Abstract
Aims
To explore the benefits of curative treatments (liver resection or local ablation) combined with splenectomy for patients with hepatocellular carcinoma (HCC) and Child grade B liver function.
Methods
We reviewed 245 patients with Child grade B liver function who underwent treatment with curative intent for HCC. Among these patients, 116 patients underwent curative treatment combined with splenectomy (the splenectomy group); the other 129 patients only underwent curative treatment (the non‐splenectomy group). A one‐to‐one matching produced 95 paired patients, perioperative and oncological outcomes were compared, and liver function changes were reassessed 1 year later.
Results
The perioperative liver failure rates were 7.4% and 6.3% (P = 1.000) and the 90‐day mortality was 4.2% and 6.3% (P = 0.747) in the splenectomy group and non‐splenectomy group, respectively. The 1‐, 3‐, and 5‐year overall survival rates were remarkably greater in the splenectomy group than in the non‐splenectomy group (92.6% vs. 79.8%, 53.4% vs. 34.7%, and 19.9% vs. 11.0%, respectively; P = 0.004). In the univariate and multivariate analyses, splenectomy was identified as a protective factor for long‐term survival. The proportion of patients whose liver function improved to Child A 1 year after surgery was also higher in the splenectomy group than in the non‐splenectomy group (95.4% vs. 83.3%; P = 0.048).
Conclusions
Compared with non‐splenectomy, curative treatments combined with splenectomy for patients with HCC and Child B grade liver function showed no different perioperative outcomes but achieved significant survival benefit. Splenectomy is a beneficial factor for patients with HCC and Child B liver function; liver function improved significantly 1 year after splenectomy.
Keywords: Child B, hepatocellular carcinoma, liver resection, local ablation, splenectomy
Introduction
Hepatocellular carcinoma (HCC) is the third tumor‐related death worldwide and primarily occurs in patients with cirrhotic background.1 Thus, two potentially lethal diseases coexist in the same patients, and both affect their prognosis.2 The Child–Pugh–Turcotte (hereinafter referred to as Child) grade, accepted by the most integrated HCC staging system, is a widely used tool to evaluate preoperative liver function.3 Before selecting the optimal treatment strategy for HCC, it is mandatory to consider not only the tumor burden but also the liver function reserve.4 Patients with well‐preserved liver function (Child A) are potentially eligible for most available treatments, from surgical resection to systemic therapy.5, 6 Decompensated liver function, a robust predictive factor for poor prognosis, is usually defined as Child grade B, precluding most useful treatments other than liver transplantation.7 According to the HCC treatment guidelines, liver resection is ineligible for patients with decompensated liver function due to high risk of liver‐related morbidity and mortality.2, 5 Theoretically, liver transplantation seems to be the optimal strategy to cure liver tumor and replace the cirrhotic liver for patients with limited tumor burden but decompensated liver function. However, only a small proportion of patients on the transplantation list receive liver transplantation due to the shortage of liver grafts. In recent years, with the improvement of surgical techniques and perioperative care, experienced surgeons can carry out liver resection in selected patients with decompensated liver function,8, 9, 10 even additional aggressive procedures, such as simultaneous splenectomy for hypersplenism or Hassab's operation (pericardial devascularization and splenectomy) for patients with variceal bleeding tendency.11, 12, 13, 14, 15 Several studies had reported that patients with HCC and impaired liver function achieved both short‐ and long‐term survival time after liver resection.7, 8, 16, 17 For single tumor or multiple small tumors that could not be a candidate for liver resection, local ablation achieved comparable survival outcomes.18 These surgical treatments (liver resection or local ablation) provide potential curative therapies for patients with HCC and Child grade B liver function that could not undergo liver transplantation.
Child grade B liver function is accompanied by portal hypertension (PH), splenomegaly, coagulopathy, hypoproteinemia, ascites, and poor performance status.19 Even though tumor recurrence after curative treatments is still the primary cause of death, other subsequent fatal episodes, including the further deterioration of liver function and variceal bleeding, also impact long‐term survival.20 Splenectomy has been regarded as a useful method to prevent variceal bleeding for more than 50 years.21 Several studies reported that patients with Child B liver function benefited from splenectomy, and liver function in most patients improved significantly 1 year after splenectomy.22, 23, 24 Other studies also indicated that splenectomy combined with hepatectomy could extend disease‐free survival and overall survival.12, 15, 25 However, these studies had some limitations, with either small sample sizes or heterogeneous populations. In addition, no research has explored the subsequent change in liver function and additional therapy after tumor recurrence. In the present study, we analyzed the short‐ and long‐term outcomes among patients with HCC and Child grade B liver function. To overcome selection bias, we used the propensity score matching (PSM) method to achieve convincing results.
Methods
Between January 2005 and December 2015, 245 patients with HCC and Child grade B liver function underwent curative treatments at the Hepatic Surgery Department of Tongji Hospital (Wuhan, China). To evaluate the effect of splenectomy on the long‐term survival and liver function change in Child B patients, these patients were divided into two groups based on the surgical procedures. This study was carried out according to the Declaration of Helsinki and approved by the Clinical Research Ethics Committee of Tongji Hospital.
Preoperative assessment
All patients underwent individual laboratory and radiological tests to evaluate their hepatic reserve and tumor burden. Laboratory tests included liver function test, complete blood count, serum α‐fetoprotein (AFP), indocyanine green retention rates at 15 min (ICG R15). The preoperative diagnosis and resectable evaluation of HCC were carried out by ultrasound, contrast‐enhanced computed tomography (CT), or magnetic resonance imaging. For patients with decompensated liver function, upper gastroenterological endoscopy was carried out to identify the presence of varices and evaluate its severity. The grade of esophageal varices was classified according to previous reports:26, 27 small, defined as straight varices not disappearing with insufflations; medium, defined as enlarged tortuous, occupying less than one‐third of the lumen; and large, defined as coil‐shaped, occupying more than one‐third of the lumen. The presence of clinically related PH was indirectly defined as the presence of varices and splenomegaly (central thickness of spleen >4 cm) and/or with a platelet count <100 000/mm3. Liver cirrhosis was assessed on the postoperative specimens by histopathology using the Laennec scoring system and was again classified as mild, moderate, and severe.28 Usually, mild cirrhosis is defined as most septa thin or one broad septum allowed. Moderate cirrhosis with at least two broad septa, and severe cirrhosis with at least one very broad septum or many minute nodules. If patients had active hepatitis, antiviral therapy was recommended to improve the perioperative safety. Patients with a decompensated liver function would not undergo liver resection until the liver function improved to Child grade A after receiving medical treatment.
Surgical indications for curative treatment and splenectomy
The resectability of the liver tumor was comprehensively assessed, considering both the liver function reserve and tumor burden. Usually, only patients with limited tumor burden but impaired liver function were considered for liver resection. Individual patients with a small tumor located deep in the liver parenchyma or with multiple small tumors that are not suitable for surgical resection should be considered for local ablation. In general, indications for simultaneous splenectomy are: (i) history of variceal bleeding; (ii) portal hypertension with serum platelet level <7.5 × 109/L and white blood cell (WBC) count <4 × 1012/L; and (iii) hypersplenism combined with moderate or large varices.
