Abstract
Aim
Evidence of the effectiveness of dietetic consultation for the management of cardiovascular disease (CVD) risk factors has not been previously synthesised. A systematic review and four meta‐analyses evaluated the effectiveness of dietetic consultation for lowering blood lipid levels in high‐risk individuals in primary health‐care settings.
Methods
Of the 4860 records identified, 10 eligible randomised controlled trials (RCTs, n = 1530) were evaluated for reporting blood lipid outcomes following dietetic consultation (DN)—defined as at least one exclusive individual face‐to‐face consultation with a dietitian and comparators (C)—defined as no nutrition intervention or usual or minimal care provided by physicians and/or nurses.
Results
DN groups were effective for lowering blood lipid levels across nine studies reporting total cholesterol (TC) and LDL; and across five of six studies reporting triglycerides (TG). Between‐group differences were not consistently assessed, with significance levels reported in four studies all in favour of DN, P < 0.05. Meta‐analyses for TC and LDL (seven studies) confirmed DN and C groups were equally effective, P > 0.05; and for TG (six studies) DN groups were significantly more effective than C groups, P < 0.05).
Conclusions
This review provides RCT evidence that dietetic counselling is effective for lowering TG levels and at least as effective as usual and minimal care for improving cholesterol levels in high‐risk individuals in primary health care. However, more adequate reporting of methods and greater consistency in timing interventions and data collection will enhance the quality of the evidence and increase confidence in the health benefits of dietetic counselling for the management of CVD risk.
Keywords: cardiovascular disease, clinical nutrition and dietetics, counselling, lipids nutrition care process, systematic literature reviews
Introduction
Dietary behaviour change is a first‐line approach to the prevention and management of cardiovascular disease (CVD) risk.1, 2, 3 Dietitians provide evidence‐based consultations consisting of individualised face‐to‐face education and counselling to facilitate dietary behaviour change and modify disease risk factors.3, 4 Guidelines for managing CVD risk focus on modifying measurable cardiometabolic risk factors, particularly in individuals at increased risk for the disease.1, 2, 3, 4, 5 Measuring clinical markers of risk can provide evidence for the effectiveness of interventions. Dietary behaviour changes aimed at improving blood lipid profiles include restricting energy intake,6 increasing dietary fibre intake, increasing fruit and vegetable intake,7 manipulating the type and amount of dietary fat and plant sterols consumed8 and adopting dietary patterns that favour the intake of whole foods such as Dietary Approaches to Stop Hypertension (DASH)9 and Mediterranean diets.10
The effectiveness of dietetic consultation for lowering cardiometabolic risk factors such as blood lipid concentrations in high‐risk individuals has not been adequately elucidated. Systematic reviews on counselling for behaviour change have been conducted but these synthesised data from studies with multiple health professional team members.11, 12 Due to the multidisciplinary nature of these teams it can be challenging to determine the effectiveness of dietetic consultation in this context. For example, there is currently no recommendation on the optimal frequency and duration of dietetic consultation to elicit a positive health outcome.13 However, it is important to clarify the health benefits of the dietetic consultation in this area of health care to inform resource planning.
In 2016, a systematic review of randomised controlled trials (RCTs) was conducted to examine the impact of dietetic consultation conducted in the primary health‐care setting.14 Primary health care refers to care delivered outside the acute hospital setting and is a key area of dietetic practice, especially for the management of chronic disease risk. This previous review was qualitative in nature and examined a range of patient outcomes to determine clear benefits for the dietetic consultation in some outcomes, such as weight loss. However, the effect on cardiometabolic risk factors, such as blood lipid concentrations, remained ambiguous and warranted a more focused review and meta‐analyses. The purpose of the current review and meta‐analyses is to evaluate the effectiveness of face‐to‐face dietetic consultation for lowering blood lipid concentrations in high‐risk individuals in primary health‐care settings. It is hoped that a review of this nature will add to the evidence‐base and help to describe an optimal frequency and duration of dietetic consultation to elicit a positive health outcome in the management of CVD risk.
Methods
The current systematic review and meta‐analyses used the same search strategy previously reported for a broader systematic review conducted in 2016 and published in 2017.14 RCTs only were included to obtain the highest level of evidence and the lowest likelihood of bias due to their study design and use of a control group. Cross‐over designs were excluded due to the lack of a valid control group (where a consultation or washout period cannot be used and removed in the same way as when testing a drug). The previous review used a range of patient outcomes to assess the effectiveness of individual dietetic consultations in the primary health‐care setting. The current review used the same search strategy to update the previous search to August 2017 and only included those studies that reported on blood lipid outcomes in individuals at high risk of CVD. The study methods and reporting comply with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA).
The literature search was conducted in the following electronic databases: ProQuest Family Health, Scopus, PubMed Central, MEDLINE, CINAHL and Cochrane databases. Keywords were combined using the Boolean operators ‘AND’ and ‘OR’ to create the following three search categories: ‘patient OR client OR client‐centred OR participant OR adult’ AND ‘dietitian OR dietetic’ AND ‘consult* OR referral OR practice OR counselling OR interview OR advice OR outpatient OR clinic’. Limits included humans, adults and English language. Additional publications were identified through hand searches of the reference lists of included publications and from systematic reviews retrieved in the search. Further details of the search strategy have been published previously.14
Study eligibility for review was based on PICOS (Population, Intervention, Comparator, Outcome, Study design) criteria presented in Table 1. Full details of the study selection process have been published previously.14 In brief, an initial selection process identified articles for further consideration if the title or abstract contained at least one keyword from each of the search categories described. The full text of all articles that met the criteria was retrieved, and a second selection process was undertaken by examining the full manuscripts to inform the final decision. A quality control training procedure was undertaken to ensure consistency of coding between reviewers. Agreement (97%) between reviewers was obtained for the coding of the first 250 abstracts.14 Where the coding differed, consensus was achieved through group discussion. Disagreements between coders were considered by a separate researcher and resolved via group discussion.
Table 1.
