Abstract
Biofilms are prevalent in non‐healing chronic wounds and implicated in delayed healing. Tolerance to antimicrobial treatments and the host's immune system leave clinicians with limited interventions against biofilm populations. It is therefore essential that effective treatments be rigorously tested and demonstrate an impact on biofilm across multiple experimental models to guide clinical investigations and protocols. Cadexomer iodine has previously been shown to be effective against biofilm in various in vitro models, against methicillin‐resistant Staphylococcus aureus biofilm in mouse wounds, and clinically in diabetic foot ulcers complicated by biofilm. Similarities between porcine and human skin make the pig a favoured model for cutaneous wound studies. Two antiseptic dressings and a gauze control were assessed against mature biofilm grown on ex vivo pig skin and in a pig wound model. Significant reductions in biofilm were observed following treatment with cadexomer iodine across both biofilm models. In contrast, silver carboxymethylcellulose dressings had minimal impact on biofilm in the models, with similar results to the control in the ex vivo model. Microscopy and histopathology indicate that the depth of organisms in wound tissue may impact treatment effectiveness. Further work on the promising biofilm efficacy of cadexomer iodine is needed to determine optimal treatment durations against biofilm.
Keywords: biofilms, cadexomer iodine, porcine, silver, wounds
1. INTRODUCTION
Initial evidence demonstrated that up to 60% of chronic non‐healing wounds contained a biofilm.1, 2 However, this number has been shown to be closer to 80% in a recent meta‐analysis of various studies examining biofilm presence in chronic wounds.3 Taking into account the heterogeneous distribution of biofilms across a wound4 and the potential to be located on surface and deeper tissues,5 this value is expected to be even higher according to experts in the field.6
The presence of biofilms in chronic wounds is not enough to establish a detrimental effect in that environment. Many chronic clinical diseases have been linked with biofilms, ranging from implant‐related infections (eg, catheters and prosthetics); chronic lung infections, particularly in cystic fibrosis patients; middle ear infections; gingivitis; sinusitis; and others.7 This clinical impact of biofilms was highlighted recently by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), with the conclusion that biofilms cause chronic infections.8 More specifically for wounds, in recent years, animal studies have clearly shown a significant impact of bacterial biofilms on wound healing in mouse,9, 10, 11 rabbit,12 and porcine models.13 This knowledge, combined with the significant clinical challenges of antimicrobial tolerance14, 15, 16 and the impaired immune response17, 18, 19 to these biofilm communities, validates a pivotal role in delayed wound healing.
To be effective against biofilms, global experts recommend that antimicrobials must reach the bacteria in an active form and not be neutralised by the biofilm extracellular polymeric substances (EPS).6 In addition, a sustained cidal concentration of antimicrobial must be supplied16 to ensure sufficient antimicrobial activity over the period of use. The main antimicrobial activity of iodine is provided by the neutrally charged I2 molecule.20 Biofilm research has highlighted that antimicrobials which are less reactive are less likely to be reactively neutralised by the negatively charged EPS of the biofilm and are hypothesised to have a greater effect against biofilm bacteria.15 Cadexomer iodine (CI) (Iodosorb, Smith & Nephew, Hull, UK) consists of small polysaccharide beads (cadexomer starch) containing 0.9% iodine, which causes the polysaccharide beads to swell in the presence of wound exudate, allowing a slow sustained release of iodine into the wound.21, 22, 23
CI has long been used effectively in chronic wounds and is supported by many clinical studies demonstrating the removal of barriers to healing, such as microbial infection,21, 24, 25, 26, 27 slough/debris,21, 28, 29, 30, 31, 32 and exudate.21, 24, 27, 28, 31, 33, 34 In addition, a recent meta‐analysis (Cochrane review) highlighted that CI generates higher healing rates than standard care in venous leg ulcers (VLUs).35, 36
CI has previously been demonstrated to be highly effective against mature biofilms in multiple in vitro models incorporating various conditions, such as low and high exudate,37 clinically relevant media,38 or clinically relevant substrates such as porcine tissue.16, 39, 40 Against biofilm, in vitro and mouse histology results suggested that the product physically dehydrates and absorbs bacteria and biofilm microcolonies to the cadexomer bead,22 and these are rapidly killed by iodine.22, 37 The sustained antimicrobial effect enables the effective disruption and killing of biofilm bacteria for up to 3 days.16 In addition, a recent study has highlighted significant efficacy against methicillin‐resistant Staphylococcus aureus (MRSA) biofilms in a mouse wound model.37 A silver carboxymethyl cellulose (CMC) dressing supplemented with a chelating agent and a surfactant was chosen for comparison with CI in these studies because of suggested anti‐biofilm activity,41 although its efficacy against biofilm has recently been called into question.37
A recent consensus paper from 10 global biofilm experts has highlighted the need for models used to assess treatments against biofilms to be as clinically relevant as possible using mature biofilms, media containing blood proteins, and a test that is able to demonstrate a measureable reduction of biofilm bacteria specifically over the treatment period.6 Biofilm models grown on animal skin provide a useful platform for the initial screening of effective biofilm treatments and can act as a valuable bridge to animal models and, ultimately, clinical outcomes following an intervention. Extensive similarities between porcine and human skin make the pig a particularly desirable model for cutaneous wound studies.42, 43
In this study, both the porcine explant model44 and a porcine wound model13 were used to assess the anti‐biofilm efficacy of CI against mature biofilms, providing valuable information for wider clinical studies and treatment guidance with this antiseptic.
2. METHODS
2.1. Ex vivo porcine skin biofilm model
2.1.1. Bacterial culture
Biofilm‐forming strains Pseudomonas aeruginosa PAO1 and Staphylococcus aureus ATCC‐35556 were streaked for isolation on tryptic soy agar (TSA; Difco, Becton, Dickinson and Company, Sparks, Maryland) plates from frozen stock cultures and were incubated overnight at 37°C for 16 to 18 hours. A culture tube containing 5 mL of tryptic soy broth (TSB; Difco, Becton Dickinson and Company) was inoculated with an isolated colony and incubated overnight at 37°C. For each organism, a separate flask containing 50 mL of TSB was inoculated with 150 μL of overnight culture and incubated at 37°C in a water bath at 150 rpm until the culture reached an optical density (OD640) between 0·2 and 0·4 (early‐log phase). The optical density of each culture was determined using a spectrophotometer (UNICO 1100, United Products & Instrument INC, Dayton, New Jersey), and the viable colony‐forming units (CFUs) per millilitre were verified by spread plate‐cultured colony counts.