Surgical procedure
All patients were placed in the supine position, and surgical resections were carried out under general anesthesia by an experienced surgical team. If the liver tumor was located in the right lobe, a bilateral subcostal incision was made for liver resection and splenectomy. If the liver tumor was in the left lobe, a left subcostal incision with upward middle extension was made. Generally, the liver resection was carried out before splenectomy. Intraoperative ultrasound was routinely undertaken to determine the relationship between major hepatic vessels and liver tumors, and it could also detect additional intrahepatic metastasis not found by preoperative radiological tests. The Pringle maneuver was occasionally used in the event of major bleeding. Parenchymal transection was done by the combination of ultrasonic scalpel and bipolar coagulation. Hepatic vessels <2 mm were coagulated with an ultrasound scalpel, whereas larger vascular structures and the intrahepatic bile duct were ligated or clipped. Liver resections were classified into major (more than two segments) and minor (two or fewer segments) according to the Couinaud's classification. In addition, if the preoperative ICG R15 rate was >30%, major hepatectomy was cautiously performed to avoid post‐hepatectomy liver failure (PHLF). Anatomical resection was defined as complete removal of Couinaud's segments involved with the tumor, whereas non‐anatomical resection included enucleation of the tumor, wedge resection, or limited resection. Local ablation was guided by ultrasound with percutaneous or direct vision before splenectomy. For splenectomy, the splenic artery was ligated first, followed by the splenic vein and the surrounding ligaments. The estimated blood loss and the volume of intraoperative transfusion were both evaluated and recorded carefully by anesthesiologists at the end of surgery for subsequent analysis. The hemostatic fiber was placed on the cut surface and splenic pedicle. The abdominal drainage tubes were routinely placed to collect postoperative fluid accumulation. The resected specimen was sent for histopathological testing.
Postoperative management
After surgery, all patients were delivered to the intensive care unit. If their basic vital signs were stable, these patients were transferred to the general ward. Intravenous antibiotics were used to prevent infectious complications on the first day of surgery. Parenteral nutrition was started immediately after surgery. Patients who underwent splenectomy received anticoagulant therapy with low molecular weight heparin (LMWH, 5 kIU/day) by s.c. injection around the umbilicus tissue on the 3rd day after surgery if no i.p. bleeding was observed. If portal vein thrombosis (PVT) was detected, we doubled the dosage of LMWH (5 kIU/12 h) until its disappearance. For some complicated cases for which LMWH was ineffective, warfarin or urokinase were given to promote dissolution of the PVT. Liver function, coagulation function, and blood cell count were routinely measured at 1, 3, 5, and 7 days after surgery. The therapeutic strategy was adjusted based on these tests. If the drainage volume wa less than 150 mL/day and no bile leakage or abdominal infection was detected, the drainage tubes were removed. The Clavien–Dindo classification categorized the postoperative complications and grade III, IV, and V complications were defined as “major” complications.29 Post‐hepatectomy liver failure was defined by the “50–50 criteria”, which is a combination of prothrombin time index <50% and serum total bilirubin levels >50 μmol/L on postoperative day 5.30
Follow‐up
For patients who underwent splenectomy, anticoagulation therapy with LMWH by daily s.c. injection, to prevent the development of PVT, lasted for 3 months after discharge. Antiviral treatment continued until serum virology was completely clear. All patients were investigated with liver function tests, serum AFP level, and abdominal ultrasound or contrast‐enhanced CT scan every 3 months during the 2 years after surgery; after which, the interval would extend to every 6 months. The liver function change 1 year after the operation was recorded and compared with the preoperative counterpart. Tumor recurrence was defined as new lesions detected by radiological test with or without increasing levels of serum AFP. The decision to treat recurrent tumors was based on the tumor burden and liver function reserve. Subsequent resection, local ablation, liver transplantation, or transarterial chemoembolization was assigned accordingly.
Statistic analysis and PSM
One‐to‐one matching using the PSM method was undertaken to overcome potential selection bias between the two groups. The PSM model was generated using possible covariables that could affect the group allocation. The following covariates were matched: age, sex, hepatitis profile, comorbidity, variceal status, liver function, prothrombin time, WBC and platelet count, AFP level, cirrhosis, Child score, ICG R15, tumor size, and tumor number. The PSM model was generated using the PSM program through the spss R‐Plugin (https://developer.ibm.com/predictiveanalytics/downloads/), which utilized a newly written R code. The analysis applied single nearest‐neighbor matching, without replacement (a single participant could not be selected multiple times). Normally distributed continuous variables were expressed as mean ± standard deviation, whereas continuous variables with a non‐normal distribution were expressed as the median with interquartile range (IQR). Differences between groups were compared using the Mann–Whitney U‐test and Wilcoxon's rank test before and after matching. Categorical variables were reported as the number of cases and prevalence was analyzed by the χ2‐test with the Yates correction or Fisher's exact test, as appropriate. Patients’ survival curves were computed using the Kaplan–Meier method and compared using the log–rank test. Disease‐free survival (DFS) was measured from the date of operation until the detection of tumor recurrence. Overall survival (OS) was defined as the interval between the date of operation and the date of tumor‐related death; patients who died from other causes were defined as censored. Factors influencing OS were analyzed using multivariate analysis with Cox's proportional hazard model. We used two‐sided P‐values of <0.05. P < 0.05 was considered statistically significant. All statistical analyses were undertaken with spss version 24.0 (IBM, Armonk, NY, USA).
Results
During the study period, a total of 245 patients with HCC and Child grade B liver function who underwent curative treatment (liver resection or local ablation) for HCC were identified. Among these patients, 116 patients underwent splenectomy combined with hepatectomy or local ablation (the splenectomy group), and 129 patients underwent hepatectomy or local ablation alone (the non‐splenectomy group). After one‐to‐one PSM, 95 paired patients were generated. Baseline characteristics of the two groups before PSM are shown in supplemental Table S1. Before PSM, more patients in the splenectomy group had poor performance status. After PSM, no significant difference in baseline variables were noted between the two subgroups, including demographic data, preoperative liver function test, cirrhosis‐related portal hypertension, tumor size, and tumor number, except that more patients in the non‐splenectomy group had undergone endoscopic therapy before receiving surgical treatment when compared with the splenectomy group (27.4% vs. 7.4%, P < 0.001). Due to decompensated liver function, most patients enrolled in the two groups had hypoleukemia, hypothrombinemia, hypoproteinemia, ascites, poor prothrombin time, severe cirrhosis, and large varices. The preoperative characteristics of the two groups are presented in Table 1.
Table 1.
