Abstract
Background
Subjects seeking facial rejuvenation want the results to appear natural. Currently, however, there is no consensus definition of, or assessment scale for, “naturalness.”
Aims
This open‐label pilot study explored evaluation techniques and criteria to assess naturalness of facial movement and expression following optimal bilateral correction of moderate‐to‐severe nasolabial folds and marionette lines with soft‐tissue hyaluronic acid fillers formulated with XpresHAn Technology™.
Methods
Primary efficacy was investigator assessed naturalness of dynamic expressions using baseline and Day 42 posttreatment 2D video. Other evaluations included investigator assessed naturalness using static images, wrinkle severity, investigator and subject Global Aesthetic Improvement Scale assessments, and subject satisfaction.
Results
Thirty Caucasian females (41‐65 years) received either Restylane ® Refyne, Restylane ® Defyne or both. Naturalness of dynamic expressions was at least maintained in all subjects. Naturalness of static expressions was not negatively affected in most subjects (96.7%). For dynamic expressions, 83.3% of subjects showed enhanced attractiveness, younger appearance and maintained naturalness.
Conclusions
Overall, nasolabial folds and marionette lines improved significantly based on severity and Global Aesthetic Improvement Scale scores, with high subject satisfaction and favorable safety profile. Based on subject satisfaction and investigator assessments, using highly flexible hyaluronic acid dermal fillers did not compromise naturalness of lower facial expressions while achieving the desired improvements in attractiveness and youthfulness. The preliminary results obtained in this pilot study suggest that dynamic and static assessments of facial animation may aid the evaluation of natural outcomes in facial rejuvenation procedures.
Keywords: dermal fillers, facial dynamics, facial rejuvenation, hyaluronic acid, natural outcomes
1. INTRODUCTION
Natural facial expressions are an important aspect of nonverbal communication and signal characteristics and patients seeking facial rejuvenation want discreet and natural‐looking outcomes.1, 2 Hyaluronic acid (HA) fillers are the gold standard for nonsurgical facial rejuvenation and discreet tissue augmentation of the face should provide aesthetic improvement, not hinder natural facial movements, and produce no obvious evidence of filler.1
Subjects describe natural results as avoiding looking unrecognizable and maintaining self‐identity.1 Currently, however, there is no consensus definition of, or assessment scale for, naturalness. Empirically, however, maintaining a subject's pretreatment appearance and expressiveness are critical in defining a natural outcome. Implicitly, natural‐looking results reflect universal notions of beauty, while being individualized based on facial appearance and movement.1
Numerous HA fillers are available, with differing characteristics.3, 4 Restylane® Refyne (HARR) and Restylane ® Defyne (HARD) (Q‐Med AB/Galderma) are injectable HA soft‐tissue fillers formulated with XpresHAn Technology™, which is characterized by flexibility and distributed product integration.5, 6
HARR and HARD have flexible gel structures able to adapt to dynamic facial expressions and are approved for injection into the mid‐to‐deep dermis for the correction of moderate‐to‐severe wrinkles and folds, such as nasolabial folds (NLFs) and marionette lines (MLs). The softer gel structure of HARR is most appropriate for less severe wrinkles and folds while the firmer texture and larger gel calibration of HARD is suitable for correction of more prominent deeper wrinkles and folds.6 In active‐controlled, split‐face studies, HARR and HARD were well tolerated and improved moderate‐to‐severe NLFs during 12 and 18 month follow‐up.7, 8, 9, 10, 11, 12 This prospective, noncomparative, open‐label, pilot clinical study conducted at two centers evaluated the perception of the naturalness of facial expressions in motion following correction of wrinkles and folds in the lower face following treatment with HARR and HARD.
2. METHODS
2.1. Subjects and inclusion and exclusion criteria
This study was approved by the Quorum Review IRB and conducted in accordance with the guidelines for standard clinical practice and the Declaration of Helsinki (Blinded for peer review). All enrolled subjects provided written consent. Subjects were Caucasian females aged from 40 to 65 years with bilateral NLFs rated as moderate (2‐3) or severe (3‐4) based on the Wrinkle Severity Rating Scale (WSRS)13 and with bilateral MLs rated as moderate (2‐3) or severe (3‐4) based on the Wrinkle Assessment Scale (WAS),14 as assessed by the treating investigator.
Exclusion criteria included the following: severe midface volume loss or severity of wrinkle or folds that required other treatments; previous tissue revitalization with neurotoxin, laser or light, mesotherapy, chemical peeling, or dermabrasion below the zygomatic arch within 6 months; previous tissue augmenting therapy or contouring with a permanent, nonpermanent, or fat‐injection in the facial area; a known hypersensitivity to lidocaine; tendency to develop keloids, hypertrophic scars, or any healing disorders; body mass index BMI < 18 or >30 kg/m2; current smokers or ex‐smokers with >10 pack‐year history within the past year.
2.2. Treatments
Subjects received bilateral treatment of NLFs and MLs using HARR (moderate wrinkles) or HARD (severe wrinkles) on Day 1 within the mid‐to‐deep dermal plane using needle injection (no cannulas). Layering the two products in the same wrinkle or fold was not permitted. An optional touch‐up was permitted at 2 weeks to achieve an optimal outcome. All treatments followed approved labeling guidelines including needle injection (HARR = 30G × ½”; HARD = 27G × ½”). The recommended maximum injection volume per session was 2 mL per NLF and 1 mL per ML.