Summary of eligibility criteria for study inclusion in the systematic review
| Domain | Inclusion criteria |
|---|---|
| Population | Adults (≥18 years) at high‐risk of cardiovascular disease who attended an individual face‐to‐face consultation with a dietitian in a primary health‐care setting |
| Intervention | Individual face‐to‐face consultations provided exclusively by a dietitian with an aim to lower cardiometabolic risk factors. The dietetic consultation was defined as at least one face‐to‐face session with a dietitian aimed at supporting an individual patient to modify dietary behaviours and could include any or all components of the dietitian's nutrition care process; assessment, diagnosis, intervention, monitoring and evaluation. Exclusions were interventions delivered to patients in hospital, via the telephone only, in a group or lecture setting, or by a multidisciplinary team where the influence of the dietitian could not be determined |
| Comparator | Usual care, where patients received usual medical care from a physician or nurse; minimal care, where patients received nutrition‐related printed material plus or minus general dietary information from a nurse; or control, where no nutrition intervention was provided. If a study had multiple study arms, only those that met the inclusion criteria were included in the review and meta‐analyses. Further detail on comparator criteria have been published previously10 |
| Outcome | Clinical measures were specifically blood lipid concentrations (total cholesterol (TC), LDL cholesterol (LDL‐C), HDL cholesterol (HDL‐C) and triglycerides (TG). Studies needed to state blood lipid moderation as an aim and/or primary outcome in the management of cardiometabolic risk factors. For inclusion in the meta‐analyses, studies needed to report at least one of the defined blood lipid concentrations at baseline and post‐intervention |
| Study design | Systematic reviews of randomised controlled trials; and randomised controlled trials using parallel design |
Two researchers independently assessed the quality of each of the included publications, published in detail elsewhere.14 Using the Cochrane Risk of Bias tool,15 eight criteria covering six domains of bias were considered: selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias. If insufficient detail was provided to adequately classify a study as either ‘low risk’ or ‘high risk’, it was classified as ‘unclear risk’, in line with the user guide.15 The overall study rating was allocated at the level of the criterion with the highest risk of bias score. No studies were excluded based on the quality assessment.
Data from the included publications were extracted by one researcher and reviewed by another researcher using a purposefully developed electronic spreadsheet. Publications reporting data on blood lipid outcomes were examined and any relevant additional data extracted. The following data were extracted: study identification (first author, year and country), primary aim of risk management, participant characteristics (gender, inclusion and exclusion criteria), study setting, sample size (intervention and comparator), description of the dietetic consultation intervention (frequency, duration), comparator (usual, minimal or no intervention), and measures of study quality as outlined above.15 Outcome methods and measures were limited to blood lipid concentrations (TC, HDL, LDL and TG), measured at baseline and nearest to the end of the intervention period for each RCT.
Mean blood lipid values and variance measures (SD and SEM or 95% CIs) were extracted. No distinction was made between serum and plasma lipid values. Blood lipid concentrations reported as mg/dL were converted to mmol/L using a standardised conversion (multiply mg/dL by 0.0259). All variance measures were converted to SDs using the Cochrane Handbook method.15 No study reported a correlation coefficient or SD of change for the outcome measures under study and these could not be imputed from other studies due to the variations in intervention duration. Instead, the SD of the within‐group mean difference was calculated (based on the mean difference from baseline to the end of the intervention period and corresponding confidence intervals) for each arm (intervention and comparator group) in the included studies.15
The absolute and relative (%) mean change and SD for blood lipid concentrations for each study group were also calculated by the researchers using baseline and post‐intervention data. Effectiveness was based on the statistical significance (P‐values) for between‐group differences in blood lipid outcomes at post‐intervention reported by the authors of each study.
Revman version 5.3 was used to conduct four meta‐analyses of lipid outcomes: TC (total cholesterol), HDL, LDL and TG (triglycerides) concentrations.16 Studies were eligible for meta‐analyses if they reported adequate data for the calculation of absolute change in TC, HDL, LDL and/or TG concentrations. The absolute change and SD for blood lipid concentrations provided the main outcome variables. Pooled effect sizes were calculated as the weighted mean difference (WMD) and 95% CI for absolute change in blood lipid concentrations. Effectiveness was based on effect sizes and P‐values for TC, HDL, LDL and TG. Heterogeneity was quantified using the I 2 statistic, which measures the between‐study variation that can be attributed to heterogeneity as opposed to random variation, with the intent to assess whether studies share a common effect size. I 2 statistic values of approximately 25%, 50% and 75% were considered to indicate a low, moderate and high‐level of heterogeneity, respectively. A random effects model was used due to the high heterogeneity of the intervention and the complexity of individual variability in response to lifestyle modification.17 Sensitivity analyses were conducted using the ‘leave‐one‐out’ method, to assess whether any single study elicited undue influence on the overall result.
The Grading of Evidence, Assessment, Development and Evaluation (GRADE) system was used to classify the quality of the evidence for each lipid outcome (TC, HDL, LDL, TG) into one of the four levels: high, moderate, low or very low.18 The final classification was based on study design and the following considerations (that may lower the grade of evidence implied by RCT design): (i) serious (−1) or very serious (−2) limitations in study quality, including high risk of reporting bias (−1); (ii) important inconsistency of results (−1); (iii) some (−1) or major (−2) uncertainty about the directness or relevance of the evidence (study population, intervention and outcome measures) to the interests of the review and (iv) imprecise or sparse data (−1).
Results
Figure 1 outlines the database search and publication selection processes. A total of 6144 publications were retrieved from electronic database searches and 302 publications from hand searches. Of these, 240 publications met the inclusion criteria based on the title or abstract and the full‐text articles obtained, to which further exclusions based on eligibility criteria were applied. As a result, 10 publications19, 20, 21, 22, 23, 24, 25, 26, 27, 28 were included in the systematic review. Where more than two intervention arms existed in a single study, only those relevant to the study criteria were included. Three of the included studies21, 22, 23 were excluded from the meta‐analyses due to insufficient data, that is, means and/or margins of error were not reported to enable the calculation of absolute mean change and CI for lipid outcomes for meta‐analysis. A fourth study24 was excluded from the meta‐analysis of TG outcomes only, as baseline TG measures were not reported.
Figure 1.