Baseline characteristics of Child B patients with hepatocellular carcinoma who underwent splenectomy and curative treatment (splenectomy group) or curative treatment alone (non‐splenectomy group), after propensity score matching
| Variable | Splenectomy group (n = 95) | Non‐splenectomy group (n = 95) | P‐value |
|---|---|---|---|
| Age, years | 51.98 ± 11.13 | 51.77 ± 8.72 | 0.885 |
| Gender | |||
| Male | 82 (86.3) | 81 (85.3) | 1.000 |
| Female | 13 (13.7) | 14 (14.7) | |
| Comorbidity | |||
| Hypertension | 23 (24.2) | 25 (26.3) | 0.867 |
| Diabetes mellitus | 12 (12.6) | 15 (15.8) | 0.678 |
| History of variceal bleeding | 25 (26.3) | 27 (28.4) | 0.871 |
| Preoperative endoscopic therapy | 7 (7.4) | 26 (27.4) | <0.001 |
| Etiology | 1.000 | ||
| HBV infection | 89 (93.7) | 90 (94.7) | |
| Others | 6 (6.3) | 5 (5.3) | |
| HBV‐DNA copy | 0.656 | ||
| Positive | 39 (41.1) | 36 (37.9) | |
| Negative | 56 (58.9) | 59 (62.1) | |
| BMI, kg/m2 | 22.37 ± 2.33 | 22.28 ± 2.18 | 0.795 |
| White blood cell count, ×1012/L | 3.02 ± 1.58 | 3.07 ± 0.71 | 0.779 |
| Platelet count, ×109/L | 48.80 ± 21.58 | 49.48 ± 12.60 | 0.791 |
| Albumin, g/L | 33.14 ± 4.16 | 32.83 ± 3.61 | 0.577 |
| Total bilirubin, μmol/L | 23.18 ± 12.50 | 24.70 ± 17.73 | 0.494 |
| PT, s | 16.23 ± 1.57 | 16.33 ± 1.24 | 0.611 |
| AFP, ng/mL | 0.461 | ||
| Positive | 59 (62.1) | 53 (55.8) | |
| Negative | 36 (37.9) | 42 (44.2) | |
| Cirrhosis† | 0.437 | ||
| Mild | 5 (5.3) | 7 (7.4) | |
| Medium | 25 (26.3) | 18 (18.9) | |
| Severe | 65 (68.4) | 70 (73.7) | |
| Esophageal varices‡ | 0.479 | ||
| Small | 25 (26.3) | 22 (23.2) | |
| Median | 21 (22.1) | 16 (16.8) | |
| Large | 49 (51.6) | 57 (60) | |
| Presence of ascites | 78 (82.1) | 75 (78.9) | 0.714 |
| Child score | 0.860 | ||
| 7 | 72 (75.8) | 69 (72.6) | |
| 8 | 20 (21.1) | 22 (23.2) | |
| 9 | 3 (3.2) | 4 (4.2) | |
| MELD score | 9.1 ± 1.7 | 9.4 ± 1.4 | 0.782 |
| ICG R15, % | 28.4 ± 10.0 | 28.0 ± 7.5 | 0.794 |
| ASA score | 1.000 | ||
| >2 | 87 (91.6) | 87 (91.6) | |
| ≤2 | 8 (8.4) | 8 (8.4) | |
| ECOG score | 0.866 | ||
| 0 | 73 (76.8) | 71 (74.7) | |
| 1 | 22 (23.2) | 24 (25.3) | |
| Spleen thickness, cm | 5.43 ± 0.92 | 5.38 ± 0.49 | 0.658 |
| Portal vein diameter, cm | 1.35 ± 0.19 | 1.34 ± 0.13 | 0.600 |
| Median tumor size, cm | 3.43 ± 1.55 | 3.42 ± 1.20 | 0.954 |
| Largest tumor size, cm | 0.885 | ||
| >3 | 47 (49.5) | 46 (48.4) | |
| ≤3 | 48 (50.5) | 49 (51.6) | |
| Tumor number | 1.000 | ||
| Solitary | 88 (92.6) | 87 (91.6) | |
| Multiple | 7 (7.4) | 8 (8.4) |
Data are expressed as n (%) or mean ± standard deviation.
Cirrhosis was graded using the following criteria: mild, most septa thin, one broad septum allowed; moderate, at least two broad septa; and severe, at least one very broad septum or many minute nodules.
Grade of varices were classified as: small, straight varices not disappearing with insufflations; medium, enlarged tortuous, occupying <1/3 of lumen; and large, coil‐shaped, occupying >1/3 of lumen.
AFP, α‐fetoprotein; ASA, American Society of Anesthesiologists; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; ICG R15, indocyanine green retention rate at 15 min; MELD, Model for End‐stage Liver Disease; PT, prothrombin time.
The surgical outcomes and postoperative complications are summarized in Table 2. Most patients in the two groups underwent open liver resection, with only a small proportion of patients undergoing laparoscopic surgery and local ablation. Among patients who received liver resection, only a small portion of patients in this study performed major resection and anatomic resection regarding a high risk of PHLF. In the splenectomy group, 47 patients (49.5%) performed additional pericardial devascularization due to large varices around the esophagus and stomach; no patient undergo this procedure in the non‐splenectomy group (P < 0.001). Given that additional procedure performed in the splenectomy group, the operating time is longer (257 vs. 185 min; P < 0.001) and the intraoperative blood loss is also greater (529 vs. 294 mL; P < 0.001) in the splenectomy group when compared with the non‐splenectomy group. Fifty‐one (53.7%) patients need intraoperative transfusion in the splenectomy group, while only 32 (33.7%) patients need intraoperative transfusion (P = 0.005). No patient died during the intraoperative procedures.
Table 2.
Comparision of surgical outcomes and postoperative complications between Child B patients with hepatocellular carcinoma who received splenectomy and curative treatment (splenectomy group) or curative treatment alone (non‐splenectomy group)
| Variable | Splenectomy group | Non‐splenectomy group | P‐value |
|---|---|---|---|
| Surgical procedure | 0.678 | ||
| Laparoscopic surgery | 12 (12.6) | 15 (15.8) | |
| Open surgery | 83 (87.4) | 80 (84.2) | |
| Surgical manner | 0.637 | ||
| Liver resection | 68 (71.6) | 64 (67.4) | |
| Local ablation | 27 (28.4) | 31 (32.6) | |
| Pericardial devascularization | 47 (49.5) | 0 (0.0) | <0.001 |
| Anatomic resection | 9 (9.5) | 12 (12.6) | 0.644 |
| Major hepatectomy | 3 (3.2) | 5 (5.3) | 0.721 |
| Operative time, min | 257 ± 71 | 185 ± 86 | <0.001 |
| Estimated blood loss, mL | 529 ± 479 | 294 ± 311 | <0.001 |
| Intraoperative blood transfusion | 51 (53.7) | 32 (33.7) | 0.005 |
| Intraoperative mortality | 0 (0.0) | 0 (0.0) | N/A |
| Total complications | |||
| Minor | 32 (33.7) | 23 (24.2) | 0.149 |
| Major | 25 (26.3) | 14 (14.7) | 0.048 |
| General complication | |||
| Respiratory | 21 (22.1) | 8 (8.4) | 0.014 |
| Renal | 4 (4.2) | 0 (0.0) | 0.121 |
| Variceal hemorrhage | 3 (3.2) | 1 (1.1) | 0.621 |
| Surgical complication | |||
| Wound infection | 6 (6.3) | 2 (2.1) | 0.279 |
| Intra‐abdominal bleeding | 3 (3.2) | 2 (2.1) | 1.000 |
| PVT | 30 (31.6) | 0 (0.0) | <0.001 |
| Pancreatic injury | 15 (15.8) | 0 (0.0) | <0.001 |
| Postoperative transfusion | 44 (46.3) | 21 (22.1) | <0.001 |
| Liver‐related | |||
| Bile leakage | 3 (3.2) | 1 (1.1) | 0.621 |
| Transient PHLF | 7 (7.4) | 6 (6.3) | 1.000 |
| Reoperation | 2 (2.1) | 1 (1.1) | 1.000 |
| Length of hospital stay, days | 18.2 ± 7.0 | 16.4 ± 5.5 | 0.069 |
| 90‐day mortality | 4 (4.2) | 6 (6.3) | 0.747 |
| Tumor recurrence | 2 (2.1) | 3 (3.2) | |
| Liver failure | 2 (2.1) | 2 (2.1) | |
| Variceal bleeding | 0 (0.0) | 1 (1.1) |
Data are expressed as n (%) or mean ± standard deviation.