The treating investigator gently massaged the injection sites to conform to the contour of the surrounding tissues. Topical cooling was permitted to reduce initial swelling, and subjects were asked to avoid exposing the treated area to any heat or extreme cold until any local inflammation resolved.
2.3. Efficacy assessments
The study's primary objective was to evaluate the naturalness of the lower face in motion 42 days after the initial treatment compared with baseline, as assessed by the treating investigator. 2D video captured the following facial expressions: big smile with closed lips; big smile with open lips and upper teeth showing; pursed lips (kissing position); and grimace (down‐turned corners of mouth with lips closed) at baseline and Day 42. The treating investigator judged posttreatment dynamic expressions as “Enhanced,” “Maintained,” or “Reduced" at Day 42 compared with baseline. A subject was considered as having “at least maintained naturalness” in motion if judged as either “Maintained” or “Enhanced.”
Secondary objectives included evaluation of the naturalness of the lower face at baseline and Day 42 using static expressions at full contraction, assessed by the treating investigator, captured using 2D photographs of a neutral (at rest) expression and those described above. The treating investigator assessed whether the filler affected naturalness of the static expressions at full contraction as: “not affected”; “affected in a positive way”; or “affected in a negative way” compared with baseline. A subject was considered as having naturalness of the expression “not negatively affected” if judged as either “not affected” or “affected in a positive way.”
At Day 42, the treating investigator used the dynamic expressions captured by 2D video to judge if treatment “Enhanced,” “Maintained,” or “Reduced" attractiveness and whether the perceived age was “Younger,” “Current Age,” or “Older" compared with baseline. A combined endpoint captured the proportion of subjects with enhanced attractiveness and who looked younger and who showed “Maintained” or “Enhanced” naturalness at Day 42 compared with baseline.
The treating investigator assessed NLF severity using a validated photonumeric WSRS scale (1 = absent; 2 = mild; 3 = moderate; 4 = severe; 5 = extreme)13 and ML severity using a validated photonumeric WAS scale (0 = no wrinkles; 1 = just perceptible wrinkle; 2 = shallow wrinkles; 3 = moderately deep wrinkle; 4 = deep wrinkle, well defined edges; 5 = very deep wrinkle, redundant fold)14 at baseline and Day 42. A reduction of at least 1 grade in NLFs or MLs on both sides of the face was considered clinically significant.
The treating investigator and subject used the Global Aesthetic Improvement Scale (GAIS) to compare a photograph of a neutral expression taken at baseline and Day 42. Subjects assessed the photographs independently of the investigator. Assessments were made using a 5‐grade scale (“Worse,” “No change,” “Somewhat improved,” “Much improved,” or “Very much improved”) with a score of “Somewhat improved,” “Much improved,” or “Very much improved” represented a clinically significant improvement.15 Subject‐reported satisfaction was evaluated at baseline and Day 42 using a 9‐item questionnaire with a 5‐point Likert scale.
2.4. Safety assessments
Safety was assessed by subject‐reported injection‐related events (IREs) following initial and optional touch‐up treatment. Local tolerability was assessed by the frequency and severity of predefined expected IREs (bruising, redness, swelling, pain [including burning], tenderness, and itching) during the first 14 days after treatment. These events were not reported as treatment‐emergent adverse events (TEAEs) if they fully resolved during that time. investigator assessed TEAEs were recorded throughout the study.
2.5. Statistical analyses
The full analysis set (FAS) was the primary population for all efficacy analyses and included all subjects treated with any amount of product who had at least one posttreatment assessment. The safety population included all subjects treated with any amount of product.
For the naturalness of the lower face in motion and static expressions, P‐values comparing efficacy assessments at Day 42 with baseline were made using a chi‐square goodness‐of‐fit test. Improvement in NLF and ML severity were summarized descriptively including a mean change from baseline based on the bilateral wrinkle scores. Improvement was also assessed by proportions of subjects with at least a 1‐grade reduction bilaterally from baseline. The P‐value for testing mean change from baseline to Day 42 was calculated using a paired t‐test. For investigator and subject‐assessed GAIS data, the number and percent of subjects with clinically significant improvement were summarized descriptively. Subject satisfaction was tabulated and summarized descriptively.
3. RESULTS
3.1. Subject demographics and product administration
Thirty Caucasian female subjects aged from 41 to 65 years (mean ± SD 55.0 ± 6.0 years) were included in the FAS and safety populations. Table 1 summarizes subject demographics and baseline NLF and ML severity. Table 2 summarizes injection techniques and volumes.
Table 1.