2.1.2. Explant preparation
The ex vivo model of biofilm on porcine skin explants used in this study is similar to that described previously.16, 44 Briefly, large sheets of fresh pig skin, approximately 20 cm by 30 cm (8 × 12 in.), were obtained from a commercial meat processing company and thoroughly cleaned, with hair closely trimmed. The subcutaneous fat layer was trimmed to leave approximately 1 to 2 mm thickness of subcutaneous fat. A large, partial‐thickness wound, approximately 0.508 mm deep (0.020 in.), was mechanically created using an electric Paget's dermatome. Individual explants, 12 mm in diameter, were punched from the wound area using 12 mm skin biopsy punches. The pig skin explants were sterilised using chlorine gas for 45 minutes and were then washed thrice in sterile saline. Explants were transferred to 90 mm diameter petri dishes containing 0.5% soft TSA supplemented with antibiotics (50 μg gentamicin per ml for P. aeruginosa or 20 μg doxycycline per ml for S. aureus) to limit the overgrowth of bacteria to the bottom of the explants. Sterile explants were inoculated with 20 μL of 107 CFU/mL of P. aeruginosa PA01 or S. aureus ATCC‐35556, and the explants were incubated at 37°C for 3 days to develop mature biofilms of P. aeruginosa or were incubated for 4 days to develop mature biofilms of S. aureus. Explants were then transferred to fresh 90 mm petri dishes with 0.5% soft TSA plus antibiotics and treated with the three test materials as described below.
2.1.3. Explant model treatment
A continuous layer of CI gel (Iodosorb gel, Smith & Nephew Medical), ~1 mm thick, was then applied to the top surface of pig skin explants; the gel was covered with moistened gauze, and a sterile glass slide was placed on top of the gauze to reduce drying. Similarly, a sheet of the CMC silver dressing (Aquacel Ag + Extra, ConvaTec, Deeside, UK) was applied to the biofilm layer of explants; sterile water was added, and the dressing was covered with a glass slide. Dressings were removed and replaced daily for 3 days. After 24, 48, and 72 hours of incubation, the CI gel or CMC dressing was removed, and explants were placed in 24‐well culture plates and washed thrice for 5 minutes with sterile distilled water. To measure CFUs of biofilm bacteria, four explants were submerged in 50× MIC antibiotic (50 μg/mL gentamicin for P. aeruginosa or 20 μg/mL doxycycline for S. aureus) for another 24 hours in a 37°C incubator to kill any remaining planktonic bacteria. The explants were then transferred into individual sterile 15 mL centrifuge tubes containing 5 mL of PBS with 5 ppm Tween 20 and were sonicated five times for 90 seconds with 1 minute between sonication cycles. Suspensions were vortexed for 10 seconds, serial dilutions were plated in triplicate, and CFUs were measured after 24 hours of incubation at 37°C. To measure the CFU of total bacterial counts (planktonic plus biofilm bacteria), the remaining five explants for each test condition were placed in 24‐well culture plates and washed thrice for 5 minutes with sterile distilled water; were then transferred to individual sterile 15 mL centrifuge tubes containing 5 mL of PBS with 5 ppm Tween 20; and were sonicated, vortexed, diluted, and plated to determine CFUs as described.
2.2. Porcine wound model
2.2.1. Animals
Four female 30 to 35 kg Yorkshire‐cross pigs were obtained from Charles Real Hog Farm. All procedures with animals were approved by the Bridge PTS IACUC committee. Animals received humane care according to the National Research Council's ‘Guide for the Care and Use of Laboratory Animals'.45
2.2.2. In vivo wounding and infection
Pigs were pre‐medicated with an intramuscular injection of atropine (0.05 mg/kg; Med‐Pharmex, Inc., Lake Forest, California) and anaesthetised with Tiletamine‐Zolazepam (4.4 mg/kg, intramuscular, Putney, Inc., Portland, Oregon) followed by inhalation of 2% to 5% Isoflurane USP mixed with oxygen.
The dorsal and lateral thorax of the pigs were clipped, washed with an antimicrobial‐free soap, and shaved with a razor. Each animal was intubated and prepared for surgery using isopropyl alcohol to disinfect the skin surface. On each pig, 24 full‐thickness wounds (1.5 cm diameter) were created using a trephine and excision, 12 per side, with wounds spaced 2 cm apart. Dilute epinephrine was applied on gauze sponges for 10 minutes to achieve haemostasis.
Inoculation of wounds was as previously described,46, 47 using a mixed inoculum including P. aeruginosa and Staphylococcus epidermidis. All 12 wounds on a side were covered with a strip of gauze; the gauze was saturated with inoculum, covered with plastic wrap, and left in place for 15 minutes, and then, the contaminated wrap and gauze were removed and discarded. The pig wounds were dressed with saline‐moistened non‐adherent dressings and secondary coverings as described below, and bacterial biofilm was allowed to form over 48 hours. Buprenorphine (0.02 mg/kg) (Hospira, Inc., Lake Forest, California) was administered to provide short‐term pain relief, and a Fentanyl patch (50 μg/h) (Fentanyl transdermal system, PAR Pharmaceutical Co. Inc, Spring Valley, New York) was secured to the shaved skin for longer‐term pain management and replaced after 3 days. At 48 hours after inoculation, punch biopsies were taken from select wounds to determine the bacterial bioburden before treatment. These wounds were excluded from future analysis.
2.2.3. In vivo treatment
At the 48‐hour point, treatment was initiated with once‐daily treatment for 2 days. CI gel was loaded into syringes, and 0.5 mL was dispensed per wound. CMC silver dressings were punched to generate 1.5 cm diameter circular swatches for application to individual wounds. All wounds were covered with sterile, moistened non‐adherent Curad dressings (Medline, Northfield, Minnesota). CI gel and control‐treated wounds received non‐adherent gauze pads moistened with saline. The Curad dressings used to cover CMC silver‐treated wounds were moistened with sterile water rather than saline to avoid potential salt inactivation of silver. Curad dressings were squeezed to remove excess fluid. The non‐adherent dressings were secured with medical tape and covered with an occlusive pad, and pigs were wrapped with a layer of elastic bandage.
After the first 24 hours of treatment, gentle removal of the CMC silver dressing and the bulk of remaining CI gel formulations were performed prior to reapplication. Use of limited saline for wetting was acceptable if it assisted in avoiding stripping of wounds (eg, by the CMC dressing being stuck to the wound). Wounds were not washed or vigorously scrubbed. Wounds were then treated and re‐dressed as described above.
