Abstract
Men with prostate cancer with positive margins, extraprostatic extension, positive lymph nodes, high prostate‐specific antigen, or high Gleason Score are at high risk of recurrence following primary therapy. Androgen deprivation therapy (ADT), which includes medical/surgical castration, antiandrogen therapy, and combined androgen blockade, can be combined with primary therapy to shrink the tumor, reduce margin positivity, and reduce the risk of recurrence. However, many problems still remain, such as optimizing the application of ADT in the treatment of prostate cancer, for example, ideal patient population and optimal timing and duration of therapy. To investigate these problems, we searched PubMed for relevant publications on clinical studies of deprivation therapy for nonmetastatic prostate cancer. In this review, we discuss our findings on the role of ADT in the treatment of castrate‐sensitive nonmetastatic prostate cancer and the adverse effects associated with ADT. We also examine the recent advances in new predictive biomarkers for ADT, many of which are currently in the exploratory phase. Overall, the addition of ADT to primary therapy improves outcomes for patients with intermediate‐ or high‐risk prostate cancer.
Keywords: androgen deprivation therapy, biomarker, nonmetastatic prostate cancer, radical prostatectomy, radiotherapy
1. INTRODUCTION
Prostate cancer is the second most common cancer affecting men, with an estimated 1.1 million new cases in the world in 2012.1, 2 Primary therapy consists of radical prostatectomy or radiotherapy. However, patients with a positive margin, extraprostatic extension, lymph node involvement, high prostate‐specific antigen (PSA), or high Gleason Score (GS) are at high risk of prostate cancer recurrence following primary therapy. In these patients, androgen deprivation therapy (ADT) can be given as neoadjuvant therapy prior to primary therapy to shrink the tumor and reduce margin positivity. Radiotherapy, ADT, or a combination of the two can also be given as adjuvant treatment following primary therapy to reduce the risk of recurrence.
There are several different ADT modalities, which aim to deplete androgen levels by suppressing testicular androgen secretion or by inhibiting circulating androgens through targeting the androgen receptor. Consequently, ADT can be delivered by medical or surgical castration, antiandrogen therapy, and combined androgen blockade (CAB).3, 4
Although ADT monotherapy is not appropriate for clinically localized prostate cancer, the addition of ADT to primary therapy has been shown to improve outcomes significantly for certain men with intermediate‐ or high‐risk prostate cancer.4 However, many questions still remain unanswered, including the ideal patient population and optimal timing and duration of therapy. Exploring these questions is complicated by long survival and observation times, leading to fewer opportunities to conduct ideal randomized clinical trials. Furthermore, diverse study endpoints make comparisons difficult and a standard comparator is lacking. A need to address this challenge in prostate cancer patients has been exemplified by the Intermediate Clinical Endpoints in Cancer of the Prostate (ICECap) working group in the development of an intermediate clinical endpoint to serve as a robust surrogate for overall survival (OS).5 This review, therefore, discusses the current findings on the role of ADT in the treatment of castrate‐sensitive nonmetastatic prostate cancer. We also propose a treatment roadmap for ADT in this setting based on the available evidence.
2. METHODS
A PubMed search of all prospective and retrospective studies or meta‐analyses evaluating the outcomes of men treated with ADT for nonmetastatic prostate cancer published since 2000 was conducted. Findings on the use of neoadjuvant and adjuvant therapy in combination with radical prostatectomy or radiotherapy, and ADT at the time of biochemical recurrence were reviewed.
Based on existing publications, long‐term ADT treatment has been defined as treatment duration ≥18 months.6
3. RESULTS
3.1. ADT for patients who received radical prostatectomy as a primary treatment
Radical prostatectomy is typically used for patients with localized disease who have an estimated life expectancy of over 10 years, and in patients with locally advanced disease.
3.1.1. Neoadjuvant ADT plus radical prostatectomy
Men with early‐stage prostate cancer with intermediate or high risk of recurrence may be considered for neoadjuvant ADT prior to primary treatment. Neoadjuvant ADT before prostatectomy has been shown to provide long‐term progression‐free survival (PFS)2 and to significantly reduce the risk of recurrence (Table 1)7; however, it has generally not been shown to extend OS.7
Table 1.
Summary of results from key clinical trials that investigated ADT as neoadjuvant, adjuvant therapy, or treatment at biochemical recurrence in patients with prostate cancer who received radical prostatectomy
| Study (years) | Level of evidencea | Study type | N | Patient characteristics | Treatment and duration | Outcomes |
|---|---|---|---|---|---|---|
| (1966–2006)7 | 1 | Meta‐analysis | 11 149 | Localized or locally advanced PC with or without lymph node involvement (T1‐4, N1, M0) | Neoadjuvant ADT + RP versus RP alone |
|
| (1966–2006)7 | 1 | Meta‐analysis | 11 149 | Localized or locally advanced PC with or without lymph node involvement (T1‐4, N1, M0) | Adjuvant ADT following RP versus RP alone |
|
| Timing Of Antigen Deprivation (TOAD) therapy in patients with prostate cancer27 (2004–2012) | 2 | Prospective, randomized, phase 3 | 293 | PSA relapse after curative treatment (RP or RT), or ineligible for curative treatment | Immediate salvage ADT or delayed salvage ADT (recommended interval ≥ 2 y, unless clinically indicated) |
|
| French Genito‐Urinary Group and the French Association of Urology (GETUG‐AFU) 1628 (2006–2010) | 2 | Prospective, randomized, phase 3 | 743 | pT2‐4a PC with rising PSA of 0.2–2.0 ng/mL following RP without evidence of clinical disease | Salvage RT (66 Gy in 33 fractions 5 d/wk for 7 wk) + 6 mo ADT (goserelin) versus salvage RT alone |
|
| Radiation Therapy Oncology Group (RTOG) 960129 (1998–2003) | 2 | Prospective, randomized, phase 3 | 760 | pT3pN0 or pT2pN0 and positive margins; rising PSA (0.2–4.0 ng/mL) following RP | ADT (bicalutamide 150 mg daily for 2 y) during and after salvage RT (64.8 Gy in 36 fractions of 1.8 Gy) versus salvage RT alone |
|
| Eastern Cooperative Oncology Group (ECOG) 388616 (1988–1993) | 2 | Prospective, randomized | 98 | Clinically localized PC (T1b or T2) and had previously undergone RP + PLND | Immediate adjuvant ADT (goserelin monthly or bilateral orchiectomy) versus RP + salvage ADT |
|
| Southwest Oncology Group (SWOG) S992117, 20 (2000–2007) | 2 | Prospective, randomized | 983 | High‐risk features at RP (GS ≥ 8; preop PSA ≥ 15 ng/mL; stage T3b, T4, or N1; or GS = 7 + preop PSA ≥ 10 ng/mL or a positive margin) | Adjuvant ADT (goserelin + bicalutamide) alone or in combination with mitoxantrone chemotherapy for 2 y |
|
| SWOG 91092 (1993–1996) | 2 | Prospective, phase 2 | 62 | Locally advanced (T3–4, N0M0) PC | Neoadjuvant ADT (goserelin [1 mo] + flutamide [4 mo]) followed by RP |
|
| (2004–2012)8 | 3 | Retrospective | 156 | High‐risk (T1c–3) PC | Neoadjuvant therapy (LHRH agonist + estramustine for 6 mo) followed by RP versus neoadjuvant ADT for ≥6 mo followed by RT (3D conformal, 70–76 Gy in 2 Gy fractions) |
|
| (2000–2014)9 | 3 | Retrospective | 518 | High‐risk PC | Neoadjuvant therapy (LHRH agonist and for 6 mo + l‐PLND and RP versus e‐PLND and RP only) |