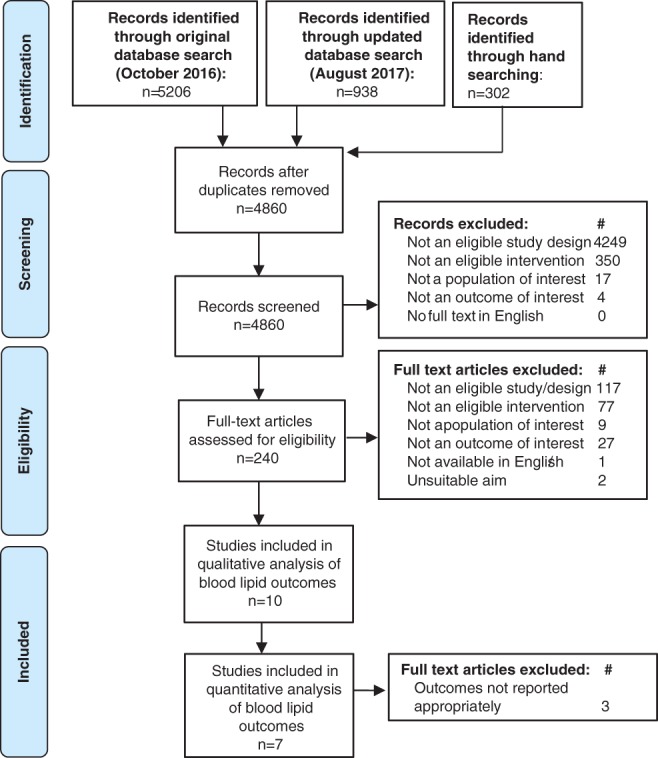
Flow diagram of the literature search and filtering results for a systematic review of the effectiveness of individual dietetic consultations for lowering blood lipid levels.
All 10 study designs were parallel RCT with participants randomised to two or more groups. Characteristics of the 10 studies are presented in Table 2. Participants attended outpatient centres in USA,19, 25, 26 Asia,20, 27, 28 Europe23, 24 and Australia.21, 22 The stated primary aim of risk management was cholesterol lowering,19, 21, 22, 26, 28 blood pressure lowering,23 diabetes management including associated CVD risk25, 27 and CVD risk factor management including blood pressure and blood lipid concentrations.20 The 10 included studies had a total of 1274 participants enrolled (dietetic consultation, DN: n = 655; comparator, C: n = 644), with a 4.1% dropout rate across studies. Five studies specifically recruited participants with existing hyperlipidaemia: TC > 5.0 mmol/L,19, 22, 24, 28 TC > 5.0 and <8.1 mmol/L,22 8.9 mmol/L19 and 9.1 mmol/L,24 LDL > 3.36 mmol/L.26 The remaining five studies recruited participants from patient populations at high‐risk of CVD: peripheral vascular disease with TC < 9.1 mmol/L,21 type 2 diabetes mellitus,27 hypertension,20, 23 or impaired fasting glucose with overweight or obesity.25 All patient populations were screened to exclude the presence of other serious medical and comorbid conditions and/or medication use that may influence outcomes or compliance. In terms of gender distribution, four studies did not specify gender;19, 20, 27, 28 one study restricted recruitment to men only;21 and five studies reported recruiting both men and women.22, 23, 24, 26 For age distribution, three studies reported an age range, with 20–81‐year‐olds included across those studies22, 24, 27 two studies reported including participants aged 18+ years,25, 28 and five studies reported limiting the age to 65 years,19, 26 70 years20, 28 and 75 years.21 Data on body mass index (BMI) were reported in eight studies, where five studies provided a mean baseline BMI that ranged from 23 to 34 kg/m2,20, 24, 26, 27, 28 and three studies were unclear when reporting baseline BMI.21, 22, 25 These studies indicated the average participant was: healthy to overweight,27, 28 overweight19, 21, 26 and obese.25 Only one study included BMI as part of the eligibility criteria, limiting BMI to <27 kg/m2.2, 23
Table 2.
Characteristics of randomised control trials assessing effectiveness of individual face‐to‐face dietitian consultations for lipid management
| First author, year, country | Primary risk management | Participant characteristics | Number analysed (number enrolled) | Dietitian intervention (number and duration of visits) and comparator | Baseline (TC) | Baseline (HDL) | Baseline (LDL) | Baseline (TG) |
|---|---|---|---|---|---|---|---|---|
| Delahanty, 2001,19 USA | Cholesterol lowering | Adults with hyperlipidaemia: Fasting TC >201.01 and <341.84 mg/dL (>5.2 and <8.84 mmol/L) | 88 (90) |
Dietitian 2–3 visits in first 2–3 months plus 2–3 follow‐up visits, if required, over a 6‐month period. Duration of visits not stated |
6.19 ± 0.73 | 1.22 ± 0.42 | 4.29 ± 0.60 | 1.46 ± 0.61 |
|
Comparator Usual care by physicians |
6.16 ± 0.75 | 1.14 ± 0.31 | 4.24 ± 0.68 | 1.17 ± 0.89 | ||||
| Heller, 1989,21 Australia | Cholesterol lowering | Men with peripheral vascular disease (ankle‐to‐brachial blood pressure ratio ≤ 0.80) or history of previous vascular surgery, and TC ≤348.03 mg/dL (<9 mmol/L) |
Dietitian 2 visits in 3 months Duration of visits not stated |
Not reported | Not reported | Not collected | Not collected | |
|
Comparator Minimal care: printed information and encouragement by a clinic nurse |
Not reported | Not reported | Not collected | Not collected | ||||
| Imai, 2008,27 Japan | Diabetes management | Adults diagnosed with T2DM | 77 (77) |
Dietitian 12 visits in 12 months. 20–30 minutes duration for each visit |
6.18 ± 0.92 | 1.53 + 0 ± 09 | 3.31 ± 0.92 | 1.81 ± 1.33 |
|
Comparator Usual care: by a doctor or nurse and printed information |
5.59 ± 0.97 | 1.58 ± 0.41 | 3.53 ± 0.92 | 1.60 ± 0.99 | ||||
| Johnston, 1995,22 Australia | Cholesterol lowering | Men and women with hyperlipidaemia: fasting plasma TC 212.68–309.36 mg/dL (5.5–8.0 mmol/L) | 131 (179) |
Dietitian 3 visits in 6 months. 1.5 hours duration for each visit |
6.35 (6.05–6.80) | Not reported | 4.05 (3.54–4.35) | Not reported |
|
Comparator Minimal care: printed information and verbal advice from a nurse |
6.30 (5.80–6.80) | Not reported | 4.00 (3.60–4.29) | Not reported | ||||
| Koopman, 1990,23 Netherlands | BP lowering | Men and women with elevated blood pressure: DBP 90–110 mm Hg on three occasions; BMI ≤27 kg/m2 | 30 (35) |
Dietitian 3 visits in 3 months. Duration of visits not stated |
Not reported | Not collected | Not collected | Not collected |
|
Comparator Control: received no intervention |
Not reported | Not collected | Not collected | Not collected | ||||
| Lim, 2008,28 South Korea | Cholesterol lowering | Adults with hyperlipidaemia: fasting serum TC ≥200 mg/dL (>5.17 mmol/L); TG ≥150 mg/dL (1.69 mmol/L) | 40 (40) |
Dietitian 5 visits in 3 months. Duration of visits not stated |
5.88 ± 0.41 | 1.15 ± 0.18 | 3.31 ± 0.92 | 1.97 ± 0.33 |