N/A, not available; PHLF, post‐hepatectomy liver failure; PVT, portal vein thrombosis.
Postoperative complications are illustrated in Table 2. Even though more patients in the splenectomy group experienced minor complications when compared with the non‐splenectomy group (33.7% vs. 24.2%), no statistical difference was identified (P = 0.149); a higher proportion of patients in the splenectomy group experienced major complications (26.3% vs. 14.7%, P = 0.048). Respiratory‐related complications were significantly high among patients in the splenectomy group, with 21 patients (22.1%), whereas only 8 patients (8.4%) in the non‐splenectomy group (P = 0.014) experienced respiratory dysfunction. Additionally, patients in the splenectomy group experienced more surgical complications. Postoperative PVT was observed in 30 patients (31.6%), and pancreatic injury in 15 patients (15.8%), whereas no patient in the non‐splenectomy group experienced these complications (P < 0.001). The postoperative transfusion rate was also higher in the splenectomy group (46.3% vs. 22.1%; P < 0.001). In contrast to patients in the non‐splenectomy group, more patients had renal dysfunction, perioperative variceal bleeding, wound infection, intra‐abdominal bleeding in the splenectomy group, but no statistical difference was found. Liver‐related complications, reoperation rate, and 90‐day mortality were similar between the two groups. Seven patients (7.4%) in the splenectomy group and six patients (6.3%) in the non‐splenectomy group (P = 1.000) developed transient PHLF due to poor liver function reserve. Three patients required reoperation due to uncontrolled intra‐abdominal bleeding, two (2.1%) in the splenectomy group and one (1.1%) in the non‐splenectomy group (P = 1.000). Ten patients died within 90 days after surgery: four patients (4.2%) in the splenectomy group (two from tumor recurrence and two from liver failure) and six patients (6.3%) in the non‐splenectomy group (three from tumor recurrence, two from liver failure, and one from variceal bleeding) (P = 0.747).
Follow‐up information and long‐term outcomes are summarized in Table 3. The median follow‐up time was 42 months (IQR, 33–51) in the splenectomy group and 38 months (IQR, 29–44) in the non‐splenectomy group. During the follow‐up period, even though there was no significant difference in the number of deaths between the groups, a statistical difference was found in time to recurrence, pattern of recurrence, subsequent treatments, the experience of variceal rebleeding, the cause of death, and liver function change within 1 year after surgery (both P < 0.05). In the non‐splenectomy group, more patients experienced tumor recurrence, recurrence within 2 years, extrahepatic metastasis, presence of variceal rebleeding, and death from liver failure and variceal rebleeding. While in the splenectomy, more patients experienced tumor recurrence beyond 2 years, more patients received reoperation, transcatheter arterial chemoembolization (TACE), and local ablation; most patients died from tumor recurrence. In contrast to patients in the non‐splenectomy group, more patients in the splenectomy group had Child grade A liver function 1 year after surgery (94.9% vs. 83.3%, P = 0.024). Long‐term survival outcomes were also statistically different between the two groups regarding DFS and OS. The 1‐, 3‐, and 5‐year DFS rates were 83.2%, 28.0%, and 0.0% in the splenectomy group, and were 65.5%, 9.7%, and 0.0% in the non‐splenectomy group (P < 0.001) (Fig. 1). The 1‐, 3‐, and 5‐year OS rates were longer in the splenectomy group than in the non‐splenectomy group (92.6% vs.79.8%, 53.4% vs. 34.7%, and 19.9% vs. 11.0%, respectively; P = 0.004) (Fig. 2).
Table 3.
Follow‐up information and long‐term outcomes among Child B patients with hepatocellular carcinoma who received splenectomy and curative treatment (splenectomy group) or curative treatment alone (non‐splenectomy group)
| Variable | Splenectomy group, n (%) | Non‐splenectomy group, n (%) | P‐value |
|---|---|---|---|
| Median follow‐up period, months | 42 (33–51) | 38 (29–44) | 0.673 |
| Death | 80 (84.2) | 76 (80.0) | 0.571 |
| Recurrence | 79 (83.2) | 89 (93.7) | 0.039 |
| Time to recurrence | <0.001 | ||
| <2 years | 43 (54.4) | 73 (82.0) | |
| ≥2 years | 36 (45.6) | 16 (18.0) | |
| Recurrence pattern | 0.014 | ||
| Intrahepatic | 78 (82.1) | 81 (85.3) | |
| Extrahepatic | 0 (0.0) | 5 (5.3) | |
| Both | 1 (1.1) | 3 (3.2) | |
| Recurrence treatment | 0.004 | ||
| Reoperation | 4 (5.1) | 1 (1.1) | |
| TACE | 32 (40.5) | 18 (20.2) | |
| Local ablation | 18 (22.8) | 11 (12.6) | |
| Transplantation | 1 (1.3) | 5 (5.6) | |
| Best support | 32 (40.5) | 48 (53.9) | |
| Experience of variceal bleeding | 5 (5.3) | 14 (14.7) | 0.047 |
| Cause of death | 0.006 | ||
| Tumor recurrence | 75 (93.8) | 58 (76.3) | |
| Liver failure | 3 (3.8) | 12 (15.8) | |
| Variceal bleeding | 1 (1.3) | 6 (7.9) | |
| Other reasons | 1 (1.3) | 0 (0.0) | |
| Liver function 1 year after surgery | 0.024 | ||
| Child grade A | 75 (94.9) | 55 (83.3) | |
| Child grade B | 4 (5.1) | 11 (16.7) |
TACE, transcatheter arterial chemoembolization.
Figure 1.