|
| (2002–2013)10 | 3 | Retrospective | 111 | High‐risk PC | Neoadjuvant hormonal therapy followed by RP |
|
| (2000–2014)11 | 3 | Retrospective | 116 | Initially inoperable PC | Neoadjuvant ADT for ≥3 mo or until PSA nadir reached followed by RP | Median OS: 10 y, comparable with that of patients with initially operable high‐risk PC |
| (2000–2006)18 | 3 | Retrospective | 128 | Locally advanced (pT3N0M0) PC | Immediate adjuvant ADT for ≥5 y |
|
| (1990–1999)19 | 3 | Matched cohort | 8290 | Pathological lymph node‐negative PC | RP + adjuvant ADT versus RP alone |
|
| (1989–2005)21 | 3 | Retrospective | 372 | High risk (PSA > 20 ng/mL, ≥T2c, or GS ≥ 8) PC | RP + adjuvant ADT (LHRH agonist, LHRH agonist/orchiectomy + oral antiandrogen, or orchiectomy alone) if seminal vesicle invasion or lymph node metastases were present versus RP alone |
|
| (2004–2012)26 | 3 | Retrospective | 132 | High‐risk PC (pelvic lymph node invasion, lymphovascular invasion, high tumor grade, or high preop PSA) | Adjuvant RT + adjuvant ADT (LHRH agonist or bicalutamide 150 mg/d) versus adjuvant RT alone following RP; duration of ADT left to the discretion of the physician |
|
ADT, androgen deprivation therapy; BCR, biochemical recurrence; BFS, biochemical progression‐free survival; DFS, disease‐free survival; DSS, disease‐specific survival; GS, Gleason score; HR, Hazards ratio; LHRH, luteinizing hormone‐releasing hormone; MFS, metastasis‐free survival; NR, not reported; OS, overall survival; OR, odds ratio; preop, preopeartive; PC, prostrate cancer; PFS, progression‐free survival; PLND, pelvic lymph node dissection; PSA, prostate‐specific antigen; RP, radical prostatectomy; RT, radiotherapy.
Level of evidence determined by study design: 1, meta‐analysis or systematic review; 2, randomized controlled trial; and 3, cohort study.
The phase 2 Southwest Oncology Group (SWOG) 9109 trial (N = 62) investigated neoadjuvant ADT plus radical prostatectomy for patients with locally advanced prostate cancer and demonstrated long survival.2 Median PFS was 7.5 years, and the 10‐year PFS rate was 40% (95% confidence interval [CI], 27–53%). Median OS was not reached, but the overall 10‐year OS rate was 68% (56–80%).
A retrospective study (N = 156) compared neoadjuvant therapy for 6 months followed by radical prostatectomy versus neoadjuvant ADT for ≥6 months followed by radiotherapy for patients with high‐risk prostate cancer.8 Biochemical PFS and OS rates were similar for both treatment groups. The 3‐year OS rate was 98.3% for neoadjuvant therapy plus radical prostatectomy versus 92.1% for neoadjuvant therapy plus radiotherapy (P = 0.156), and the 3‐year biochemical PFS rate was 86.4% versus 89.4% (P = 0.878). A larger retrospective analysis (N = 518) assessed whether neoadjuvant therapy with extended (e‐) pelvic lymph node dissection (PLND) conferred a benefit for high‐risk prostate cancer patients compared with neoadjuvant ADT with luteinizing hormone‐releasing hormone agonist (LHRHa) plus estramustine.9 Five‐year biochemical recurrence‐free survival rates were 84.9% and 54.7% for ADT and e‐PLND, respectively (P < 0.0001).
Neoadjuvant ADT can also eradicate high‐risk prostate cancer. A retrospective analysis of men with high‐risk prostate cancer (N = 111) treated with neoadjuvant ADT followed by radical prostatectomy found that 57.1% of men with non‐pT0 disease (residual tumor) developed biochemical relapse within a median of 14 months.10 However, among the six patients who had pT0 disease, none of them experienced recurrence after a median follow‐up of 59 months. Another retrospective analysis of men with initially inoperable prostate cancer (N = 116) treated with neoadjuvant ADT for ≥3 months, or until PSA nadir was reached (whichever was the sooner), found that median OS was 10 years, which is comparable to that of patients with initially operable high‐risk prostate cancer.11
A longer duration (either 6 or 8 months) of neoadjuvant hormonal therapy, compared with short‐term (usually 3 months) treatment, prior to radical prostatectomy, has demonstrated increased clinical benefit. This benefit usually presents as lower positive margin rates after prostatectomy and decreased PSA recurrence risk after 2–5 years.7, 12, 13, 14, 15 Between 3 and 8 months of neoadjuvant ADT therapy, prostate tumors may still undergo pathological and biochemical regression, which might result from prolonged duration of apoptosis of prostate tumor cells.12 However, due to the absence of long‐term survival results, the optimal duration of neoadjuvant therapy before radical prostatectomy is still to be elucidated.
Summary of findings for neoadjuvant ADT plus radical prostatectomy
Neoadjuvant ADT followed by radical prostatectomy is feasible in patients with localized or locally advanced prostate cancer with intermediate‐ or high‐risk features, or men with initially inoperable prostate cancer. Neoadjuvant ADT significantly reduced recurrence and positive surgical margin rates. A moderate level of evidence currently suggests that 6‐8 months of neoadjuvant ADT before radical prostatectomy provide clinical benefit but no studies have yet demonstrated an OS benefit. Neoadjuvant ADT plus radical prostatectomy warrants further exploration, particularly to determine the optimal duration of treatment. As of July 2018, no clinical trials have been listed on Clinicaltrials.gov comparing short‐term and long‐term neoadjuvant ADT prior to radical prostatectomy. However, there are currently seven studies that include the use of neoadjuvant ADT followed by radical prostatectomy (Clinicaltrials.gov identifiers: NCT01696877, NCT01409200, NCT01542021, NCT03358563, NCT00589472, NCT03228810, and NCT00430183), each using neoadjuvant ADT for different durations. A meta‐analysis of these studies in the future may provide more evidence for the optimal duration of neoadjuvant ADT.
3.1.2. Adjuvant ADT following radical prostatectomy
A number of studies have demonstrated that adjuvant ADT following radical prostatectomy results in excellent PFS, OS, and disease‐specific survival in patients with high‐risk localized or locally advanced prostate cancer (Table 1).15, 16, 17, 18
Several of these studies investigated the optimal timing and duration of ADT following radical prostatectomy. A matched cohort study (N = 8290) compared outcomes of patients with lymph node‐negative prostate cancer who were treated with radical prostatectomy with or without adjuvant ADT. Adjuvant ADT improved 10‐year rates for systemic PFS (95% vs 90%; P < 0.001) and disease‐specific survival (98% vs 95%; P = 0.009) compared with ADT following PSA increase; however, 10‐year OS was similar (84% vs 83%; P = 0.427).19
The Eastern Cooperative Oncology Group study EST 3886 study (n = 98) found that immediate, continuous adjuvant ADT following prostatectomy and lymphadenectomy significantly improved outcomes in men with node‐positive prostate cancer compared with ADT at clinical recurrence.16 Median PFS was 13.9 years for men who received immediate ADT versus 2.4 years for those who received salvage ADT (hazard ratio [HR], 3.42; P < 0.0001). Median disease‐specific survival was 12.3 years with salvage ADT and not reached with immediate ADT (HR, 4.09; P = 0.0004). OS was also significantly improved, with a median of 13.9 years versus 11.3 years with immediate ADT versus salvage ADT (HR, 1.84; P = 0.04). The duration of adjuvant ADT varied across studies; however, key studies have demonstrated excellent survival with long‐term adjuvant ADT.
