplants. The authors concluded that NRP facilitated organ recovery 11. The same group built upon this initial encouraging experience and recently published the outcomes from 43 livers transplanted after NRP in controlled DCD donation 12. Compared to a contemporaneous control group, livers subjected to NRP demonstrated a significant reduction in ischaemic cholangiopathy (with no cases in the 43 NRP livers) and a strong trend towards increased graft survival. In 2017, the Spanish group reported their first experience with at least 60 min NRP in controlled DCD donation. They transplanted 55 organs, with a 1‐year death censored kidney survival rate of 91%. The 1‐year liver survival rate was 90.1% and as observed by the UK group, there were no cases of ischaemic cholangiopathy. The recovered and transplanted lungs and pancreata developed primary organ function. Hessheimer et al. 13 compared NRP with super‐rapid recovery in controlled DCD liver transplantation in an observational cohort study including all controlled DCD liver transplants performed in Spain between June 2012 and December 2016. This study highlighted the superiority of NRP compared to a super‐rapid recovery procedure with reduced biliary complications and graft loss from livers transplanted in the NRP cohort 13. The Spanish group also published their experience of more than 500 kidney transplants from uncontrolled DCD donors. These analyses also showed an improved graft survival after using NRP or hypothermic regional perfusion and preferable to in situ cooling of kidneys from uncontrolled DCD donors. In a prospective study by Demiselle et al. 14, NRP in uncontrolled DCD donation led to a lower DGF rate and a better renal graft function 2 years after transplantation compared with cold stored DCD kidneys and extended criteria DBD kidneys.
In order to explore a standardized NRP approach and protocol, French researchers recently published their experimental work. Kerforne et al. performed NRP for 2, 4 or 6 h in a DCD porcine model and compared it with a control group without NRP. Before being transplanted, the kidneys underwent HMP. During HMP, NRP kidneys showed clearly lower intrarenal resistance. Kidneys transplanted after 4 and 6 h of NRP displayed better function than the control group 15.
Clinically, for the future of NRP in solid organ transplantation, prospective randomized trials are of utmost importance. The technique is often discussed as very encouraging and has been used widely clinically for several years. However, in order to clearly establish the extent of any benefit and determine if there is an option to use the technique for treating the donor to avoid or to reduce the damage caused by IRI in the recipient, prospective randomized trials for several organ groups are needed. To gain the most information, groups should include NRP and no‐NRP followed by either SCS, HMP or NMP. Depending on the experience with NRP and organ perfusion post organ recovery, a planned comparison of NRP and no‐NRP in liver and pancreas transplantation, for example, would be a good initiative to start.
After organ recovery: ex‐situ kidney preservation
Moers et al. 9 pioneered the use of HMP, without oxygen supplementation, in kidney transplantation in 2009. They demonstrated that the rate of DGF was significantly lower and 1‐year graft survival was significantly higher after HMP compared with SCS. This finding was exclusive of donor type in their analysis 9. This trial was followed immediately by HMP applied solely to DCD kidneys by Jochmans et al. 16. The trial revealed that HMP reduced the rate of DGF and produced better kidney function up to 1 month after transplantation although the long‐term outcome was not influenced. Therefore, it is advisable to store DCD kidneys dynamically, nonstatic, by HMP instead of SCS 16. To complete the circle, a prospective investigation was performed to analyse the impact of HMP on extended criteria DBD kidneys. Surprisingly, in this cohort, HMP did not show a significant impact on the occurrence of DGF. In fact, “traditional” markers such as CIT, re‐transplantation and duration of dialysis were independent factors influencing initial function after transplanting an ECD kidney 17. However, despite the small numbers there was a significant advantage for ECD kidneys with regards to 1‐year survival, especially in those which developed DGF after HMP compared with DGF after SCS 17. The trial by Treckmann et al. provided an insight into the importance of CIT in the field of HMP and was strengthened with the study results from Kox et al. 18 who investigated the benefits of HMP and short CIT in deceased donor kidney transplantation. The publication revealed that HMP is beneficial, with a significantly lower DGF rate, in kidneys with a CIT shorter than 10 h but this positive effect diminishes with the prolongation of CIT 18. However, CIT still remains one of the most powerful, independent and relevant risk factors for DGF even after dynamically cold stored deceased donor kidneys by using HMP.
The duration of CIT and its impact are precisely the target where NMP could, potentially, make a major impact. The clinical and experimental experience gained so far have shown promising results suggesting that NMP is a viable alternative to SCS. The clinical evidence demonstrating the superiority of NMP to SCS in kidney preservation is primarily based upon clinical and experimental studies from Hosgood and Nicholson from the University of Cambridge who have pioneered this field 19, 20, 21, 22, 23, 24. Their team has, over the last decade, developed the concept of a period of normothermic perfusion in kidney transplantation at the end of a period of cold ischaemia. Hosgood and Nicholson apply 1 h of NMP to kidneys after SCS, thereby restoring function and improving early graft function of extended criteria donor kidneys. Their first clinical trial resulted in a significantly lower DGF rate in normothermically preserved kidneys compared with SCS allografts 19. They are currently leading a large multi‐centre trial in the United Kingdom to formally investigate the impact of normothermic perfusion on delayed graft function in a trial of DCD kidneys 25. The decision to transplant an NMP‐kidney is based upon a scoring system developed by Hosgood and Nicholson 26. The score consists of three parameters: macroscopic assessment of the kidney; renal blood flow and total urine output. The score comprises five levels of kidney quality; 1 the best and 5 the worst quality. The scoring 1–5 is a composite of the main parameters which are: (i) macroscopic assessment of the kidney: excellent perfusion (1 point), moderate perfusion (2 points), poor perfusion (3 points); (ii) renal blood flow (ml/min/100 g): ≥50 (0 points), ≤50 (1 point); (iii) total urine output (ml): ≥43 (0 points), ≤43 (1 point) 26, 27.
For clinical transplantation, no prolonged normothermic kidney preservation method has yet been implemented. Encouraging data about long‐term NMP in the kidney have been reported by Selzner's group at the University of Toronto using an experimental DBD and DCD porcine model. They demonstrated that longer periods of up to 16‐h of NMP are superior to both SCS alone and short‐duration NMP in terms of both tubular injury and post‐transplant organ function 28, 29, 30, 31, 32, 33.