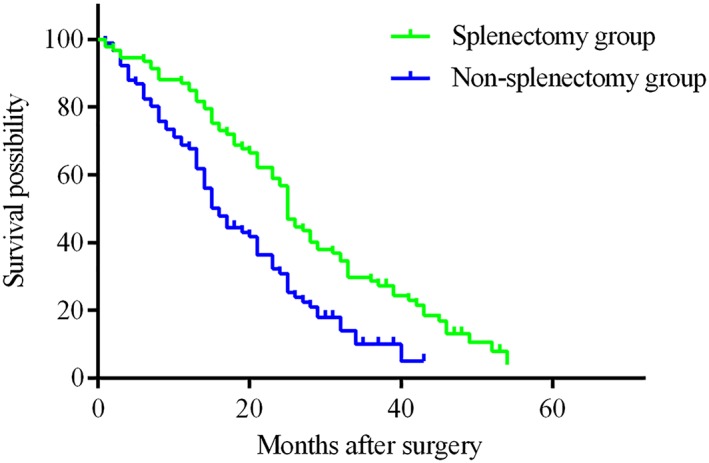
Disease‐free survival (DFS) in Child B patients with hepatocellular carcinoma who underwent splenectomy and liver resection or local ablation (splenectomy group) was significantly longer than in those who underwent liver resection or local ablation only (non‐splenectomy group). The 1‐, 3‐, and 5‐year DFS rates were 83.2%, 28.0%, and 0.0% in the splenectomy group, and 65.5%, 9.7%, and 0.0% in the non‐splenectomy group, respectively (P < 0.001). [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 2.
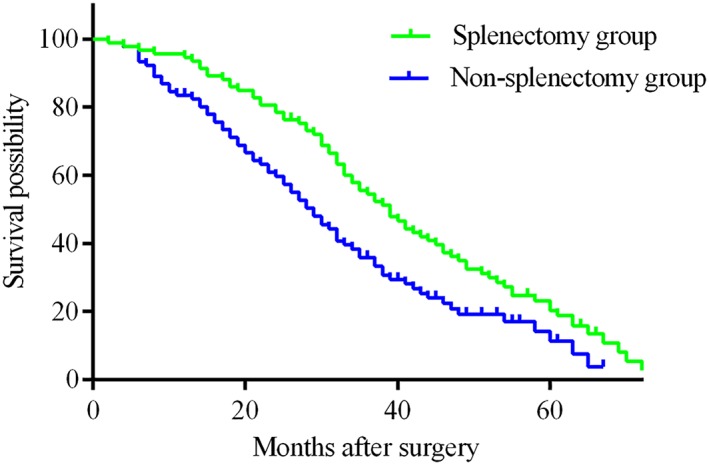
There was a significant difference in overall survival (OS) between Child B patients with hepatocellular carcinoma who underwent splenectomy and liver resection or local ablation (splenectomy group) was significantly longer than in those who underwent liver resection or local ablation only (non‐splenectomy group). The 1‐, 3‐, and 5‐year OS rates were longer in the splenectomy group than in the non‐splenectomy group (92.6% vs.79.8%, 53.4% vs. 34.7%, and 19.9% vs. 11.0%, respectively; P = 0.004). [Color figure can be viewed at http://wileyonlinelibrary.com]
In univariate Cox hazard analysis, Child score 8 (hazard ratio [HR], 0.406; 95% confidence interval [CI], 0.179–0.920; P = 0.031), Child score 9 (HR, 0.304; 95% CI, 0.140–0.656; P = 0.002), ECOG score more than 0 point (HR, 1.933; 95% CI, 1.087–3.438; P = 0.025), splenectomy (HR, 0.633; 95% CI, 0.459–0.873; P = 0.005), tumor size >3 cm (HR, 1.663; 95% CI, 1.205–2.294; P = 0.002), multiple tumors (HR, 5.585; 95% CI, 3.994–8.591; P < 0.001), and postoperative liver failure (HR, 2.190; 95% CI, 1.181–4.058; P = 0.013) were significant factors correlated with OS. Furthermore, Child score 8 (HR, 0.348; 95% CI, 0.149–0.813; P = 0.015), Child score 9 (HR, 0.198; 95% CI, 0.087–0.451; P < 0.001), ECOG score >0 (HR, 2.452; 95% CI, 1.338–4.492; P = 0.004), splenectomy (HR, 0.432; 95% CI, 0.306–0.610; P < 0.001), tumor size >3 cm (HR, 1.284; 95% CI, 0.870–1.896; P = 0.029), multiple tumor (HR, 6.875; 95% CI, 4.254–11.111; P < 0.001), and postoperative liver failure (HR, 2.627; 95% CI,1.351–5.109; P = 0.004) remained survival prognosticators in multivariate analysis. Splenectomy was identified as a significant protective factor for long‐term survival (Table 4).
Table 4.
Univariate and multivariate analyses of prognostic factors for overall survival among Child B patients with hepatocellular carcinoma who received splenectomy and curative treatment, or curative treatment alone, using the Cox hazard model
| Variables | Univariate analysis | P‐value | Multivariate analysis | P‐value |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| History of variceal bleeding (yes vs. no) | 1.013 (0.704–1.458) | 0.944 | ||
| Endoscopic therapy (yes vs. no) | 0.934 (0.599–1.458) | 0.763 | ||
| Child score | ||||
| 7 vs. 8 | 0.406 (0.179–0.920) | 0.031 | 0.348 (0.149–0.813) | 0.015 |
| 7 vs. 9 | 0.304 (0.140–0.656) | 0.002 | 0.198 (0.087–0.451) | <0.001 |
| Cirrhosis | ||||
| Mild vs. severe | 0.477 (0.222–1.026) | 0.058 | 0.454 (0.197–1.044) | 0.063 |
| Mild vs. medium | 1.072 (0.738–1.556) | 0.716 | 1.295 (0.862–1.945) | 0.213 |
| Varices | ||||
| Small vs. large | 0.671 (0.441–1.021) | 0.063 | 0.956 (0.610–1.499) | 0.845 |
| Small vs. medium | 0.770 (0.526–1.128) | 0.179 | 1.317 (0.851–2.040) | 0.217 |
| ASA score (≤2 vs. >2) | 1.082 (0.585–2.001) | 0.804 | ||
| ECOG score (0 vs. 1) | 1.933 (1.087–3.438) | 0.025 | 2.452 (1.338–4.492) | 0.004 |
| Pericardial devascularization (yes vs. no) | 0.888 (0.620–1.272) | 0.517 | ||
| Splenectomy (yes vs. no) | 0.633 (0.459–0.873) | 0.005 | 0.432 (0.306–0.610) | <0.001 |
| Presence of ascites (yes vs. no) | 1.096 (0.731–1.096) | 0.658 | ||
| Therapeutic manner (Resection vs. ablation) | 1.061 (0.758–1.484) | 0.730 | ||
| Intraoperative transfusion (yes vs. no) | 0.881 (0.637–1.218) | 0.442 | ||
| Tumor diameter (>3 cm vs. ≤3 cm) | 1.663 (1.205–2.294) | 0.002 | 1.284 (0.870–1.896) | 0.029 |
| Tumor number (multiple vs. solitary) | 5.858 (3.994–8.591) | <0.001 | 6.875 (4.254–11.111) | <0.001 |
| HBV‐DNA copy (positive vs. negative) | 1.093 (0.790–1.512) | 0.593 | ||
| AFP level (negative vs. positive) | 0.935 (0.678–1.289) | 0.680 | ||
| Postoperative liver failure (yes vs. no) | 2.190 (1.181–4.058) | 0.013 | 2.627 (1.351–5.109) | 0.004 |
AFP, α‐fetoprotein; ASA, American Society of Anesthesiologists; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HR, hazard ratio.