|
Comparator Control: received no intervention |
5.94 ± 1.34 | 1.13 ± 0.23 | 3.53 ± 0.92 | 2.05 ± 1.22 | ||||
| Neil, 1995,24 Great Britain | Cholesterol lowering | Men and women with hyperlipidaemia: TC, 251.35–348.03 mg/dL (6.5–9.0 mmol/L) and repeat fasting TC 6.0–8.5 mmol/L; TC: HDL ratio ≥154.68 mg/dL1 (≥4.0 mmol/L); LDL ≥135.34 mg/dL1 (≥3.5 mmol/L); fasting TG ≤495.99 mg/dL (≤5.6 mmol/L) | 205 (205) |
Dietitian 2 visits in 8 weeks. 30 minutes duration for each visit |
7.01 ± 0.61 | 1.18 ± 0.26 | 5.11 ± 0.6 | Not Collected |
|
Comparator Minimal care: printed information |
7.23 ± 0.63 | 1.23 ± 0.28 | 5.25 ± 0.65 | Not collected | ||||
| Parker, 2014,25 USA | Diabetes risk factors | Overweight or obese men and women BMI ≥25 kg/m2 with impaired fasting glucose or HbA1c 5.7–6.4% and no previous history or treatment of diabetes | 76 (81) |
Dietitian 4 visits in 3 months. 60 minutes duration for each visit |
5.26 ± 0.95 | 1.33 ± 0.32 | 3.19 ± 0.91 | 1.71 ± 0.64 |
|
Comparator Control: received no intervention |
4.93 ± 1.18 | 1.21 ± 0.32 | 2.90 ± 0.95 | 1.88 ± 0.95 | ||||
| Rhodes, 1996,26 USA | Cholesterol lowering | Men and women with hyperlipidaemia: 2 readings within 8 weeks of LDL‐C ≥160.09 mg/dL (≥4.14 mmol/L) or >129.93 mg/dL1 (>3.36 mmol/L) + CHD or 2 other risk factors for CHD | 97 (104) |
Dietitian Up to 3 visits in 3 months. 1‐hour duration for initial visit; 30 minutes duration for subsequent visits |
6.93 ± 0.71 | 1.22 ± 0.28 | 5.11 ± 0.67 | 1.53 ± 0.47 |
|
Comparator Usual care: by a physician |
6.75 ± 0.79 | 1.21 ± 0.26 | 4.96 ± 0.77 | 1.57 ± 0.50 | ||||
| Wong, 2015,20 Hong Kong | Cardiovascular risk factors | Adults with newly diagnosed Grade 1 hypertension, not taking anti‐hypertensive medications | 504 (556) |
Dietitian A one‐off visit of 35‐minutes duration |
5.57 ± 0.86 | 1.55 ± 0.43 | 3.39 ± 0.81 | 1.40 ± 0.81 |
|
Comparator Usual care: by a physician and printed information |
5.25 ± 0.82 | 1.55 ± 0.43 | 3.25 ± 0.76 | 1.33 ± 0.70 |
BMI, body mass index; BP, blood pressure; CHD, Coronary Heart Disease; DBP, diastolic blood pressure; HbA1c, glycolated haemoglobin; T2DM, type 2 diabetes mellitus; TG, triglyceride.
Converted using factor of 38.67.
Studies reported an intervention duration of 3,21, 23, 26, 28 622, 24 and 12 months19, 20, 25, 27 and wide variations in frequency and duration of the dietetic consultation. The number of face‐to‐face consultations with the dietitian also varied and included a one‐off consultation in one study,20 two to three consultations in 3 months in three studies,19, 21, 23 and more frequent consultations in the remaining studies to a maximum of 12 visits over a 12‐month period in one study.27 The duration of dietetic consultation ranged from 20 minutes to 1.5 hours in the six studies reporting this measure;20, 22, 24, 25, 26, 27 four studies did not report the duration of dietetic consultation.19, 21, 23, 28 Comparator groups were determined to be: usual care by a doctor or nurse which may or may not have included printed dietary information (four studies);19, 20, 26, 27 minimal care based on printed dietary information with or without advice from a nurse (three studies)21, 22, 24 or controls who received no alternative dietary intervention during the intervention period (three studies).23, 25, 28
Quality assessment determined three studies had at least one quality criterion at the level of high‐risk of bias19, 21, 27 and the remaining seven studies had at least one criterion considered to be an unclear risk of bias. No study met all eight quality criteria for deeming the level of risk to be low, mainly due to the lack of reporting of allocation concealment (nine studies),19, 20, 27 blinding of participants and personnel delivering the intervention (nine studies)19, 21, 22, 23, 24, 25, 26, 27, 28 and blinding of assessment personnel (eight studies).20, 21, 23, 24, 25, 26, 27, 28
The relative changes in blood lipid concentrations for dietetic and comparator groups in each of the 10 studies under review are shown in Table 3. TC levels: nine studies reported significant reductions in all dietetic and comparator groups; a statistically significant between‐group difference in favour of the dietetic consultation was reported in two studies;19, 21 five studies reported no between‐group differences and two studies did not report a between‐group comparison.22, 28 HDL: seven studies reported variable results with no statistically significant between‐group differences in six studies; one study did not report a between‐group comparison.28 LDL: nine studies reported significant reductions in all dietetic groups and in all but one comparator groups;23 a statistically significant between‐group difference in that study by Koopman et al. was in favour of the dietetic consultation;23 six studies reported no between‐group differences and two studies did not report a between‐group comparison.22, 28 TG: six studies reported significant reductions in five dietetic and four comparator groups and a statistically significant between‐group difference in the study by Rhodes et al. in favour of the dietetic consultation;26 one study reported increases in both dietetic and comparator groups;19 and one study did not report a between group comparison.28
Table 3.