Although ADT has been shown to significantly improve OS following radical prostatectomy, patients with high‐risk prostate cancer still experience worse disease progression and shorter OS than patients with lower‐risk disease. Chemotherapy may improve outcomes in a number of solid tumors, and the addition of chemotherapy to adjuvant ADT following radical prostatectomy was investigated in the SWOG S9921 study. Patients with high‐risk features at radical prostatectomy (N = 961) received 2 years of CAB alone or in combination with mitoxantrone chemotherapy and prednisone.17, 20 Survival results were greater than expected and similar for both treatment regimens. Ten‐year disease‐free survival was 72% in both treatment groups (HR, 1.01; P = 0.94), and 10‐year OS was 87% in the CAB group and 86% in the CAB plus mitoxantrone group (HR, 1.06; P = 0.70). However, mitoxantrone and prednisone when added to CAB significantly increased the risk of leukemia and other cancers, making CAB alone preferable.
A retrospective study of men with pT3N0M0 prostate cancer who received adjuvant ADT following radical prostatectomy (N = 128) found that immediate long‐term ADT for ≥5 years is feasible in patients with locally advanced prostate cancer.18 The 10‐year disease‐specific survival and hormone‐refractory biochemical PFS rates were 96.3% and 88.3%, respectively.
Another study retrospectively analyzed patients with high‐risk prostate cancer (N = 372) who received radical prostatectomy; patients with seminal vesicle invasion or lymph node metastases also received adjuvant ADT.21 Five‐ and 10‐year biochemical PFS rates were 76.6% and 56.2%, respectively. Despite having more advanced prostate cancer, patients who received adjuvant ADT had significantly longer biochemical PFS (P = 0.0019). Five‐ and 10‐year OS rates were 84.3% and 72.1%, respectively. OS was similar between the two patient groups (P = 0.0821). These findings demonstrate that stage‐dependent adjuvant therapy for patients receiving radical prostatectomy is a viable therapeutic option.
Summary of findings for adjuvant ADT following radical prostatectomy
Findings from these studies show that long‐term adjuvant ADT immediately following radical prostatectomy can benefit men with high‐risk prostate cancer. Several studies have demonstrated progression‐free and disease‐specific survival benefits, and one study showed that immediate, continuous adjuvant ADT significantly prolonged OS compared with delaying ADT until progression. Adjuvant ADT following radical prostatectomy is supported by strong evidence (meta‐analysis of over 10 000 patients) for men with high‐risk localized or locally advanced prostate cancer, particularly those with positive lymph nodes. Further study is needed to determine the optimal duration of treatment; however, selecting a control arm and conducting randomized clinical trials with very long follow‐up times is challenging. Clinical studies are also needed to determine whether adjuvant ADT following radical prostatectomy benefits patients with intermediate‐risk prostate cancer.
3.1.3. Adjuvant radiotherapy plus ADT following radical prostatectomy
Radiotherapy as adjuvant therapy following radical prostatectomy was also reported to improve outcomes in a number of studies.22, 23, 24 Compared with radical prostatectomy alone, adjuvant radiotherapy has been shown to significantly improve biochemical PFS (10‐year PFS: 56% vs 35%; P < 0.0001),22, 23, 24 median metastasis‐free survival (14.7 years vs 12.9 years; HR, 0.71; P = 0.016),25 and median OS (15.2 years vs 13.3 years; HR, 0.72; P = 0.023).25
The combination of adjuvant ADT plus adjuvant radiotherapy following radical prostatectomy was analyzed in a retrospective study (N = 132), which found that men with high‐risk prostate cancer who received adjuvant radiotherapy plus ADT following prostatectomy had excellent outcomes (Table 1).26 Five‐year biochemical relapse‐free, metastasis‐free, disease‐specific, and OS rates were 90.5, 95.9, 100, and 90.6%, respectively. The median duration of ADT was 24 months (6–36). Further investigation into this combination regimen is therefore warranted.
Summary of findings for adjuvant radiotherapy plus ADT following radical prostatectomy
A strong level of evidence supports combining adjuvant ADT with adjuvant radiotherapy following radical prostatectomy, which meta‐analysis has shown to improve outcomes compared with radical prostatectomy alone, particularly in men with high‐risk prostate cancer. Five‐year biochemical relapse‐free, metastasis‐free, disease‐specific, and OS rates were >90% with long‐term ADT in combination with adjuvant radiotherapy.
3.1.4. Biochemical recurrence following radical prostatectomy
The standard of care for patients with biochemical recurrence is ADT. However, the optimal timing of ADT (early or late) and the optimal adjuvant regimen remain controversial. Three phase 3 trials have investigated these issues (Table 1).
The timing of antigen deprivation (TOAD) study (n = 293) investigated immediate treatment with ADT versus delayed ADT for men with PSA relapse following radical prostatectomy or radiotherapy, and men with incurable prostate cancer.27 The investigated timing of delayed ADT was ≥2 years after biochemical recurrence, unless earlier treatment was clinically indicated. Immediate ADT significantly improved OS (5‐year OS was 91.2% vs 86.4%; P = 0.047) compared with delayed ADT.
The French genito‐urinary group and the French association of urology (GETUG‐AFU) 16 study (n = 743) compared salvage radiotherapy plus short‐term ADT versus salvage radiotherapy alone for men with rising PSA following radical prostatectomy.28 This study showed that 6 months of ADT plus salvage radiotherapy significantly improved PFS (5‐year PFS was 80% vs 62%; HR, 0.50; P < 0.0001). However, OS was similar between the two treatment groups; 5‐year OS was 96% (93‐98%) vs 95% (92‐97%; HR, 0.7; P = 0.18).
The RTOG 9601 study (n = 760) investigated long‐term ADT plus salvage radiotherapy versus salvage radiotherapy alone in patients with rising PSA following radical prostatectomy.29 The addition of 24 months of ADT improved 12‐year OS (76.3% vs 71.3%; HR, 0.77; P = 0.04) and reduced the 12‐year rates of metastatic prostate cancer (14.5% vs 23.0%; HR, 0.63; P = 0.005) and prostate cancer‐specific mortality (5.8% vs 13.4%; HR, 0.49; P < 0.001). In addition, subgroup analyses indicated that men with a GS ≥7, PSA = 0.7‐4.0 ng/mL, or positive surgical margins were most likely to benefit.
Summary of findings for biochemical recurrence following radical prostatectomy
ADT with or without radiotherapy at the time of biochemical relapse significantly improved outcomes for patients who previously received radical prostatectomy as a primary therapy, which is strongly supported by three phase 3, randomized, prospective trials. Among the salvage treatment options that have been studied, radiotherapy plus long‐term ADT provided an OS benefit. Thus, radiotherapy plus long‐term ADT is recommended at the time of biochemical recurrence, especially for men with GS ≥7, PSA = 0.7‐4.0 ng/mL, or positive surgical margins.