The first long‐term NMP preservation studies on human kidneys (not transplanted) were published by Weissenbacher et al. 34 in spring 2018. The Oxford research group published the feasibility of 24‐h NMP using a fully automated, transportable preclinical prototype for normothermic kidney preservation. Kidney parenchyma quality was maintained or even improved over 24 h of NMP. Urine recirculation was applied to maintain volume ionic homeostasis of the perfusate and volume of the blood‐based perfusion solution 34.
The future of kidney perfusion and re‐conditioning is multifarious. Hypothermic machine perfusion will have its place undoubtedly; it decreases DGF rates and ECD and DCD kidneys seem to benefit from HMP up to 1 year. However, the CIT must be kept short.
The remaining question is how to optimally place HMP within the entire process of kidney preservation, most likely in combination with NRP or followed by NMP. According to experimental work published by the Clavien group 35, rodent DCD kidneys treated with oxygenated HMP (HOPE) showed drastically better function after transplantation than SCS grafts with regards to nuclear injury, macrophage activation, endothelium activation, tubular damage and graft function. Schlegel and Dutkowski 35 concluded that the improved outcome after transplantation was because of oxygen as a supplement during HMP. Kron et al. 36 developed the HOPE‐perfusion model in the rodent further and showed for the first time a beneficial effect of HOPE on the T‐cell immune response following experimental kidney transplantation. Whether oxygenated HMP will change the clinical world of kidney preservation should be reported in the near future. The results of two prospective trials conducted by the Consortium for Organ Preservation in Europe (COPE), are due to be presented in 2019 37. The “POMP” study addressed oxygenated HMP re‐conditioning of ECD kidneys after SCS. The “COMPARE” trial is designed to compare oxygenated HMP of Maastricht III DCD kidneys (50 years or older) with nonoxygenated HMP 37, 38.
Independent of the impact HMP has made to date, improvement of utilization of marginal kidneys, including those from DCD donors, has remained elusive. This is where NMP may have most potential. As previously described, NMP offers the possibility of assessing kidney function ex‐situ under near‐physiological conditions enabling viability assessment to be performed in real‐time during preservation. Moreover, CIT stops as soon as the organ is perfused at 37 °C and oxygenated at physiological levels. Eliminating CIT and its detrimental effects on graft outcome with the option to assess viability may aid clinical decision making regarding the “transplantability” of an organ.
Normothermic organ perfusion may address many contemporary challenges in transplantation. Transplant logistics will become more demanding in future as more organs are transplanted into more complex recipients (both surgically and immunologically) within more stringent working regulations. For these reasons, not only are transplant professionals and researchers interested in NMP, but also hospital management may endorse this technology which could offer a shift away from out of hours operating and allow enhanced operative planning. These implications of NMP in routine clinical practice are of considerable interest.
The introduction of novel agents during NMP may also impact on other emerging issues in transplantation: repair of injured organs and immunomodulation of the donor kidney. Machine‐based normothermic perfusion could be effective in the treatment and repair of damaged kidneys before transplantation as it offers a near‐physiological environment. In addition, it could be a platform to study the immunogenicity of an organ and to treat it according to the recipient's sensitization status. Approaches like this will require the application of biological molecules, stem cells or genes. In relation to IRI, NMP might serve as an optimal system to prevent this damaging cascade of molecular events with targeted, drug‐loaded nanoparticles for example 39.
For centres performing autotransplantations of kidneys after removing malignancies from native kidneys, removing ureteral pathologies or restoring a functioning renal anatomy, NMP could be of particular interest as the back‐table reconstruction after tumour removal could be done under physiological circumstances and allow assessment of the perfusion characteristics of the remodelled kidney as well as to prevent IRI in the recipient of the autotransplanted organ.
Another area of interest which should be highlighted along with liver NMP is the pharmacological testing of newly developed drugs and the pretransplant treatment of the organ with stem cells. In future, it could be possible to replace phase‐1 clinical trials in healthy subjects by perfusing kidneys and livers on NMP devices with the possibility to test the biology of the drug and its pharmacokinetics meticulously in tissue, the perfusate and in the urine/bile at the same time at multiple time points.
To go even a step further, having an organ “alive” ex situ opens up new vistas regarding treating neoplasms of the organ in the meaning of individualized and absolutely locally applied chemotherapeutics to improve action efficacy of the drug and to minimize systemic side effects in the patient.
Preservation of the liver
In recent years, liver transplantation has benefited from a rapid and impressive development in machine perfusion technologies. Machine perfusion has become a well‐established method of organ preservation in liver transplantation, largely driven by the need to increase organ utilization and improve outcomes from marginal organs. This is of great importance when considering a 12–20% waiting list mortality 40, 41, 42 and an increasing proportion of marginal organs in the donor pool 43. Furthermore, mitigating the risks associated with DCD liver transplantation, particularly ischaemic cholangiopathy 44, are of high priority.
Normothermic machine perfusion has been the subject of most interest in liver transplantation 10, 45, 46, 47, 48, 49 and recently, Jassem et al. 50 have provided a much needed mechanistic insight into NMP in liver transplantation by demonstrating an altered gene expression profile in NMP compared with cold‐stored livers from pro‐inflammation to pro‐healing and regeneration. It's safety and feasibility was first demonstrated in 2016 by the Oxford group 45 and the first randomized controlled trial by the same group was recently published 10. In this trial by Nasralla et al., outcomes from 121 NMP and 101 SCS livers were compared. The trial met its primary outcome, demonstrating a significant reduction in recipient peak serum aspartate aminotransferase (AST) in the NMP group (a surrogate marker for long term patient and graft survival 10, 51). The improvement in early biochemical function in NMP livers was in the context of significantly longer preservation times; indeed one of the benefits of NMP is extended graft preservation 52. As with the kidney, prolonged preservation could improve operating room logistics and may also provide the potential to provide liver‐directed therapeutic interventions to further enhance the graft during preservation. Another important finding was that of increased organ utilization with a 50% lower discard rate in the normothermic group 10. This is most likely attributable to the ability to obtain an objective assessment of liver function during NMP, increasing the surgeon's confidence in implanting higher‐risk livers. Viability assessment during NMP has been explored by both the Birmingham and Cambridge groups 49, 53. Lactate clearance, glucose metabolism, pH maintenance, bile production and transaminase levels during NMP have been postulated to predict post‐transplant outcome 54. The Cambridge group have most recently explored bile composition and demonstrated that bile pH, glucose and bicarbonate concentrations are more predictive of biliary complications than the volume of bile produced 49. These findings were recently corroborated by Matton et al. 55. The criteria adopted by the Cambridge group 49, based on their extensive experience in perfusing high‐risk livers, are summarized in Table 1.