Relative mean change (%)(a) in blood lipid concentrations for patients receiving individual face‐to‐face consultations with a dietitian and comparator groups
| Study author | TC | LDL | HDL | TG | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DN | C | DN | C | DN | C | DN | C | DN | C | |
| n | n | % change | % change | % change | % change | % change | % change | % change | % change | |
| Delahanty, 200119 | 44 | 44 | −6.79 | −2.11(b) | −7.23 | −2.59 | −6.56 | −4.39 | 0.68 | 8.55 |
| Heller, 198921 | 31 | 28 | −8.57 | −1.97(b) | — | — | — | — | — | — |
| Imai, 200827 | 29 | 30 | −6.32 | −6.80 | −4.92 | −8.46 | 3.39 | 0.00 | −13.75 | −4.93 |
| Johnston, 199522 | 44 | 47 | −7.94 | −14.17(c) | −8.75 | −9.14(c) | — | — | — | — |
| Koopman, 199023 | 17 | 18 | — | — | −11.43 | 8.3(b) | — | — | — | — |
| Lim, 200828 | 20 | 20 | −20.90 | −3.87(c) | −14.53 | −4.39(c) | 3.14 | −3.21(c) | −37.97 | −6.15(c) |
| Neil, 199524 | 102 | 38 | −1.43 | −1.80 | −2.15 | −3.62 | −0.85 | 1.63 | — | — |
| Parker, 201425 | 43 | 52 | −4.94 | −2.03 | −7.07 | −3.98 | −0.04 | 2.64 | −4.60 | −5.39 |
| Rhodes, 199626 | 52 | 250 | −9.96 | −7.26 | −10.96 | −8.87 | −8.20 | −9.92 | −4.46 | 11.76(b) |
| Wong, 201520 | 254 | 44 | −3.05 | −2.59 | −4.42 | −3.69 | 0.65 | −0.65 | −5.00 | −1.50 |
C, comparator group; DN, dietitian intervention; n, number of participants; % change, relative change from baseline at 6 months post‐intervention.
The relative mean change (%) in lipid concentration from baseline was calculated by the researchers for each study group.
The authors of the publication report a significant between‐group difference in the absolute change.
An analysis of between‐group difference was not reported.
Seven out of the 10 studies under review were eligible for inclusion in the meta‐analyses,19, 20, 24, 25, 26, 27, 28 that is, provided adequate baseline data for the calculation of absolute change in blood lipid concentrations following intervention. Therefore, baseline data were available on TC, HDL and LDL from 1081 participants (DN = 544; C = 537), with an average dropout rate of 9.3% across six of the studies, where one study did not state the number of dropouts.27 Baseline TG data were available from 876 participants (DN = 442; C = 434), where the study by Neil et al.24 was excluded from the TG meta‐analysis as TG values were not reported in those studies.
Figure 2 illustrates the meta‐analyses performed for absolute TC, HDL, LDL and TG outcomes. Between‐study heterogeneity was low and not statistically significant for TC (I 2 = 28%, P = 0.22), HDL (I 2 = 0%, P = 0.95), LDL (I 2 = 0%, P = 0.81) and TG (I 2 = 38%, P = 0.15). The pooled mean difference in absolute change in values following intervention did not reach statistical significance and, therefore, did not favour dietetic or comparator groups for TC (pooled mean difference −0.12 with 95% CI [−0.30, 0.05], P = 0.16), HDL (pooled mean difference −0.01 with 95% CI [−0.06, 0.05], P = 0.54) and LDL (pooled mean difference −0.04 with 95% CI [−0.16, 0.08], P = 0.54). In contrast, the pooled mean difference in TG values following intervention was statistically significant in favour of the dietitian (pooled mean difference −0.22 with 95% CI [−0.43, −0.02], P = 0.03).
Figure 2.
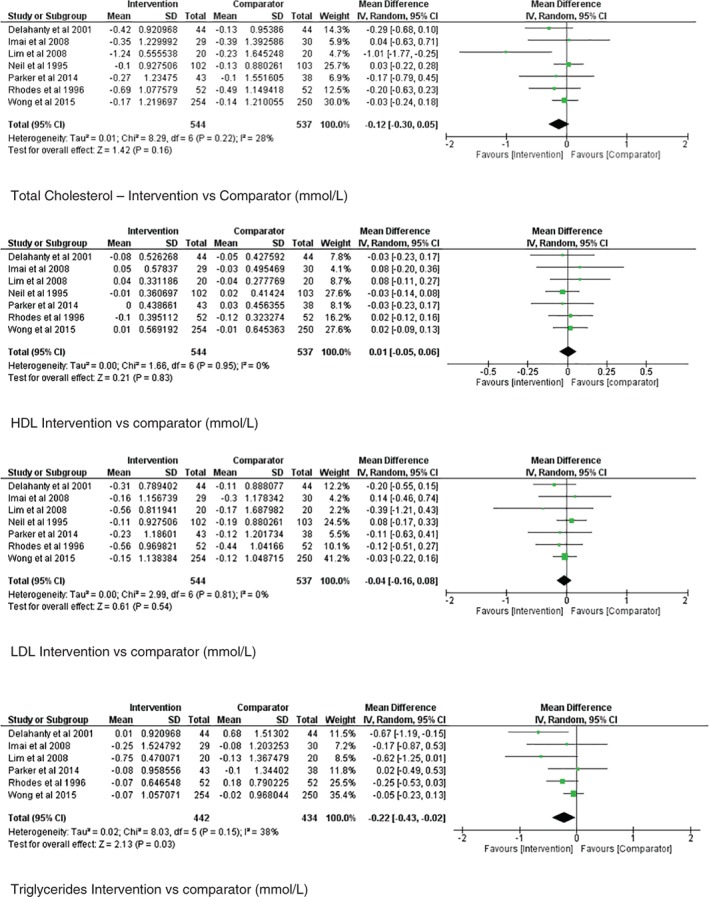
Forest plots showing comparisons for individual counselling versus minimal or usual care for reducing (a) TC, (b) HDL, (c) LDL and (d) TG in mmol/L.
