Abstract
Background
In the Mediterranean and Black Sea Region, arbovirus infections are emerging infectious diseases. Their surveillance can benefit from one health inter‐sectoral collaboration; however, no standardized methodology exists to study One Health surveillance.
Methods
We designed a situation analysis study to document how integration of laboratory/clinical human, animal and entomological surveillance of arboviruses was being implemented in the Region. We applied a framework designed to assess three levels of integration: policy/institutional, data collection/data analysis and dissemination. We tested the use of Business Process Modelling Notation (BPMN) to graphically present evidence of inter‐sectoral integration.
Results
Serbia, Tunisia and Georgia participated in the study. West Nile Virus surveillance was analysed in Serbia and Tunisia, Crimea‐Congo Haemorrhagic Fever surveillance in Georgia. Our framework enabled a standardized analysis of One Health surveillance integration, and BPMN was easily understandable and conducive to detailed discussions among different actors/institutions. In all countries, we observed integration across sectors and levels except in data collection and data analysis. Data collection was interoperable only in Georgia without integrated analysis. In all countries, surveillance was mainly oriented towards outbreak response, triggered by an index human case.
Discussion
The three surveillance systems we observed prove that integrated surveillance can be operationalized with a diverse spectrum of options. However, in all countries, the integrated use of data for early warning and inter‐sectoral priority setting is pioneeristic. We also noted that early warning before human case occurrence is recurrently not operationally prioritized.
Keywords: arboviruses, Crimean Congo Haemorrhagic Fever, One Health, surveillance, vector‐borne infections, West Nile virus
Impacts.
Tunisia, Serbia and Georgia are addressing the prevention and surveillance of arbovirus infections with a One Health/multidisciplinary approach (involving human, animal and entomological sectors) providing lessons learned for other contexts.
This multidisciplinary approach was assessed by identifying the inter‐sectoral integrations in the surveillance systems and by investigating processes and procedures across the sectors involved.
Business Process Modelling Notation provided a formalized, visual and easy to understand approach for the identification and analysis of the multidisciplinary interactions (triggering event, tasks sequence, interdependencies and potential outcomes) in the surveillance system.
1. INTRODUCTION
The Mediterranean Region, defined here as a vast area populated by over 500 million people and distributed in about 30 countries of Africa, Asia and Europe, (including those bordering with the Mediterranean Sea and the surrounding areas), is characterized by similar ecosystems and relevant vulnerability to climate change (Gualdi et al., 2013) that finally results in similar disease epidemiology as well as common priorities for disease prevention and control.
Surveillance and control of vector‐borne diseases is a well known priority for the Region (World Health Organization [WHO], 2005) and, more specifically, arbovirus infections are a recognized endemic or emerging priority in several countries that share favourable environmental drivers and climatic features (Failloux et al., 2017; Gasperi et al., 2012; Negev et al., 2015; Paz & Semenza, 2013; WHO, 2005).
Arbovirus infections are characterized by complex cycles that often involve both human and animal hosts and are transmitted by vectors whose fecundity and survival depend on environmental characteristics such as temperature, relative humidity and vegetation (McIntyre et al., 2017). For this reason, the surveillance and control of these infections are thought to benefit from inter‐sectoral collaborations involving, among others, human health, animal health and medical entomology.
The concept of One Health, as developed especially in the last decade, is defined as the collaborative effort of multiple disciplines to attain optimal health for people, animals and our environment (Grace, 2014; Marcotty et al., 2013; One Health Commission, 2015).
It is therefore not surprising that the One Health strategy is receiving attention in the Mediterranean Region where arboviral infections are prioritized.
Given the frequency of human pathogen emergence from animal reservoirs, the rationale behind the application of One Health, namely through the promotion of a more harmonized and integrated approach to monitor, investigate, plan and react to zoonotic disease risks, is considered promising (Bueno‐Marí, Almeida, & Navarro, 2015; Conrad, Meek, & Dumit, 2013; Faburay, 2015; Häsler et al., 2012).
One Health surveillance, or integrated surveillance, is the latest conceptual tool being proposed to prove the added value of the One Health concept and to ultimately reduce the risks of infectious diseases at the animal–human–ecosystem interfaces. One Health surveillance consists of the systematic collection, validation, analysis, interpretation of data and of the dissemination of the acquired information on humans, animals and the environment to inform decisions for more effective, evidence‐ and system‐based health interventions (Stärk et al., 2015). It is considered one of the four types of inter‐sectoral collaboration (the other three being: One Health to share and save operational costs; One Health risk mitigation programmes for endemic zoonotic diseases; and One Health activities to prevent zoonotic disease emergence and establishment), which can support improvements in technical and/or economic efficiency of One Health programmes (Häsler et al., 2012).
New interesting initiatives are ongoing (Network for Evaluation of One Health (NEOH) (2018); United States Agency–International Development (UNAIDS) 2018), but criteria and methods to describe and assess existing levels of integration of surveillance for specific pathogens to facilitate the evaluation of the impact and the added value of One Health, are still to be defined and tested (Baum, Machalaba, Daszak, Salerno, & Karesh, 2017).
This article describes the implementation of a situation analysis study, carried out in the framework of the MediLabSecure Project (MediLabSecure website http://www.medilabsecure.com), to contribute to the development of methods to assess One Health impact by assessing integration in surveillance of arbovirus infections in the Mediterranean Region. To perform this study, we used a set of criteria aimed at investigating processes and procedures underlying surveillance integration that we proposed in prior studies (Dente et al., 2016, 2018). We also tested the use of Business Process Modelling Notation (BPMN) to graphically present evidence of inter‐sectoral integration within surveillance systems for arboviral diseases.
2. METHODS
The situation analysis that we performed in the Mediterranean Region (hereby called the MeSA Study) is a qualitative study involving the human, animal and medical entomology sectors (hereby called sectors) of vector‐borne disease surveillance.
For this study, we refer to integrated surveillance as per the previously cited definition of One Health Surveillance provided by K.D.C. Stärk et al.
The objective of the MeSA study was to document how the integration of surveillance of arboviruses across sectors was being implemented in Mediterranean countries and to identify recurring elements enabling inter‐sectoral collaboration in diverse settings on the basis of a set of criteria, reported in Table 1.
Table 1.