3.2. ADT for patients who received radiotherapy as a primary treatment
Radiotherapy, either external beam or brachytherapy, can be administered alone, following radical prostatectomy, or in combination with ADT to patients with prostate cancer. In patients with low‐risk localized prostate cancer, radiotherapy alone has been shown to provide durable control, with 73% each disease‐free survival rates at 15, 20, and 25 years.30
Many studies have shown that radiotherapy plus adjuvant ADT provides a benefit for patients with intermediate‐risk, high‐risk, or locally advanced disease (Table 2). Results from a meta‐analysis indicated that adjuvant ADT following radiotherapy significantly improves 5‐year OS (odds ratio [OR], 1.46; P = 0.0009), disease‐specific survival (OR, 2.10; P = 0.00001), and disease‐free survival (OR, 2.53; P < 0.00001).7
Table 2.
Summary of results from important clinical trials that investigated radiotherapy as primary therapy with or without neoadjuvant or adjuvant ADT
| Study (years) | Level of evidencea | Study type | N | Patient characteristics | Treatment and duration | Outcomes (95% CI) |
|---|---|---|---|---|---|---|
| 1966–20067 | 1 | Meta‐analysis | 11 149 | Localized or locally advanced PC with or without lymph node involvement (T1–4 N1, M0) | Adjuvant ADT following RT versus RT alone |
|
| DFCI 9509631 (1995–2001) | 2 | Prospective, randomized | 206 | Localized (T1b‐T2b) but unfavorable‐risk PC | RT plus 6 mo ADT (LHRHa + flutamide) versus RT alone |
|
| EORTC 2299132 (2001–2008) | 2 | Prospective, randomized | 819 | Localized (T1b‐T2aN0M0) or locally advanced (T2b‐T4) PC | RT + concomitant/adjuvant ADT for 6 mo (goserelin) versus RT alone |
|
| RTOG 94–0833 (1994–2001) | 2 | Prospective, randomized, phase 3 | 1979 | T1b‐T2b PC with PSA ≤20 ng/mL | 4 mo CAB beginning 2 mo before RT (46.8 Gy to pelvis and 19.8 Gy to prostate) versus RT alone |
|
| RTOG 991034 (2000–2004) | 2 | Prospective, randomized | 1579 | Intermediate‐risk PC | Neoadjuvant CAB (8 wk vs 28 wk) + 8 wk CAB during RT |
|
| TROG 96.0135 (1996–2000) | 2 | Prospective, randomized | 818 | T2b, T2c, T3, or T4, N0, M0 PC | Neoadjuvant ADT (goserelin + flutamide, 6 mo vs 3 mo) + RT (66 Gy in 33 fractions over 6.5‐7 wk) versus RT alone |
|
| DART01/05 GICOR36 (2005–2010) | 2 | Prospective, randomized, phase 3 | 355 | Clinical stage T1c‐T3b N0M0 PC with intermediate‐ or high‐risk factors | Short‐term ADT: Neoadjuvant and concomitant ADT for 4 mo +radiotherapy (3D conformal) versus long‐term ADT: the same treatment + adjuvant ADT for 24 mo |
|
| EORTC 2286337 (1987–1995) | 2 | Prospective, randomized, phase 3 | 415 | High‐risk T1–4 PC | Long‐term ADT: 36 mo goserelin plus external RT (5 days/wk for 7 wk, total dose 50 Gy to whole pelvis plus additional 20 Gy to prostate and seminal vesicles) versus RT alone |
|
| RTOG 861038 (1987–1991) | 2 | Prospective, randomized, phase 3 | 456 | Locally advanced (T2‐4) PC with or without lymph node involvement | EBRT + neoadjuvant CAB (goserelin + flutamide) for 2 mo before and concurrent with EBRT versus EBRT alone |
|
| EORTC 2296139 (1997–2001) | 2 | Prospective, randomized | 1113 | Locally advanced (T1c–T2a–b, pN1–2, M0 or T2c–4, cN0–2, M0) PC | EBRT (3D conformal, 50 Gy for first target volume, an additional 20 Gy for the second target volume, 5 d/wk for 7 wk) + ADT (LHRH analog) for 6 mo or 3 y | 5‐y overall mortality: 19.0% versus 15.2%; HR, 1.42 |
| Trials 23, 24, and 2540 (NR) | 2 | Three prospective, randomized trials | 8113 | Localized (T1–2 N0/Nx M0) or locally advanced (T3–4 and any N, or any T and N+; M0) PC | Standard care plus either bicalutamide 150 mg daily or placebo |
|
| RTOG 85–3141 (1987–1992) | 2 | Prospective, randomized, phase 3 | 945 | Locally advanced (T3 or regional lymphatic involvement) PC | RT (1.8‐2.0 Gy) daily for 4‐5 times/wk for a total of 44–46 Gy plus additional 20‐25 Gy to prostate) plus adjuvant ADT (goserelin) until progression or RT alone followed by salvage ADT |
|
| RTOG 92‐0242 (1992–1995) | 2 | Prospective, randomized | 1554 | Locally advanced (T2c‐T4, N0‐X) PC with no extra pelvic lymph node involvement and PSA < 150 ng/mL | ADT (goserelin + flutamide) for 4 mo before and during RT (45 Gy to pelvic nodes and 65‐70 Gy to the prostate) with or without an additional 2 y goserelin after RT |
|
| SWOG‐JPR743 (1999–2005) | 2 | Prospective, randomized | 1386 | Rising PSA > 3 ng more than 12 mo after primary or salvage RT | Intermittent salvage ADT (LHRHa + nonsteroidal antiandrogen in 8‐mo cycles versus continuous salvage ADT (LHRHa + nonsteroidal antiandrogen or orchiectomy) |
|
ADT, androgen deprivation therapy; BFS, biochemical progression‐free survival; CAB, combined androgen blockade; DFS, disease‐free survival; DSM, disease‐specific mortality; DSS, disease‐specific survival; EBRT, external beam radiation therapy; GS, Gleason score; LHRH, luteinizing hormone‐releasing hormone; NR, not reported; OS, overall survival; PFS, progression‐free survival; PSA, prostate‐specific antigen; RP, radical prostatectomy; RT, radiotherapy.
Level of evidence determined by study design: 1, meta‐analysis or systematic review and 2, randomized controlled trial.
3.2.1. Radiotherapy plus ADT for patients with intermediate‐risk disease
The DFCI 95096 study (N = 206) showed that 6 months of CAB plus radiotherapy significantly improved OS compared with radiotherapy alone in men with intermediate‐risk localized prostate cancer (8‐year OS was 74% [64–82%] vs 61% [49–71%]; P = 0.01).31 Men with no or minimal comorbidities benefited from ADT; however, ADT may have a negative impact on survival for men with moderate or severe comorbidities.
In the EORTC 22991 study (N = 819), radiotherapy plus 6 months of concomitant and adjuvant ADT improved biochemical and disease‐free survival compared with radiotherapy alone.32 Five‐year biochemical disease‐free survival was 82.6% (78.4–86.1%) vs 69.8% (64.9–74.2%); HR, 0.52 (0.41–0.66); P < 0.001. Five‐year clinical disease‐free survival was 88.7% (82.1–85.2%) vs 80.8% (76.5–84.3%); HR, 0.63; P = 0.001.