Sensitivity analyses for TC, HDL and LDL meta‐analyses resulted in minimal changes to effect sizes and statistical P‐values. These results indicate that no single study exerted a greater influence than other included studies and provides a high degree of certainty for pooled outcomes in each of the meta‐analyses. With respect to the TG meta‐analysis, removal of the study by Wong et al.20 resulted in an increase in the overall effect size and an increase in the significance attributable to the remaining studies (Z = 2.66; P = 0.008), indicating the largest included study was exerting a less favourable effect of the dietetic consultation than the other smaller studies. However, removal of each of the other studies from the TG meta‐analysis resulted in decreased overall effect sizes and non‐significant P‐values, suggesting individual studies were exerting a greater influence on the outcome than pooled data.
The GRADE approach enabled reviewers to rate the quality of evidence for each lipid outcome (TC, HDL, LDL, TG) (see Appendix A). All 10 studies were graded as high quality based on RCT design. However, the quality rating was down‐graded for all lipid outcomes based on the following modifiers: (i) serious limitation of reporting bias (−1), that is, lack of reporting on allocation concealment and blinding of participants and personnel across nine studies; (ii) no serious limitation due to important inconsistencies in results, that is, while just four studies reported a significant between‐group difference and two studies failed to report a between‐group analysis, endpoints for absolute change and meta‐analyses were able to be calculated from reported data that demonstrated consistent improvements in blood lipid outcomes across intervention and comparator groups; (iii) some uncertainty in the directness of the intervention (−1), that is, wide variations between studies for frequency and duration of interventions with four studies failing to report these parameters and (iv) no modifications to the quality rating were made due to imprecision or sparse data or publication bias. In conclusion, the reviewers considered the likely benefits of dietetic consultation to outweigh the potential harms in high‐risk populations and determined a final GRADE recommendation of ‘low’ for lowering cholesterol and TG concentrations under review, that is, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Discussion
The current systematic review of RCTs found that dietetic consultation was at least as effective as usual and minimal care for bringing about positive relative changes in blood lipid concentrations of TC, HDL, LDL and TG in high‐risk individuals in primary health‐care settings. Meta‐analyses of pooled mean values for TC, HDL and LDL outcomes from seven eligible studies confirmed, with a high degree of certainty and low heterogeneity, that dietetic consultation produced improvements that were similar to those achieved by comparator groups, consisting of general dietary advice and/or written information provided by physicians and nurses. Notably, the meta‐analysis of pooled mean values for TG outcomes from six eligible studies suggests that dietetic consultation was significantly more effective than comparators. However, this latter result should be interpreted with caution due to disproportionate influences of the six individual studies determined by sensitivity analysis.
The focus of review was on dietetic consultations aimed at reducing clinical indicators of CVD risk in high‐risk individuals. The results are comparable to those observed in pharmacotherapy studies conducted in high‐risk populations29 and provide evidence of the effectiveness of dietary therapies for reducing cardiometabolic risk factors. Dietary advice has previously been shown to be effective in the prevention of chronic disease,30 and dietetic consultation has been shown to elicit improvements in diet quality, weight loss and diabetes outcomes.14 However, this study is the first to synthesise the effectiveness of dietetic consultation for the management of CVD risk factors.
The 10 eligible studies consistently reported reductions in TC, LDL and TG post‐intervention for dietetic and comparator groups. However, the magnitude of change varied widely between studies. For example, relative mean changes in TC ranged from −1.43% to −20.9% for dietetic consultation and from −1.8% to −14.17% for comparators. In addition, 4 of the 10 studies under review did not report values for one or more of the blood lipid concentrations under analysis (TC, HDL, LDL, TG)21, 22, 23, 24 and two studies did not report a between‐group comparison at post‐intervention,22, 28 despite significant changes in the dietetic group that were not seen in the control group.28 Nevertheless, four studies were able to demonstrate superior effect of dietetic consultation where all studies reporting a statistically significant between‐group difference in post‐intervention blood lipid values were in favour of the dietetic consultation for lowering TC,19, 21 LDL,23 or TG.26 Results for HDL were the most variable, which may reflect the known inverse correlation with CVD31 and the need for targeted advice to positively influence HDL metabolism.
To provide the highest level of available evidence from the current literature, included studies were restricted to RCT design and the dietetic consultation was well defined as a relatively homogenous intervention for review. Despite this rigour for review, variations in methods likely contributed to the wide ranges observed for blood lipid outcomes. For example, variations in the timing of outcome data collection up to 3, 6 and 12 months after intervention appear problematic and may have contributed to a significant between‐group difference for TG in comparison to cholesterol outcomes, where TG are more reactive to dietary changes in the short term and dietary adherence may reduce over the longer term. Equally, there were wide variations and inadequate reporting regarding the frequency and duration of intervention (from a once only consultation to regular monthly visits over 12 months) that effectively limited any attempt to evaluate a dose response gradient or to describe the optimal parameters for dietetic consultation to achieve a positive health benefit. Similarly, comparator interventions varied in duration and frequency between studies, as did the amount and type of input from other health professionals, usually physicians and nurses who in many cases followed the same National Cholesterol Education Program (NCEP) guidelines32 used by the dietitians in the same study, providing no clear control group. Furthermore, the variations in duration among the included studies made it unfeasible to calculate SD of change in lipid outcomes for comparison. Instead, the SD of the within‐group difference of each study arm at the end of each intervention period was considered appropriate but is acknowledged as a limitation of this review.
The GRADE approach to rate the quality of evidence to support dietetic consultation in lowering blood lipid concentrations confirmed the main reasons for downrating the quality of evidence beyond study design were serious limitations due to reporting bias (lack of reporting on allocation concealment and blinding) and some uncertainty in the directness of the intervention due to the variations in frequency and duration.
Other potential influences of mention were factors known to be associated with reductions in blood lipid levels. For example, baseline blood lipid concentrations are known to result in greater absolute reductions in response to intervention. However, the study designs of the included studies appeared adequate to support reductions, where five studies recruited based on hyperlipidaemia and five studies recruited high‐risk populations and all studies reported elevated baseline LDL >2.5 mmol/L and TG >1.5 mmol/L. The known association between increasing age and increasing LDL33 was difficult to assess due to inadequate reporting, however, the number of elderly participants were likely limited due to exclusion criteria used in five studies to cap participant age at or above 65 years.19, 20, 21, 26, 28 While it is acknowledged that weight loss is known to influence metabolic processes,34 a lack of reporting of consistent weight measures for comparison made it difficult to assess any contribution of weight loss to changes in blood lipid outcomes and was not an outcome of this study.