Criteria to describe existing levels of integration between human, animal and entomological surveillance
| Level of integration | Sublevels of integration | Criteria |
|---|---|---|
| Policy and institutional level | Policy level |
1. Existence of a National policy addressing integrated surveillance for this specific exposure 2. Existence of a policy addressing integrated surveillance for this specific exposure at subnational level |
| Institutional level |
3. Existence of agreements among the institutions involved in human/animal/entomological surveillance for the specific exposure 4. Existence of a coordination mechanisms among the institutions involved 5. Existence of identified focal points for each of human/animal/entomological surveillance for the specific exposure |
|
| Data collection and analysis level | Interoperability mechanisms at data collection level |
6. Existence of integrated data collection tools 7. Existence of activation mechanisms of human surveillance based on signals from animal/entomological surveillance 8. Other interoperability mechanisms at data collection level |
| Interoperability mechanisms at data analysis level |
9. Presence of DB exchange/merging/other mechanisms to facilitate joint analysis among sectors 10. Performance of joint/integrated data analysis among the different surveillance sectors 11. Other interoperability mechanisms at data analysis level |
|
| Dissemination level | 12. Existence of joint result dissemination mechanisms (e.g. bulletins, reports, papers, media reports, websites) | |
Source: Dente et al. (2016).
In particular, this study was designed to enable investigators to describe how the collection, analysis and dissemination/exchange of information were organized within and between sectors for surveillance of arboviruses; to identify formal procedures and informal practices for integrated surveillance and inter‐sectoral collaboration; and to discuss main challenges and success stories. We studied three integrated surveillance systems in the Region: Serbia, Tunisia and Georgia.
The MeSA study was guided and performed by a team of investigators including experts from the Istituto Superiore di Sanità, the University of Cassino and from the Public Health Institutes/Ministries of Health of the countries involved.
The study was structured in four phases. Firstly, the three countries to involve in the study (hereby “participating countries”) were selected; secondly, available data and documents for each country were collected to build a country portfolio and common interview checklists were designed; thirdly, a site visit was performed in each participating country to investigate processes and procedures in the field of arbovirus surveillance integration between sectors and, finally, a report for each visited country was produced in agreement with all the involved national authorities.
We identified the following criteria for the selection of the participating countries (for details, refer to the Study design available in Study Reports (The MeSA Study Reports, 2018):
the three countries had to reflect the diversity of the Region;
the level of integration of surveillance in the country had to be high; this was assessed on the basis of a previous study (Dente et al., 2016);
the countries had to be willing to share national lessons learned and experiences;
there was internal national capacity to meet the study's organization requirements.
Each site visit included a briefing meeting involving the human health sector on the first day, site visits to laboratories and public health institutions of each concerned sector and a debriefing meeting involving representatives of all visited institutions on the last day.
Meetings with each concerned sector institution were organized with the support of the involved country experts responsible for human surveillance, who had the responsibility of engaging with, and ensuring the availability of, all stakeholders from the other sectors.
The investigators conducted the interviews with the support of a checklist that was specifically designed for the MeSA Study (see the annex to the Country Portfolio available in Study Reports (The MeSA Study Reports, 2018). The checklist was conceived to assess and document the level of integration between sectors, and the involvement and role of each sector in case of a potential outbreak. During the interviews, we proposed an early stage outbreak scenario and we marked down, in the checklist, the flow of information, data and communications within and between the involved sectors, as reported by the interviewed stakeholders.
A preliminary informal analysis of integrated surveillance was performed during the site visits and presented during the debriefing meetings by the investigator team. During each debriefing, the investigators shared the preliminary results based on the data collected with all the involved stakeholders in order to allow immediate amendments, integrations and further discussions.
In addition to describing the integrated surveillance systems on the basis of the information captured by investigators (informal analysis), we decided to apply a standardized comprehensive and well‐established method to discuss and share the processes with the stakeholders involved (hereby formal analysis). To this effect, we decided to test the applicability of one of the most widely accepted standards for process analysis and representation: the Business Process Modelling Notation (BPMN). Among the many Business Process Modelling (BPM) standards, BPMN provides a comprehensive and easy to read visual modelling methodology, and its shared representation techniques and symbols are specifically aimed at allowing a better understanding, analysis and dissemination of complex processes (Chinosi & Trombetta, 2012). Considering the informative power of the approach, and on the basis of previously successful experiences in using BPMN to represent and analyse health‐related processes (e.g. in Huang, Tseng, Hsu, Lee, & Chu, 2015), we thought that BPMN could be a potentially appropriate tool to study One Health surveillance.
Specifically, for this study we used BPMN to present visual evidence of inter‐sectoral integration within surveillance systems of arboviral diseases and to provide the description of the interactions between the various sectors involved. In particular, during the Georgian site visit, in which this approach was more comprehensively applied, we articulated the BPMN analysis in two phases. phase I: During the meetings mentioned above, we specifically asked questions to map intra‐ and inter‐sectoral activities including triggering events and expected results. A first draft of the BPMN diagram was then discussed among the researchers that had participated in the interviews comparing notes and acquiring a common understanding of the process. phase II: We scheduled a meeting with representatives of all the involved units (debriefing meeting) where we presented a comprehensive BPMN diagram, with all the interactions and flows (of information and materials) among all the sectors. With the visual diagram on the screen, the actors discussed the processes within and across sectors and validated the study findings.
This allowed us to assess the understanding of the visualizations produced with this method and its capacity to illustrate and capture the features and complexity of the integrated surveillance processes.
For the graphic presentation of the BPMN diagram, in order to simplify the BPMN notation, we highlighted only information flow among units of different sectors (hiding all the communication flows emerging from units in the same sector). We identified two types of information channels: bidirectional (the information trigger can have two sectors as input) and mono‐directional (one sector sending the info to one or more sectors).
3. RESULTS
On the basis of the identified selection criteria, Serbia and Tunisia were selected for their surveillance system of West Nile Virus (WNV) and Georgia for its surveillance system of Crimean Congo Haemorrhagic Fever (CCHF).
The three study visits were carried out between July and December 2016, and all the sectors and related institutions involved in the surveillance systems were met first individually and then collectively during the final debriefing meetings (Table 2).
Table 2.