Table 1.
The ‘Cambridge Criteria’ of variables associated with successful transplantation of normothermic perfused livers
| Maximum bile pH > 7.5 |
| Bile glucose concentration ≤3 mmol/l or ≥10 mmol/l less than perfusate glucose |
| Able to maintain perfusate pH > 7.2 without >30 mmol bicarbonate supplementation |
| Falling glucose beyond 2 h or perfusate glucose under 10 mmol/l which, on challenge with 2.5 g glucose, does subsequently fall |
| Peak lactate fall ≥4.4 mmol/l/kg/h |
| Alanine aminotransferase (ALT) <6000 U/l at 2 h |
Although formal viability criteria are yet to be validated, NMP's role in increasing liver utilization has been formally assessed by the Birmingham group. All livers declined by the seven UK transplant centres, that met the inclusion criteria, were perfused and transplanted if functional criteria were fulfilled 53. This study aimed to assess the organ recovery rate which can be achieved by NMP with the combined end‐point of success rate of NMP to produce a transplantable liver and 90‐day patient survival 53.
As NMP has now become an accepted method of liver preservation and is a well‐established practice in several centres, exploiting the technology to further enhance its potential in re‐conditioning and enhancing organs should be explored. Steatotic livers are associated with poorer outcomes following transplantation owing to their increased susceptibility to IRI 56, 57, 58. It is estimated that steatosis exists in 13–28% of deceased donor livers 59 and therefore salvaging these livers for transplantation may help bridge the gap between organ supply and demand. NMP provides the potential to reduce IRI by avoiding the deleterious effects of cooling possibly by inhibiting inflammation and promoting graft regeneration 50. NMP also provides a platform to treat the liver ex situ in an attempt to remove the fat during preservation which may further improve outcomes. Few studies have explored the structural and functional effects of machine perfusion on steatotic livers. Jamieson et al. 60 explored the effects of NMP on steatotic porcine livers. In this study, the authors observed a reduction in steatosis from 28% to 15% over the course of the 48 h perfusion and also observed a decrease in lipid droplet size 60. Nagrath et al. 61 tested the effect of several de‐fatting agents on normothermically perfused fatty livers isolated from Zucker rats. They observed a 65% reduction in hepatic triglyceride content after only 3 h of perfusion 61. Although the ability to reduce liver fat appears to be possible in animal models 60, 61, 62, where steatosis has been artificially induced for experimental purposes, it is unclear whether these findings are applicable to steatotic human livers; the limited available data would suggest not. Liu et al. 63 perfused 10 discarded livers with variable degrees of baseline steatosis for 24 h and demonstrated a significant increase in perfusate triglyceride levels over the duration of the perfusion but did not observe any reduction in steatosis 63. Banan et al. 64 have reported results from two human livers which were preserved normothermically with the addition of de‐fatting agents, namely l‐carnitine and exendin‐4. However, in the two treated livers, only one showed a minimal reduction in the degree of macrovesicular steatosis (10%) after 8 h NMP 64. In order to further explore NMP's role in liver de‐fatting, a better understanding of the pathways of human liver fat metabolism which could be manipulated are required and it may be necessary to perfuse livers for longer.
The Toronto group demonstrated significantly increased uptake of Miravirsen [an oligonucleotide which inhibits hepatitis C virus (HCV) replication] in porcine livers preserved via NMP compared with cold storage 65. This fascinating work suggests that Miravirsen administration during NMP could be a potential strategy to prevent HCV reinfection after liver transplantation. Gene therapy is another exciting avenue which has been explored to enhance the graft in the context of attenuating IRI 66. RNA interference (RNAi) is a naturally occurring mechanism which downregulates gene expression by specifically targeting messenger RNA (mRNA) transcripts 67. Administering RNAi therapeutics during NMP could potentially down‐regulate IRI‐associated pathways during preservation, removing the need to treat the donor (with its associated limitations). This method is likely to be less expensive, with a lower dose required to be administered to a single organ. Furthermore, one can be confident that the therapeutic intervention can be successfully delivered to the target organ, without other systemic uptake and associated effects. Thijssen et al. 66 have demonstrated that small interfering RNA, together with lipid‐based nanoparticles, successfully silenced the apoptotic gene p53 in a murine NMP model.
Hypothermic machine perfusion in liver transplantation has also shown promising results 68, 69, 70, 71. Guarrera et al. 68 transplanted 31 extended criteria livers preserved via nonoxygenated HMP and showed significantly fewer biliary complications and a significantly shorter hospital stay compared with matched SCS controls. Dutkowski et al. 69 also reported a significant reduction in ischaemic cholangiopathy and a significant improvement in graft survival in DCD livers undergoing oxygenated HMP compared with matched SCS controls. However, in this study, the control group had a high incidence of ischaemic cholangiopathy (22%) and poor 1‐year graft survival (69%) 69. Interestingly, van Rijn et al. 70 demonstrated an 11‐fold increase in cellular ATP during 2 h of oxygenated dual HMP (arterial and portal perfusion) which was associated with improved early biochemical function compared with SCS controls. The most recent report by Schlegel et al. 71, 72 demonstrated excellent 5‐year graft survival (94%) in DCD livers preserved via oxygenated HMP. Nonanastomotic biliary strictures were identified in 8% of HMP grafts compared with 22% of SCS controls 71. These studies, therefore, demonstrate the potential for HMP to improve post‐transplant outcomes in higher risk grafts. However, these studies are small and are limited by the use of retrospective matched control groups. Results from prospective randomized controlled trials are awaited and a head‐to‐head comparison of liver HMP ± oxygen with liver NMP would be of particular interest to the entire field. It may, however, be important to combine HMP and NMP to optimize outcomes as suggested by Boteon et al. who demonstrated in a discarded human liver model that livers benefited from 2 h of hypothermic oxygenated machine perfusion (HOPE) before NMP. Although these livers were not transplanted, HOPE + NMP livers achieved lower tissue expression markers of oxidative injury and inflammation as well as enhanced metabolic recovery compared with livers subjected directly to NMP 73.