Subject demographics and baseline nasolabial fold and marionette line assessments
| Variable, statistic or category | All subjects |
|---|---|
| n | 30 |
| Mean age, years (SD; range) | 55.0 (6.01; 41‐65) |
| Gender, n (%) | |
| Female | 30 (100.0) |
| Race, ethnicity, n (%) | |
| White, not Hispanic or Latino | 30 (100.0) |
| Baseline NLF WSRS scores, n (%) | |
| Left NLF | |
| Grade 3 (moderate) | 21 (70.0%) |
| Grade 4 (severe) | 9 (30.0%) |
| Right NLF | |
| Grade 3 (moderate) | 19 (63.3%) |
| Grade 4 (severe) | 11 (36.7%) |
| Baseline ML WAS scores, n (%) | |
| Left ML | |
| Grade 3 (moderately deep wrinkle) | 17 (56.7%) |
| Grade 4 (deep wrinkle, well defined edges) | 13 (43.3%) |
| Right ML | |
| Grade 3 (moderately deep wrinkle) | 17 (56.7%) |
| Grade 4 (deep wrinkle, well defined edges) | 13 (43.3%) |
Abbreviations: ML, marionette line; NLF, nasolabial fold; WAS, Wrinkle Assessment Scale; WSRS, Wrinkle Severity Rating Scale.
Table 2.
Injection technique and volume administered
| Filler | HARR | HARD | Overall | |||
|---|---|---|---|---|---|---|
| Area | NLFs | MLs | NLFs | MLs | NLFs | MLs |
| Injection technique: Initial treatment n (%) | ||||||
| Number of subjects | 14 | 16 | 16 | 14 | 30 | 30 |
| Linear threading | 14 (100) | 15 (93.8) | 14 (87.5) | 13 (92.9) | 28 (93.3) | 28 (93.3) |
| Fanning | 1 (7.1) | 3 (18.8) | 0 (0.0) | 1 (7.1) | 1 (3.3) | 4 (13.3) |
| Serial depot | 14 (100) | 16 (100) | 16 (100) | 14 (100) | 30 (100) | 30 (100) |
| Injection technique: Touch‐up treatment n (%) | ||||||
| Number of subjects | 10 | 12 | 11 | 13 | 21 | 25 |
| Linear threading | 6 (42.9) | 3 (18.8) | 4 (25.0) | 4 (28.6) | 10 (33.3) | 7 (23.3) |
| Fanning | 0 (0.0) | 0 (0.0) | 1 (6.3) | 0 (0.0) | 1 (3.3) | 0 (0.0) |
| Serial depot | 9 (64.3) | 12 (75.0) | 11 (68.8) | 13 (92.9) | 20 (66.7) | 25 (83.3) |
| Injection volume: Initial treatment (mL) | ||||||
| Number of subjects | 14 | 16 | 16 | 14 | 30 | 30 |
| Mean (SD) | 1.43 (0.186) | 1.43 (0.380) | 1.61 (0.362) | 1.54 (0.397) | 1.52 (0.302) | 1.48 (0.386) |
| Median (min, max) | 1.40 (1.2, 1.8) | 1.50 (0.8, 2.0) | 1.70 (1.0, 2.2) | 1.70 (1.0, 2.0) | 1.40 (1.0, 2.2) | 1.60 (0.8, 2.0) |
| Injection volume: Touch‐up treatment (mL) | ||||||
| Number of subjects | 10 | 12 | 11 | 13 | 21 | 25 |
| Mean (SD) | 0.54 (0.295) | 0.56 (0.281) | 0.54 (0.280) | 0.72 (0.334) | 0.54 (0.280) | 0.64 (0.314) |
| Median (min, max) | 0.50 (0.2, 1.2) | 0.50 (0.2, 1.1) | 0.50 (0.2, 0.9) | 0.60 (0.4, 1.6) | 0.50 (0.2, 1.2) | 0.60 (0.2, 1.6) |
| Injection volume: Total (initial plus touch‐up treatment; mL) | ||||||
| Number of subjects | 14 | 16 | 16 | 14 | 30 | 30 |
| Mean (SD) | 1.81 (0.474) | 1.84 (0.604) | 1.98 (0.585) | 2.20 (0.701) | 1.90 (0.533) | 2.01 (0.665) |
| Median (min, max) | 1.80 (1.2, 2.9) | 1.70 (1.1, 3.0) | 2.00 (1.0, 2.9) | 2.30 (1.1, 3.6) | 1.95 (1.0, 2.9) | 1.80 (1.1, 3.6) |
Abbreviations: Max, maximum; Min, minimum; ML, marionette line; NLF, nasolabial folds; SD, Standard deviation.
3.2. Naturalness
The naturalness of the lower face in motion at Day 42 was “at least maintained” compared with baseline in all subjects (Figure 1). Naturalness was “Enhanced” in 24 subjects (80.0%) and “Maintained” in 6 subjects (20.0%). The P‐value comparing “At least maintained” versus “Not at least maintained” could not be estimated due to all subjects having only one categorical response for “At least maintained.”
Figure 1.