In the phase 3 RTOG 94‐08 study (n = 1979), short‐term CAB for 4 months before and during radiotherapy was associated with a decreased disease‐specific mortality and increased OS.33 Patients with intermediate‐risk prostate cancer benefited from neoadjuvant and concurrent ADT; however, there was no benefit for those with low‐ or high‐risk disease.
A fourth study, the RTOG 9910 study (n = 1,579), investigated treatment with 8 weeks versus 28 weeks of neoadjuvant CAB therapy plus 8 weeks of CAB during radiotherapy.34 Outcomes were similar for the two treatment groups, suggesting that CABs for 8 weeks before and 8 weeks during radiotherapy are preferred for patients with intermediate‐risk prostate cancer.
Summary of findings for men with intermediate‐risk disease
Compelling evidence supports the use of short‐term ADT in combination with an LHRHa and an antiandrogen to provide benefit to men with intermediate‐risk prostate cancer, particularly, those with unfavorable intermediate‐risk disease with no or minimal comorbidities. Short‐term ADT (neoadjuvant/concomitant ADT for 4 months, concomitant/adjuvant ADT for 4‐6 months, or adjuvant ADT for 6 months) is recommended for intermediate‐risk patients who received radiotherapy as a primary therapy.
3.2.2. Radiotherapy plus ADT for high‐risk localized and locally advanced prostate cancer
For high‐risk disease, several studies have investigated various lengths of ADT therapy in combination with radiotherapy. The results demonstrate better outcomes with long‐term use of ADT, which has become the standard of care.
The TROG 96.01 study (N = 818) compared three different treatment groups: radiotherapy preceded by 6 months of neoadjuvant ADT, radiotherapy plus 3 months of neoadjuvant ADT, and radiotherapy alone in patients with high‐risk, localized prostate cancer.35 The study showed that 6 months of ADT significantly improved outcomes compared with radiotherapy alone, and also improved outcomes compared with 3 months of ADT.
In the phase 3 DART01/05 GICOR study (N = 355), men with intermediate‐ or high‐risk prostate cancer were treated with 4 months of neoadjuvant plus concurrent ADT combined with radiotherapy (short‐term ADT), or with the same treatment followed by 24 months of adjuvant ADT (long‐term ADT).36 Long‐term ADT plus radiotherapy improved biochemical control and OS compared with short‐term ADT. The 5‐year OS rate was 95% (93–97%) in the long‐term ADT group versus 86% (83–89%) in the short‐term ADT group (HR, 2.48; P = 0.009).
The phase 3 EORTC 22863 clinical trial in patients with high‐risk prostate cancer (N = 415) showed that adjuvant ADT with an LHRHa during, and for 3 years following, radiotherapy significantly improved 10‐year clinical disease‐free survival and OS compared with radiotherapy alone.37 The 10‐year OS rate was 58.1% (49.2–66.0%) in the group that received adjuvant ADT versus 39.8% (31.9–47.5%) in the group that received radiotherapy alone (HR, 0.60; P = 0.0004). In addition, 10‐year prostate cancer mortality was decreased in high‐risk patients receiving adjuvant ADT.
For locally advanced disease, many studies have demonstrated that the addition of long‐term adjuvant therapy following radiotherapy improved outcomes. The phase 3 RTOG 8610 study (N = 456) investigated 2 months of neoadjuvant CAB plus 2 months of ADT during external beam radiotherapy versus radiotherapy alone for men with locally advanced prostate cancer with or without lymph node involvement.38 The addition of ADT improved 10‐year disease‐free survival, disease‐specific mortality, distant metastases, and biochemical failure. There was also a trend toward improved OS.
Furthermore, results from the EORTC 22961 study in patients with locally advanced prostate cancer (N = 970) demonstrated that radiotherapy plus 3 years of adjuvant ADT decreased mortality compared with radiotherapy plus 6 months of adjuvant ADT.39 The 5‐year overall mortality rate was 19.0% (15.5–23.0%) for short‐term ADT versus 15.2% (12.1–18.9%) for long‐term ADT (HR, 1.42). The 5‐year prostate cancer‐specific mortality rate was 4.7% (2.7–6.7%) versus 3.2% (1.6–4.8%; HR, 1.71; P = 0.002).
Trials 23, 24, and 25 of the bicalutamide Early Prostate Cancer program investigated treatment with radiotherapy, radical prostatectomy, or watchful waiting followed by 150 mg bicalutamide or placebo in men with localized or locally advanced prostate cancer (N = 8113). A combined analyses showed that, among patients treated with radiotherapy, the addition of bicalutamide improved PFS (HR, 0.62; P = 0.001) and OS (HR, 0.70; P = 0.031) in men with locally advanced disease.40
Results from the RTOG 85‐31 study in locally advanced prostate cancer (N = 945) patients showed that OS improved with radiotherapy plus adjuvant goserelin until progression compared with radiotherapy and goserelin at relapse.41 The 10‐year OS rate was 49% in the adjuvant ADT group versus 39% in the ADT at relapse group (P = 0.002); the benefit was significant in men with GS 7 or GS 8‐10 but not those with GS 2‐6.
In the RTOG 92‐02 study of men with locally advanced disease (N = 1554), 10‐year disease‐free survival, disease‐specific survival, local progression, distant metastasis, and biochemical failure rates were all improved with 4 months of goserelin and flutamide neoadjuvant therapy, radiotherapy, and 24 months of goserelin adjuvant therapy compared with neoadjuvant therapy plus radiotherapy without long‐term adjuvant therapy.42
Summary of findings for men with high‐risk localized and locally advanced disease
The results from these studies strongly indicate that the higher the patient's risk of recurrence, the longer the duration of ADT should be used as adjuvant therapy in combination with radiotherapy. Long‐term use of ADT (2–3 years) concomitant or adjuvant with LHRHa, with or without an antiandrogen, is correlated with improved PFS and OS in patients with high‐risk or locally advanced nonmetastatic prostate cancer. Alternatively, a shorter course of ADT (4–6 months) may be sufficient for patients with intermediate‐risk prostate cancer.
3.2.3. Biochemical recurrence following radiotherapy
The SWOG‐JPR7 study investigated intermittent versus continuous ADT in men with elevated PSA following primary or salvage radiotherapy (N = 1386; Table 2).43 Patients were treated with an LHRHa plus a nonsteroidal antiandrogen either continuously or in 8‐month cycles. Nontreatment periods were determined by PSA levels. Median OS was similar: 8.8 years in the intermittent group and 9.1 years in the continuous group (HR, 1.02). However, certain quality‐of‐life issues improved with intermittent ADT. Thus, intermittent ADT was noninferior to continuous therapy at the time of biochemical relapse following radiotherapy but improved quality of life, particularly, during nontreatment phases. Further research is warranted to determine the optimal PSA level to undergo intermittent ADT. The difficulties in determining the optimal PSA level for reinitiating ADT may be resolved by conducting studies with more stringent patient stratification in terms of prognostic parameters. However, this presents a difficulty in limiting the number of patients who fit the specified criteria, resulting in a small sample size.