Finally, a key focus of the dietetic consultation is to support patients in achieving dietary behaviour changes to improve health outcomes. Therefore, the measurement of the diet is important for determining the effects of dietary change.35 Only three of the studies in this review reported an analysis of dietary change,19, 26, 28 including reductions in fat but not fibre,19 reductions in macronutrients and dietary cholesterol but not energy intake,26 and one study reported no significant dietary change.28 However, no association between diet and blood lipid levels was able to be determined. Future studies need to adequately assess dietary modifications and associations with biomarkers.
Strengths of this review include the rigorous nature of a systematic process with an explicit and reproducible protocol that complied with PRISMA recommendations. The inclusion of meta‐analyses of pooled data enhanced the power to detect a difference in the outcomes under review and allowed statistical explorations of heterogeneity and sensitivity. Limitations of the review include: publication bias where potential sources of grey literature were not included in the search; the choice of outcomes were not inclusive of other measures of CVD risk such as blood pressure; and the application, interpretation and generalisation of results using GRADE required subjective judgements to arrive at the final quality recommendations. Furthermore, the inability to calculate SD of change for each study resulted in conservative (larger) margins of error, limiting the ability of meta‐analysis to detect an influence of dietetic consultations. Future studies in dietetics should report error of the mean change, or correlation coefficients to allow for more accurate pooled analysis.
The results of the current systematic review and meta‐analyses provide evidence for the effectiveness of the dietetic consultation in the primary care setting for reducing blood lipid concentrations, as important measurable cardiometabolic risk factors in high‐risk individuals. Counselling from a dietitian was found to be more effective for lowering TG concentrations than comparator groups across six eligible studies (GRADE: low quality), while reductions in cholesterol concentrations were similar to those achieved by comparator groups in at least seven studies (GRADE: low quality). Inadequate reporting and variations in the methods likely confounded results and reduced the effects of intervention over time. The implications for dietetic researchers are to collect consistent, high quality, long‐term outcome data to enhance the overall grade of the body of evidence to support policy changes and funding for dietetic counselling in the management of chronic diseases in primary health‐care settings.
Funding source
Funding was provided for the initial literature search from Griffith University 2016 New Researchers Grant Scheme. The funders placed no restrictions on the study and had no involvement in the study.
Conflict of interest
The authors declare that six of the seven authors are Accredited Practising Dietitians. No other potential conflicts of interest either personally or in relation to their affiliations are reported.
Authorship
Each author participated in planning, search and review processes as well as data extraction and quality assessment of individual articles. PL and KAB undertook the meta‐analyses. LJR led the synthesis of extracted data and wrote the first draft of the manuscript. All authors contributed to writing the manuscript and are in agreement with the final manuscript being submitted. All authors declare that the content of the manuscript has not been published elsewhere.
GRADE Evidence Profile Table: effectiveness of individual face‐to‐face Dietitian consultations versus comparator groups for lipid management
| Quality assessment | Summary of findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | Absolute change | ||||||||||
| No of studies (design) | Limitation: risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Intervention | Comparator | Pooled mean difference | (95% CI) | Significance, P‐value | Quality |
| Total cholesterol | |||||||||||
| 7 (RCT) | Serious limitation | No serious inconsistency | Serious indirectness | No serious imprecision | Undetected | 544 | 537 | −0.12 | −0.30, 0.05 | 0.16 | −1, −1 (low) |
| HDL‐cholesterol | |||||||||||
| 7 (RCT) | Serious limitation | No serious inconsistency | Serious indirectness | No serious imprecision | Undetected | 544 | 537 | −0.01 | −0.06, 0.05 | 0.83 | −1, −1 (low) |
| LDL‐cholesterol | |||||||||||
| 7 (RCT) | Serious limitation | No serious inconsistency | Serious indirectness | No serious imprecision | Undetected | 544 | 537 | −0.04 | −0.16, 0.08 | 0.54 | −1, −1 (low) |
| Triglycerides | |||||||||||
| 6 (RCT) | Serious limitation | No serious inconsistency | Serious indirectness | No serious imprecision | Undetected | 442 | 434 | −0.22 | −0.43, 0.02 | 0.03 | −1, −1 (low) |
L.J. Ross, PhD, AdvAPD, Senior Lecturer
K.A. Barnes, PhD, APD, Research Assistant
L.E. Ball, PhD, APD, Research Fellow
L.J. Mitchell, PhD, AdvAPD, Lecturer
I. Sladdin, BNutrDiet, APD, Research Assistant
P. Lee, PhD, Senior Lecturer
L.T. Williams, PhD, FDAA, Department Head of Nutrition & Dietetics
The legal statement for this article was changed on 15 February 2019 after original online publication.