Institutions involved in surveillance of arboviruses met in Serbia, Tunisia and Georgia
| Serbia |
| Ministry of Health—http://www.zdravlje.gov.rs/ |
| Ministry of Agriculture and Environmental Protection—http://www.eko.minpolj.gov.rs/en/ |
| Institute of Public Health of Serbia "Dr Milan Jovanović Batut” (Batut Institute) http://www.batut.org.rs/index.php?lang=2 |
| Institute of Virology, Vaccines and Sera Torlak—National Reference Laboratory for ARBO viruses and hemorrhagic fevers Torlak Institute)—http://www.torlakinstitut.com/en/home/index/Home |
| Institute for Biocides and Medical Ecology—http://www.biocidi.org.rs/ |
| Institute of Veterinary Medicine of Serbia Virology Department—http://nivs.rs/category/referentne-laboratorije/ |
| Scientific Veterinary Institute “Novi Sad”—http://niv.ns.ac.rs/?lang=en |
| Faculty of Agriculture, University of Novi Sad Laboratory for Medical and Veterinary Entomology—https://www.uns.ac.rs/index.php/en/laboratories/faculty-of-agriculture |
| Serbian Clinical of Infectious Disease of Human, Belgrade |
| Tunisia |
| Ministry of Health of Tunisia— http://www.santetunisie.rns.tn/fr/ |
| Primary Health Care Direction (DSSB) |
| Directorate for Environmental Health and Environmental Protection (DHMPE)—http://www.santetunisie.rns.tn/fr/direction-de-l%E2%80%99hygi%C3%A8ne |
| Observatory of New and Emerging Diseases (ONMNE)—http://www.onmne.tn/en/ |
| Ministère de l'Agriculture, des Ressources Hydrauliques et de la Pêche— http://www.agriculture.tn/ |
| Directorate of Veterinary Health (DGSV) |
| National Center for Animal Health Surveillance (CNVZ)—http://cnvz.agrinet.tn/index.php/fr/ |
| Veterinary Research Center of Tunis (IRVT)—http://www.irvt.agrinet.tn/index.php/fr/ |
| Pasteur Institute of Tunis— http://www.pasteur.tn/ |
| Regional Health Directorate of Monastir |
| Georgia |
| National Center for Disease Control and Public Health of Georgia (NCDC)—http://www.ncdc.ge/ |
| Laboratory of Lugar Center of NCDC (virology and entomology department)—http://www.ncdc.ge/en-US/LaboratoryNetworksAndBS |
| National Food Agency of Georgia (NFA)—Ministry of Agriculture—http://nfa.gov.ge/en/ |
| Laboratory of the Ministry of Agriculture—http://www.lma.gov.ge/index.php?lang=en&Itemid=107 http://www.moa.gov.ge/En/ |
The surveillance of human cases of WNVD was started in Tunisia in 2004 and in Serbia in 2012, following relevant outbreaks in humans. Human cases of CCHF were notified in Georgia since 2009, although the first relevant CCHF outbreak in humans occurred in 2014.
A synopsis of the information on the three systems collected through the MeSA is summarized in Table 3; additional information is available in the Study's Reports (The MeSA Study Reports, 2018).
Table 3.
The characteristics of the integrated surveillance systems for arbovirus infections in Serbia, Tunisia and Georgia
| Country | Arbovirus | inter‐sectoral collaboration (see also Table 4 and Figure 1) | Human surveillance | Animal surveillance | Vector surveillance |
|---|---|---|---|---|---|
| Serbia |
WNV The WNV surveillance system developed in Serbia after the first outbreak in humans in August 2012 (Petric et al., 2012; Petrović et al., 2014) |
The WNV outbreaks stimulated the establishment of an inter‐sectoral Committee in 2014 (which includes representatives of all the sectors involved in the WNV Surveillance and Response) on the basis of a Ministry of Health 2014 Law. The inter‐sectoral Committee is under the coordination of the Batut Public Health Institute and started to meet also for other emerging infections (e.g. in 2016 for Zika virus) Plans for WNV surveillance have been drafted since 2013 by the Ministry of Health (human surveillance) and by the Ministry of Agriculture (animals/vector surveillance), but officially released and funded from April 2014 (Petric et al., 2012). So far, these plans were released with distinct official communications. In 2016 the Ministry of Agriculture did not release the Plan due to other emerging priorities and lack of funds. The 2016 surveillance Plan for human cases has been released with identical procedures for endemic and not endemic areas |
Surveillance of human cases started in 2012 based on EC WNVD case definitiona (27/9/2012) WNVD is notifiable since 2016b, with notification due within 24 hr from laboratory confirmation. Measures for the safety of blood products are taken in line with the EU directive for blood safety. Enhanced Surveillance is triggered by the first human WNV case detected in the area. Enhanced Surveillance is activated 24 hr after the identification of a cluster of WNV human cases in the same area |
The Veterinary Directorate of the Ministry of Agriculture and Environmental Protection launched and funded the national WNV monitoring programme, Veterinary plus Entomological surveillance, starting from April 2014. Active surveillance includes: (a) serological testing of sentinel horses (ELISA WNV IgM Ab test) (b) testing on virus presence in samples of found dead/alive captured susceptible wild birds (RT‐PCR). Passive surveillance includes the following: (c) serological testing (paired sera samples) and testing of virus presence in samples of horses with clinical signs of neurological disorders. The active and passive surveillance encompassed all municipalities in the Republic of Serbia with distinction by endemicity areas: distribution of sampling points is determined based on risk assessment of exposure to WNV |
An Entomological Surveillance system reporting to the MoH was started in 2013 with a Programme financed by the MoH and the Beograd Municipality in Beograd and other urban areas in Serbia Active surveillance includes the following: (a) testing on virus presence in mosquito vectors (RT‐PCR) in risky areas In 2014 and 2015, the Faculty of Agriculture, University of Novi Sad Laboratory for Medical and Veterinary Entomology (LME) was indirectly involved (subcontracted by Scientific Veterinary Institute of Novi Sad)in Ministry of Agriculture National WNV surveillance programme |
| Tunisia |
WNV After the epidemic in humans in 2003, the WNV surveillance system was enhanced with a multidisciplinary approach (human, veterinarian and entomological surveillance) (Observatoire National des Maladies Nouvelles et Emergentes, 2012) |
The WNV multidisciplinary Surveillance Committee, in place since 2008, has been reinforced especially for the assessment of risk. In case of emergency, many stakeholders can take part in the SC including the Ministry of Interior and the Ministry of Equipment. An integrated 2010–2015 strategic plan for WNV surveillance has been developed. In 2015, it focused mainly on human surveillance activities. The plan is developed at national level but the Regions are free to adapt it to local needs |
Human surveillance for meningitis and meningoencephalitis (WNVD) due to WNV aims at the early detection of WNV circulation. It is based on the notification of any clinically suspected cases of WNVD (national case definition)c from all hospitals (public and private) to the Observatory of New and Emerging Diseases (ONMNE). The surveillance is activated every year from 1 April to 30 November (Circulaire no 36 of 27/06/2011) |
On the regulatory level, WNV is on the list of notifiable diseases in Tunisia.d Surveillance/monitoring is based on risk assessments (areas at risk of epidemics) for WNV as for other diseases and is coordinated by the national centre for animal surveillance (Centre National de Veille Zoosanitaire—CNVZ) To be noted, at the time of the study, WNV disease had been notified only in one horse in Tunisia (in 2015), although research has shown viral circulation in animals, and very high serological prevalence in equidae. Horses resulted negative to suspected rabies are tested for WNV. Active surveillance on migratory birds is in place |