3.3. Unfit or unwilling to receive primary treatment
ADT can be used in men with localized or locally advanced prostate cancer who refuse, or are not candidates for, primary treatment, including those with limited life expectancy, advanced tumor stage, or other serious comorbidities. In the EORTC 30891 study, men with newly diagnosed localized or locally advanced prostate cancer who were not suitable for primary treatment were treated with buserelin, an LHRH analog, immediately or at symptomatic disease progression (n = 985).44 Immediate treatment significantly improved OS (HR, 1.25; noninferiority P > 0.1). Trials 23, 24, and 25, as described above, found that patients with locally advanced disease derived a greater improvement in PFS from 150 mg bicalutamide compared to watchful waiting patients (HR, 0.67; P < 0.001).39
3.4. Real‐life implications of ADT
There is a lack of ongoing studies investigating the real‐life implications of ADT in prostate cancer patients with regard to overall benefit of the therapy. Of the 4033 ongoing prostate cancer studies listed in Clinicaltrials.gov, only one (Clinicaltrials.gov identifier NCT02895230) is an observational study investigating the real‐life implications of ADT, enrolling all men receiving treatment for prostate cancer over an 18‐month period. While the primary objective of this study is to investigate the association between ADT and vascular stroke, it would also be of interest to determine whether different modalities of ADT have an impact on survival or not. Previous studies have established a survival benefit associated with the use of ADT in patients with high‐risk or locally advanced disease, with up to 26% and 24% more patients still alive at 5 and 10 years, respectively.45 There are two ongoing prostate cancer studies taking place in China (Clinicaltrials.gov identifier NCT03177551 and NCT03507597), but these do not focus on real‐life implications of ADT. However, the Asian Prostate Cancer Study, a database established through an alliance of 12 countries and regions in Asia, is a promising source of real‐world data on the implications of prostate cancer, including ADT.46
3.5. Adverse events associated with ADT
While neoadjuvant and/or adjuvant ADT offers significant improvements to survival, it can cause significant morbidity and negatively affect quality of life. Most adverse events are not dose limiting and can be managed through pharmacological or other interventions. ADT is associated with a decrease in bone mineral density, resulting in an increased risk of bone fractures.47 Denosumab and toremifene have both been shown to increase bone mineral density and decrease risk of fractures in randomized controlled studies with over 1000 participants.48, 49 Prospective and population‐based studies have associated the use of ADT with the development of metabolic syndrome, a group of cardiovascular risk factors related to insulin resistance. An observational study by Keating et al analyzed a population‐based cohort of 73 196 men and found an increased risk of insulin resistance and development of diabetes following ADT (HR, 1.44; P < 0.001).50 Exercise and prophylactic use of metformin have been trialed and are promising strategies to prevent the development of metabolic syndrome in men undergoing ADT.51, 52 However, this has only been trialed in small populations and further research with larger cohorts is required to validate these preliminary findings. Increased risk of cardiovascular disease (CVD) and CVD‐related death has been associated with ADT.50 CVD events can differ between different modalities of ADT. A study involving 3578 Chinese patients found that orchiectomy results in more ischemic events than gonadotropin‐releasing hormone agonists.53
Sexual dysfunction affects over 90% of men receiving ADT, due to the reduction in testosterone. This results in loss of libido, as well as erectile dysfunction. For men with rising PSA but no metastases, intermittent ADT may be a feasible option. In a randomized, noninferiority study involving 1386 patients, there was no difference in OS between men receiving continuous versus intermittent ADT (HR, 1.03; P = 0.009), and the group receiving intermittent ADT experienced significant improvements in libido, fatigue, and overall quality of life.43 Other effects of ADT affecting quality of life include gynecomastia, fatigue, and hot flashes.54 Gynecomastia and breast pain are common adverse events, affecting up to 85% of men receiving high‐dose ADT, and strategies to mitigate these effects are currently being investigated. Although ADT is associated with a number of adverse side effects, a cohort study of 13 368 patients from the Taiwan National Health Insurance Research Database identified a reduced risk of dementia in men with prostate cancer who underwent chemical castration (HR, 0.79; P < 0.001).55
3.6. New predictive biomarkers for ADT and personalized therapy
Prognostic and predictive biomarkers have the potential for optimizing therapy through personalization of treatment regimens. The use of blood or urine‐based prognostic markers, present a minimally invasive method for determining treatment response, allowing more responsive adjustments to therapy when necessary.
Inactivating phosphate and tensin homolog (PTEN) mutations are commonly detected in prostate cancers and are associated with a poorer prognosis. PTEN expression may also be able to predict response to ADT; this is currently being investigated in patients with intermediate‐ and high‐risk prostate cancer (Clinicaltrials.gov identifier NCT01542021). What's more, subtyping based on luminal and basal lineage could also potentially serve as a predictive biomarker.56 Results from an analysis of 1567 prostate cancer samples from high‐risk patients treated with prostatectomy showed that luminal B prostate cancers were significantly associated with response to ADT: 10‐year metastasis occurred in 33% for those treated with ADT versus 55% for those untreated (P = 0.006), suggesting that luminal/basal subtyping may be useful in the selection of patient treatment.56
Circulating tumor cells (CTCs) are increasingly investigated prognostic biomarkers in many cancers, including prostate cancer. High levels of CTCs are correlated with unfavorable prognosis and worse clinical outcomes.57, 58 Protein expression in CTCs can also be used as a predictive biomarker of treatment response, where epidermal growth factor receptor (EGFR) expression in CTCs is associated with poor response to ADT (time to progression of 5 months [EGFR+] compared to 11 months [EGFR−]; P < 0.05).59 By using a blood test instead of radiographic imaging, monitoring can be performed more frequently, allowing prompt treatment before metastatic tumors become clinically detectable.
Currently, ongoing clinical trials are investigating the use of urine metabolomic profiling, as well as the expression of tumor markers in CTCs as biomarkers for predicting response to therapy. These studies are being explored in small populations and will require further validation before they can be applied in a clinical setting.
4. CONCLUSIONS
The addition of ADT to primary therapy significantly improves outcomes for certain men with intermediate‐ or high‐risk prostate cancer and is recommended in these settings in our proposed roadmap for treatment (Figure 1). Among men who undergo radical prostatectomy as primary therapy, neoadjuvant ADT is feasible in those with localized or locally advanced prostate cancer and warrants further exploration. Long‐term adjuvant ADT is recommended immediately following radical prostatectomy for men with high‐risk localized or locally advanced prostate cancer, particularly those with positive lymph nodes. Radiotherapy plus long‐term ADT provided an OS benefit and is recommended at the time of biochemical recurrence, particularly for men with GS ≥7, PSA 0.7–4.0 ng/mL, or positive surgical margins. ADT with an LHRHa and an antiandrogen is recommended for 4–6 months following radiotherapy as a primary therapy, for patients with intermediate‐risk disease, while ADT with an LHRHa with or without an antiandrogen is recommended for 2–3 years in high‐risk localized or locally advanced disease. Intermittent ADT was noninferior to continuous therapy following biochemical recurrence but it improved quality of life, particularly during nontreatment phases. Further research is warranted to determine the optimal PSA level at which to begin intermittent ADT; however, the inherent challenges associated with undertaking such studies makes identifying optimal PSA levels an elusive target. Several adverse events are frequently associated with ADT. While they are not dose limiting, some of these events can cause serious morbidity such as death from CVD or bone fractures due to loss of bone mineral density. As such, careful monitoring of patients is required during use of ADT. A predictive biomarker may be helpful to identify patients who would benefit from ADT. It is still at the stage of exploration and research. Further clinical studies are needed to confirm and validate the clinical value of these predictive biomarkers, so as to guide clinical practice in future.