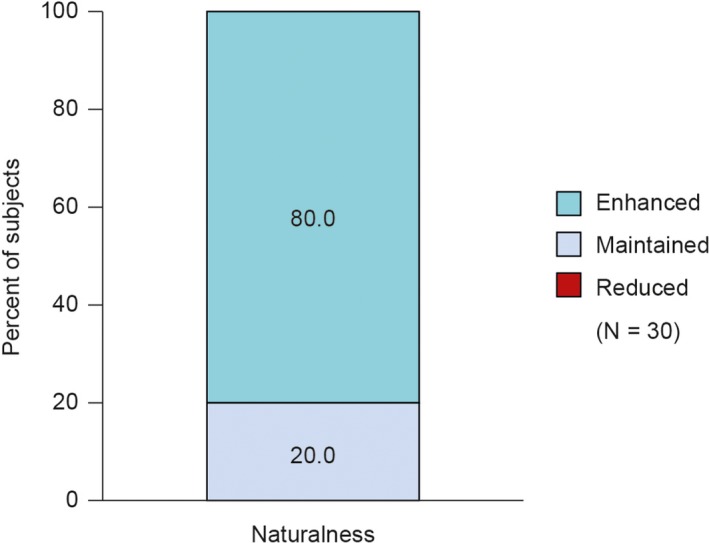
Treatment impact on naturalness of facial expression of lower face in motion based on 2D video assessment by treating investigator at Day 42 compared with baseline
The naturalness of the lower face using static expressions at full contraction was “not negatively affected” compared with baseline for 100% of subjects during neutral, open smile, and grimace expressions (Figure 2). For the closed smile and lip purse expressions, naturalness was not negatively affected in 29 subjects (96.7%).
Figure 2.
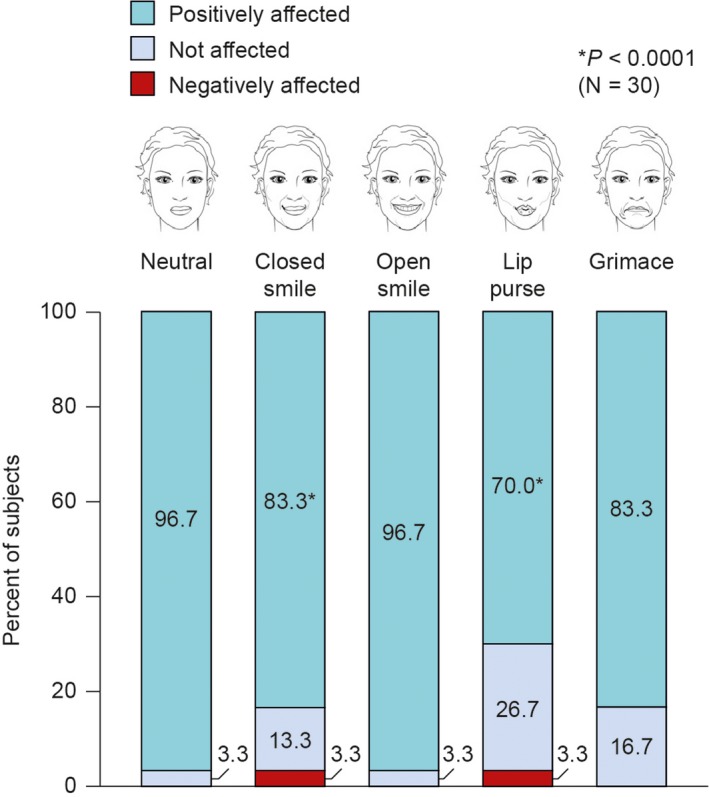
Naturalness of expression in the lower face at full contraction based on 2D photo assessment by treating investigator at Day 42 compared with baseline
3.3. Attractiveness, age and the composite endpoint
Attractiveness of the lower face in motion at Day 42 was assessed as “Enhanced” compared with baseline in 86.7% of subjects (Figure 3). No subjects were assessed as having “Reduced” attractiveness. The perception of age at Day 42 was assessed as being “Younger” for 25 subjects (83.3%, P < 0.001) with 5 subjects (17%) rated as current age (Figure 3). Twenty‐five subjects (83.3%) were judged to have met the composite endpoint (Figure 3).
Figure 3.
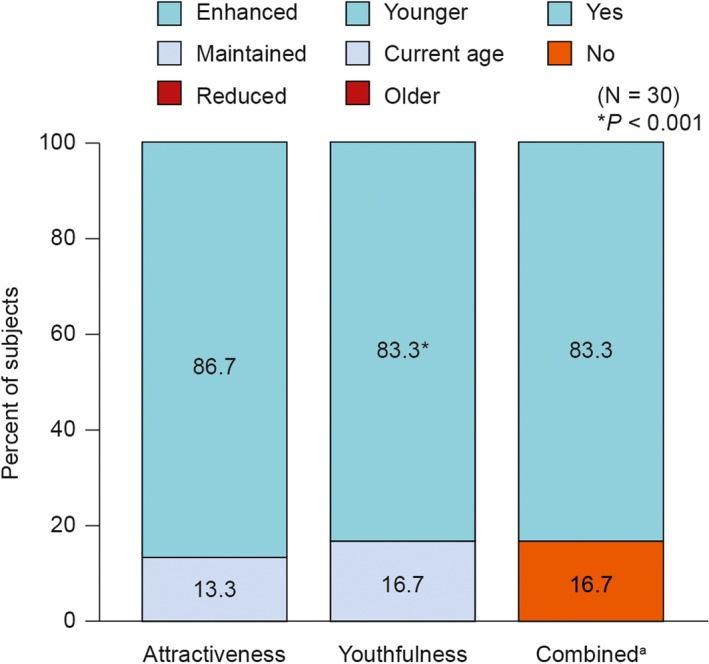
Perception of attractiveness and age of lower face in motion based on 2D video assessment by treating investigator at Day 42 compared with baseline
3.4. NLF and ML wrinkle severity improvement
All subjects showed at least a 1‐grade improvement in NLF severity bilaterally based on WSRS scores at Day 42 compared with baseline. Ten subjects (33.3%) had at least a 2‐grade improvement. The mean (SD) change from baseline in bilateral WSRS scores at Day 42 was −1.42 (0.53) (P <0.001).