2.2.4. Endpoints
After the second 24‐hour treatment, central biopsies were taken for quantitative microbiology using a 4‐mm biopsy punch, and an adjacent strip of tissue was also cut through each wound for histology analysis. Histology tissue samples were fixed, processed, paraffin‐embedded, sectioned, Gram‐stained, and evaluated by a certified histopathologist. For microbiological processing, biopsy tissues were each placed into a pre‐weighed vessel containing neutralising DE broth, tared, homogenised, serially diluted, drop‐plated on agar media, and incubated to determine the bacterial counts. Each sample was evaluated on TSA, Mannitol Salts Agar (MSA), and Pseudomonas Isolation Agar (PIA) to determine total bacterial, staphylococcal, and pseudomonal counts (CFU/g), respectively.
Analysis by the histopathologist included a description of the infection and wound bed, as well as a scaled score of biofilm character: 1—no significant bacteria observed; 2—individual bacteria observed; 3—some microcolony(ies) of bacteria; 4—larger colony of bacteria observed or extensive microcolonies; 5—connected microcolonies, many large colonies, or extended segments of bacterial communities. Based on the histopathologist's biofilm scores of the Gram‐stained tissue sections, 10 samples for each treatment, with biofilm scores encompassing the median score for each treatment, were selected and examined by light microscopy. Images were taken for these 10 samples (per treatment) of representative regions showing substantial biofilm characteristics or the presence of bacteria in the cases where biofilm was not clearly observed in the sample.
2.3. Statistical analysis
For the ex vivo pig skin biofilm model, values for each of the test conditions were compared for significant differences with all other test conditions using anova (with Tukey post‐hoc test) at each time point (Day 1, 2, or 3) for each count type (Total or Biofilm).
For the in vivo pig wound model, quantitative counts after completion of treatment were analysed by anova with Tukey HSD comparison of pairs, histopathology biofilm scores by the Steel‐Dwass method, and bacterial category results (eg, presence of Gram‐positive bacteria) by Pearson contingency table χ 2 tests. As χ 2 tests indicated significant differences among the three groups for the bacterial categories examined, post hoc χ 2 tests were also performed on the treatment pairs to examine which treatments differed.
3. RESULTS
3.1. Ex vivo porcine explant model
The effect of CI dressing (Iodosorb gel, Smith & Nephew Medical) and CMC silver dressing (Aquacel Ag + Extra, ConvaTec) compared with a gauze control was assessed against mature biofilms grown on porcine tissue explants. The CI dressing significantly reduced levels of P. aeruginosa biofilm from greater than 7 Log10 CFU/sample to the detection limit of the test (0.7 log10 CFU/sample) within 24 hours. This extent of biofilm kill was sustained across the 72‐hour test period. This reduction in biofilm counts was highly significant (P < 0.0001) versus both the CMC silver dressing and the gauze control at each time point over the 72‐hour test. In contrast, biofilm counts for the gauze control and the CMC silver dressing showed minimal reductions over the 3‐day period, with significantly higher biofilm counts observed following incubation with the CMC silver dressing compared with the gauze control by 48 and 72 hours (P = 0.0015 and 0.0134, respectively) (Figure 1).
Figure 1.
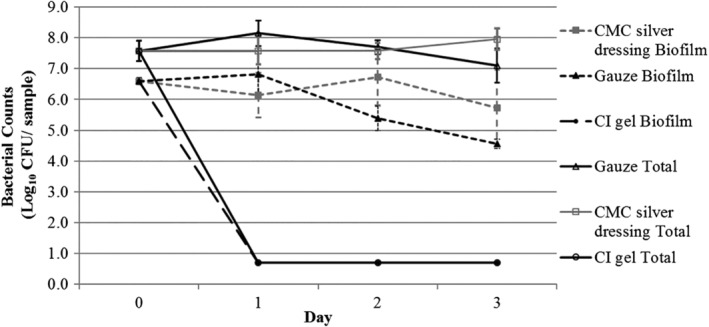
Pseudomonas aeruginosa biofilm counts and total bacterial counts (planktonic and biofilm) following treatment with cadexomer iodine (CI) dressing, carboxymethyl cellulose (CMC) silver dressing, and gauze control over a 3‐day period (n = 5 for total bacterial counts, n = 4 for biofilm counts). Detection limit of test is 0.7 log CFU/sample; Day 0 = 3 day biofilm growth counts on porcine tissue before treatment. Values shown are mean counts with 95% confidence intervals
Similar reductions were observed using CI against total bacterial counts (planktonic and biofilm), with kill to detection limit observed by 24 hours and maintained over the 3‐day test. All reduction values were highly significant compared with both the CMC silver dressing and the gauze control (P < 0.0001). Conversely, a minimal effect was observed using the CMC silver dressing on total counts compared with the non‐antimicrobial control, with a slight increase observed by 3 days of treatment (P = 0.0022).
Against S. aureus, a >4 log mean reduction in biofilm level was observed following 24 hours of CI treatment, and this reduction was highly significant (P < 0.0001) compared with the gauze control and the CMC silver dressing. Further CI treatment produced biofilm kill to the detection limit (>5 log reduction) by 48 hours, and this efficacy was maintained at 72 hours, with a highly significant difference compared with the CMC dressing and the gauze control (P < 0.0001). Sterile gauze or CMC silver dressings did not substantially reduce CFUs at any time point over the 3‐day treatment and did not differ significantly at 3 days (Figure 2).
Figure 2.
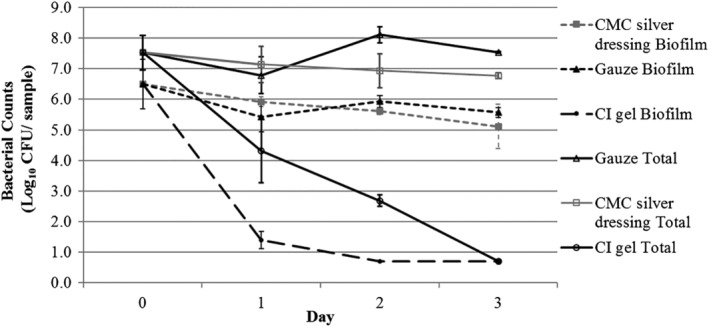
Staphylococcus aureus biofilm counts and total bacterial counts (planktonic and biofilm) following treatment with cadexomer iodine (CI) dressing, carboxymethyl cellulose (CMC) silver dressing, and gauze control over a 3‐day period (n = 5 for total bacterial counts, n = 4 for biofilm counts). Detection limit of test is 0.7 log CFU/sample; Day 0 = 4 day biofilm growth counts on porcine tissue before treatment. Values shown are mean counts with 95% confidence intervals
Total bacterial counts were reduced markedly following CI treatment over the 3‐day period; however, the rate of kill was not as rapid as that seen against P. aeruginosa, with kill to the detection limit by 3 days only. The reductions observed were highly significant (P = 0.0001 or greater) compared with both the CMC dressing and the gauze control at all time points. As observed with P. aeruginosa, the CMC dressings had a limited impact on the total bacterial counts over the test period compared with the control, although the difference was statistically significant on the final 2 days.