Entomological surveillance for WNV is not presently implemented in Tunisia. However, non‐disease‐specific entomological monitoring is carried out under the Directorate for Environmental Health and Environmental Protection (DHMPE) of MoH to: (a) Categorize governorates according to their vulnerability to WNV potential vectors, (b) Manage critical situations (transmission of WNV), (c) Predict epidemics (Early Warning System), (d) Promote the rational use of insecticides. In coordination with DHMPE, Institut Pasteur provides entomological expertise and training, implements entomological surveillance and vector control in collaboration with relevant stakeholders. The Veterinary Research Center of Tunis implements entomological surveillance and provides data to ONMNE |
| Georgia |
CCHFV CCHF surveillance started in Georgia in 2009, when the disease reporting tool, the EIDSS, was established nationally.e |
Since 2009, for surveillance purposes, Center and public health municipal units are using the Electronic Integrated Disease Surveillance System (EIDSS). It provides real‐time biosurveillance throughout Georgia and is being used as a registration, notification and reporting system for the notifiable diseases/conditions of human cases, and also for veterinary diseases (by the respective structural units of the Ministry of Agriculture). The EIDSS has 190 data entry points that constitute the Public Health Network of Georgia. 72 notifiable diseases are under surveillance within the system |
Suspected CCHF cases are notified to the National Center for Disease Control (NCDC) based on a national case definition |
Animal surveillance is not conducted routinely for CCHFV. During the 2014 epidemic in humans, National Food Agency state veterinarians collected samples from the cows of infected owners. The laboratory of the Ministry of Agriculture performed PCR on 2,221 collected samples which resulted all negative. Tests for serology were, and are, not available. State veterinary surveillance includes clinical inspection of animals at slaughterhouses but does not include sample collection and laboratory analysis of animals |
The Entomology Department of Lugar Center (NCDC) is in charge for vector monitoring: (a) seasonal monitoring of ticks (early spring–late summer) as per the field surveillance plan prepared by NCDC and approved by MoLSA. In endemic areas, ticks are tested for CCHFV. (b) Ad hoc monitoring: during outbreak investigations triggered by any CCHF human case. Monitoring and control plans are developed by the National Food Agency with support of the MoA. Vector control actions are implemented in case of human infection as well as in villages where human cases were notified the previous year. Villages at risk and villages with human cases in the previous years, to avoid re‐emergence, are the target each year |
eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012D0506&qid=1428573336660&from=EN#page=38
under the law on protection of the population against communicable diseases, Official Gazette of the RS no 15/2016.
Decree No. 2009– 22000 of 14 July 2009, establishing the nomenclature of regulated animal diseases and enacting the general measures applicable to these diseases, amended by Decree No. 2010–1207 of 24 May 2010.
3.1. Level of integration of the surveillance systems
As reported in Table 4, the analysed surveillance systems show some degree of integration for all except two identified levels. The data collection level was interoperable across sectors only in Georgia with the Electronic Integrated Disease Surveillance System (EIDSS) established nationally in 2009. No country was interoperable at the data analysis level.
Table 4.
Level of integration for surveillance of arbovirus infections in Serbia, Tunisia and Georgia
| Level of integration | Sublevels of integration | Criteria | ||
|---|---|---|---|---|
| Serbia‐WNV | Tunisia‐WNV | Georgia‐CCHFV | ||
| Policy and institutional level | Policy level |
‐Legislation issued (2014) by the Ministry of Health has created an inter‐sectoral Committee in order to share information across sectors to recognize early circulation of WNV and make decisions (coordination/communication role of the PH sector) ‐ National and district level projects supported financially by the Ministry of Agriculture and by the Ministry of Health have sustained inter‐sectoral integration of entomological with veterinary and human surveillance of WNV. ‐A unique reporting system legislation for entomological and veterinary surveillance is in place ‐A strategic plan was developed after 2014 epidemic |
‐ Legislation issued by the Ministry of Health (2004) has created inter‐sectoral committees at regional/local level in order to rapidly respond to WNV human cases ‐ Human Health and Entomology both refer to the Ministry of Health and show coordination at central and subcentral levels. ‐A strategic plan for WNV control with protocols for all sectors (not backed by formal legislation) is available. |
‐Legislation issued by the Government (2015) has created the One Health inter‐sectoral committee at national level. ‐Human Health and Entomology refer both to the Ministry of Health. ‐Presence of a strategic plan developed after the CCHF epidemic in 2014 which was, at the time of the study, being developed in a generic preparedness plan |
| Institutional level |
‐Presence of formal institutional collaboration mechanisms within sectors (e.g. bilateral agreements in place for the entomological surveillance in Vojvodina province‐northern Serbia) and of informal collaboration mechanisms (across sectors). ‐Existence of identified focal points for each of sector |
‐Presence of informal collaboration mechanisms (across sectors and within the human health sector) ‐Presence of formal institutional collaboration mechanisms with other sectors (e.g. role of regional councils) ‐Existence of identified focal points for each of sector |
‐Presence of informal collaboration mechanisms (across sectors and within the human health sector) ‐Presence of formal institutional collaboration mechanisms within other sectors (as during the 2014 outbreak). ‐Existence of identified focal points for each of sector |
|
| Data collection and analysis level | Interoperability mechanisms at data collection level |
‐Data sharing is in place within sectors with distinct databases. A unique web‐based database across all administrative level exists for veterinary surveillance since 2013 |
‐A database on animal data at Directorate General of Veterinary Health (Ministry of Agriculture) |
‐An Electronic Integrated Disease Surveillance System (EIDSS) is available across all sectors |
| Interoperability mechanisms at data analysis level | ‐Not available | ‐Not available | ‐Potential with the EIDSS, but presently used across human epi and virology | |
| Dissemination level | ‐Information and weekly reports are shared across sectors. Each institution might deliver information to the public autonomously | ‐Information and reports are shared across sectors during coordination meetings (e.g. periodic meetings of permanent committee for vector control). An integrated annual report, including the annual report of the Directorate General of Veterinary Health, is published regularly on the website of the MoH. | ‐Information and reports are shared across sectors during One Health Meetings organized by NCDC every 3 months | |
3.2. Interactions between sectors in the surveillance systems
The informal analysis is fully described in the Study Reports; the formal analysis presented in Georgia is available in Figure 1.