One year after surgery, we successfully tested liver function in 145 patients, 79 patients in the splenectomy group and 66 patients in the non‐splenectomy group. The liver function changes of 66 newly paired patients were reassessed. Table 5 shows the comparison of liver function change between the two groups. In contrast to patients in the non‐splenectomy group, white blood cell count and platelet count in the splenectomy group was significantly elevated, and aspartate transaminase, total bilirubin, prothrombin time, and Child score decreased remarkably (P < 0.001). More patients with preoperative Child grade B liver function improved to Child grade A after splenectomy. Albumin levels in the non‐splenectomy group were also significantly elevated, but the elevation was greater in the splenectomy group.
Table 5.
Comparison of liver function changes at 1 year after surgery in Child B patients with hepatocellular carcinoma who received splenectomy and curative treatment (splenectomy group) or curative treatment alone (non‐splenectomy group)
| Before surgery | One year after surgery | P‐value | |
|---|---|---|---|
| Splenectomy group (n = 66) | |||
| White blood cell count, ×1012/L | 2.84 ± 1.26 | 6.15 ± 1.15 | <0.001 |
| Platelet count, ×109/L | 45.2 ± 19.8 | 155.3 ± 33.9 | <0.001 |
| Aspartate transaminase, U/L | 45.0 ± 25.1 | 31.3 ± 21.8 | <0.001 |
| Total bilirubin, μmol/L | 22.5 ± 9.2 | 18.6 ± 4.4 | <0.001 |
| Albumin, g/L | 33.2 ± 3.8 | 41.8 ± 3.9 | <0.001 |
| Prothrombin time, s | 16.3 ± 1.4 | 12.9 ± 1.1 | <0.001 |
| Presence of ascites | 60 (90.9) | 3 (4.6) | <0.001 |
| Child score | 7.2 ± 0.4 | 5.2 ± 0.5 | <0.001 |
| Child grade A | 0 (0.0) | 63 (95.4) | <0.001 |
| Non‐splenectomy group (n = 66) | |||
| White blood cell count, ×1012/L | 3.22 ± 0.65 | 3.38 ± 0.47 | 0.091 |
| Platelet count, ×109/L | 50.7 ± 13.4 | 52.4 ± 13.0 | 0.523 |
| Aspartate transaminase, U/L | 42.5 ± 17.7 | 31.3 ± 11.5 | <0.001 |
| Total bilirubin, μmol/L | 23.8 ± 15.4 | 20.1 ± 3.0 | 0.070 |
| Albumin, g/L | 33.1 ± 3.8 | 37.6 ± 3.6 | <0.001 |
| Prothrombin time, s | 16.3 ± 1.2 | 12.8 ± 1.5 | <0.001 |
| Presence of ascites | 62 (93.9) | 11 (16.7) | <0.001 |
| Child score | 7.3 ± 0.5 | 5.4 ± 0.8 | <0.001 |
| Child grade A | 0 (0.0) | 55 (83.3) | <0.001 |
Data are expressed as n (%) or mean ± standard deviation.
Discussion
Liver transplantation is the optimal treatment option for patients with limited tumor burden and Child grade B liver function. However, the number of patients on the transplantation list is greater than the number of potential organ donors, making it essential to adopt alternative treatments for these patients, even if these options are inferior to liver transplantation. Our study, not the first attempt, provided a challenging alternative that some selective HCC patients with decompensated liver function could benefit from simultaneous splenectomy and curative treatments. The DFS and OS rates in splenectomy patients were significantly higher than in patients who underwent liver resection or local ablation alone, and liver function in most patients improved 1 year after splenectomy. The postoperative complications in our study are relatively high compared with other studies, but the lethal comorbidity is acceptable. We suggest that simultaneous splenectomy combined with liver resection or local ablation are safe and beneficial for some selected HCC patients with Child grade B liver function.
Patients with decompensated liver function usually experience portal hypertension, hypersplenism, and thrombocytopenia, which could cause decreases in WBC and platelet counts and coagulopathy, precluding subsequent curative treatment for HCC.7 Unlike most countries in Europe and North America, where HCC and hepatic decompensation is regarded as a contradiction to surgical resection,2, 31 liver resection or local ablation combined with other aggressive surgical procedures, such as splenectomy with or without Hassab's operation, are still carried out in some Asian countries, mainly due to the lack of liver donors and no better alternatives.13, 14, 32, 33, 34 Splenectomy alone has been reported as a useful measure to extend the surgical indication, as this procedure could improve the liver function within a short time.22, 33 In the past, some surgeons thought that, for patients with HCC complicated with the decompensated liver function, splenectomy should be carried out first, then hepatectomy after the improvement of liver function.35 This two‐stage splenectomy and hepatectomy was used to decrease the high risk of bleeding with liver resection and postoperative complications, whereas repeated abdominal surgery within a short time also increased the risk of other complications and inevitably prolonged the treatment period. The recent improvements in preoperative evaluation, postoperative care, and minimally invasive surgery have made it feasible to undertake surgical resection in some selected patients with Child grade B liver function.10, 36 For small liver tumors located deep in the liver parenchyma or the center of the liver, local ablation provides an alternative curative treatment option, achieving comparable oncological outcomes but remarkable low complication rates.18 Some patients with small tumors located on the peripheral surface of the liver are also recommended for liver resection instead of local ablation. In the present study, most patients underwent liver resection, except for some patients with tumor size <3 cm and multiple tumors that were not considered candidates for liver resection.
Decompensated liver function and the presence of large varices are usually considered as contraindications for liver resection due to a high risk of perioperative mortality and PHLF rates.7 Most of the patients enrolled in our study had severe cirrhosis and large varices, half of whom underwent additional splenectomy. Even though endoscopic therapy (sclerotherapy or band ligation) and transjugular intrahepatic portosystemic shunt are the standard treatments for variceal patients in Europe and North America,20 splenectomy combined with or without Hassab's procedure is more common in our center. Unsurprisingly, the proportion of postoperative complications was relatively high, but fatal complications were similar to that in other studies. A recent meta‐analysis concluded that there is no difference in terms of perioperative mortality and PHLF between simultaneous surgery and liver resection alone for patients with HCC and PH.15 Major complications in the two groups are also comparable in our study.