The potential role of HMP in assessing liver viability during preservation remains unproven although Guarrera et al. 68 have shown that recipient AST correlated with 2‐h effluent AST, ALT and lactate dehydrogenase. Hypothermic machine perfusion may not be as effective in delivering ex situ therapies during preservation. This has certainly been demonstrated in the quest for liver de‐fatting; Liu et al. 74 explored the de‐fatting potential of machine perfusion at sub‐normothermic temperatures (20 °C) and no significant changes in steatosis were observed. This finding was corroborated in a murine model, and findings suggested that machine perfusion's de‐fatting potential was temperature‐dependent, favouring normothermia 62.
This is an exciting time for liver transplantation, where dynamic perfusion technologies are already demonstrating a transformative, beneficial impact. In the not too distant future, we will hopefully have a better understanding for the precise roles of both NMP and HMP once the results from more trials become available.
Perfusion and preservation of the pancreas after organ retrieval
There is growing interest to implement perfusion and re‐conditioning techniques in to the preservation process for pancreatic allografts. In the pancreas transplant community this is happening for a slightly different reason compared with the liver and kidney transplant area. In the field of pancreas and islet transplantation, donor organ shortage is not the major issue. The limiting factors are the assessment of the organ prior to transplantation with regard to steatosis and the likelihood of post‐transplant pancreatitis and to estimate the β‐cell viability and function of the organ.
Hamaoui et al. 75 demonstrated the feasibility of pancreatic hypothermic machine perfusion in a small number of porcine and human pancreata. They compared SCS preserved organs with those which underwent 5 h of HMP after SCS. After HMP, perfusion flow indices were stable throughout viability assessment of the organ using oxygenated normothermic reperfusion, whereas the flow indices deteriorated in SCS‐only grafts. Insulin secretion representing β‐cell function was measurable in the porcine and the human model. Exocrine function could, however, be detected only in human grafts. The authors concluded that HMP could be beneficial in improving pancreas preservation 75. The French group of Branchereau et al. 76 perfused discarded human pancreata in a pulsatile fashion hypothermically for 24 h. Seven pancreatic allografts underwent hypothermic pulsatile perfusion and were monitored including duodenal and pancreas‐parenchyma biopsies throughout the perfusion duration. The most encouraging findings from a clinical perspective, was the complete absence of oedema of the perfused organ at any time during HMP, decreasing resistance indices and normal stainings for insulin, glucagon and somatostatin suggesting maintained parenchyma quality 76. Leemkuil et al. also investigated HMP in discarded human pancreata with the addition of oxygenating the perfusate. Overall, five DBD and five DCD pancreata were preserved by oxygenated HMP for 6 h. The pancreata were perfused homogenously and no signs of cellular injury or oedema were detected. After HMP, islets of Langerhans could be isolated which showed good viability and function 77. Another option to shorten standard SCS and to minimize its deleterious effect would be oxygen persufflation as published by Kelly et al. 78. When this research group compared persufflated pancreata with SCS‐only pancreata, the islets had higher glucose‐stimulated insulin secretion and reduced inflammatory responses 78. In summary, the evidence to date suggests that HMP for pancreatic allografts results in improved β‐cell function with the limitation that none of these perfused organs were transplanted and CIT, one of the factors thought to increase graft pancreatitis, is still not able to be extended.
The only option to shorten CIT and possibly reduce IRI with all its associated complications such as peri‐pancreatic fluid collections, vascular thrombosis, bleeding and risk of graft loss, is NMP of the pancreas. So far, only Barlow et al. 79 demonstrated the feasibility of ex situ pancreas NMP in which they could correlate amylase levels with fat infiltration of the organ and exocrine function. However, this approach needs more refinement and is not yet ready for clinical application 80. For future research and clinical progression, it may be important to invest in the normothermic preservation technology as has been done in liver and kidney transplantation. Attempts thus far have not shown much promise, most likely because of the susceptibility of the pancreatic parenchyma to ischaemic injury and the ensuing pancreatitis that follows as well as the (vascular) anatomy of the organ and the low‐flow situation. Nevertheless, with the future development of portable NMP devices delivering optimal temperature and oxygen, CIT can be minimized as the organ does not need to travel. Another important step would be a solution to deal with the exocrine component of the pancreas as this seems to be one of the limiting factors. The huge advantage of an “actively working” pancreas on an NMP circuit would be facilitating the treatment of the organ prior to transplantation instead of treating the recipient. One drug‐group could the family of protease inhibitors which are still controversially discussed for the treatment of pancreatitis; NMP offers an optimal platform and the opportunity to test their effect with the aim to avoid pancreatitis after transplant in the recipient.
Preservation of the intestine
Similar to pancreas transplantation, in the field of intestinal transplantation we are not fighting against a shortage of donor organs. The main issue remains to find the immunological balance between rejection and infection and to deal with the post‐transplant difficulties induced by IRI. The intestine is a pretty unique, hollow and contaminated abdominal organ, where IRI, with the subsequent mucosal barrier damage, can trigger bacterial translocation, as well as rejection 81. Intestinal teams strive to maintain short CIT (below 10 h); however, as shown by Tesi et al. 82, 83, mucosal injury is already evident within 1 h of cold storage and progresses fast to subepithelial oedema (4 h). Despite the detrimental early effects of IRI to the intestine, little innovation has been achieved over the years to minimize IRI. Currently, the standard of practice involves in situ vascular flush with either UW or HTK, followed by SCS. UW and HTK have been found to be favourably comparable in terms of function, graft and patient survival, in the intestinal transplant setting with fairly short CIT and are both used equally 84.
Intraluminal preservation solutions before SCS have been introduced in an attempt to reach and protect the enterocytes and theoretically prevent fluid and electrolyte shifts. The luminal membrane can be used for the uptake of nutrients and electrolytes and the intestinal lumen provides direct access 85. The optimal content of luminal preservation solution remains unclear 86. UW has been used for both vascular and luminal preservation with promising results 87 however, there have been concerns regarding the safety of such a potassium rich solution in clinical practice. Roskott et al. 88 used WME plus (Williams Medium E plus additional buffering, impermeants and a colloid) for luminal preservation in a rodent model and found reduced preservation and reoxygenation injury, by assessing the histomorphologic integrity, ATP levels, and mRNA expression of several stress‐responsive genes. The Swedish group have been working on intraluminal preservation solutions in rodents and have, most recently, used polyethylene glycol (PEG 3350) and amino acid (l‐glutamine) solutions. They found that PEG solutions improved the mucosal morphology, maintained the tight junction structure longer (14 h) and preserved the mucin stores in the mucosal goblet cells. They speculated that PEG act as protective coating for the luminal membrane and prevent the degradation of tight junctions by the luminal proteases. Glutamine, which has previously been found to protect cell lines by modulating intracellular pathways and inhibiting apoptosis, was rapidly absorbed by the enterocytes 86, 89.