3.2. Porcine full‐thickness infection model
3.2.1. Quantitative microbiology
Using an established model,13 P. aeruginosa and Staphylococcus epidermidis biofilm was allowed to form over 2 days in porcine full‐thickness wounds after an initial inoculation, followed by 2 days of treatment with antimicrobial dressings. Significant differences were seen between CI gel, CMC silver dressings, and control in reducing quantitative bacterial wound tissue counts. Figure 3 shows the quantitative microbiology results for samples prior to treatment (2‐day‐old biofilm) and control, CMC silver, and CI‐treated samples after 2 days of treatment. The total, pseudomonal, and staphylococcal counts were similar prior to treatment. A trend of increasing P. aeruginosa and decreasing staphylococci with control treatment suggests that, in the absence of antimicrobial treatment, P. aeruginosa may have competed more effectively in the wound environment than S. epidermidis. Treatment with CI gel resulted in substantial log10 CFU/g reductions versus the control in total counts (2.3) and pseudomonal counts (3.3), both highly significant effects (P < 0.001). CMC silver dressing had lesser but also statistically significant (P < 0.001) log10 CFU/g reductions versus control in total counts (1.5) and pseudomonal counts (1.85). However, the effect of CI gel was significantly greater than that of the CMC silver dressing for both total counts (P < 0.05) and pseudomonal counts (P < 0.01). CI gel had a modest but significant effect versus control for staphylococcal counts (P < 0.05). Staphylococcal counts following CMC silver dressing treatment did not differ significantly from control (P > 0.05).
Figure 3.
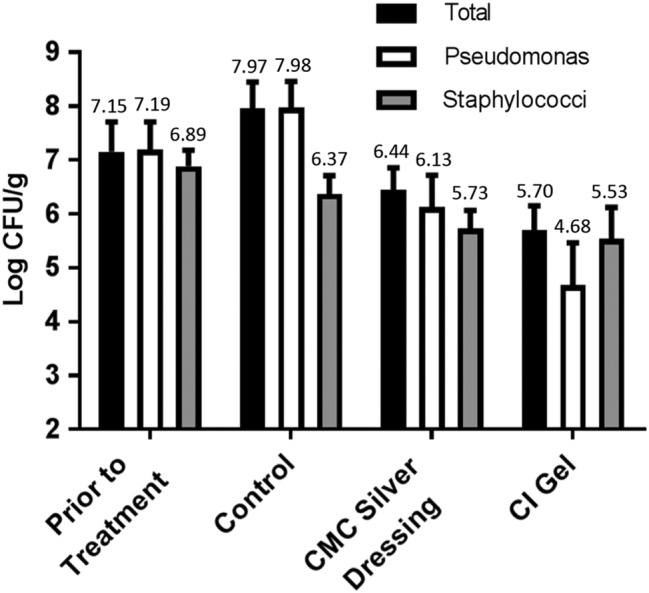
Wound bioburden (total, pseudomonal, and staphylococcal log10 CFU/g) prior to treatment (2‐day‐old biofilm, n = 16) and after 2 days of daily therapy with control dressing, carboxymethyl cellulose (CMC) silver dressing, or cadexomer iodine (CI) gel (n = 20). Values shown are means with 95% confidence intervals, with mean numerical values also indicated
3.2.2. Histopathology biofilm and bacteria scoring
A certified histopathologist examined Gram‐stained tissues for the same 20 wounds used for each treatment to evaluate quantitative counts, scoring each sample on a 5‐point scale for biofilm character. Control and CMC silver dressing had median scores indicating substantial levels of biofilm, in contrast to CI gel, for which the median score only indicated the presence of bacteria (Figure 4). The reduction in biofilm score for CI gel was highly significant versus CMC silver dressing and control (P < 0.0001). CMC silver dressing was not found to differ significantly from control (P > 0.05).
Figure 4.
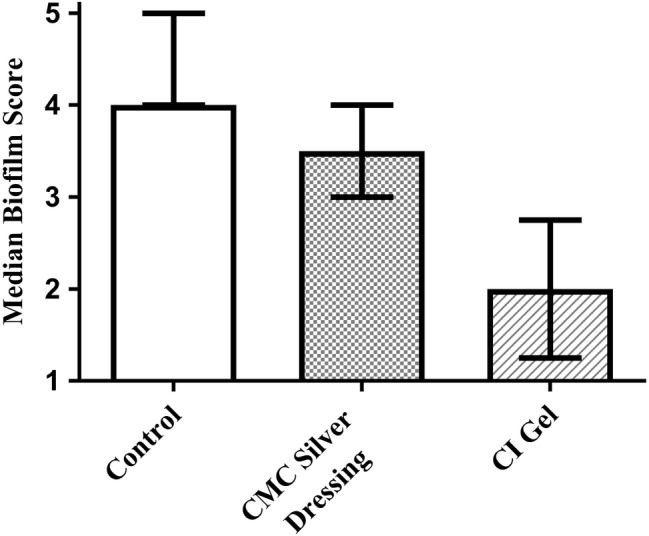
The median values and interquartile range of histopathology scoring for biofilm character of the wound infection (n = 20)
The histopathologist also provided an indication of the presence of Gram‐negative and Gram‐positive bacteria in each sample. Table 1 tabulates the percentage of different types of bacteria observed for each treatment, as well as statistical analysis (χ 2) of which groups differed. Nearly all of the control samples (90%) had both Gram‐positive and Gram‐negative bacteria present, and 100% contained bacteria. Samples treated with CMC silver dressing also had bacteria in 100% of samples and had Gram‐negative bacteria present in 90% of the samples (P = 0.548 versus control), with 50% of samples having both Gram‐positive and Gram‐negative bacteria present (P = 0.006 versus control). CI gel alone, of all the treatments, had samples where no bacteria were present, at 25% (P = 0.017 versus CMC silver and control). In addition, CI gel significantly reduced categories of bacteria versus the other treatments as only 30% of CI‐treated samples contained Gram‐negative bacteria (P ≤ 0.0001 versus CMC silver and control), and only 20% had both Gram‐positive and Gram‐negative bacteria (P = 0.047 versus CMC silver and P < 0.0001 versus control). CI gel and CMC silver dressing had a similar number of samples where gram‐positive bacteria were observed at 60% to 65% (P = 0.744), a significant decrease relative to 95% for control (P < 0.02 for CMC silver and CI gel).