All subjects showed at least a 1‐grade improvement in ML severity bilaterally based on WAS scores at Day 42 compared with baseline. Twenty‐five subjects (83.3%) had at least a 2‐grade improvement. The mean (SD) change from baseline in bilateral WAS scores at Day 42 was −2.15 (0.59) (P <0.001). Figure 4 shows representative 2D subject photographs of “open smile” at baseline and Day 42.
Figure 4.
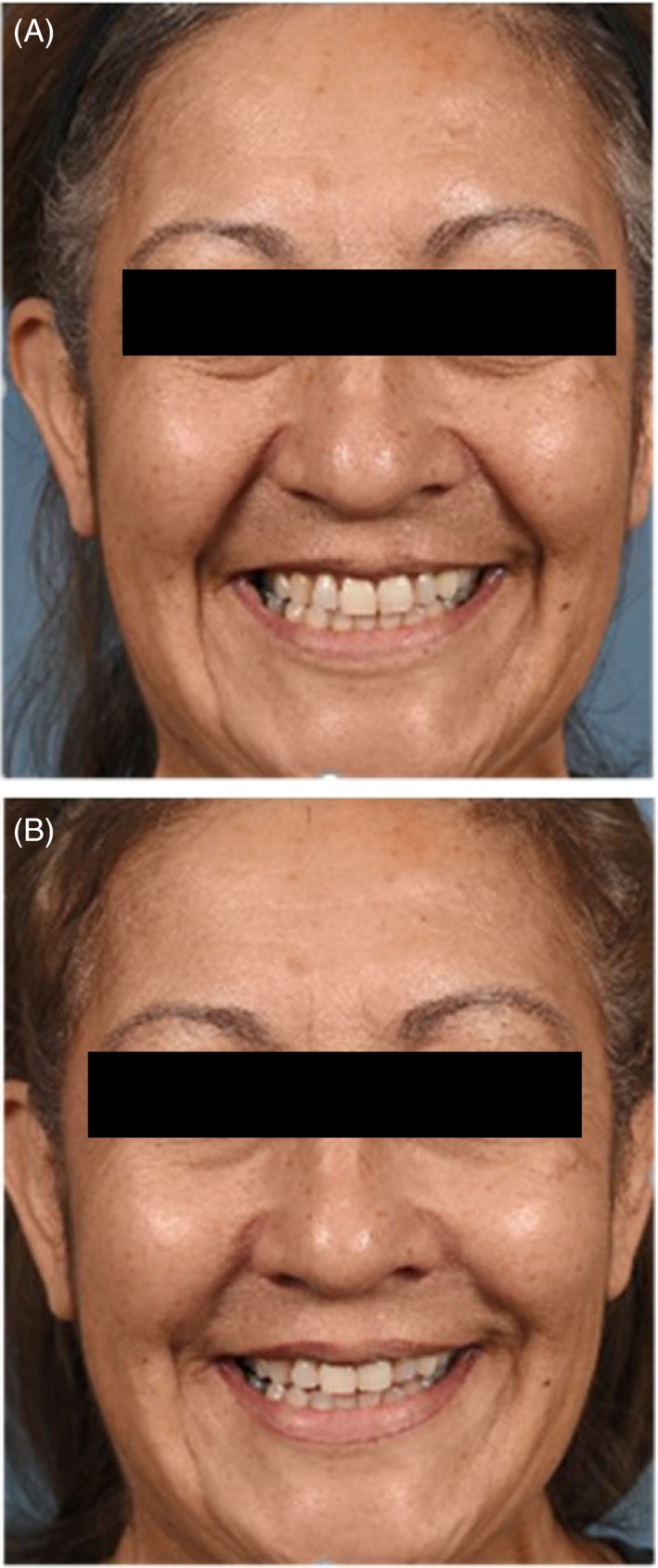
Representative subject photographs of (A) “Open smile” at baseline and (B) 42 d after treatment. Subject 134‐19, age 53 y. Treatment HARD. Total injection volume = 3.8 mL (2.2 mL NLF; 1.6 mL ML)
3.5. Global aesthetic improvement
The treating investigator assessed that all subjects showed a clinically significant aesthetic improvement at Day 42 (Figure 5). The treating investigator rated 30.0% of subjects overall as "very much improved" and 63.3% as “much improved.” Subject‐reported GAIS, assessed independently of the investigator, showed that all reported some improvement posttreatment (Figure 5). “Very much improved” at Day 42 was reported by 40% of subjects. P‐values comparing clinically significant improvement versus no clinically significant improvement could not be estimated as all subjects had one categorical response.
Figure 5.