Figure 1.
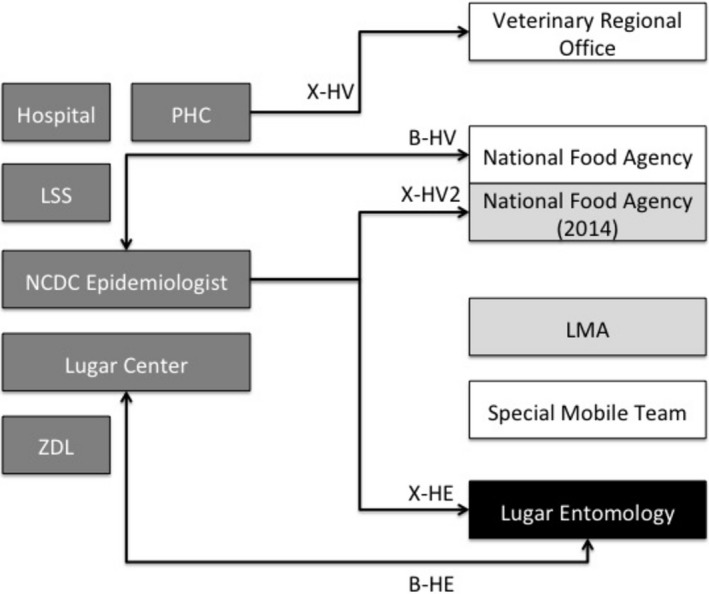
Inter‐sectoral interactions as per BPMN visualization. We highlighted only information flow among units of different sectors (hiding all the communication flows emerging from units in the same sector). We identified five different information channels: 2 are bidirectional (meaning the information trigger can have both the units as input and are identified by the first letter “B”) and 3 are mono‐directional (identified by the initial letter “X”). In details: B‐HV is a bidirectional flow between Human (H) and Veterinarian sector (V); B‐HE is a bidirectional between Human (H) and Entomological sector (E); X‐HV is an information flow originating from Human sector and targeting the Veterinarian one; X‐HV2 is another information flow originating from Human sector and targeting the Veterinarian one; X‐HE is an information flow originating from Human sector and targeting the Entomological one. LSS: Local health authorities; LMA: Laboratory of the Ministry of Agriculture; NCDC: National Centre for Disease Control; NFA: National Food Agency; PHC: Primary Health Care; ZDL: Zonal health authorities [Colour figure can be viewed at http://wileyonlinelibrary.com]
During the debriefing, all experts found the BPMN readily understandable and useful in guiding detailed discussions on the processes described.
Both the informal and formal analyses highlight that collaboration between sectors is well established. However, the inter‐sectoral interactions aimed at early warning, communication, data exchanging and analysis are often emergent and not consolidated by shared procedures and tools. This reflects the general finding that sector‐specific internal communication flows are more structured than inter‐sectoral interactions.
Using BPMN in Georgia (Figure 1), we identified 6 main players (defined “lanes”) for the Human health sector comprising the following clinical, laboratory and public health actors: Hospital, Primary Health Care (PHC); Local (LSS) and Zonal (ZDL) health authorities, National Centre for Disease Control (NCDC) Epidemiologists, Lugar Centre. For the Veterinary Surveillance sector, we identified 4 lanes: the National Food Agency (NFA), the Laboratory of the Ministry of Agriculture (LMA), the Veterinary Regional Office, and the Special Mobile Team. Finally, we identified one lane for the entomology sector through interviews conducted at the Lugar Centre integrated with documentation and comments provided during the debriefing meeting at the NCDC. A brief process description by lane is available in the Study Report. We identified five different information channels: two are bidirectional (meaning the information trigger can have both the units as input and are identified by the first letter “B”) and three are mono‐directional (identified by the initial letter “X”).
3.3. Recurrent characteristics of the three surveillance systems
The Study has highlighted that some features are common across all the three countries. In particular:
Animal and entomological surveillance are integral part of the systems, but the central role of the human surveillance is underlined by several factors:
it is always the detection/notification of suspected human case/s that triggers the response of the systems and starts the flow of communication between sectors;
a strategic plan and a multisector committee have been established in the three countries and are always under the coordination of the Directorates in charge for human surveillance;
the human sector can be delegated by the other sectors in the dissemination of data and information and in the communication to the public, but the opposite is rare.
The multisector committee is perceived as a strategy to reinforce integration and, even if the committee might be established for specific pathogen/s, in all three countries we found it was called again for any threat that may need an integrated approach, including preparedness.
In all the three countries, the surveillance strategy includes distinction by endemicity areas: Distribution of sampling points and monitoring are determined by risk assessment of exposure (areas at risk of epidemics).
Medical entomology activities are under the responsibility of more than one Institution (Ministry of Health, Ministry of Agriculture, Research Institutes/Universities), with relevant tasks in terms of planning and coordination.
4. DISCUSSION
As reported, we based this study on a set of criteria from a conceptual framework aimed at describing and assessing surveillance integration.
The checklist, developed for the interviews on the basis of these criteria, allowed us to carry out structured interviews which provided information able to describe the systems. By analysing, with all concerned actors, procedures and processes in place we began understanding the drivers that led to the systems being as they are.
The BPMN methodology, fully tested in Georgia, led to the development of a graphical representation that was easily and univocally understandable and conducive to a detailed and clear discussion among all the involved actors/institutions about their processes, especially the inter‐sectoral ones. Specifically, in the Georgian example, we found that the human health sector was the largest sector of the integrated surveillance system and the one with the majority of inter‐sectoral interactions with the other existing sectors (veterinarian and entomological). This is in line with the fact that CCHF is commonly perceived as a predominantly human health issue, because animals do not show symptoms of infection.
This study showed that the diagram can be used to investigate One Health surveillance processes and procedures. However, we also found that during the debriefing the diagram was used as a nexus for the negotiation of meanings: Each actor was able to see and understand every activity performed by each sector, also discussing in detail if and when their interactions would start or end (and under which conditions). The BPMN was adopted in the study as a co‐production methodological approach, where every actor was able to reach a deeper understanding of its direct and indirect interactions with every other unit, reducing the risks of misunderstandings and misalignment.
Our findings suggest that its use on a broader scale could allow investigators to produce standardized and easily understandable diagrams. Considering prior applications of this methodology to other sectors, BPMN could be a way to describe and compare different integrated surveillance systems, also including quantitative process assessments.
The analysis of the three systems suggests that integration in surveillance, although conceived in accordance with specific components and criteria, is operationalized with a spectrum of options, where not all components need to be always in place. The health system organization, the resources available and the local epidemiology of the disease under surveillance all influence the final architecture of this integration.