The specific mechanism of improvement of liver function after splenectomy is still unknown. Several studies have found that splenectomy could improve liver function among patients with Child grade B liver function.22, 24, 37 In our research, hepatic function in most patients was ameliorated 1 year after surgery, especially among patients who underwent splenectomy. One possible factor contributes this effect is that these patients received additional medical treatments or changed their lifestyles, such as antiviral therapy, smoking cessation, and alcohol withdrawal. Other potential beneficial factors could be the increased platelet count after splenectomy. It has been reported that platelets play a vital role in liver regeneration after partial hepatectomy in animal models.38, 39 The rapidly elevated platelet count after splenectomy could promote liver regeneration, which is beneficial to the recovery of liver function. Platelets can also delay fibrosis of chronic intoxication in an animal model.40, 41 The difference in liver function improvement between the two groups makes us believe that splenectomy can significantly improve the liver function of Child grade B patients.
The possible reasons for the combined procedure contributing to prolonged DFS and OS after the operation are as follows. First, the increased platelet count and improvement of liver function improved the quality of life, making it feasible to receive available treatments after tumor recurrence. Second, splenectomy or Hassab's operation could decrease the portal inflow, reducing episodes of variceal bleeding, as well as mortality from non‐tumorigenic causes. Furthermore, a series of studies reported that splenectomy could promote the antitumor effect through restoring lymphocyte function,42 increasing the number of natural killer cells,35, 43 and decreasing myeloid‐derived suppressor cells in vivo.44 However, compared with patients without splenectomy, patients receiving splenectomy had a longer overall survival. In terms of the limited tumor burden in our study, survival time is not satisfactory in either group, and most patients died from tumor recurrence, suggesting that the cirrhosis background could not be reversed to normal even if the liver function of most patients was significantly improved after splenectomy. Moreover, liver resection or local ablation is the only strategy to achieve a potentially curative treatment when compared with other therapeutic options like TACE or sorafenib if liver transplantation is unavailable.2 In addition, univariate and multivariate Cox hazard analyses both identified that splenectomy is a protective factor for long‐term survival in Child B patients with HCC. Considering that China is the biggest developing country and accounts for almost half of all HCC patients worldwide, any non‐transplantation attempt that could achieve improved long‐term survival should be encouraged. Liver resection or local ablation combined with splenectomy could be an alternative, but not the perfect, option for patients with limited tumor burden and decompensated liver function if economic factors and subsequent unavailability of liver transplantation were considered.
Several limitations of this study should be mentioned. First, although the PSM analysis is applied in this study, potential selection bias still exists. Second, the sample size is relatively small and originates from a single center. A prospective randomized control trial is required to identify the real role of splenectomy in decompensated HCC patients to overcome these shortcomings. In addition, the liver function changes in this study were followed up in 1 year after surgery due to the inconsistency of follow‐up compliance in the same center. Long‐term outcomes of liver function and immunobiological changes should be further followed and investigated. Also, the primary etiology in our study is HBV infection; whether patients with liver cirrhosis caused by other etiology can benefit from simultaneous splenectomy is still unknown. Furthermore, most patients who were candidates for liver transplantation underwent alternative treatments in this study, and the actual survival gap between liver transplantation and alternative therapy is still unknown. Considering the survival difference in our research, future researchers focusing on these decompensated HCC patients should compare the long‐term outcomes of this surgical procedure to liver transplantation and identify those who can benefit the most from simultaneous splenectomy and liver resection or local ablation.
In conclusion, hepatectomy or local ablation combined with splenectomy can be safely carried out in some selected patients with HCC and Child grade B liver function. The combined procedure shows acceptable mortality and major complications, and favorable survival benefit. Simultaneous splenectomy achieved improved OS time and provided a higher proportion of Child grade B liver function conversion to Child grade A 1 year after splenectomy.
Supporting information
Table S1 Baseline characteristics of each treatment subgroup before propensity score matching.
Acknowledgments
Some clinical data were collected from the Large‐scale Data Analysis Center of Cancer Precision Medicine‐LinkDoc database. Funding was provided by the State Key Project on Infectious Disease of China (grant no. 2018ZX10723204‐003‐002).
Pei, Y. , Zhang, Z. , Mba'nbo‐koumpa, A. , Chen, X. , and Zhang, W. (2019) Improved survival following splenectomy combined with curative treatments for hepatocellular carcinoma in Child B patients: A propensity score matching study. Hepatol Res, 49: 177–188. 10.1111/hepr.13276.
Conflict of interest: The authors have no conflict of interest.
Financial support: Funding was provided by the State Key Project on Infectious Disease of China (grant no. 2018ZX10723204‐003‐002).
Contributor Information
Xiaoping Chen, Email: chenxpchenxp@163.com.
Wanguang Zhang, Email: wgzhang@tjh.tjmu.edu.cn.
References
- 1. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. The Lancet 2012; 379: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 2. Bruix J, Reig M, Sherman M. Evidence‐based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 2016; 150: 835–853. [DOI] [PubMed] [Google Scholar]
- 3. Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child–Pugh versus MELD. J Hepatol 2005; 42: S100–S107. [DOI] [PubMed] [Google Scholar]
- 4. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999; 19: 329–338. [DOI] [PubMed] [Google Scholar]
- 5. Liver EAFTSOT . EASL‐EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: 908–943. [DOI] [PubMed] [Google Scholar]
- 6. Heimbach JK, Kulik LM, Finn RS et al AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018; 67: 358–380. [DOI] [PubMed] [Google Scholar]
- 7. Granito A, Bolondi L. Non‐transplant therapies for patients with hepatocellular carcinoma and Child‐Pugh‐Turcotte class B cirrhosis. Lancet Oncol 2017; 18: e101–e112. [DOI] [PubMed] [Google Scholar]
- 8. Piscaglia F, Terzi E, Cucchetti A et al Treatment of hepatocellular carcinoma in Child‐Pugh B patients. Digest Liver Dis 2013; 45: 852–858. [DOI] [PubMed] [Google Scholar]
- 9. Ishizawa T, Hasegawa K, Aoki T et al Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology 2008; 134: 1908–1916. [DOI] [PubMed] [Google Scholar]