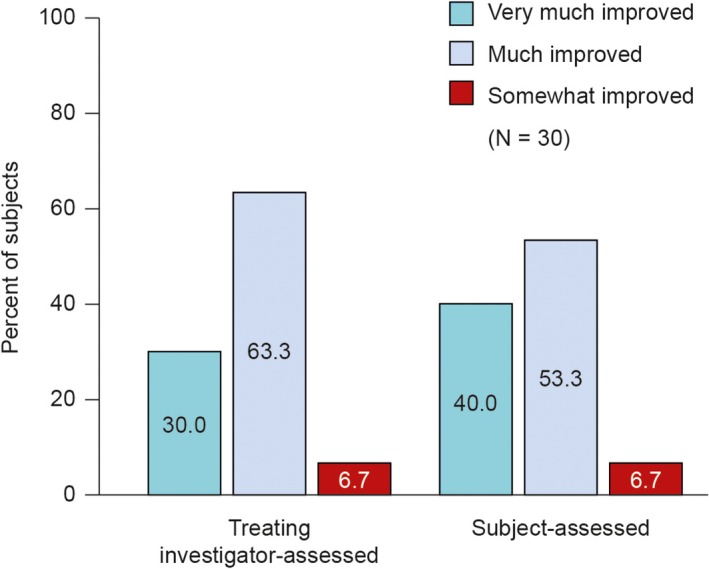
Global Aesthetic Improvement Scale at Day 42 relative to baseline
3.6. Subject satisfaction
Subjects reported high levels of satisfaction with their aesthetic outcome at Day 42 using a 5‐point Likert scale (Figure 6). Regarding attractiveness and youthfulness, most subjects strongly agreed or agreed with the statements: “The overall appearance of my face has a pleasing appearance” (90.0%) and “I look younger than my actual age” (83.3%). Regarding naturalness, most or all subjects strongly agreed or agreed with the statements: “My face looks natural when it is relaxed,” (96.7%) “My face looks natural when smiling,” (93.3%) and “The overall appearance of my face looks natural” (100.0%).
Figure 6.
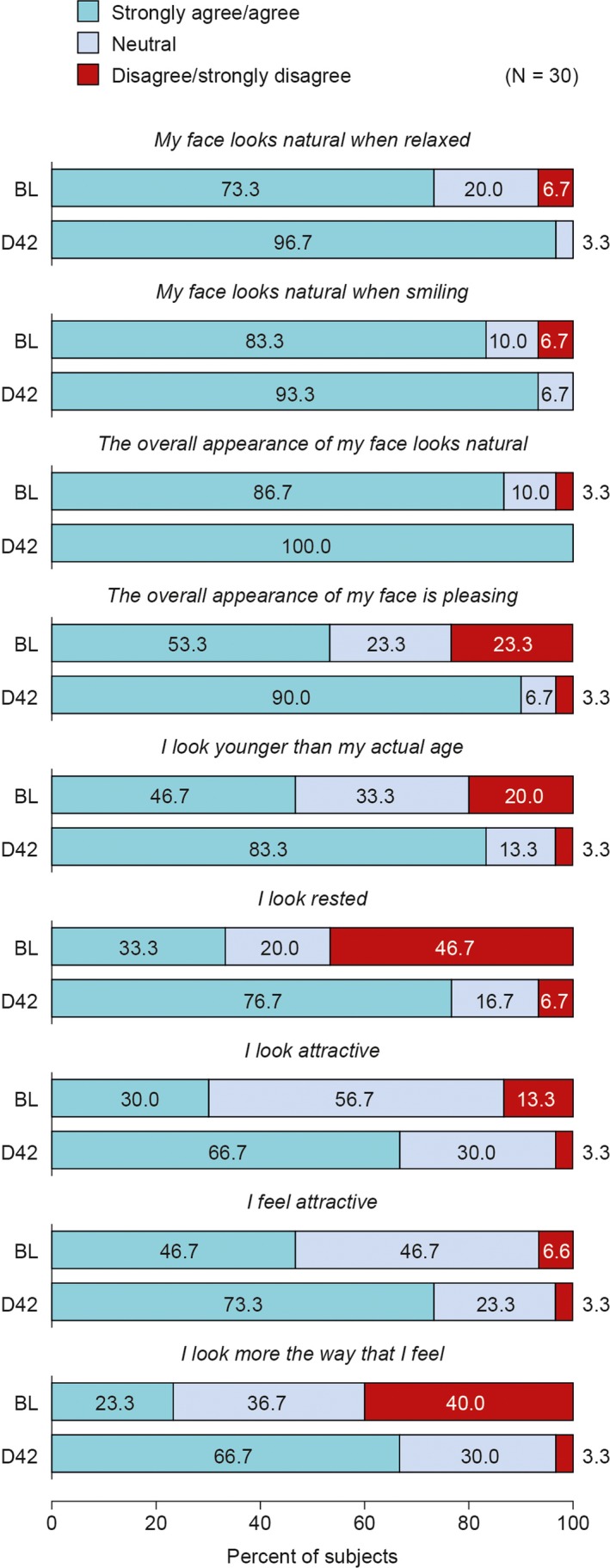
Subject satisfaction with aesthetic outcomes
3.7. Safety
Twenty‐nine subjects (96.7%) experienced at least one IRE (Table 3) associated with the initial or touch‐up treatment; 26 subjects (86.7%) reported at least one IRE with the treatment of NLFs and 27 subjects (90.0%) with the treatment of MLs. The most frequently reported IREs were bruising (86.7%) and redness (63.3%) and most were mild or moderate in intensity. All IREs resolved within 2 weeks of treatment. Two subjects (6.7%) experienced a TEAE, which were mild in severity and assessed as not being related to either the study procedure or the study product.
Table 3.