References
- 1. Stone N, Robinson J, Lichtenstein ABM et al ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014; 129: S1–S45. [DOI] [PubMed] [Google Scholar]
- 2. National Vascular Disease Prevention Alliance . Guidelines for the Management of Absolute Cardiovascular Disease Risk. Melbourne: The National Stroke Foundation, 2012. [Google Scholar]
- 3. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand . Reducing Risk in Heart Disease: An Expert Guide to Clinical Practice for Secondary Prevention of Coronary Heart Disease. Melbourne: National Heart Foundation of Australia, 2012. [Google Scholar]
- 4. Dietetians Association of Australia . Chronic Disease Prevention and Management in Primary Health Care. Canberra: Dietetians Association of Australia, 2015. [Google Scholar]
- 5. Pedersen T, Olsson A, Færgeman O et al Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S). Circulation 1998; 97: 1453–60. [DOI] [PubMed] [Google Scholar]
- 6. Varady K, Hellerstein M. Alternate‐day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr 2007; 86: 7–13. [DOI] [PubMed] [Google Scholar]
- 7. He F, Nowson C, Lucas M, MacGregor GA. Increased consumption of fruit and vegetables is meta‐analysis of cohort study. J Hum Hypertens 2007; 21: 717–28. [DOI] [PubMed] [Google Scholar]
- 8. National Heart Foundation of Australia . Position Statement: Dietary Fats and Dietary Sterols for Cardiovascular Health. Melbourne: National Heart Foundation of Australia, 2009. [Google Scholar]
- 9. Asemi Z, Samimi M, Tabassi Z, Shakeri H, Sabihi S‐S, Esmaillzadeh A. Effects of DASH diet on lipid profiles and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: a randomized clinical trial. Nutrition 2014; 30: 1287–93. [DOI] [PubMed] [Google Scholar]
- 10. Martinez‐Gonzalez MA, Bes‐Rastrollo M. Dietary patterns, Mediterranean diet, and cardiovascular disease. Curr Opin Lipidol 2014; 25: 20–6. [DOI] [PubMed] [Google Scholar]
- 11. Ball L, Leveritt M, Cass S, Chaboyer W. Effect of nutrition care provided by primary health professionals on adults’ dietary behaviours: a systematic review. Fam Pract 2015; 32: 605–17. [DOI] [PubMed] [Google Scholar]
- 12. Bhattarai N, Prevost AT, Wright AJ, Charlton J, Rudisill C, Gulliford MC. Effectiveness of interventions to promote healthy diet in primary care: systematic review and meta‐analysis of randomised controlled trials. BMC Public Health 2013; 13: 1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ball L, Sladdin I, Mitchell L, Barnes K, Ross L, Williams L. Quality of development and reporting of dietetic intervention studies in primary care: a systematic review of randomised controlled trials. J Hum Nutr Diet 2018; 31: 47–57. [DOI] [PubMed] [Google Scholar]
- 14. Mitchell L, Ball L, Ross L, Barnes K, Williams L. Effectiveness of dietetic consultations in primary health care: a systematic review of randomized controlled trials. J Acad Nutr Diet 2017; 117: 941–1962. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Deeks J. Selecting studies and collecting data. In: Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1, Chapter 7). The Cochrane Collaboration, 2011. Available from: http://www.handbook.cochrane.org. [Google Scholar]
- 16. The Cochrane Collaboration . Review Manager (RevMan). [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- 17. DerSimonian R, Kacker R. Random‐effects model for meta‐analysis of clinical trials: an update. Contemp Clin Trials 2007; 28: 105–14. [DOI] [PubMed] [Google Scholar]
- 18. Guyatt G, Oxman AD, Akl EA et al GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64: 383–94. [DOI] [PubMed] [Google Scholar]
- 19. Delahanty LM, Sonnenberg LM, Hayden D, Nathan DM. Clinical and cost outcomes of medical nutrition therapy for hypercholesterolemia: a controlled trial. J Am Diet Assoc 2001; 101: 1012–23. [DOI] [PubMed] [Google Scholar]
- 20. Wong MC, Wang HH, Kwan MW et al Dietary counselling has no effect on cardiovascular risk factors among Chinese Grade 1 hypertensive patients: a randomized controlled trial. Eur Heart J 2015; 36: 2598–607. [DOI] [PubMed] [Google Scholar]
- 21. Heller RF, Elliott H, Bray AE, Alabaster M. Reducing blood cholesterol levels in patients with peripheral vascular disease: dietitian or diet fact sheet? Med J Aust 1989; 151: 566–8. [DOI] [PubMed] [Google Scholar]
- 22. Johnston HJ, Jones M, Ridler‐Dutton G, Spechler F, Stokes GS, Wyndham LE. Diet modification in lowering plasma cholesterol levels. A randomised trial of three types of intervention. Med J Aust 1995; 162: 524–6. [PubMed] [Google Scholar]
- 23. Koopman H, Spreeuwenberg C, Westerman RF, Donker AJ. Dietary treatment of patients with mild to moderate hypertension in a general practice: a pilot intervention study (1). The first three months. J Hum Hypertens 1990; 4: 368–71. [PubMed] [Google Scholar]
- 24. Neil HA, Roe L, Godlee RJ et al Randomised trial of lipid lowering dietary advice in general practice: the effects on serum lipids, lipoproteins, and antioxidants. BMJ 1995; 310: 569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parker AR, Byham‐Gray L, Denmark R, Winkle PJ. The effect of medical nutrition therapy by a registered dietitian nutritionist in patients with prediabetes participating in a randomized controlled clinical research trial. J Acad Nutr Diet 2014; 114: 1739–48. [DOI] [PubMed] [Google Scholar]
- 26. Rhodes KS, Bookstein LC, Aaronson LS, Mercer NM, Orringer CE. Intensive nutrition counseling enhances outcomes of National Cholesterol Education Program dietary therapy. J Am Diet Assoc 1996; 96: 1003–10. [DOI] [PubMed] [Google Scholar]
- 27. Imai S, Kozai H, Matsuda M et al Intervention with delivery of diabetic meals improves glycemic control in patients with type 2 diabetes mellitus. J Clin Biochem Nutr 2008; 42: 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lim HJ, Choi YM, Choue R. Dietary intervention with emphasis on folate intake reduces serum lipids but not plasma homocysteine levels in hyperlipidemic patients. Nutr Res 2008; 28: 767–74. [DOI] [PubMed] [Google Scholar]
- 29. Nissen SE, Tuzcu EM, Schoenhagen P et al Statin therapy, LDL cholesterol, C‐reactive protein, and coronary artery disease. N Engl J Med 2005; 352: 29–38. [DOI] [PubMed] [Google Scholar]
- 30. Brunner E, White I, Thorogood M. Can dietary interventions change diet and cardiovascular risk factors? A meta‐analysis of randomized controlled trials. Am J Public Health 1997; 87: 1415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barter P. HDL‐C: role as a risk modifier. Atheroscler Suppl 2011; 12: 267–70. [DOI] [PubMed] [Google Scholar]
- 32. National Cholesterol Education Program Expert Panel . Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143. [PubMed] [Google Scholar]
- 33. Kronmal R, Cain K, Ye Z, Omenn G. Total serum cholesterol levels and mortality risk as a function of age. A report based on the Framingham data. Arch Intern Med 1993; 153: 1065–73. [PubMed] [Google Scholar]
- 34. Jensen MD, Ryan DH, Apovian CM et al 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society. Circulation 2014; 129: S102–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Willett W. Nutritional Epidemiology, 3rd edn. New York, USA:Oxford University Press, 2012. [Google Scholar]