The type and number of institutions and stakeholders in charge for surveillance in the countries, and therefore involved in the study (Table 2), give the idea of the complexity of relations and interactions involved in the integration. As we already pointed out, step‐up of inter‐sectoral regional capacities in the face of emerging viral threats is a methodological, rather than disease‐driven approach (Escadafal et al., 2016), which may lead to much greater efficiencies in the long term, though it may dilute efforts, increase costs and complicate capacity building in the immediate term (WHO, 2016).
Therefore, it is understandable that countries retain and consolidate those integration components that proved to improve their surveillance systems and may enhance long‐lasting cost‐effectiveness also in the light of their national health systems, local situations and available resources.
Notwithstanding this driver‐driven diversity, we observed characteristics which were recurrently present in all the integrated surveillance systems we studied.
One of those was the establishment of a multisector committee. This seems an indication of the willingness for the implementation of an inter‐sector coordinated surveillance and response system and a possible opportunity to harmonize activities and programmes to increase efficiency. It provides a formally established environment for the exchange of information and a way to strengthen existing informal contacts (telephone calls, emails) that can favour rapid exchanges for early warning purposes between sectors. In addition, we found evidence that multisector committees originally established for one arboviral disease can rapidly be called to inter‐sectorally manage other emerging arboviral diseases, as was the case for the management of the Zika virus alert.
The elaboration of surveillance plans released annually was another recurring element that shows the effort of identifying targets, schedules, protocols and resources and of strengthening integration procedures between sectors. Although plans might be not always annual, due to scarce resources and additional priorities, acquired inter‐sector procedures seem to last and consolidate in new priorities.
We found evidence of how the erratic availability of resources may force towards “coping strategies.” For example, leading to the choice of conducting risk assessments instead of setting up an early warning system based on entomological surveillance (i.e. virus detection in vectors for early warning ahead of human case occurrence) to guide public health actions. These risk assessments were based on at‐risk areas identified and monitored through vector mapping (i.e. presence/absence of the vectors; breeding sites) and/or geo‐localized data on human cases occurring in the previous years.
We also observed what appear to be common challenges for the systems in the three countries. While early warning for timely action is typically identified as an area for which integration could provide an added value, it is recurrently not prioritized as one of the main aspects of integration.
Although the level of risk of an outbreak occurrence might have been assessed high, institutional and integrated operative measures are generally triggered by the first human case. For example, in Serbia where entomological monitoring activities for WNV date back to 2005 in Vojvodina province in the north of the country, with a programme supported by the Ministry of Education and Science and Ministry of Agriculture, a well‐structured integrated surveillance system for WNV was set up only when the first human outbreak of WNVD occurred in 2012. Response also starts typically upon confirmation of a human case/s (what we could call a sentinel human) in the countries we studied. This is performed in the framework of an outbreak investigation aiming to assess the number of people involved and the geographical area/s affected through active case finding and an entomological investigation (of breeding sites and vector presence and density). This reflects on the integration between sectors, which appear more consolidated towards response oriented activities (including vector control) compared with surveillance and early warning oriented ones.
The establishment of an interoperable data collection system, like the EIDSS in Georgia, seems the first step to promote data sharing between sectors, but this remains a rare feature in the Mediterranean and Black Sea Region, as we recently highlighted (Dente et al., 2016). Even when well established, like in Georgia, the use of these data for integrated early warning, analysis, inter‐sectoral priority setting and multisector risk assessments is still pioneeristic.
Because both WNV and CCHF cause human disease and death, but preventable (WNV) or absent (CCHF) animal disease, early warning is a priority mostly for human surveillance and this finally determines the relevant role of the human sector in the surveillance of these pathogens. Probably because of this perceived importance for humans, the human sector is also predominant in driving integration, as showed by its coordinating role in the multisector committee and in communicating to the public.
In our view, enhancing identification of common risks and priorities across sectors would help to better define the role and relevance of each sector. For example, animal surveillance activities for diseases with perceived low or absent impact on animal health or as secondary priorities for early warning are either not prioritized or prioritized for a limited time (e.g. after a major outbreak).
If each sector could perceive the global impact of a disease at national level, beyond their specific sector, roles and resources could be allotted to inter‐sectoral priorities. For example, to ensure early warning through animal surveillance for a national priority pathogen which has no impact on animal health could be considered as essential as early warning to prevent human cases.
The selection of arbovirus infections (WNV and CCHF) particularly relevant for humans might have misrepresented the results and therefore constitute a limit of this study. An additional limit might be the testing of the BPMN: Although the method was used for the analysis of all the three systems, the obtained visualizations were shared only with the Georgian stakeholders.
5. CONCLUSION
Surveillance is at the core of all public health activities and is essential to prevent, detect and respond to health threats effectively (M’ikanatha & Iskander, 2015). It is conceivable that One Health surveillance may lead to faster disease detection, more efficient disease control and tangible financial savings when formally compared against separated surveillance streams especially in the case of arbovirus infections (Stärk et al., 2015).
To contribute to the need of describing and assessing integrated surveillance systems, we designed and implemented the MeSA study on the basis of a previously developed ad hoc conceptual framework with specific criteria.
The analysis of system‐wide levels of integration was complemented by assessing the type of interactions, both within and between sectors, with the support of the BPMN methodology. For this study, this method provided us a set of tools for a visual, easily understandable and shared description of the ownership of the tasks involved in the process, their sequence, their interdependencies, as well as their triggering points (what activates a task) and relative output (what is the expected result of every task). We deem that BPMN could represent a valuable analytical tool for reducing the risk of gap activities (that have no clear owner) and overlapping tasks, easing a seamless coordination of inter‐sector and intrasector interdependencies.
The operationalization of One Health in terms of inter‐sectoral data collection and analysis that are strategic for early warning and risk assessments was shown to be particularly challenging.
These are the areas in which multisectoral integration needs to be further developed. This will promote data sharing and analysis across sectors and increase awareness on inter‐sectoral priorities, including cross‐border ones.
CONFLICT OF INTEREST
The authors have no conflict of interests to declare.
ACKNOWLEDGEMENTS
We are particularly thankful to Dr. Dragan Ilic (Institute of Public Health of Serbia “Dr Milan Jovanović Batut”), unexpectedly passed away during this study, for his motivation and support since the starting of our collaboration in 2007. We are also glad to Alessia Ranghiasci e Gloria Nacca for their constant support to the implementation of the study. This study was conducted in the framework of MediLabSecure Project (European Union ‐DEVCO: IFS/21010/23/‐194).