Table 1.
Presence of bacterial classes within the wound tissue for the three treatments as determined by the histopathologist and testing for χ2 statistical significance
| Bacteria observed | ||||
|---|---|---|---|---|
| Gram + | Gram − | Gram + and Gram − | No bacteria | |
| Control | 95% | 95% | 90% | 0% |
| CMC silver dressing | 60% | 90% | 50% | 0% |
| CI gel | 65% | 30% | 20% | 25% |
| χ2 P value all three treatments | 0.026 | <0.0001 | <0.0001 | 0.003 |
| χ2 P value CMC silver versus control | 0.008 | 0.548 | 0.006 | NA |
| χ2 P value CI gel versus control | 0.018 | <0.0001 | <0.0001 | 0.017 |
| χ2 P value CI gel versus CMC silver | 0.744 | 0.0001 | 0.047 | 0.017 |
CI, cadexomer iodine; CMC, carboxymethyl cellulose.
3.2.3. Imaging of Gram‐stained tissues
In an effort to obtain images presenting the typical bacterial presence/biofilm level for each treatment, we examined a significant number of samples (10/treatment) encompassing the median biofilm score. Images were taken for each sample of regions showing substantial biofilm character or the presence of bacteria in the cases where biofilm was not clearly observed in the sample. Figure 5 shows representative images for each treatment to indicate key observations. Nearly all control samples exhibited extended segments and/or large colonies of Gram‐negative biofilm near the surface of the wound tissue (Figure 5A‐C, black arrows). Colonies of Gram‐positive bacteria were also observed in nearly all control samples, often at somewhat greater depth within the wound tissue (Figure 5B,C, orange arrows). The majority of wounds treated with CMC silver dressing likewise exhibited extended segments of Gram‐negative biofilm or large colonies of Gram‐negative biofilm near the surface of the wound tissue (Figure 5D,E, black arrows). Colonies of Gram‐positive bacteria were also observed in the majority of CMC silver‐treated samples, often at somewhat greater depth within the wound tissue (Figure 5E,F, orange arrows). CMC silver dressing material was also observed in some samples (Figure 5F, white arrows). For CI gel, samples exhibited limited or no clear presence of Gram‐negative bacteria (Figure 5G, black arrow). Gram‐positive bacteria were present in the majority of samples, often occurring at substantial depth within the wound tissue (Figure 5H, orange arrow). Cadexomer beads were observed in some samples (Figure 5I, yellow arrows).
Figure 5.
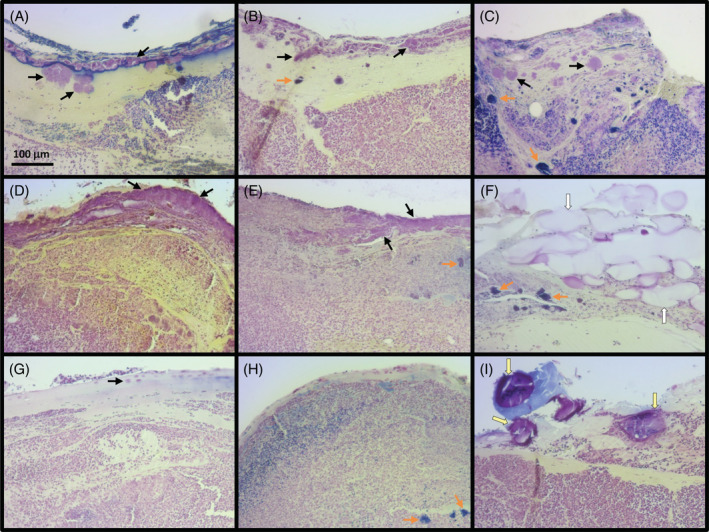
Representative images of biofilm/bacteria observed for Gram‐stained tissue from wounds treated with control (A‐C), carboxymethyl cellulose (CMC) silver dressing (D‐F), or cadexomer iodine (CI) gel (G‐I). Arrows by colour point to Gram‐negative bacteria (black), Gram‐positive bacteria (orange), carboxymethylcellulose silver dressing material (white), or cadexomer iodine beads (yellow)
4. DISCUSSION
Biofilms are present in up to 78% of chronic wounds3 and are linked to delayed wound healing.9, 10, 13 Multifaceted strategies combining aggressive debridement and, where possible, cleansing and treatment with effective anti‐biofilm antiseptics are recommended to treat these biofilms,6, 48, 49 which are normally tolerant to antimicrobial intervention.14, 16
This study demonstrates the superior effect of CI gel compared with CMC silver dressing in both ex vivo and in vivo porcine biofilm models and is in agreement with previous studies using similar models.16, 37, 50, 51 The efficacy in vivo is reduced in absolute magnitude compared with the ex vivo model, a common occurrence with transition towards more clinically relevant and biologically complex contexts. Such data showing effects in the laboratory, combined with animal models, may start to bridge the gap between experimental findings and impact of treatments clinically, thus helping to guide clinician treatment decisions were biofilm is suspected. Furthermore, in the clinic, CI has been reported to show a significant reduction in biofilm using both microscopy and quantitative counts by polymerase chain reaction (PCR), along with a concurrent reduction in MMP‐2 and MMP‐9.52
The CMC dressing was developed to specifically address wound biofilm41; however, the minimal effect reported across all models in this study following treatment with the CMC silver dressing concurs with other published data using clinically relevant biofilm models37 and, more widely, the suggestion that silver may have little impact on biofilms at the concentrations found in current dressings53 despite the addition of supplementary surfactants. Furthermore, case studies using the dressing on wounds with suspected biofilm do not measure the impact on biofilm specifically, highlighting the need for more appropriately designed clinical studies to demonstrate effective anti‐biofilm strategies.54
Most experts in the field agree that biofilms are not present uniformly across wounds and that biofilms may be located both on the surface of wounds and deeper in the tissue.4 In this study, images were taken of 10 Gram‐stained tissue samples from the pig model encompassing the median value of biofilm score for each treatment group, where each image represented the significant biofilm/bacteria observed in the sample. These images agree with the histopathology scores and quantitative microbiology, showing substantial reduction in the biofilm character of the infection and level of bacteria between the CI gel and the other two treatment groups in these pig wounds. These findings align with previous biofilm microscopy and quantification findings following clinical CI use in DFU wounds with suspected biofilm,52 where a reduction in biofilm was observed microscopically in addition to a reduction in bacterial counts analysed by quantifiable PCR.