Figure 1.
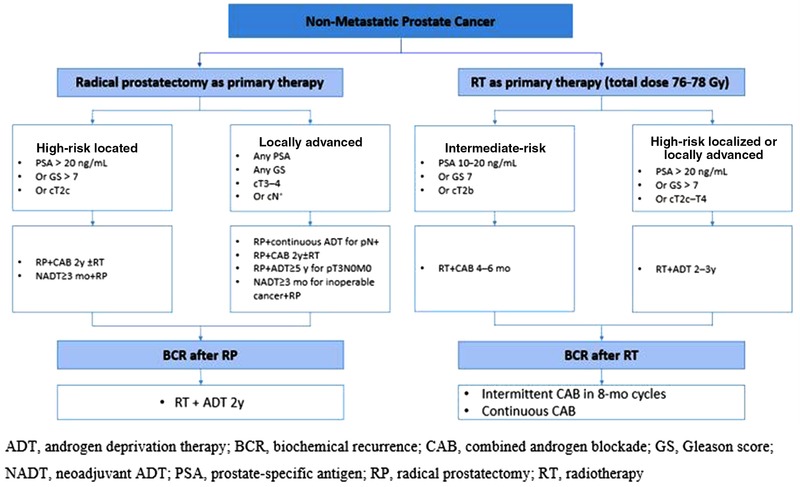
ADT treatment roadmap [Color figure can be viewed at http://wileyonlinelibrary.com]
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
ACKNOWLEDGMENTS
All authors contributed to the study design and were responsible for the final interpretation of the data and development of the manuscript. All authors read and approved the final manuscript. Editorial assistance in manuscript preparation and data collation for this review was provided by Julie Shilane, PhD, of Articulate Science LLC and Joyce Lee, PhD, of Nucleus Global, Shanghai, China, funded by AstraZeneca.
Fang D, Zhou L. Androgen deprivation therapy in nonmetastatic prostate cancer patients: Indications, treatment effects, and new predictive biomarkers. Asia‐Pac J Clin Oncol. 2019;15:108–120. 10.1111/ajco.13108
REFERENCES
- 1. Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, eds. SEER Cancer Statistics Review, 1975–2013. Bethesda, MD: National Cancer Institute; 2015. http://seer.cancer.gov/csr/1975_2013/ [Google Scholar]
- 2. Berglund RK, Tangen CM, Powell IJ, et al. Ten‐year follow‐up of neoadjuvant therapy with goserelin acetate and flutamide before radical prostatectomy for clinical T3 and T4 prostate cancer: update on southwest oncology group study 9109. Urology. 2012;79:633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mottet N, Bellmunt J, Bolla M, et al. EAU‐ESTRO‐SIOG guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–629. [DOI] [PubMed] [Google Scholar]
- 4. Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. [DOI] [PubMed] [Google Scholar]
- 5. ICECaP Working Group , Sweeney C, Nakabayashi M, et al. The development of intermediate clinical endpoints in cancer of the prostate (ICECaP). J Natl Cancer Inst. 2015;107:djv261 10.1093/jnci/djv261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nabid A, Carrier N, Martin A, et al. Duration of androgen deprivation therapy in high‐risk prostate cancer: a randomized phase III trial. Eur Urol. 2018; 74:432–441. [DOI] [PubMed] [Google Scholar]
- 7. Kumar S, Shelley M, Harrison C, Coles B, Wilt TJ, Mason MD. Neo‐adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer. Cochrane Database Syst Rev. 2006:CD006019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koie T, Ohyama C, Yamamoto H, et al. Both radical prostatectomy following treatment with neoadjuvant LHRH agonist and estramustine and radiotherapy following treatment with neoadjuvant hormonal therapy achieved favorable oncological outcome in high‐risk prostate cancer: a propensity‐score matching analysis. World J Surg Oncol. 2014;12:134 10.1186/1477-7819-12-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Narita T, Koie T, Ookubo T, et al. The impact of extended lymph node dissection versus neoadjuvant therapy with limited lymph node dissection on biochemical recurrence in high‐risk prostate cancer patients treated with radical prostatectomy: a multi‐institutional analysis. Med Oncol. 2017;34:e543. [DOI] [PubMed] [Google Scholar]
- 10. Joung JY, Kim JE, Kim SH, et al. The prevalence and outcomes of pT0 disease after neoadjuvant hormonal therapy and radical prostatectomy in high‐risk prostate cancer. BMC Urol. 2015;15:82 10.1186/s12894-015-0079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hajili T, Ohlmann C, Linxweiler J, Siemer S, Stoeckle M, Saar M. Neoadjuvant androgen deprivation in primarily inoperable prostate cancer: consecutive assessment of peri‐ and postoperative outcomes. J Urol. 2016;195:MP50–04. [Google Scholar]
- 12. Gleave ME, Goldenberg SL, Chin JL, et al . Randomized comparative study of 3 versus 8‐month neoadjuvant hormonal therapy before radical prostatectomy: biochemical and pathological effects. J Urol. 2001;166:500–506. [PubMed] [Google Scholar]
- 13. Gleave ME, La Bianca SE, Goldenberg SL, Jones EC, Bruchovsky N, Sullivan LD. Long‐term neoadjuvant hormone therapy prior to radical prostatectomy: evaluation of risk for biochemical recurrence at 5‐year follow‐up. Urology 2000;56:289–294. [DOI] [PubMed] [Google Scholar]
- 14. Naiki T, Kawai N, Okamura T, et al. Neoadjuvant hormonal therapy is a feasible option in laparoscopic radical prostatectomy. BMC Urol. 2012;12:36 10.1186/1471-2490-12-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Selli C, Montironi R, Bono A, et al. Effects of complete androgen blockade for 12 and 24 weeks on the pathological stage and resection margin status of prostate cancer. J Clin Pathol. 2002;55:508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node‐positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–479. [DOI] [PubMed] [Google Scholar]
- 17. Dorff TB, Flaig TW, Tangen CM, et al. Adjuvant androgen deprivation for high‐risk prostate cancer after radical prostatectomy: sWOG S9921 study. J Clin Oncol. 2011;29:2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsurumaki Sato Y, Fukuhara H, Suzuki M, et al. Long‐term results of radical prostatectomy with immediate adjuvant androgen deprivation therapy for pT3N0 prostate cancer. BMC Urol. 2014;14:13 10.1186/1471-2490-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siddiqui SA, Boorjian SA, Inman B, Bagniewski S, Bergstralh EJ, Blute ML. Timing of androgen deprivation therapy and its impact on survival after radical prostatectomy: a matched cohort study. J Urol. 2008;179:1830–1837. [DOI] [PubMed] [Google Scholar]
- 20. Glode L, Tangen C, Hussain M, et al. Adjuvant androgen deprivation (ADT) versus mitoxantrone plus prednisone (MP) plus ADT in high‐risk prostate cancer (PCa) patients following radical prostatectomy: A phase III intergroup trial (SWOG S9921). Paper presented at 2017 Genitourinary Cancers Symposium February 16–18, 2017; Orlando, FL.