Intraluminal gaseous insufflation has been attempted with oxygen, carbon monoxide, hydrogen and nitrogen. Oxygen insufflation was found to improve tissue energetics; however, mucosal integrity was superior with only a brief 1‐h period of luminal perfusion. The authors suggested that there could be a delicate balance between the luminal delivery of oxygen and the physical injury incurred as a direct result of mechanical perfusion 90. Nakao et al. used CO supplementation to the UW intraluminal solution in a rodent model and demonstrated ameliorated IRI. The authors suggested that the soluble form of CO in UW preservation solution would minimize the concerns about possible toxicity induced by in vivo CO inhalation 91. Hydrogen‐enriched preservation solutions (UW and lactated Ringers) were used by Buchholtz et al. and proved to significantly ameliorate graft damage and ultimately facilitate recipient survival. Mechanistically, hydrogen appreciably reduced graft oxidative stress, maintained immune homeostasis and limited proinflammatory molecular responses. Nitrogen‐enriched solutions were used by the same group and did not show any improvement compare to the control group 92.
The Japanese group from Kobe, have developed a hybrid method by utilizing perfluorocarbons (PFC), which carry and release high amounts of oxygen. The group uses a cavitary 2‐layer method (cTLM), which they developed as a pancreas preservation technique, and first successfully achieved 24‐h safe preservation time in a canine model 93. Following this success, they added glutamine to their cTLM model and achieved a remarkable 40‐h preservation cold storage.
Most recently, the Yale‐New Haven group developed an extracorporeal hypothermic dual perfusion device (vasculature and intestinal lumen) and published their experience of fie human intestines, in which they used either UW or HTK as preservation solutions. They performed this project as proof of concept that extracorporeal intestinal perfusion is feasible and achieved viability of human intestine, and favourable histopathologic evaluation of perfused intestine 94.
It is becoming increasingly evident that current practice is being hindered by the limitations of intestinal SCS. A variety of animal research projects have shown promising results so far. The refinement of current intraluminal preservation techniques or the development of new concepts for the intestine, such as subnormothermic or normothermic preservation, following their success in other organs, might result into clinical translation.
Conclusion and perspectives
After many decades with little change, the field of organ preservation is experiencing a resurgence of interest. Greatest progress has been made in the field of kidney and liver transplantation although there is still great scope for further improvements. For pancreas and intestinal transplantation, we still have to work on the refinement of HMP and NMP techniques with the logical consequence and indisputable need of prospective clinical trials and mechanistic studies. Within the areas of kidney and liver perfusion/preservation we can expect the development of a parallel stream, independent from organ remodelling and re‐conditioning for transplantation. This evolution will go towards pharmacological testing including drug delivery and individualized treatment, ex vivo high‐precision tumour targeting 95 to avoid systemic therapies of the patient and will build the basis for studies and analyses in several areas of medicine dealing with metabolic disorders and diseases. Prospective clinical trials combining and comparing several preservation techniques are desperately needed.
The procedure in the donor, NRP, prior to organ recovery, will be either the link between several preservation techniques or used to antagonize factors and mediators harming the organ because of the process of death in both DBD and DCD.
Funding
The authors have declared no funding.
Conflicts of interest
The authors have declared no conflicts of interest.
References
- 1. Organ Preservation [Internet]. Defined Term – A dictionary of defined terms for the legal profession. [Cited 2018 Dec 26]. Available from: https://definedterm.com
- 2. Organ Preservation (definition) [Internet]. [Cited 2018 Dec 26]. Available from: http://www.reference.md/files/D009/mD009926.html
- 3. Legallois C. (1770–1814) A du texte. Expériences sur le principe de la vie, notamment sur celui des mouvemens du coeur, et sur le siège de ce principe ; suivies du Rapport fait à l'Institut sur celles relatives aux mouvemens du coeur, par M. Le Gallois,… [Internet], 1812. [Cited 2018 Aug 15]. Available from: https://gallica.bnf.fr/ark:/12148/bpt6k1511249w
- 4. Carrel A, Lindbergh CA. The culture of whole organs. Science 1935; 81: 621. [DOI] [PubMed] [Google Scholar]
- 5. Belzer FO, Ashby BS, Gulyassy PF, Powell M. Successful seventeen‐hour preservation and transplantation of human‐cadaver kidney. N Engl J Med 1968; 278: 608. [DOI] [PubMed] [Google Scholar]
- 6. Collins GM, Bravo‐Shugarman M, Terasaki PI. Kidney preservation for transportation. Initial perfusion and 30 hours’ ice storage. Lancet 1969; 2: 1219. [DOI] [PubMed] [Google Scholar]
- 7. Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia‐reperfusion injury. Anesthesiology 2001; 94: 1133. [DOI] [PubMed] [Google Scholar]
- 8. Chouchani ET, Pell VR, Gaude E, et al Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014; 515: 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moers C, Smits JM, Maathuis M‐HJ, et al Machine perfusion or cold storage in deceased‐donor kidney transplantation. N Engl J Med 2009; 360: 7. [DOI] [PubMed] [Google Scholar]
- 10. Nasralla D, Coussios CC, Mergental H, et al A randomized trial of normothermic preservation in liver transplantation. Nature 2018; 557: 50. [DOI] [PubMed] [Google Scholar]
- 11. Oniscu GC, Randle LV, Muiesan P, et al In situ normothermic regional perfusion for controlled donation after circulatory death–the United Kingdom experience. Am J Transplant 2014; 14: 2846. [DOI] [PubMed] [Google Scholar]