S. epidermidis was observed deeper in tissue than P. aeruginosa. This result was surprising given past clinical sample studies of P. aeruginosa versus S. aureus 5 and lesser virulence/invasiveness expected of S. epidermidis versus S. aureus. However, this localisation may be more of an aspect of the model, and less representative of human clinical wounds, because the healthy young pigs exhibited robust granulation during the pre‐treatment, biofilm‐formation phase of the study despite the presence of substantial microbial bioburden. Thus, the presence of S. epidermidis deeper in tissue may represent more occurrences of granulation tissue formation on top of this organism rather than penetration into tissue. Perhaps the localisation difference actually represents greater host tolerance for a less pathogenic organism.
The observed depth of the different bacterial species is still important in interpreting the results. A strong clearance of dense Gram‐negative biofilm at the wound surface was observed following CI treatment, similar to strong clearance of MRSA biofilm at the wound surface seen previously in a mouse model.37 This data, combined with the detected depth of S. epidermidis in the pig tissue, may explain the reduced impact of CI on this organism compared with the pseudomonads. The cross‐model results demonstrate the potential of CI for addressing biofilm with a broad spectrum of coverage near the wound surface.
Duration of treatment is also an area needing greater exploration with regard to biofilm treatment. CI demonstrated a significant impact on both Gram‐negative and Gram‐positive biofilm in the porcine ex vivo model with only 3 days of treatment, albeit slightly slower against the S. aureus populations. However, the dynamic nature of animal models (and therefore human wounds) obviously adds complexity and a greater challenge to any treatment as indicated by the somewhat smaller reductions observed in the pig model counts. This has also previously been demonstrated in a mouse biofilm model compared with in vitro testing.37 The recent paper by Malone and colleagues highlighted that three applications of CI over 7 days of treatment may not be sufficient to address biofilm completely in all clinical cases,52 emphasising that more studies are required to ascertain the optimum duration of antiseptic treatment to provide the most benefit against biofilm and that successful protocols for planktonic infections may not be applicable to biofilms, with longer treatment durations required.
ACKNOWLEDGEMENTS
Histopathology processing and analysis was performed by Dr. Scott Estep, DACVP. The animal study design was executed at Bridge PTS Inc. under the leadership of Dr. Paul Attar, Kan Lam, and Sarah Korn. We thank Dan Fitzgerald, Chris Saunders, Paul Renick, and Shannon Tetens for technical assistance/advice. Both E.W. and E.R. are employees of Smith & Nephew. G.S. is a paid consultant of Smith & Nephew. This work was funded by Smith & Nephew plc.
Roche ED, Woodmansey EJ, Yang Q, Gibson DJ, Zhang H, Schultz GS. Cadexomer iodine effectively reduces bacterial biofilm in porcine wounds ex vivo and in vivo. Int Wound J. 2019;16:674–683. 10.1111/iwj.13080
Funding information Smith & Nephew plc
REFERENCES
- 1. James GA, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16(1):37‐44. http://www.ncbi.nlm.nih.gov/pubmed/18086294 Accessed October 29, 2012. [DOI] [PubMed] [Google Scholar]
- 2. Bjarnsholt T, Kirketerp‐Møller K, Jensen PØ, et al. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen. 2008;16(1):2‐10. http://www.ncbi.nlm.nih.gov/pubmed/18211573 Accessed March 16, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Malone M, Bjarnsholt T, McBain AJ, et al. The prevalence of biofilms in chronic wounds: a systematic review and meta‐analysis of published data. J Wound Care. 2017;26(1):20‐25. http://www.ncbi.nlm.nih.gov/pubmed/28103163. Accessed January, 2017. [DOI] [PubMed] [Google Scholar]
- 4. Thomsen TR, Aasholm MS, Rudkjøbing VB, et al. The bacteriology of chronic venous leg ulcer examined by culture‐independent molecular methods. Wound Repair Regen. 2010;18(1):38‐49. 10.1111/j.1524-475X.2009.00561.x. [DOI] [PubMed] [Google Scholar]
- 5. Fazli M, Bjarnsholt T, Kirketerp‐Møller K, et al. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J Clin Microbiol. 2009;47(12):4084‐4089. 10.1128/JCM.01395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schultz G, Bjarnsholt T, James GA, et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017;25:744‐757. http://www.ncbi.nlm.nih.gov/pubmed/28960634. Accessed September 20, 2017. [DOI] [PubMed] [Google Scholar]
- 7. del Pozo JL, Patel R. The challenge of treating biofilm‐associated bacterial infections. Clin Pharmacol Ther. 2007;82(2):204‐209. [DOI] [PubMed] [Google Scholar]
- 8. Høiby N, Bjarnsholt T, Moser C, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2014;21(suppl 1):S1‐S25. http://www.ncbi.nlm.nih.gov/pubmed/25596784. Accessed July 17, 2018. [DOI] [PubMed] [Google Scholar]
- 9. Schierle CF, De la Garza M, Mustoe TA, Galiano RD. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen. 2009;17(3):354‐359. http://www.ncbi.nlm.nih.gov/pubmed/19660043 Accessed October 29, 2012. [DOI] [PubMed] [Google Scholar]