- 21. Spahn M, Weiss C, Bader P, et al. Long‐term outcome of patients with high‐risk prostate cancer following radical prostatectomy and stage‐dependent adjuvant androgen deprivation. Urol Int. 2010;84:164–173. [DOI] [PubMed] [Google Scholar]
- 22. Wiegel T, Bartkowiak D, Bottke D, et al. Adjuvant radiotherapy versus wait‐and‐see after radical prostatectomy: 10‐year follow‐up of the ARO 96‐02/AUO AP 09/95 trial. Eur Urol. 2014;66:243–250. [DOI] [PubMed] [Google Scholar]
- 23. Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high‐risk prostate cancer: long‐term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380:2018–2027. [DOI] [PubMed] [Google Scholar]
- 24. Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate‐specific antigen: aRO 96‐02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–2930. [DOI] [PubMed] [Google Scholar]
- 25. Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long‐term followup of a randomized clinical trial. J Urol. 2009;181:956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Omrcen T, Hrepic D, Boraska Jelavic T, Vrdoljak E. Combination of adjuvant radiotherapy and androgen deprivation therapy after radical prostatectomy in high risk prostate cancer patients—results from retrospective analysis. J BUON. 2015;20:1061–1067. [PubMed] [Google Scholar]
- 27. Duchesne GM, Woo HH, Bassett JK, et al. Timing of androgen‐deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01–03 [TOAD]): a randomised, multicentre, non‐blinded, phase 3 trial. Lancet Oncol. 2016;17:727–737. [DOI] [PubMed] [Google Scholar]
- 28. Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short‐term hormone therapy for rising prostate‐specific antigen concentration after radical prostatectomy (GETUG‐AFU 16): a randomised, multicentre, open‐label phase 3 trial. Lancet Oncol. 2016;17:747–756. [DOI] [PubMed] [Google Scholar]
- 29. Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Critz FA, Benton JB, Shrake P, Merlin ML. 25‐year disease‐free survival rate after irradiation for prostate cancer calculated with the prostate specific antigen definition of recurrence used for radical prostatectomy. J Urol. 2013;189:878–883. [DOI] [PubMed] [Google Scholar]
- 31. D'Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299:289–295. [DOI] [PubMed] [Google Scholar]
- 32. Bolla M, Maingon P, Carrie C, et al. Short androgen suppression and radiation dose escalation for intermediate‐ and high‐risk localized prostate cancer: results of EORTC trial 22991. J Clin Oncol. 2016;34:1748–1756. [DOI] [PubMed] [Google Scholar]
- 33. Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short‐term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–118. [DOI] [PubMed] [Google Scholar]
- 34. Pisansky TM, Hunt D, Gomella LG, et al. Duration of androgen suppression before radiotherapy for localized prostate cancer: radiation therapy oncology group randomized clinical trial 9910. J Clin Oncol. 2015;33:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Denham JW, Steigler A, Lamb DS, et al. Short‐term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10‐year data from the TROG 96.01 randomised trial. Lancet Oncol. 2011;12:451–459. [DOI] [PubMed] [Google Scholar]
- 36. Zapatero A, Guerrero A, Maldonado X, et al. High‐dose radiotherapy with short‐term or long‐term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16:320–327. [DOI] [PubMed] [Google Scholar]
- 37. Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long‐term androgen suppression for prostate cancer with high metastatic risk: 10‐year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–1073. [DOI] [PubMed] [Google Scholar]
- 38. Roach M, 3rd , Bae K, Speight J, et al. Short‐term neoadjuvant androgen deprivation therapy and external‐beam radiotherapy for locally advanced prostate cancer: long‐term results of RTOG 8610. J Clin Oncol. 2008;26:585–591. [DOI] [PubMed] [Google Scholar]
- 39. Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–2527. [DOI] [PubMed] [Google Scholar]
- 40. Iversen P, McLeod DG, See WA, Morris T, Armstrong J, Wirth MP, Casodex Early Prostate Cancer Trialists' Group . Antiandrogen monotherapy in patients with localized or locally advanced prostate cancer: final results from the bicalutamide early prostate cancer programme at a median follow‐up of 9.7 years. BJU Int. 2010;105:1074–1081. [DOI] [PubMed] [Google Scholar]
- 41. Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma—long‐term results of phase III RTOG 85‐31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. [DOI] [PubMed] [Google Scholar]
- 42. Horwitz EM, Bae K, Hanks GE, et al. Ten‐year follow‐up of radiation therapy oncology group protocol 92‐02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504. [DOI] [PubMed] [Google Scholar]
- 43. Crook JM, O'Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Studer UE, Whelan P, Albrecht W, et al. Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European organisation for research and treatment of cancer (EORTC) trial 30891. J Clin Oncol. 2006;24:1868–1876. [DOI] [PubMed] [Google Scholar]
- 45. Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. [DOI] [PubMed] [Google Scholar]
- 46. Kim C, Lee JY, Chung BH, et al. Report of the second Asian prostate cancer (A‐CaP) study meeting. Prostate Int. 2017;5:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang A, Karunasinghe N, Plank L, et al. Effect of androgen deprivation therapy on bone mineral density in a prostate cancer cohort in New Zealand: a pilot study. Clin Med Insights Oncol. 2017;11:1179554917733449 10.1177/1179554917733449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smith MR, Egerdie B, Toriz NH, et al. Denosumab in men receiving androgen‐deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith MR, Morton RA, Barnette KG, et al. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2010;184:1316–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. [DOI] [PubMed] [Google Scholar]
- 51. Galvão DA, Spry N, Taaffe DR, et al. A randomized controlled trial of an exercise intervention targeting cardiovascular and metabolic risk factors for prostate cancer patients from the RADAR trial. BMC Cancer. 2009;9:419 10.1186/1471-2407-9-419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Newton RU, Taaffe DR, Spry N, et al. Can exercise ameliorate treatment toxicity during the initial phase of testosterone deprivation in prostate cancer patients? Is this more effective than delayed rehabilitation? BMC Cancer. 2012;12:432 10.1186/1471-2407-12-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen D, See L, Liu J, et al. Risk of cardiovascular ischemic events after surgical castration and gonadotropin‐releasing hormone agonist therapy for prostate cancer: a nationwide cohort study. J Clin Oncol. 2017;35:3697–3705. [DOI] [PubMed] [Google Scholar]
- 54. Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67:825–836. [DOI] [PubMed] [Google Scholar]
- 55. Hong J, Liao C, Huang C, Lu Y. Chemical castration decreased the risk of dementia in patients with prostate cancer from 13368 patients, taiwan national health insurance research database. Eur Urol Suppl. 2017;16:e86. [Google Scholar]
- 56. Zhao SG, Chang SL, Erho N, et al. Associations of luminal and basal subtyping of prostate cancer with prognosis and response to androgen deprivation therapy. JAMA Oncol. 2017;3:1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Okegawa T, Nutahara K, Higashihara E. Prognostic significance of circulating tumor cells in patients with hormone refractory prostate cancer. J Urol. 2009;181:1091–1097. [DOI] [PubMed] [Google Scholar]
- 58. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration‐resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. [DOI] [PubMed] [Google Scholar]
- 59. Josefsson A, Linder A, Flondell Site D, et al. Circulating tumor cells as a marker for progression‐free survival in metastatic castration‐naïve prostate cancer. Prostate. 2017;77:849–858. [DOI] [PubMed] [Google Scholar]