- 10. Brytska N, Han HS, Shehta A, Yoon YS, Cho JY, Choi Y. Laparoscopic liver resection for hepatitis B and C virus‐related hepatocellular carcinoma in patients with Child B or C cirrhosis. Hepatobil Surg Nutrit 2015; 4: 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin MC, Wu CC, Ho WL, Yeh DC, Liu TJ, P'eng FK. Concomitant splenectomy for hypersplenic thrombocytopenia in hepatic resection for hepatocellular carcinoma. Hepatogastroenterology 1999; 46: 630–634. [PubMed] [Google Scholar]
- 12. Chen XP, Wu ZD, Huang ZY, Qiu FZ. Use of hepatectomy and splenectomy to treat hepatocellular carcinoma with cirrhotic hypersplenism. Br J Surg 2005; 92: 334–339. [DOI] [PubMed] [Google Scholar]
- 13. Shi R, Zhang YM, Zhu ZJ et al Synchronous splenectomy and hepatectomy in patients with hepatocellular carcinoma, hypersplenism and liver cirrhosis. Hepatogastroenterology 2014; 61: 1363–1367. [PubMed] [Google Scholar]
- 14. Yang T, He H, Yuan J et al Surgery for hepatocellular carcinoma presenting with variceal bleeding: the Eastern experience. J Surg Oncol 2016; 113: 165–174. [DOI] [PubMed] [Google Scholar]
- 15. Li W, Shen SQ, Wu SM, Chen ZB, Hu C, Yan RC. Simultaneous hepatectomy and splenectomy versus hepatectomy alone for hepatocellular carcinoma complicated by hypersplenism: a meta‐analysis. Onco Targets Ther 2015; 8: 2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paquet KJ. Surgery for cirrhotic patients with hepatocellular carcinoma and hypersplenism. Surg Endosc 2001; 15: 104–105. [DOI] [PubMed] [Google Scholar]
- 17. Duan YF, Li XD, Sun DL, Chen XM, An Y, Zhu F. A preliminary study on surgery for hepatocellular carcinoma patients with portal hypertension. Am J Surg 2015; 210: 129–133. [DOI] [PubMed] [Google Scholar]
- 18. Lucchina N, Tsetis D, Ierardi AM et al Current role of microwave ablation in the treatment of small hepatocellular carcinomas. Ann Gastroenterol 2016; 29: 460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. D'Amico G, Garcia‐Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006; 44: 217–231. [DOI] [PubMed] [Google Scholar]
- 20. Garcia‐Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017; 65: 310–335. [DOI] [PubMed] [Google Scholar]
- 21. Hassab MA, Younis MT. el‐Kilany MS. Gastroesophageal decongestion and splenectomy in the treatment of esophageal varices secondary to bilharzial cirrhosis: anatomical and experimental studies. Surgery 1968; 63: 731–737. [PubMed] [Google Scholar]
- 22. Yamamoto N, Okano K, Oshima M et al Laparoscopic splenectomy for patients with liver cirrhosis: improvement of liver function in patients with Child‐Pugh class B. Surgery 2015; 158: 1538–1544. [DOI] [PubMed] [Google Scholar]
- 23. Murata K, Ito K, Yoneda K, Shiraki K, Sakurai H, Ito M. Splenectomy improves liver function in patients with liver cirrhosis. Hepatogastroenterology 2008; 55: 1407–1411. [PubMed] [Google Scholar]
- 24. Leite LA, Pimenta Filho AA, Ferreira Rde C et al Splenectomy improves hemostatic and liver functions in hepatosplenic Schistosomiasis mansoni . PloS One 2015; 10: e0135370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu CC, Cheng SB, Ho WM et al Appraisal of concomitant splenectomy in liver resection for hepatocellular carcinoma in cirrhotic patients with hypersplenic thrombocytopenia. Surgery 2004; 136: 660–668. [DOI] [PubMed] [Google Scholar]
- 26. Jensen DM. Endoscopic screening for varices in cirrhosis: findings, implications, and outcomes. Gastroenterology 2002; 122: 1620–1630. [DOI] [PubMed] [Google Scholar]
- 27. Reliability of endoscopy in the assessment of variceal features. The Italian Liver Cirrhosis Project. J Hepatol 1987; 4: 93–98. [DOI] [PubMed] [Google Scholar]
- 28. Kim MY, Cho MY, Baik SK et al Histological subclassification of cirrhosis using the Laennec fibrosis scoring system correlates with clinical stage and grade of portal hypertension. J Hepatol 2011; 55: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 29. Clavien PA, Barkun J, de Oliveira ML et al The Clavien‐Dindo classification of surgical complications: five‐year experience. Ann Surg 2009; 250: 187–196. [DOI] [PubMed] [Google Scholar]
- 30. Balzan S, Belghiti J, Farges O et al The “50‐50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 2005; 242: 824–828 discussion 8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. European Association for the Study of the Liver . EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018; 69: 182–236. [DOI] [PubMed] [Google Scholar]
- 32. Ryu T, Takami Y, Tsutsumi N et al Simultaneous microwave coagulo‐necrotic therapy (MCN) and laparoscopic splenectomy for the treatment of hepatocellular carcinoma with cirrhotic hypersplenism. Surg Today 2017; 47: 548–554. [DOI] [PubMed] [Google Scholar]
- 33. Zhang XY, Li C, Wen TF et al Synchronous splenectomy and hepatectomy for patients with hepatocellular carcinoma and hypersplenism: a case‐control study. World J Gastroenterol 2015; 21: 2358–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hu K, Lei P, Yao Z et al Laparoscopic RFA with splenectomy for hepatocellular carcinoma. World J Surg Oncol 2016; 14: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shimada M, Hashizume M, Shirabe K, Takenaka K, Sugimachi K. A new surgical strategy for cirrhotic patients with hepatocellular carcinoma and hypersplenism. Performing a hepatectomy after a laparoscopic splenectomy. Surg Endosc 2000; 14: 127–130. [DOI] [PubMed] [Google Scholar]
- 36. Noda T, Eguchi H, Iwagami Y et al Minimally invasive liver resection for hepatocellular carcinoma of patients with liver damage B: a propensity score‐based analysis. Hepatol Res 2018; 48: 539–548. [DOI] [PubMed] [Google Scholar]
- 37. Inagaki Y, Sugimoto K, Shiraki K et al The long‐term effects of splenectomy and subsequent interferon therapy in patients with HCV‐related liver cirrhosis. Mol Med Rep 2014; 9: 487–492. [DOI] [PubMed] [Google Scholar]
- 38. Matsuo R, Nakano Y, Ohkohchi N. Platelet administration via the portal vein promotes liver regeneration in rats after 70% hepatectomy. Ann Surg 2011; 253: 759–763. [DOI] [PubMed] [Google Scholar]
- 39. Lisman T, Porte RJ. Mechanisms of platelet‐mediated liver regeneration. Blood 2016; 128: 625–629. [DOI] [PubMed] [Google Scholar]
- 40. Watanabe M, Murata S, Hashimoto I et al Platelets contribute to the reduction of liver fibrosis in mice. J Gastroenterol Hepatol 2009; 24: 78–89. [DOI] [PubMed] [Google Scholar]
- 41. Takahashi K, Murata S, Fukunaga K, Ohkohchi N. Human platelets inhibit liver fibrosis in severe combined immunodeficiency mice. World J Gastroenterol 2013; 19: 5250–5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ugel S, Peranzoni E, Desantis G et al Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep 2012; 2: 628–639. [DOI] [PubMed] [Google Scholar]
- 43. Karakantza M, Mouzaki A, Theodoropoulou M, Bussel JB, Maniatis A. Th1 and Th2 cytokines in a patient with Evans’ syndrome and profound lymphopenia. Br J Haematol 2000; 110: 968–970. [DOI] [PubMed] [Google Scholar]
- 44. Long X, Wang J, Zhao JP et al Splenectomy suppresses growth and metastasis of hepatocellular carcinoma through decreasing myeloid‐derived suppressor cells in vivo. J Huazhong Univ Sci Technolog Med Sci 2016; 36: 667–676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Baseline characteristics of each treatment subgroup before propensity score matching.