- 10. Zhao G, Hochwalt PC, Usui ML, et al. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge—a model for the study of chronic wounds. Wound Repair Regen. 2010;18(5):467‐477. 10.1111/j.1524-475X.2010.00608.x/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao G, Usui M, Underwood R, et al. Time course study of delayed wound healing in a biofilm‐challenged diabetic mouse model. Wound Repair Regen. 2012;20(3):342‐352. 10.1111/j.1524-475X.2012.00793.x/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gurjala AN, Geringer MR, Seth AK, et al. Development of a novel, highly quantitative in vivo model for the study of biofilm‐impaired cutaneous wound healing. Wound Repair Regen. 2011;19(3):400‐410. http://www.ncbi.nlm.nih.gov/pubmed/21518094 Accessed March 9, 2014. [DOI] [PubMed] [Google Scholar]
- 13. Roche ED, Renick PJ, Tetens SP, Ramsay SJ, Daniels EQ, Carson DL. Increasing the presence of biofilm and healing delay in a porcine model of MRSA‐infected wounds. Wound Repair Regen. 2012;20(4):537‐543. http://www.ncbi.nlm.nih.gov/pubmed/22672311 Accessed March 26, 2014. [DOI] [PubMed] [Google Scholar]
- 14. Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358(9276):135‐138. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=11463434&retmode=ref&cmd=prlinks%5Cnpapers3://publication/uuid/80E756B3‐3A98‐4F81‐8A7F‐7401B2CF5836. Accessed July 17, 2018. [DOI] [PubMed] [Google Scholar]
- 15. Stewart PS, Rayner J, Roe F, Rees WM. Biofilm penetration and disinfection efficacy of alkaline hypochlorite and chlorosulfamates. J Appl Microbiol. 2001;91(3):525‐532. http://www.ncbi.nlm.nih.gov/pubmed/11556920 Acessed October 21, 2014. [DOI] [PubMed] [Google Scholar]
- 16. Phillips PL, Yang Q, Davis S, et al. Antimicrobial dressing efficacy against mature Pseudomonas aeruginosa biofilm on porcine skin explants. Int Wound J. 2013;12(4):469‐483. http://www.ncbi.nlm.nih.gov/pubmed/24028432 Accessed October 16, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jesaitis AJ, Franklin MJ, Berglund D, et al. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J Immunol. 2003;171(8):4329‐4339. http://www.ncbi.nlm.nih.gov/pubmed/14530358. Accessed November 17, 2015. [DOI] [PubMed] [Google Scholar]
- 18. Bjarnsholt T, Jensen PØ, Burmølle M, et al. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum‐sensing dependent. Microbiology. 2005;151(2):373‐383. [DOI] [PubMed] [Google Scholar]
- 19. Cochrane DM, Brown MR, Anwar H, Weller PH, Lam K, Costerton JW. Antibody response to Pseudomonas aeruginosa surface protein antigens in a rat model of chronic lung infection. J Med Microbiol. 1988;27(4):255‐261. http://www.ncbi.nlm.nih.gov/pubmed/3143837. Accessed July 17, 2018. [DOI] [PubMed] [Google Scholar]
- 20. McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12(1):147‐179. http://www.ncbi.nlm.nih.gov/pubmed/9880479. Accessed July 18, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skog E, Arnesjö B, Troëng T, et al. A randomized trial comparing cadexomer iodine and standard treatment in the out‐patient management of chronic venous ulcers. Br J Dermatol. 1983;109(1):77‐83. http://www.ncbi.nlm.nih.gov/pubmed/6344906. Accessed July 18, 2018. [DOI] [PubMed] [Google Scholar]
- 22. Akiyama H, Oono T, Saito M, Iwatsuki K. Assessment of cadexomer iodine against Staphylococcus aureus biofilm in vivo and in vitro using confocal laser scanning microscopy. J Dermatol. 2004;31(7):529‐534. http://www.ncbi.nlm.nih.gov/pubmed/15492416. Accessed July 18, 2018. [DOI] [PubMed] [Google Scholar]
- 23. Zhou LH, Nahm WK, Badiavas E, Yufit T, Falanga V. Slow release iodine preparation and wound healing: in vitro effects consistent with lack of in vivo toxicity in human chronic wounds. Br J Dermatol. 2002;146(3):365‐374. http://www.ncbi.nlm.nih.gov/pubmed/11952535 Accessed October 21, 2014. [DOI] [PubMed] [Google Scholar]
- 24. Sundberg J, Pharm MS, Meller R, Se B, Pharm MS. A retrospective review of the use of cadexomer iodine in the treatment of chronic wounds. Wounds A Compend Clin Res Pract. 1997;9(3):68‐86. [Google Scholar]
- 25. Schwartz JA, Lantis JC, Gendics C, Fuller AM, Payne W, Ochs D. A prospective, non comparative, multicenter study to investigate the effect of cadexomer iodine on bioburden load and other wound characteristics in diabetic foot ulcers. Int Wound J. 2013;10(2):193‐199. http://www.ncbi.nlm.nih.gov/pubmed/23136838. Accessed July 18, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Danielsen L, Cherry GW, Harding K, Rollman O. Cadexomer iodine in ulcers colonised by Pseudomonas aeruginosa . J Wound Care. 1997;6(4):169‐172. [DOI] [PubMed] [Google Scholar]
- 27. Troëng T, Skog E, Arnesjö B, Gjöres JE, Bergljung L, Gundersen JEA. A randomised multicentre trial to compare the efficacy of cadexomer iodine and standard treatment in the management of chronic venous ulcers in out patients. In: Fox J, Fisher H, eds. Cadexomer Iodine. Stuttgart, Gemanay: Schattauer Verlag; 1983:43‐50. [Google Scholar]
- 28. Hansson C, Persson LM, Stenquist B, et al. The effects of cadexomer iodine paste in the treatment of venous leg ulcers compared with hydrocolloid dressing and paraffin gauze dressing. Int J Dermatol. 1998;37(5):390‐396. [DOI] [PubMed] [Google Scholar]
- 29. Harcup JW, Saul PA. A study of the effect of cadexomer iodine in the treatment of venous leg ulcers. Br J Clin Pract. 1986;40(9):360‐364. http://www.ncbi.nlm.nih.gov/pubmed/3542001 Accessed October 22, 2014. [PubMed] [Google Scholar]
- 30. Laudanska H, Gustavson B. In‐patient treatment of chronic varicose venous ulcers. a randomized trial of cadexomer iodine versus standard dressings. J Int Med Res. 1988;16(6):428‐435. http://www.ncbi.nlm.nih.gov/pubmed/2466712 Accessed January 9, 2015. [DOI] [PubMed] [Google Scholar]