- 12. Watson CJE, Hunt F, Messer S, et al In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am J Transplant 2018; 10.1111/ajt.15241. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13. Hessheimer AJ, Coll E, Torres F, et al Normothermic regional perfusion vs. super‐rapid recovery in controlled donation after circulatory death liver transplantation. J Hepatol 2019; 70: 658. [DOI] [PubMed] [Google Scholar]
- 14. Demiselle J, Augusto J‐F, Videcoq M, et al Transplantation of kidneys from uncontrolled donation after circulatory determination of death: comparison with brain death donors with or without extended criteria and impact of normothermic regional perfusion. Transpl Int 2016; 29: 432. [DOI] [PubMed] [Google Scholar]
- 15. Kerforne T, Allain G, Giraud S, et al Defining the optimal duration for normothermic regional perfusion in the kidney donor: a porcine preclinical study. Am J Transplant 2019; 19: 737. [DOI] [PubMed] [Google Scholar]
- 16. Jochmans I, Moers C, Smits JM, et al Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann Surg 2010; 252: 756. [DOI] [PubMed] [Google Scholar]
- 17. Treckmann J, Moers C, Smits JM, et al Machine perfusion versus cold storage for preservation of kidneys from expanded criteria donors after brain death. Transpl Int 2011; 24: 548. [DOI] [PubMed] [Google Scholar]
- 18. Kox J, Moers C, Monbaliu D, et al The benefits of hypothermic machine preservation and short cold ischemia times in deceased donor kidneys. Transplantation 2018; 102: 1344. [DOI] [PubMed] [Google Scholar]
- 19. Nicholson ML, Hosgood SA. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant 2013; 13: 1246. [DOI] [PubMed] [Google Scholar]
- 20. Hosgood SA, Nicholson ML. The first clinical case of intermediate ex vivo normothermic perfusion in renal transplantation. Am J Transplant 2014; 14: 1690. [DOI] [PubMed] [Google Scholar]
- 21. Hosgood SA, Nicholson ML. First in man renal transplantation after ex vivo normothermic perfusion. Transplantation 2011; 92: 735. [DOI] [PubMed] [Google Scholar]
- 22. Hosgood SA, Nicholson ML. The evolution of donation after circulatory death donor kidney repair in the United Kingdom. Curr Opin Organ Transplant 2018; 23: 130. [DOI] [PubMed] [Google Scholar]
- 23. Hosgood SA, Thompson E, Moore T, Wilson CH, Nicholson ML. Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. Br J Surg 2018; 105: 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hosgood SA, Nicholson ML. Ex vivo normothermic perfusion of declined human kidneys after inadequate in situ perfusion. Am J Transplant 2014; 14: 490. [DOI] [PubMed] [Google Scholar]
- 25. Hosgood SA, Saeb‐Parsy K, Wilson C, Callaghan C, Collett D, Nicholson ML. Protocol of a randomised controlled, open‐label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open 2017; 7: e012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hosgood SA, Barlow AD, Hunter JP, Nicholson ML. Ex vivo normothermic perfusion for quality assessment of marginal donor kidney transplants. Br J Surg 2015; 102: 1433. [DOI] [PubMed] [Google Scholar]
- 27. Weissenbacher A, Hunter J. Normothermic machine perfusion of the kidney. Curr Opin Organ Transplant 2017; 22: 571. [DOI] [PubMed] [Google Scholar]
- 28. Hamar M, Urbanellis P, Kaths MJ, et al Normothermic ex vivo kidney perfusion reduces warm ischemic injury of porcine kidney grafts retrieved after circulatory death. Transplantation 2018; 102: 1262. [DOI] [PubMed] [Google Scholar]
- 29. Kaths JM, Hamar M, Echeverri J, et al Normothermic ex vivo kidney perfusion for graft quality assessment prior to transplantation. Am J Transplant 2018; 18: 580. [DOI] [PubMed] [Google Scholar]
- 30. Kaths JM, Cen JY, Chun YM, et al Continuous normothermic ex vivo kidney perfusion is superior to brief normothermic perfusion following static cold storage in donation after circulatory death pig kidney transplantation. Am J Transplant 2017; 17: 957. [DOI] [PubMed] [Google Scholar]
- 31. Kaths JM, Echeverri J, Goldaracena N, et al Eight‐hour continuous normothermic ex vivo kidney perfusion is a safe preservation technique for kidney transplantation: a new opportunity for the storage, assessment, and repair of kidney grafts. Transplantation 2016; 100: 1862. [DOI] [PubMed] [Google Scholar]
- 32. Kaths JM, Spetzler VN, Goldaracena N, et al Normothermic ex vivo kidney perfusion for the preservation of kidney grafts prior to transplantation. J Vis Exp 2015; 101: e52909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaths JM, Echeverri J, Linares I, et al Normothermic ex vivo kidney perfusion following static cold storage‐brief, intermediate, or prolonged perfusion for optimal renal graft reconditioning? Am J Transplant 2017; 17: 2580. [DOI] [PubMed] [Google Scholar]
- 34. Weissenbacher A, Lo Faro L, Boubriak O, et al Twenty‐four‐hour normothermic perfusion of discarded human kidneys with urine recirculation. Am J Transplant 2019; 19: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schlegel A, Dutkowski P. Role of hypothermic machine perfusion in liver transplantation. Transpl Int 2015; 28: 677. [DOI] [PubMed] [Google Scholar]
- 36. Kron P, Schlegel A, Muller X, Gaspert A, Clavien P‐A, Dutkowski P. Hypothermic Oxygenated Perfusion (HOPE) – a simple and effective method to modulate the immune response in kidney transplantation. Transplantation 2019; 10.1097/TP.0000000000002634. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 37. COPE – trials [Internet]. [Cited 2018 Aug 15]. Available from: http://cope-eu.com/work%20programme/trials.html
- 38. Jochmans I, Akhtar MZ, Nasralla D, et al Past, present, and future of dynamic kidney and liver preservation and resuscitation. Am J Transplant 2016; 16: 2545. [DOI] [PubMed] [Google Scholar]
- 39. DiRito JR, Hosgood SA, Tietjen GT, Nicholson ML. The future of marginal kidney repair in the context of normothermic machine perfusion. Am J Transplant 2018; 18: 2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laing RW, Mergental H, Yap C, et al Viability testing and transplantation of marginal livers (VITTAL) using normothermic machine perfusion: study protocol for an open‐label, non‐randomised, prospective, single‐arm trial. BMJ Open 2017; 7: e017733 [Cited 2018 Dec 26]. Available from: https://www.nature.com/magazine-assets/d41586-018-04458-w/d41586-018-04458-w.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim WR, Lake JR, Smith JM, et al OPTN/SRTR 2013 annual data report: liver. Am J Transplant 2015; 15(Suppl. 2): 1. [DOI] [PubMed] [Google Scholar]