Injection‐related and treatment‐emergent adverse events
| Variable, statistic, symptom | NLF (n = 30) | ML (n = 30) | All (N = 30) |
|---|---|---|---|
| Subjects with ≥1 IRE, n (%) | 26 (86.7) | 27 (90.0) | 29 (96.7) |
| Bruising | 20 (66.7) | 20 (66.7) | 26 (86.7) |
| Redness | 16 (53.6) | 17 (56.7) | 19 (63.3) |
| Swelling | 1 (3.3) | 2 (6.7) | 3 (10.0) |
| Tenderness | 2 (6.7) | 1 (3.3) | 2 (6.7) |
| Itching | 1 (3.3) | – | 1 (3.3) |
| Pain (including burning) | 1 (3.3) | – | 1 (3.3) |
| Proportion of IRE by Severity, n (%) | |||
| Mild | 17 (56.7) | 16 (53.3) | 15 (50.0) |
| Moderate | 9 (30.0) | 10 (33.3) | 13 (43.3) |
| Severe | – | 1 (3.3) | 1 (3.3) |
| Subject with ≥ 1 TEAE, n (%) | 2 (6.7) | ||
| Related | – | ||
| Unrelated |
2 (6.7) 1‐nasopharyngitis 1‐urinary tract infection |
||
Adverse events are coded using MedDRA version 18.1.
Abbreviations: IRE, injection‐related event; ML, marionette line; NLF, nasolabial folds; TEAE, treatment‐emergent adverse events.
4. DISCUSSION
Currently there is no consensus definition of, or assessment scale for, the naturalness of facial rejuvenation. Indeed, the perception of naturalness is subjective and may be discernable only when facial movements appear unnatural following treatment with fillers. This pilot study was undertaken as the researchers were concerned that subjects would rate their naturalness as worse following treatment with fillers. The findings that investigators and, independently, subjects reported a maintained impression of naturalness were unexpected.
Outcomes depend partly on the filler's physicochemical properties and the injection technique and the highly flexible fillers selected for use in this study accommodate natural dynamic facial movements. The study confirmed that naturalness of the lower face in motion was at least maintained in all subjects at Day 42 following optimal treatment of NLF and ML (Figure 1) across a range of expressions.
In paired baseline and posttreatment photographs, naturalness of the lower face was not negatively affected in any subject during three of the five expressions: neutral, open smile, and grimace (Figure 2). One subject was assessed as having the naturalness of a closed smile negatively affected by treatment with HARR. In another subject, the combination of HARR + HARD was assessed as having negatively affected the naturalness of a lip purse. However, 83.3% to 96.7% of all subjects were assessed as having the naturalness of their expressions positively affected by treatment. Additionally, a post hoc analysis (data not shown) showed a high level of agreement between assessments made by the investigators at the two centers using photographic comparisons (neutral expression, 83.3%; closed smile, 80.0%; open smile, 90.0%; lip purse, 63.4%; and grimace, 86.7%).
Video comparisons confirmed that naturalness was not compromised while achieving desired aesthetic improvements: 25 (83.3%) subjects were rated with enhanced attractiveness and looked younger, while at least maintaining naturalness, compared with baseline (Figure 3). A post hoc analysis (data not shown) showed a high level of agreement between the assessments made by the investigators at the two centers using 2D video comparisons (naturalness in motion, 80.0%; attractiveness in motion, 83.3%; and age of the lower face in motion, 70.0%).
Notably, subjects’ GAIS self‐assessments were consistent with those of the treating investigators. Subjects’ satisfaction scores were consistent with investigators’ assessments of attractiveness, youthfulness, and naturalness. Taken together, the video and photographic results, the significant improvements in NLF WSRS and ML WAS (Figure 4), GAIS (Figure 5), high subject satisfaction (Figure 6), and favorable safety profile support the natural‐looking aesthetic benefits associated with treating NLFs and MLs using HARR and HARD.
There is, however, neither a standardized definition of naturalness nor a “gold standard” assessment method. This study, therefore, relied on clinical judgement of pre‐ versus posttreatment images to evaluate naturalness and appearance. However, the results were consistent across a range of standardized expressions captured as 2D video and 2D photographs and between the investigators at both the centers.16
A limitation of this study is that the protocol restricted treatments to the lower face and enrolled female subjects. As this was a pilot study designed to explore and evaluate the dynamic strain assessment, the trial enrolled a small number of patients, did not include a control group and used a relatively homogenous cohort of Caucasian women, with moderate‐to‐severe wrinkles. The cohort enrolled in this pilot study was intended to represent the patients that present in our clinical practice, rather than being a definitive clinical trial population.
The open‐label nature of the assessments, while appropriate for a pilot study, means that the results require confirmation in prospective blinded evaluation. The investigators used linear threading, fanning and serial depot to inject the filler (Table 2). Future studies could assess whether the technique influences outcome and determine any correlation between the amount of filler used and GAIS or subject satisfaction.
5. CONCLUSIONS
In this pilot study, naturalness of facial expressions was not compromised while achieving desired aesthetic improvements in attractiveness and youthfulness using highly flexible gel HA fillers. Subject‐reported satisfaction reinforced investigator assessed attractiveness, youthfulness, and naturalness. The preliminary results obtained suggest that dynamic and static assessments of facial animation may aid evaluation of natural outcomes in rejuvenation procedures, although further studies are needed. Until a validated assessment is available, assessments such as those used in this study complemented by subject‐reported outcomes on naturalness, can be used to evaluate whether facial rejuvenation treatments produce subjectively natural outcomes.