- 31. Lindsay G, Latta D, Lyons KGB. A study in general practice of the efficacy of cadexomer iodine in venous leg ulcers treated on alternate days. Acta Ther. 1986;12:141‐148. [Google Scholar]
- 32. Moberg S, Hoffman L, Grennert ML, Holst A. A randomized trial of cadexomer iodine in decubitus ulcers. J Am Geriatr Soc. 1983;31(8):462‐465. http://www.ncbi.nlm.nih.gov/pubmed/6688261. Accessed July 18, 2018. [DOI] [PubMed] [Google Scholar]
- 33. Ormiston MC, Fox JA. Controlled trial of Iodosorb. Br Med J (Clin Res Ed). 1985;291(6506):308‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson A. A combative healer with no ill‐effect. Iodosorb in the treatment of infected wounds. Prof Nurse. 1991;7(1):60 62, 64. [PubMed] [Google Scholar]
- 35. O'Meara S, Al‐Kurdi D, Ologun Y, Ovington LG, Martyn‐St James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev. 2014;1(12):CD003557. http://www.ncbi.nlm.nih.gov/pubmed/24408354 Accessed November 4, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O'Meara S, Al‐Kurdi D, Ologun Y, Ovington LG. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev. 2010;(1):CD003557. http://www.ncbi.nlm.nih.gov/pubmed/20091548 Accessed June 26, 2014. [DOI] [PubMed] [Google Scholar]
- 37. Fitzgerald DJ, Renick PJ, Forrest EC, et al. Cadexomer iodine provides superior efficacy against bacterial wound biofilms in vitro and in vivo. Wound Repair Regen. 2017;25(1):13‐24. http://www.ncbi.nlm.nih.gov/pubmed/27859922. Accessed February 20, 2017. [DOI] [PubMed] [Google Scholar]
- 38. Alhede M, Woodmansey E. Antibiofilm Efficacy of a Silver Hydrofiber Antibiofilm Dressing and Cadexomer Iodine Dressing on Mature Biofilms (In Vitro). San Antonio, TX: SAWC; 2015. p. 1. [Google Scholar]
- 39. Schultz G, Yang Q. Microbicidal Effects of Three Daily Treatments of a Carboxymethylcellulose Silver Dressing or a Cadexomer Iodine Gel on Mature Bacterial Biofilms Grown on Pig Skin Explants. Florence, Italy: WUWHS; 2016. p. 1. [Google Scholar]
- 40. Phillips PL, Yang Q, Sampson E, Schultz G. Effects of antimicrobial agents on an in vitro biofilm model of skin wounds. Adv Wound Care. 2010;1:299‐304. [Google Scholar]
- 41. Bowler PG, Parsons D. Combatting wound biofilm and recalcitrance with a novel anti‐biofilm Hydrofiber® wound dressing. Wound Med. 2016;14:6‐11. [Google Scholar]
- 42. Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001;9(2):66‐76. [DOI] [PubMed] [Google Scholar]
- 43. Seaton M, Hocking A, Gibran NS. Porcine models of cutaneous wound healing. ILAR J. 2015;56(1):127‐138. [DOI] [PubMed] [Google Scholar]
- 44. Yang Q, Phillips PL, Sampson EM, et al. Development of a novel ex vivo porcine skin explant model for the assessment of mature bacterial biofilms. Wound Repair Regen. 2013;21(5):704‐714. http://www.ncbi.nlm.nih.gov/pubmed/23927831 Accessed August 13, 2013. [DOI] [PubMed] [Google Scholar]
- 45. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 46. Wright JB, Lam K, Buret AG, Olson ME, Burrell RE. Early healing events in a porcine model of contaminated wounds: effects of nanocrystalline silver on matrix metalloproteinases, cell apoptosis, and healing. Wound Repair Regen. 2002;10(3):141‐151. http://www.ncbi.nlm.nih.gov/pubmed/12100375. Accessed July 18, 2018. [DOI] [PubMed] [Google Scholar]
- 47. Serena T, Lee SK, Lam K, Attar P, Meneses P, Ennis W. The impact of noncontact, nonthermal, low‐frequency ultrasound on bacterial counts in experimental and chronic wounds. Ostomy Wound Manage. 2009;55(1):22‐30. http://www.ncbi.nlm.nih.gov/pubmed/19174586 Accessed September 15, 2014. [PubMed] [Google Scholar]
- 48. Wolcott RD, Kennedy JP, Dowd SE. Regular debridement is the main tool for maintaining a healthy wound bed in most chronic wounds. J Wound Care. 2009;18(2):54‐56. http://www.ncbi.nlm.nih.gov/pubmed/19418781. Accessed July 18, 2018. [DOI] [PubMed] [Google Scholar]
- 49. Wolcott RD, Rumbaugh KP, James G, et al. Biofilm maturity studies indicate sharp debridement opens a time‐ dependent therapeutic window. J Wound Care. 2010;19(8):320‐328. http://www.ncbi.nlm.nih.gov/pubmed/20852503. Accessed July 18, 2018. [DOI] [PubMed] [Google Scholar]
- 50. Mertz P, Davis S, Brewer L, Franzen L. Can antimicrobials be effective without impairing wound healing? The evaluation of a cadexomer iodine ointment. Wounds. 1994;6(6):184‐193. [Google Scholar]
- 51. Mertz PM, Oliveira‐Gandia MF, Davis SC. The evaluation of a cadexomer iodine wound dressing on methicillin resistant Staphylococcus aureus (MRSA) in acute wounds. Dermatol Surg. 1999;25(2):89‐93. http://www.ncbi.nlm.nih.gov/pubmed/10037509. Accessed January 31, 2017. [DOI] [PubMed] [Google Scholar]
- 52. Malone M, Johani K, Jensen SO, et al. Effect of cadexomer iodine on the microbial load and diversity of chronic non‐healing diabetic foot ulcers complicated by biofilm in vivo. J Antimicrob Chemother. 2017;72(7):2093‐2101. http://www.ncbi.nlm.nih.gov/pubmed/28402558. Accessed September 6, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bjarnsholt T, Kirketerp‐Møller K, Kristiansen S, et al. Silver against Pseudomonas aeruginosa biofilms. APMIS. 2007;115(8):921‐928. http://www.ncbi.nlm.nih.gov/pubmed/17696948. Accessed May 2, 2018. [DOI] [PubMed] [Google Scholar]
- 54. Metcalf D, Parsons D, Bowler P. A next‐generation antimicrobial wound dressing: a real‐life clinical evaluation in the UKand Ireland. J Wound Care. 2016;25(3):132, 134–138 http://www.ncbi.nlm.nih.gov/pubmed/26947693. Accessed November 29, 2016. [DOI] [PubMed] [Google Scholar]