- 42. Kim WR, Lake JR, Smith JM, et al OPTN/SRTR 2015 annual data report: liver. Am J Transplant 2017; 17(Suppl. 1): 174. [DOI] [PubMed] [Google Scholar]
- 43. Tisone G, Manzia TM, Zazza S, et al Marginal donors in liver transplantation. Transplant Proc 2004; 36: 525. [DOI] [PubMed] [Google Scholar]
- 44. Taner CB, Bulatao IG, Willingham DL, et al Events in procurement as risk factors for ischemic cholangiopathy in liver transplantation using donation after cardiac death donors. Liver Transplant 2012; 18: 100. [DOI] [PubMed] [Google Scholar]
- 45. Ravikumar R, Jassem W, Mergental H, et al Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (first‐in‐man) clinical trial. Am J Transplant 2016; 16: 1779. [DOI] [PubMed] [Google Scholar]
- 46. Selzner M, Goldaracena N, Echeverri J, et al Normothermic ex vivo liver perfusion using steen solution as perfusate for human liver transplantation: first North American results. Liver Transplant 2016; 22: 1501. [DOI] [PubMed] [Google Scholar]
- 47. Bral M, Gala‐Lopez B, Bigam D, et al Preliminary single‐center Canadian experience of human normothermic ex vivo liver perfusion: results of a clinical trial. Am J Transplant 2017; 17: 1071. [DOI] [PubMed] [Google Scholar]
- 48. Watson CJE, Kosmoliaptsis V, Randle LV, et al Normothermic perfusion in the assessment and preservation of declined livers before transplantation: hyperoxia and vasoplegia‐important lessons from the first 12 cases. Transplantation 2017; 101: 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Watson CJE, Kosmoliaptsis V, Pley C, et al Observations on the ex situ perfusion of livers for transplantation. Am J Transplant 2018; 18: 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jassem W, Xystrakis E, Ghnewa YG, et al Normothermic machine perfusion (NMP) inhibits proinflammatory responses in the liver and promotes regeneration. Hepatology 2018; 15: 20. [DOI] [PubMed] [Google Scholar]
- 51. Eisenbach C, Encke J, Merle U, et al An early increase in gamma glutamyltranspeptidase and low aspartate aminotransferase peak values are associated with superior outcomes after orthotopic liver transplantation. Transplant Proc 2009; 41: 1727. [DOI] [PubMed] [Google Scholar]
- 52. Watson CJE, Randle LV, Kosmoliaptsis V, Gibbs P, Allison M, Butler AJ. 26‐hour storage of a declined liver before successful transplantation using ex vivo normothermic perfusion. Ann Surg 2017; 265: e1. [DOI] [PubMed] [Google Scholar]
- 53. Laing RW, Mergental H, Yap C, et al Viability testing and transplantation of marginal livers (VITTAL) using normothermic machine perfusion: study protocol for an open‐label, non‐randomised, prospective, single‐arm trial. BMJ Open 2017; 7: e017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Watson CJE, Jochmans I. From ‘gut feeling’ to objectivity: machine preservation of the liver as a tool to assess organ viability. Curr Transplant Rep 2018; 5: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Matton APM, de Vries Y, Burlage LC, et al Biliary bicarbonate, pH and glucose are suitable biomarkers of biliary viability during ex situ normothermic machine perfusion of human donor livers. Transplantation 2018; 10.1097/TP.0000000000002500. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Spitzer AL, Lao OB, Dick AAS, et al The biopsied donor liver: incorporating macrosteatosis into high‐risk donor assessment. Liver Transplant 2010; 16: 874. [DOI] [PubMed] [Google Scholar]
- 57. Selzner N, Selzner M, Jochum W, Amann‐Vesti B, Graf R, Clavien P‐A. Mouse livers with macrosteatosis are more susceptible to normothermic ischemic injury than those with microsteatosis. J Hepatol 2006; 44: 694. [DOI] [PubMed] [Google Scholar]
- 58. Berthiaume F, Barbe L, Mokuno Y, MacDonald AD, Jindal R, Yarmush ML. Steatosis reversibly increases hepatocyte sensitivity to hypoxia‐reoxygenation injury. J Surg Res 2009; 152: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Koneru B, Dikdan G. Hepatic steatosis and liver transplantation current clinical and experimental perspectives. Transplantation 2002; 73: 325. [DOI] [PubMed] [Google Scholar]
- 60. Jamieson RW, Zilvetti M, Roy D, et al Hepatic steatosis and normothermic perfusion‐preliminary experiments in a porcine model. Transplantation 2011; 92: 289. [DOI] [PubMed] [Google Scholar]
- 61. Nagrath D, Xu H, Tanimura Y, et al Metabolic preconditioning of donor organs: defatting fatty livers by normothermic perfusion ex vivo. Metab Eng 2009; 11: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vairetti M, Ferrigno A, Carlucci F, et al Subnormothermic machine perfusion protects steatotic livers against preservation injury: a potential for donor pool increase? Liver Transplant 2009; 15: 20. [DOI] [PubMed] [Google Scholar]
- 63. Liu Q, Nassar A, Buccini L, et al Lipid metabolism and functional assessment of discarded human livers with steatosis undergoing 24 hours of normothermic machine perfusion. Liver Transplant 2018; 24: 233. [DOI] [PubMed] [Google Scholar]
- 64. Banan B, Watson R, Xu M, Lin Y, Chapman W. Development of a normothermic extracorporeal liver perfusion system toward improving viability and function of human extended criteria donor livers. Liver Transplant 2016; 22: 979. [DOI] [PubMed] [Google Scholar]
- 65. Goldaracena N, Spetzler VN, Echeverri J, et al Inducing hepatitis C virus resistance after pig liver transplantation – a proof of concept of liver graft modification using warm ex vivo perfusion. Am J Transplant 2017; 17: 970. [DOI] [PubMed] [Google Scholar]
- 66. Thijssen MF, Brüggenwirth IMA, Gillooly A, Khvorova A, Kowalik TF, Martins PN. Gene silencing with siRNA (RNA interference): a new therapeutic option during ex‐vivo machine liver perfusion preservation. Liver Transpl 2019; 25: 140. [DOI] [PubMed] [Google Scholar]
- 67. Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double‐stranded RNA in Caenorhabditis elegans . Nature 1998; 391: 806. [DOI] [PubMed] [Google Scholar]