APPENDIX 1.
MeSA Working Group
Vesna Knjeginic, Borka Stojkovic (Ministry of Health of Serbia), Tatjana Labus (Veterinary Directorate, Ministry of Agriculture), Vesna Milicevic, Ljubisa Veljovic, Jelena Maksimovic‐Zoric (Institute of Veterinary Medicine Belgrade, Virology Department), Vera Stoiljkovic, Svetlana Filipovic Vignjevic, Jelena Protic (Institute of Virology, Vaccines and Sera Torlak), Marija Zgomba, Dusan Petric (Faculty of Agriculture, University of Novi Sad), Dragana Despot, Branislav Pesic, Katarina Serovic, Ivan Aleksic, Ivana Djuric (Institute for Biocides and Medical Ecology), Dragan Ilic, Svetlana Vrga, Ljiljana Pavlovic, Dragana Plavsa, Edita Grego (Institute of Public Health of Serbia "Dr Milan Jovanović Batut"); Kaouther Harabech (Ministère de la Santé Publique/Direction des soins de santé de base, Tunis—Tunisia), Nissaf Ben Alaya and Souha Bougatef—National Observatory of New and Emerging Deseaes/Observatoire national des maladies nouvelles et émergentes (ONMNE); Henda Triki and Ali Bouattour—Pasteur Institute of Tunis/Institut Pasteur de Tunis (IPT); Mohamed Rebhi, Jabeur Daaboub and Lamia Somai—Directorate for Environmental Health and Environmental Protection/Direction de l'hygiène du milieu et de protection de l'environnement (DHMPE); Malek Zerlli, Kaouther Oukaili and Heni Haj Ammar—General Directorate of Veterinary Health/Direction Générale des Services Vétérinaires (DGSV); Chedia Sghaier, Naouel Fatnassi, Sana Kalthoum, Anissa Dhaouadi—National Center for Animal Health Surveillance/Centre national de veille zoosanitaire; Abdelhak Ben Youness—Veterinary Research Institute of Tunis/Institut de recherche vétérinaire de Tunis; Issam Mahale, Mongi Marzouk, Sassi Ben Bdira, Samia Grira, Sonia Ayadi, Maha Mastouri, Allad Faten, Ben Abdelkader (Regional Health Directorate of Monastir); Giorgi Chakhunashvili, Giorgi Babuadze, Nana Mamuchishvili, Gvantsa Chanturia, Ekaterine Adeishvili (National Center for Disease Control and Public Health), Lasha Avaliani, Lena Ninidze, Natia Kartskhia (National Food Agency), Ana Gulbani, Maka Kokhreidze, Marina Donduashvili, Anna Kekelidze (Laboratory of the Ministry of Agriculture).
Dente MG, Riccardo F, Bolici F, et al. Implementation of the One Health approach to fight arbovirus infections in the Mediterranean and Black Sea Region: Assessing integrated surveillance in Serbia, Tunisia and Georgia. Zoonoses Public Health. 2019;66:276–287. 10.1111/zph.12562
Contributor Information
Maria Grazia Dente, Email: mariagrazia.dente@iss.it.
the MeSA Working Group:
Vesna Knjeginic, Borka Stojkovic, Tatjana Labus, Vesna Milicevic, Ljubisa Veljovic, Jelena Maksimovic‐Zoric, Vera Stoiljkovic, Svetlana Filipovic Vignjevic, Jelena Protic, Marija Zgomba, Dusan Petric, Dragana Despot, Branislav Pesic, Katarina Serovic, Ivan Aleksic, Ivana Djuric, Dragan Ilic, Svetlana Vrga, Ljiljana Pavlovic, Dragana Plavsa, Edita Grego, Dr Milan Jovanović Batut, Kaouther Harabech, Ministère de la Santé Publique, Nissaf Ben Alaya, Souha Bougatef, Henda Triki, Ali Bouattour, Mohamed Rebhi, Jabeur Daaboub, Lamia Somai, Malek Zerlli, Kaouther Oukaili, Heni Haj Ammar, Chedia Sghaier, Naouel Fatnassi, Sana Kalthoum, Anissa Dhaouadi, Abdelhak Ben Youness, Issam Mahale, Mongi Marzouk, Sassi Ben Bdira, Samia Grira, Sonia Ayadi, Mastouri, Allad Faten, Ben Abdelkader, Giorgi Chakhunashvili, Giorgi Babuadze, Nana Mamuchishvili, Gvantsa Chanturia, Ekaterine Adeishvili, Lasha Avaliani, Lena Ninidze, Natia Kartskhia, Ana Gulbani, Maka Kokhreidze, Marina Donduashvili, and Anna Kekelidze
REFERENCES
- Baum, S. E. , Machalaba, C. , Daszak, P. , Salerno, R. H. , & Karesh, W. B. (2017). Evaluating one health: Are we demonstrating effectiveness? One Health, 3, 5–10. 10.1016/j.onehlt.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno‐Marí, R. , Almeida, A. P. G. , & Navarro, J. C. (2015). Editorial: Emerging zoonoses: Eco‐epidemiology, involved mechanisms, and public health implications. Front Public Health, 3, 157 10.3389/fpubh.2015.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinosi, M. , & Trombetta, A. (2012). BPMN: An introduction to the standard. Computer Standards & Interfaces, 34(1), 124–134. https://pdfs.semanticscholar.org/7204/efae043bb9b338f63982a667998e66d6bd40.pdf [Google Scholar]
- Conrad, P. A. , Meek, L. A. , & Dumit, J. (2013). Operationalizing a One Health approach to global health challenges. Comparative Immunology, Microbiology and Infectious Diseases, 36, 211–216. 10.1016/j.cimid.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Dente, M. G. , Riccardo, F. , Nacca, G. , Ranghiasci, A. , Manuguerra, J.‐C. , Escadafal, C. , Declich, S. (2016). Strengthening integrated surveillance for arboviruses in the Mediterranean and Black Sea regions in the framework of the One Health approach . Quaderni Della Società Italiana Di Medicina 19tropicale E Salute Globale N. 1, 498. Retrieved from http://www.simetweb.eu/document/3847.