CONFLICT OF INTEREST
Study funded by Galderma Laboratories, LP, Fort Worth, Texas. Dr Solish is paid consultant, speaker, and clinical trial investigator for Galderma. Dr Bertucci is a paid consultant, speaker and clinical trial investigator for Galderma. Dr Percec is a paid consultant and speaker for Galderma. Mr Wagner, Dr Nogueira, and Dr Mashburn are employees of Galderma Laboratories, LP
ACKNOWLEDGMENTS
The authors wish to thank Erika von Grote, PhD, Marian East BA (hons) and Mark Greener, BSc (hons), MRSB for their writing assistance and figure design on this manuscript. The authors would also like to acknowledge Premier Research International LLC for managing the clinical trial and providing statistical analysis support.
Solish N, Bertucci V, Percec I, Wagner T, Nogueira A, Mashburn J. Dynamics of hyaluronic acid fillers formulated to maintain natural facial expression. J Cosmet Dermatol. 2019;18:738–746. 10.1111/jocd.12961
REFERENCES
- 1. Michaud T, Gassia V, Belhaouari L. Facial dynamics and emotional expressions in facial aging treatments. J Cosmet Dermatol. 2015;14:9‐21. [DOI] [PubMed] [Google Scholar]
- 2. Jack RE, Schyns PG. The human face as a dynamic tool for social communication. Curr Biol. 2015;25:R621‐R634. [DOI] [PubMed] [Google Scholar]
- 3. Micheels P, Sarazin D, Tran C, Salomon D. Effect of different crosslinking technologies on hyaluronic acid behavior: a visual and microscopic study of seven hyaluronic acid gels. J Drugs Dermatol. 2016;15:600‐606. [PubMed] [Google Scholar]
- 4. Molliard GS, Albert S, Mondon K. Key importance of compression properties in the biophysical characteristics of hyaluronic acid soft‐tissue fillers. J Mech Behav Biomed Mater. 2016;61:290‐298. Restylane https://www.restylaneusa.com/ Accessed January 2019. [DOI] [PubMed] [Google Scholar]
- 5. Segura S, Anthonioz L, Fuchez F, Herbage B. A complete range of hyaluronic acid filler with distinctive physical properties specifically designed for optimal tissue adaptations. J Drugs Dermatol. 2012;11(1 Suppl):s5‐S8. [PubMed] [Google Scholar]
- 6. Rzany B, Bayerl C, Bodokh I, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of moderate nasolabial folds: 6‐month interim results of a randomized, evaluator‐blinded, intra‐individual comparison study. J Cosmet Laser Ther. 2011;13:107‐112. [DOI] [PubMed] [Google Scholar]
- 7. Ascher B, Bayerl C, Brun P, et al. Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines: 6‐month interim results of a randomized, evaluator‐blinded, intra‐individual comparison study. J Cosmet Dermatol. 2011;10:94‐98. [DOI] [PubMed] [Google Scholar]
- 8. Rzany B, Bayerl C, Bodokh I, et al. An 18‐month follow‐up, randomized comparison of effectiveness and safety of two hyaluronic acid fillers for treatment of moderate nasolabial folds. Dermatol Surg. 2017;43:58‐65. [DOI] [PubMed] [Google Scholar]
- 9. Ascher B, Bayerl C, Kestemont P, Rzany B, Edwartz C, Podda M. A 12‐month follow‐up, randomized comparison of effectiveness and safety of two hyaluronic acid fillers for treatment of severe nasolabial folds. Dermatol Surg. 2017;43:389‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fagien S, Monheit G, Jones D, et al. Hyaluronic acid gel with (HARRL) and without lidocaine (HAJU) for the treatment of moderate‐to‐severe nasolabial folds: a randomized, evaluator‐blinded, phase III study. Dermatol Surg. 2018;44:549‐556. [DOI] [PubMed] [Google Scholar]
- 11. Baumann L, Weiss RA, Grekin S, et al. Comparison of hyaluronic acid gel with (HARDL) and without lidocaine (HAJUP) in the treatment of moderate‐to‐severe nasolabial folds: a randomized, evaluator‐blinded study. Dermatol Surg. 2018;44:833‐840. [DOI] [PubMed] [Google Scholar]
- 12. Day DJ, Littler CM, Swift RW, Gottlieb S. The wrinkle severity rating scale. Am J Clin Dermatol. 2004;5:49‐52. [DOI] [PubMed] [Google Scholar]
- 13. Lemperle G, Holmes RE, Cohen SR, Lemperle SM. A classification of facial wrinkles. Plast Reconstr Surg. 2001;108:1735‐1750. [DOI] [PubMed] [Google Scholar]
- 14. Narins RS, Brandt F, Leyden J, Lorenc ZP, Rubin M, Smith S. A randomized, double‐blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29:588‐595. [DOI] [PubMed] [Google Scholar]
- 15. Ekman P. Darwin, deception, and facial expression. Ann N Y Acad Sci. 2003;1000:205‐221. [DOI] [PubMed] [Google Scholar]