- 68. Guarrera JV, Henry SD, Samstein B, et al Hypothermic machine preservation facilitates successful transplantation of ‘orphan’ extended criteria donor livers. Am J Transplant 2015; 15: 161. [DOI] [PubMed] [Google Scholar]
- 69. Dutkowski P, Polak WG, Muiesan P, et al First comparison of hypothermic oxygenated perfusion versus static cold storage of human donation after cardiac death liver transplants: an international‐matched case analysis. Ann Surg 2015; 262: 764; discussion 770–1. [DOI] [PubMed] [Google Scholar]
- 70. van Rijn R, Karimian N, Matton APM, et al Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br J Surg 2017; 104: 907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schlegel A, Muller X, Kalisvaart M, et al Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J Hepatol 2019; 70: 50. [DOI] [PubMed] [Google Scholar]
- 72. Muller X, Schlegel A, Würdinger M, et al Can hypothermic oxygenated perfusion (HOPE) rescue futile DCD liver grafts? HPB 2019; pii: S1365‐182X(19)30058‐9. [DOI] [PubMed] [Google Scholar]
- 73. Boteon YL, Laing RW, Schlegel A, et al Combined hypothermic and normothermic machine perfusion improves functional recovery of extended criteria donor livers. Liver Transplant 2018; 24: 1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu Q, Berendsen T, Izamis M‐L, Uygun B, Yarmush ML, Uygun K. Perfusion defatting at subnormothermic temperatures in steatotic rat livers. Transplant Proc 2013; 45: 3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hamaoui K, Gowers S, Sandhu B, et al Development of pancreatic machine perfusion: translational steps from porcine to human models. J Surg Res 2018; 223: 263. [DOI] [PubMed] [Google Scholar]
- 76. Branchereau J, Renaudin K, Kervella D, et al Hypothermic pulsatile perfusion of human pancreas: preliminary technical feasibility study based on histology. Cryobiology 2018; 85: 56. [DOI] [PubMed] [Google Scholar]
- 77. Leemkuil M, Lier G, Engelse MA, et al Hypothermic oxygenated machine perfusion of the human donor pancreas. Transplant Direct 2018; 4: e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kelly AC, Smith KE, Purvis WG, et al Oxygen perfusion (persufflation) of human pancreata enhances insulin secretion and attenuates islet proinflammatory signaling. Transplantation 2019; 103: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hosgood SA, Barlow AD, Dormer J, Nicholson ML. The use of ex‐vivo normothermic perfusion for the resuscitation and assessment of human kidneys discarded because of inadequate in situ perfusion. J Transl Med 2015; 13: 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Barlow AD, Hamed MO, Mallon DH, et al Use of ex vivo normothermic perfusion for quality assessment of discarded human donor pancreases. Am J Transplant 2015; 15: 2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kawai M, Kitade H, Koshiba T, Waer M, Pirenne J. Intestinal ischemia reperfusion and lipopolysaccharide transform a tolerogenic signal into a sensitizing signal and trigger rejection. Transplantation 2009; 87: 1464. [DOI] [PubMed] [Google Scholar]
- 82. Tesi RJ, Jaffe BM, McBride V, Haque S. Histopathologic changes in human small intestine during storage in Viaspan organ preservation solution. Arch Pathol Lab Med 1997; 121: 714. [PubMed] [Google Scholar]
- 83. Olson DW, Jijon H, Madsen KL, et al Human small bowel storage: the role for luminal preservation solutions. Transplantation 2003; 76: 709. [DOI] [PubMed] [Google Scholar]
- 84. Mangus RS, Tector AJ, Fridell JA, Kazimi M, Hollinger E, Vianna RM. Comparison of histidine‐tryptophan‐ketoglutarate solution and University of Wisconsin solution in intestinal and multivisceral transplantation. Transplantation 2008; 86: 298. [DOI] [PubMed] [Google Scholar]
- 85. Oltean M, Churchill TA. Organ‐specific solutions and strategies for the intestinal preservation. Int Rev Immunol 2014; 33: 234. [DOI] [PubMed] [Google Scholar]
- 86. Oltean M. Intestinal preservation for transplantation: current status and alternatives for the future. Curr Opin Organ Transplant 2015; 20: 308. [DOI] [PubMed] [Google Scholar]
- 87. DeRoover A, De Leval L, Gilmaire J, et al Luminal contact with University of Wisconsin solution improves human small bowel preservation. Transplant Proc 2004; 36: 273. [DOI] [PubMed] [Google Scholar]
- 88. Roskott AM, Nieuwenhuijs VB, Leuvenink HGD, et al Reduced ischemia‐reoxygenation injury in rat intestine after luminal preservation with a tailored solution. Transplantation 2010; 90: 622. [DOI] [PubMed] [Google Scholar]
- 89. Oltean M, Hellström M, Ciuce C, Zhu C, Casselbrant A. Luminal solutions protect mucosal barrier during extended preservation. J Surg Res 2015; 194: 289. [DOI] [PubMed] [Google Scholar]
- 90. Zhu JZJ, Castillo EG, Salehi P, Avila J, Lakey JRT, Churchill TA. A novel technique of hypothermic luminal perfusion for small bowel preservation. Transplantation 2003; 76: 71. [DOI] [PubMed] [Google Scholar]
- 91. Nakao A, Toyokawa H, Tsung A, et al Ex vivo application of carbon monoxide in University of Wisconsin solution to prevent intestinal cold ischemia/reperfusion injury. Am J Transplant 2006; 6: 2243. [DOI] [PubMed] [Google Scholar]
- 92. Buchholz BM, Masutani K, Kawamura T, et al Hydrogen‐enriched preservation protects the isogeneic intestinal graft and amends recipient gastric function during transplantation. Transplantation 2011; 92: 985. [DOI] [PubMed] [Google Scholar]
- 93. Tsujimura T, Suzuki Y, Takahashi T, et al Successful 24‐h preservation of canine small bowel using the cavitary two‐layer (University of Wisconsin solution/perfluorochemical) cold storage method. Am J Transplant 2002; 2: 420. [DOI] [PubMed] [Google Scholar]
- 94. Muñoz‐Abraham AS, Patrón‐Lozano R, Narayan RR, et al Extracorporeal hypothermic perfusion device for intestinal graft preservation to decrease ischemic injury during transportation. J Gastrointest Surg 2016; 20: 313. [DOI] [PubMed] [Google Scholar]
- 95. Lyon PC, Gray MD, Mannaris C, et al Safety and feasibility of ultrasound‐triggered targeted drug delivery of doxorubicin from thermosensitive liposomes in liver tumours (TARDOX): a single‐centre, open‐label, phase 1 trial. Lancet Oncol 2018; 19: 1027. [DOI] [PMC free article] [PubMed] [Google Scholar]