- Dente, M. G. , Riccardo, F. , Nacca, G. , Ranghiasci, A. , Escadafal, C. , Gaayeb, L. , … Declich, S. (2018). Strengthening preparedness for arbovirus infections in Mediterranean and Black Sea countries: A conceptual framework to assess integrated surveillance in the context of the One Health Strategy. International Journal of Environmental Research and Public Health, 15, 489 10.3390/ijerph15030489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escadafal, C. , Gaayeb, L. , Riccardo, F. , Pérez‐Ramírez, E. , Picard, M. , Dente, M. G. , Robert, V. (2016). Risk of Zika virus transmission in the Euro‐Mediterranean area and the added value of building preparedness to arboviral threats from a One Health perspective. BMC Public Health 16(1),1219 10.1186/s12889-016-3831-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faburay, B. (2015). The case for a “one health” approach to combating vector‐borne diseases. Infection Ecology & Epidemiology, 5, 10.3402/iee.v5.28132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failloux, A. B. , Bouattour, A. , Faraj, C. , Gunay, F. , Haddad, N. , Harrat, Z. , … Robert, V. (2017). Surveillance of arthropod‐borne viruses and their vectors in the Mediterranean and Black Sea regions within the MediLabSecure Network. Current Tropical Medicine Reports, 4, 27 10.1007/s40475-017-0101-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperi, G. , Bellini, R. , Malacrida, A. R. , Crisanti, A. , Dottori, M. , & Aksoy, S. (2012). A new threat looming over the Mediterranean Basin: Emergence of viral diseases transmitted by Aedes albopictus mosquitoes. PLoS Neglected Tropical Diseases, 6(9), e1836 10.1371/journal.pntd.0001836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace, D. (2014). The business case for One Health. Onderstepoort Journal of Veterinary Research 81(2), Art. #725, 6 pages. 10.4102/ojvr.v81i2.725. [DOI] [PubMed] [Google Scholar]
- Gualdi, S. , Somot, S. , Li, L. , Artale, V. , Adani, M. , Bellucci, A. , Braun, A. , … Navarra, A. (2013). The CIRCE simulations: regional climate change projections with realistic representation of the Mediterranean Sea . American Meteorological Society, http://journals.ametsoc.org/doi/abs/10.1175/BAMS-D-11-00136.1. [Google Scholar]
- Häsler, B. , Gilbert, W. , Jones, B. A. , Pfeiffer, D. U. , Rushton, J. , & Otte, M. J. (2012). The economic value of one health in relation to the mitigation of zoonotic disease risks. Current Topics in Microbiology and Immunology, 365, 127–151. 10.1007/82_2012_239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, W. R. , Tseng, R. C. , Hsu, Y. F. , Lee, T. Y. , & Chu, W. C. (2015). Using BPMN to model a patient safety promulgation service based on a clinical process. Proceedings of Engineering and Technology Innovation, 1, 36–39. [Google Scholar]
- M’ikanatha, N. M., Iskander, J. (2015). Surveillance as a foundation for infectious disease prevention and control Concepts and methods in infectious disease surveillance (1st ed.). Hoboken, NJ: Wiley‐Blackwell. [Google Scholar]
- Marcotty, T. , Thys, E. , Conrad, P. , Godfroid, J. , Craig, P. , Zinsstag, J. , … Bouleard, M. (2013). Intersectoral collaboration between the medical and veterinary professions in low‐resource societies: The role of research and training institutions Comparative Immunology. Microbiology and Infectious Diseases, 36(2013), 233–239. 10.1016/j.cimid.2012.10.009 [DOI] [PubMed] [Google Scholar]
- Network for Evaluation of One Health (NEOH) (2018). Retrieved from http://neoh.onehealthglobal.net/
- McIntyre, K. M. , Setzkorn, C. , Hepworth, P. J. , Morand, S. , Morse, A. P. , & Baylis, M. (2017). Systematic assessment of the climate sensitivity of important human and domestic animals pathogens in Europe. Scientific Reports, 7, Article Number, 7134 10.1038/s41598-017-06948-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MeSA (2018). MeSA Study Reports . Retrieved from http://www.medilabsecure.com/events_mesa.html.
- Observatoire National des Maladies Nouvelles et Emergentes (2012). Bulletin n.2 2012. Retrieved from http://www.onmne.tn/fr/images/Bulletin2WN.pdf.
- Negev, M. , Paz, S. , Clermont, A. , Noemie Groag, P.‐O. , Uri, S. , Yeger, T. , & Green, M. S. (2015). Impacts of climate change on vector borne diseases in the Mediterranean Basin — Implications for preparedness and adaptation policy. International Journal of Environmental Research and Public Health, 12, 6745–6770. 10.3390/ijerph120606745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- One Health Commission (2015). Retrieved from https://www.onehealthcommission.org/
- Paz, S. , & Semenza, J. C. (2013). Environmental drivers of West Nile Fever Epidemiology in Europe and Western Asia—A review. International Journal of Environmental Research and Public Health, 10, 3543–3562. 10.3390/ijerph10083543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petric, D. , Cvjetkovic, I. H. , Radovanov, J. , Cvjetkovic, D. , Jerant Patic, V. J. , Milosevic, V. , … Sánchez‐Seco, M. P. (2012). West Nile virus surveillance in humans and mosquitoes and detection of cell fusing agent virus in Vojvodina province (Serbia). Healthmed 6(2),462–468, http://niv.ns.ac.rs/StariSajt/tr31084/fajlovi/12/11.pdf. [Google Scholar]
- Petrović, T. , Šekler, M. , Petrić, D. , Lazić, S. , Lupulović, D. , Lazić, G. , … Plavšić, B. (2014). West Nile virus Surveillance Programme in Serbia Arhiv veterinarske . Medicine, 7(2), 29–45 https://www.cabdirect.org/cabdirect/abstract/20153323036. [Google Scholar]
- Stärk, K. D. C. , Kuribreña, A. M. , Dauphin, G. , Vokaty, S. , Ward, M. P. , Wieland, B. , & Lindberg, A. (2015). One Health surveillance ‐ More than a buzz word? Preventive Veterinary Medicine, 120(1), 124–130. 10.1016/j.prevetmed.2015.01.019 [DOI] [PubMed] [Google Scholar]
- United States Agency–International Development (UNAIDS) (2018). One health multisectoral self‐assessment tool. Retrieved from http://preparednessandresponse.org/self-assessment-feedback/. [Google Scholar]
- World Health Organization (2016). Zika Strategic Response Plan. WHO/ZIKV/SRF/16.3 © World Health Organization Retrieved fromhttp://apps.who.int/iris/bitstream/10665/246091/1/WHO-ZIKV-SRF-16.3-eng.pdf.
- World Health Organization (2005). Mediterranean Zoonoses Control Programme (MZCP) of the World Health Organization . Presented at the 16th JCC-MZCP in Athens 20-22 December 2005. Retrieved from http://www.who.int/zoonoses/institutions/MZCP_workplan.pdf?ua=1.


